Microstructural Evolution and Gas-Tight Properties of Yttria-Stabilized Zirconia/Crofer 22H Stainless Steel Brazed Joints with the Ag-Ge-Si Filler for Use in Solid-Oxide Fuel Cells
Abstract
:1. Introduction
2. Material and Experimental Procedures
2.1. Brazing Filler Preparation
2.2. Characterization of the Brazing Filler
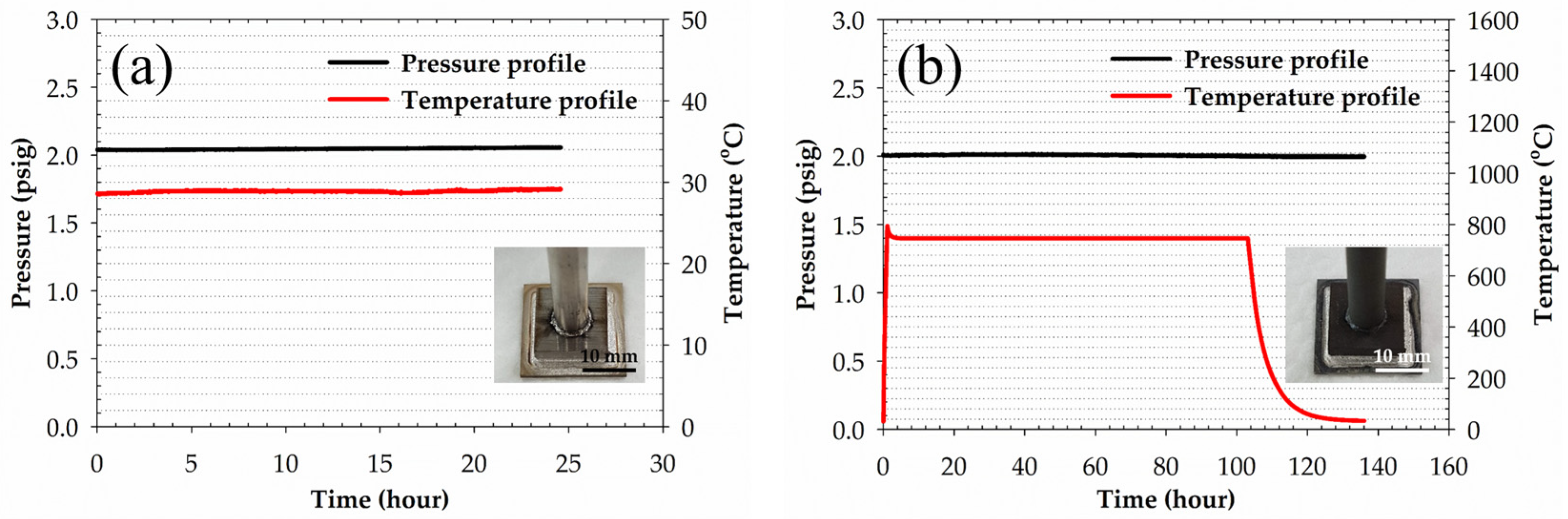
2.3. Wetting Tests and Brazing of the YSZ Electrolyte and Crofer 22H Stainless Steel
2.4. The Microstructural Observation of YSZ/Crofer 22H Brazed Joints
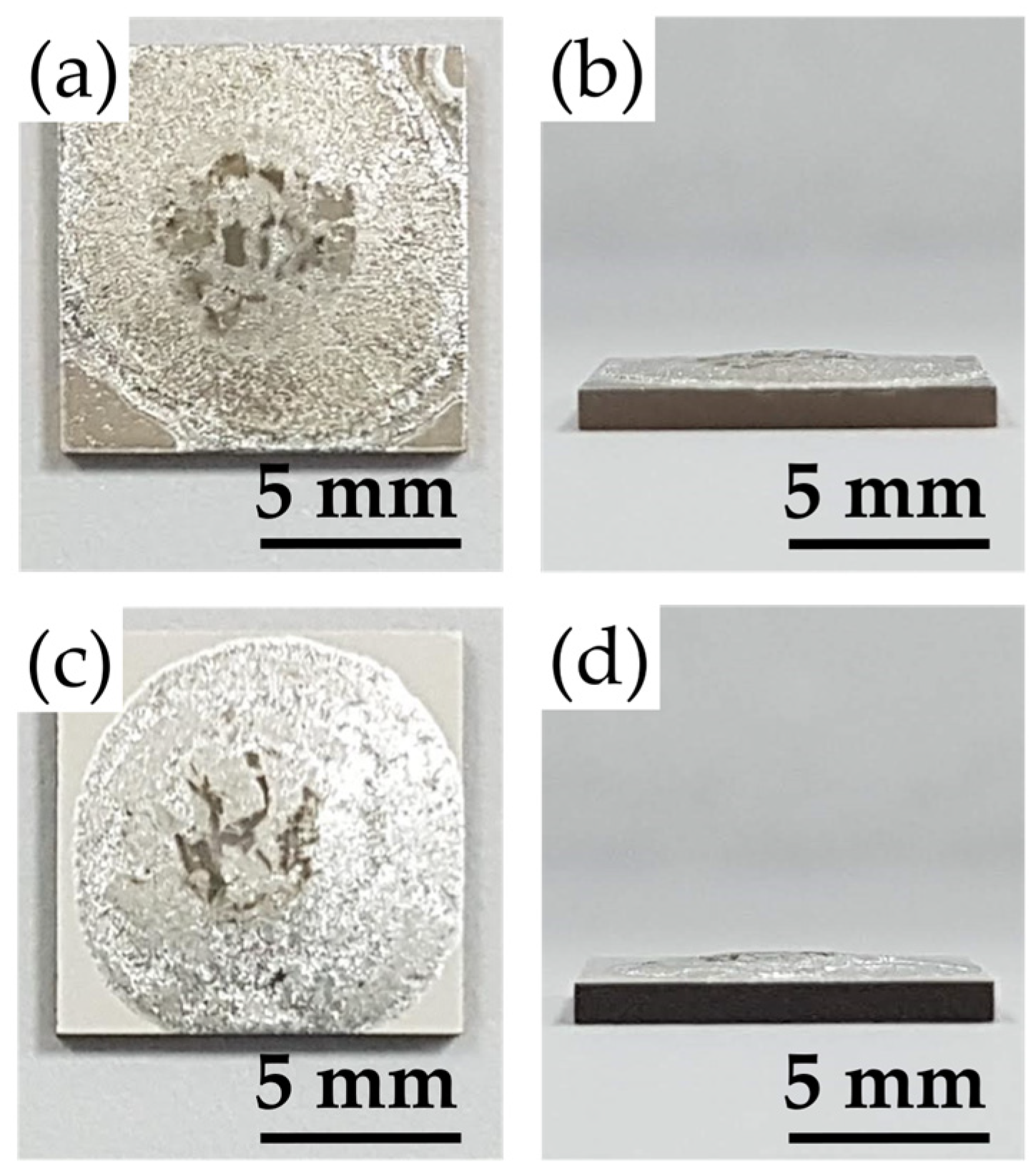
2.5. The Gas-Tightness Tests of YSZ/Crofer 22H Brazed Joints
3. Results and Discussion
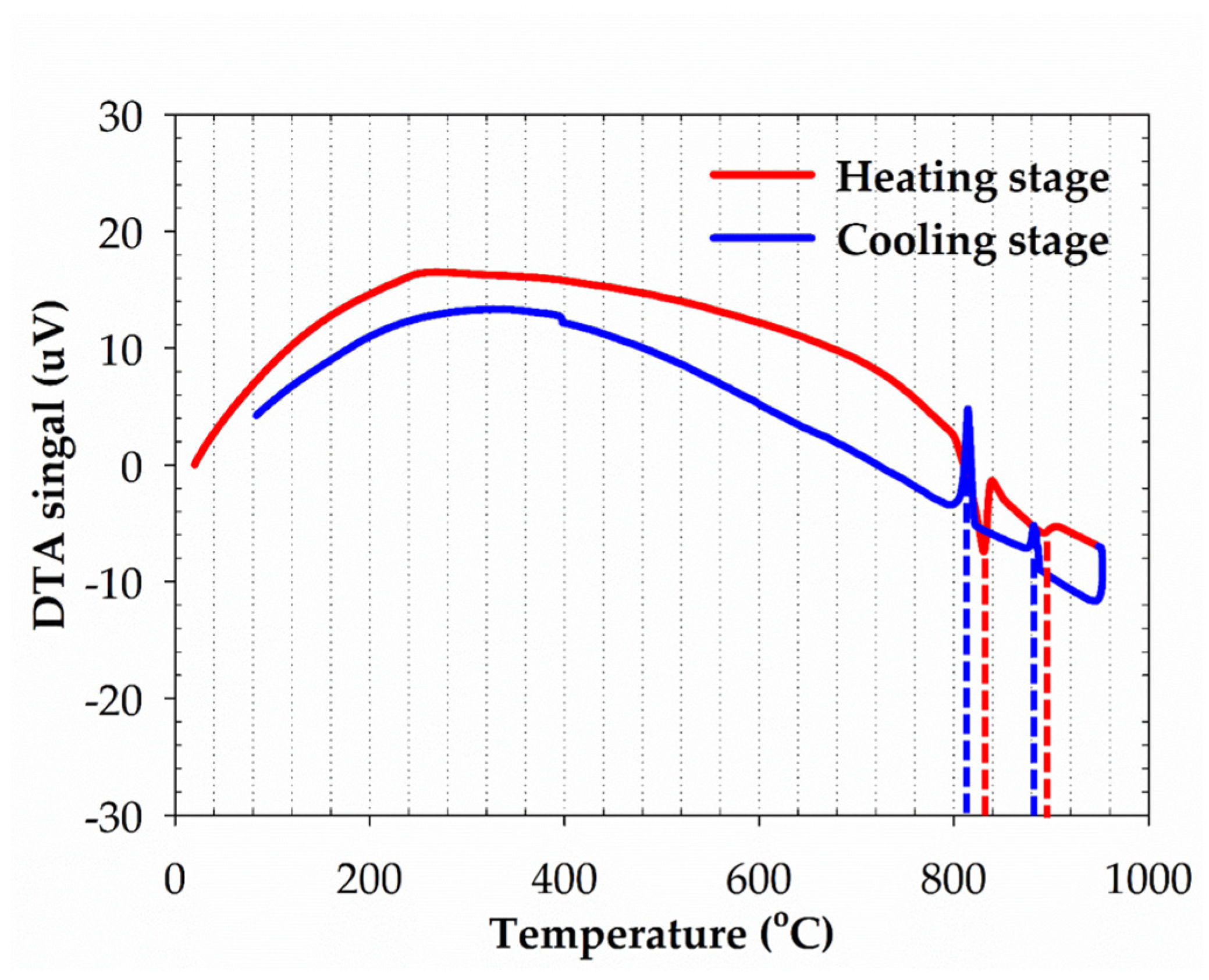
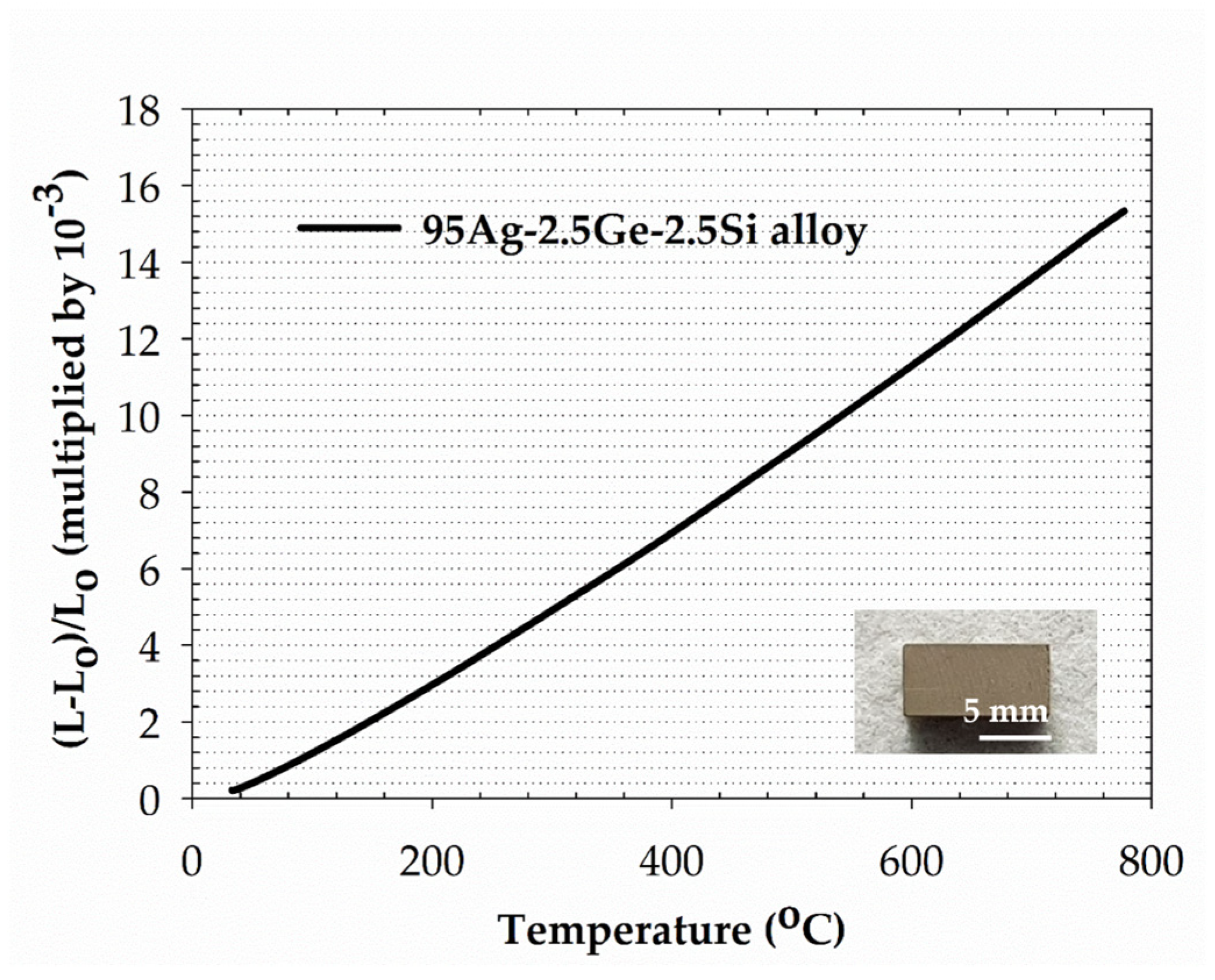
3.1. The Physical Characterization of the 95Ag-2.5Ge-2.5Si Filler
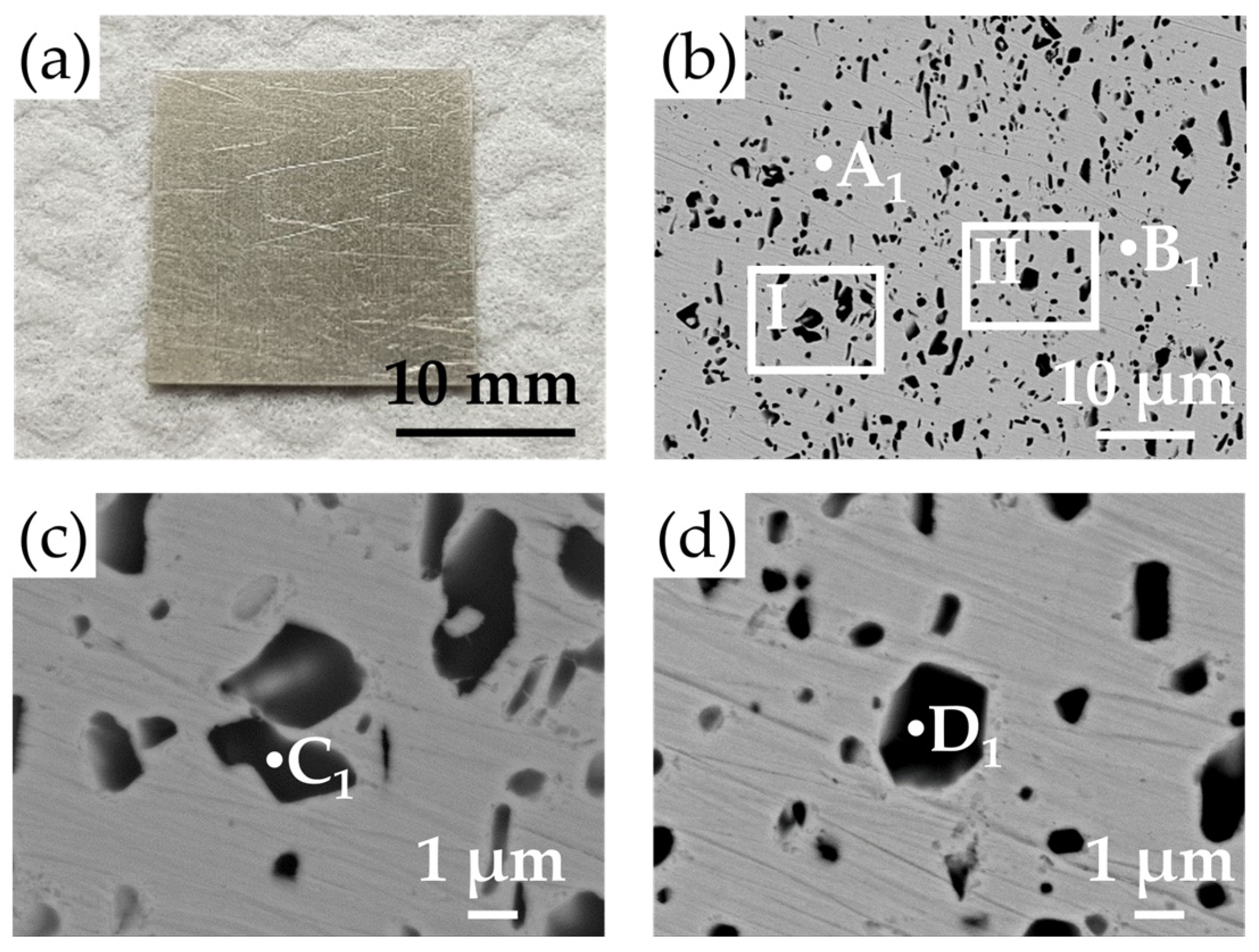
3.2. The Microstructure and Phase Identification of the 95Ag-2.5Ge-2.5Si Filler
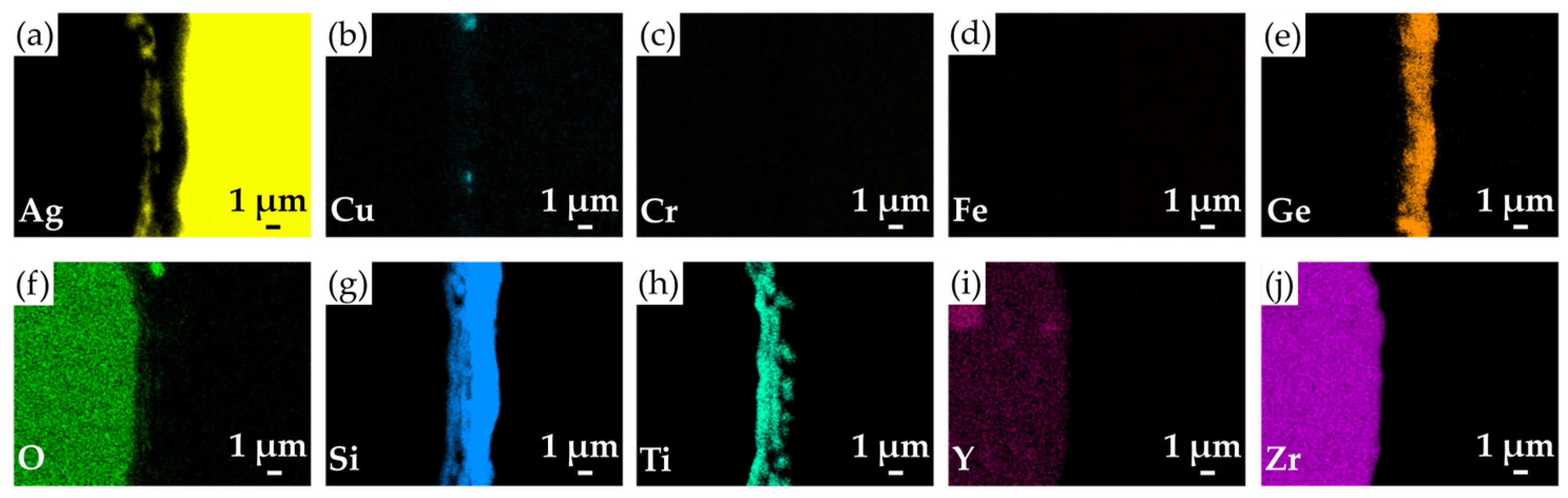
3.3. The Wetting Tests and Cross-Sectional Microstructure of As-Brazed Joints
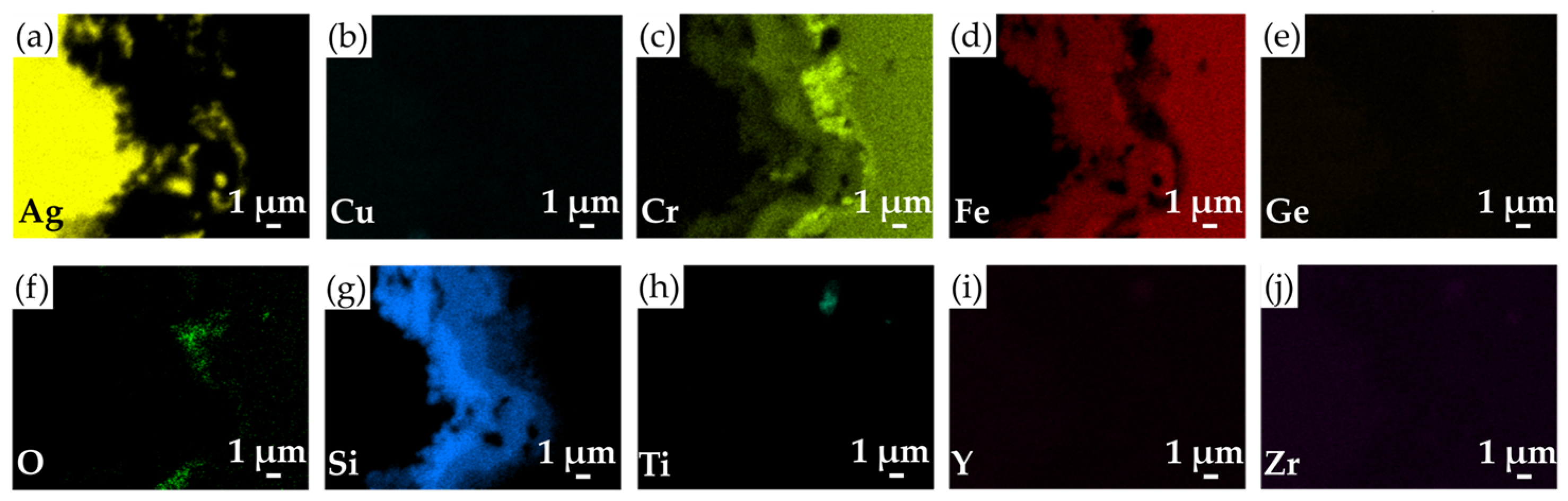
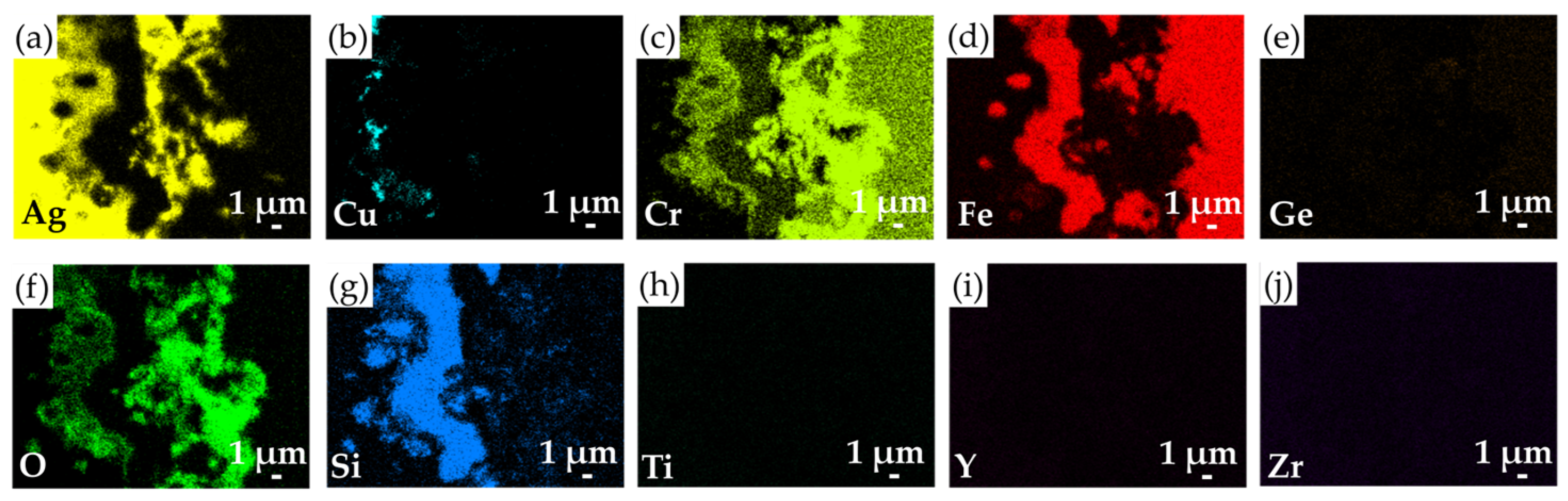
3.4. The Microstructural Evolution and Gas Tightness of the Thermal-Aged Joints
4. Conclusions
- The microstructure of the filler is composed of Ag-rich and Si-rich phases. The melting range of the filler can be decreased to 830–890 °C through Ge and Si addition with a stoichiometric ratio of 2.5 wt %, which meets the requirement of IT-SOFC sealing. The correlation between alloy composition and liquidus temperature is consistent with the data from the Ag-Ge-Si ternary phase diagram. The total CTE increases with temperature and reaches around 22.7 ppm/K at IT-SOFC operational temperatures.
- The wetting performance of the filler on parent materials can be improved through the metallization process with Ti, Cu, and Ag coating layers. From microstructural observations and EPMA quantitative chemical analyses, Si-rich and Si/Ti-rich oxide layers were found at the YSZ/filler interface of YSZ/Crofer 22H brazed joints. The excellent bonding with YSZ is attributed to the Ti reactive wetting. As for the filler/Crofer 22H interface, an Fe-Cr-Si alloying area was formed, which results from Si dissolution into an Fe-Cr solid solution.
- The microstructural evolution of the brazed joints occurred after exposure to air at 750 °C for 100 h. At the YSZ/filler interface, the Si-rich phase was oxidized into a SiO2 layer, and the Si/Ti-rich oxide layer was still well maintained. Similarly, the oxidation of Cu and Si contents could also be observed in the brazed zone. At the filler/Crofer 22H interface, Cr diffusion formed an AgCrO2 oxide and a Cr-rich oxide. In addition, the original Fe-Cr-Si alloy was preserved without suffering from oxidation.
- Although the 95Ag-2.5Ge-2.5Si filler owns higher CTE than that of YSZ and Crofer 22H substrates, the brazed joint still demonstrates excellent gas-tight properties and withstands the thermal stress induced at the cooling stage of the pressure-drop test. This is because the interfacial integrity of the joints can be maintained well even after being tested at elevated temperatures. The phenomenon is also consistent with that described in the previous report, i.e., the thermal strain can be mitigated through plastic deformation of the metal layer.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hwang, J.; Maharjan, K.; Cho, H. A review of hydrogen utilization in power generation and transportation sectors: Achievements and future challenges. Int. J. Hydrogen Energy 2023, 48, 28629–28648. [Google Scholar]
- Song, K.E.; Schlegl, H.; Kang, H.; Choi, W.; Kim, J.H. Electrochemical characteristic of non-stoichiometric SmBa0.45Sr0.5Co2O5+d layered perovskite oxide system for IT-SOFC cathode. Int. J. Hydrogen Energy 2023, 48, 17664–17676. [Google Scholar]
- Han, Z.; Bai, J.; Chen, X.; Zhu, X.; Zhou, D. Novel cobalt-free Pr2Ni1−xNbxO4 (x = 0, 0.05, 0.10, and 0.15) perovskite as the cathode material for IT-SOFC. Int. J. Hydrogen Energy 2021, 46, 11894–11907. [Google Scholar]
- Schilm, J.; Rost, A.; Javed, H.; Poetschke, M. Stability of glass ceramic sealants in atmospheres with increased water contents. ECS Trans. 2023, 111, 2341–2350. [Google Scholar]
- Li, R.; Lu, Y.; Yu, Y.; Ren, X.; Ding, F.; Guan, C.; Wang, J. Investigation on long-term stability of vermiculite seals for reversible solid oxide cell. Molecules 2023, 28, 1462–1472. [Google Scholar] [PubMed]
- Cao, J.; Wang, Z.; Li, C.; Si, X.; Yang, B.; Guo, X.; Huang, Y.; Qi, J. Microstructure evolution and mechanical properties of Co coated AISI 441 ferritic stainless steel/YSZ reactive air brazed joint. Int. J. Hydrogen Energy 2021, 46, 8758–8766. [Google Scholar]
- Ritucci, I.; Agersted, K.; Zielke, P.; Wulff, A.C.; Khajavi, P.; Smeacetto, F.; Sabato, A.G.; Kiebach, R. A Ba-free sealing glass with a high coefficient of thermal expansion and excellent interface stability optimized for SOFC/SOEC stack applications. Int. J. Appl. Ceram. Technol. 2018, 15, 1011–1022. [Google Scholar]
- Li, R.; Peng, L.; Wang, X.; Yang, J.; Yan, D.; Pu, J.; Chi, B.; Li, J. Investigating the performance of Glass/Al2O3 composite seals in planar solid oxide fuel cells. Compos. Part B 2020, 192, 107984–107991. [Google Scholar]
- Wang, S.F.; Hsu, Y.F.; Liao, Y.L.; Yang, Y.J.; Jasinski, P. Physical and sealing properties of BaO-Al2O3-SiO2-CaO-V2O5 glasses for solid oxide fuel cell applications. Int. J. Hydrogen Energy 2022, 47, 10044–10055. [Google Scholar]
- Li, R.; Liang, X.; Wang, X.; Zeng, W.; Yang, J.; Yan, D.; Pu, J.; Chi, B.; Li, J. Improvement of sealing performance for Al2O3 fiber-reinforced compressive seals for intermediate temperature solid oxide fuel cell. Ceram. Int. 2019, 45, 21953–21959. [Google Scholar]
- Li, R.; Wang, X.; Peng, L.; Jiang, B.; Yang, J.; Yan, D.; Pu, J.; Chi, B.; Li, J. Thermal cycling stability of novel hexagonal boron nitride (h-BN)/glass compressive seals for planar intermediate temperature solid oxide fuel cells. J. Alloys Compd. 2020, 843, 155620–155626. [Google Scholar]
- Wang, Z.Q.; Li, C.; Si, X.Q.; Yang, B.; Huang, X.Y.; Qi, J.L.; Feng, J.C.; Cao, J. Brazing YSZ ceramics by a novel SiO2 nanoparticles modified Ag filler. Ceram. Int. 2020, 46, 16493–16501. [Google Scholar]
- Huang, L.W.; Liu, C.K.; Cheng, Y.N.; Lee, R.Y. Characterization of Ag-based filler for electrolyte/metallic-interconnect joint in metal-supported solid oxide fuel cells applications. ECS Trans. 2021, 103, 701–711. [Google Scholar]
- Yang, Z.W.; Xiong, Z.; Wang, J.L.; Wang, Y.; Wang, D.P. Microstructural evolution and high-temperature oxidation resistance of YSZ/Crofer 22H brazed joints using Ag-based filler for solid-oxide fuel cell applications. Mater. Charact. 2023, 200, 112888–112901. [Google Scholar]
- Singh, M.; Shpargel, T.P.; Asthana, R. Brazing of stainless steel to yttria-stabilized zirconia using gold-based brazes for solid oxide fuel cell applications. Int. J. Appl. Ceram. Technol. 2007, 4, 119–133. [Google Scholar]
- Lin, K.L.; Singh, M.; Asthana, R. TEM characterization of Au-based alloys to join YSZ to steel for SOFC applications. Mater. Charact. 2012, 63, 105–111. [Google Scholar]
- Lin, K.L.; Singh, M.; Asthana, R. Characterization of yttria-stabilized-zirconia/stainless steel joint interfaces with gold-based interlayers for solid oxide fuel cell applications. J. Eur. Ceram. Soc. 2014, 34, 355–372. [Google Scholar]
- Lin, K.L.; Singh, M.; Asthana, R.; Lin, C.H. Interfacial and mechanical characterization of yttria-stabilized zirconia (YSZ) to stainless steel joints fabricated using Ag–Cu–Ti interlayers. Ceram. Int. 2014, 40, 2063–2071. [Google Scholar]
- Lin, K.L.; Singh, M.; Asthana, R. Effect of short-term aging on interfacial and mechanical properties of yttria-stabilized zirconia (YSZ)/stainless steel joints. J. Eur. Ceram. Soc. 2015, 35, 1041–1053. [Google Scholar]
- Kiebach, R.; Engelbrecht, K.; Grahl-Madsen, L.; Sieborg, B.; Chen, M.; Hjelm, J.; Norrman, K.; Chatzichristodoulou, C.; Hendriksen, P.V. An Ag based brazing system with a tunable thermal expansion for the use as sealant for solid oxide cells. J. Power Sources 2016, 315, 339–350. [Google Scholar]
- Si, X.; Wang, X.; Li, C.; Lin, T.; Qi, J.; Cao, J. Joining 3YSZ electrolyte to AISI 441 interconnect using the Ag particle interlayer: Enhanced mechanical and aging properties. Crystals 2021, 11, 1573–1586. [Google Scholar]
- Wang, Z.; Li, C.; Cao, J.; Ritucci, I.; Khajavi, P.; Kiebach, R. Ag-SiO2—An optimized braze for robust joining of commercial coated stainless steel to ceramic solid oxide cells. Ceram. Int. 2022, 48, 32740–32747. [Google Scholar]
- Lee, S.; Kang, K.H.; Hong, H.S.; Yun, Y.; Ahn, J.H. Interfacial morphologies between NiO-YSZ fuel electrode/316 stainless steel as the interconnect material and B-Ni3 brazing alloy in a solid oxide fuel cell system. J. Alloys Compd. 2009, 488, L1–L5. [Google Scholar]
- Lee, S.; Kang, K.H.; Hong, H.S.; Yun, Y.; Woo, S.K. Microstructure and interfacial morphologies of brazed NiO-YSZ/316 stainless steel using B-Ni2 brazing alloy. Mater. Test 2010, 52, 257–262. [Google Scholar]
- Chung, D.Y.; Heo, Y.H.; Lee, S.B.; Lim, T.H.; Song, R.H.; Shin, D.R. Induction brazing for gas sealing of anode-supported tubular solid oxide fuel cells using the nickel-based brazing alloy modified by TiH2. Int. J. Hydrogen Energy 2011, 36, 1890–1896. [Google Scholar]
- Weil, K.S.; Kim, J.Y.; Hardy, J.S. Reactive air brazing: A novel method of sealing SOFCs and other solid-state electrochemical devices. Electrochem. Solid-State Lett. 2005, 8, A133–A136. [Google Scholar]
- Si, X.Q.; Cao, J.A.; Song, X.G.; Qu, Y.; Feng, J.C. Reactive air brazing of YSZ ceramic with novel Al2O3 nanoparticles reinforced Ag-CuO-Al2O3 composite filler: Microstructure and joint properties. Mater. Des. 2017, 114, 176–184. [Google Scholar]
- Si, X.; Cao, J.; Talic, B.; Ritucci, I.; Li, C.; Qi, J.L.; Feng, J.C.; Kiebach, R. A novel Ag-based sealant for solid oxide cells with a fully tunable thermal expansion. J. Alloys Compd. 2020, 831, 154608–154617. [Google Scholar]
- Sun, Z.; Zhang, L.X.; Li, X.; Zhang, S.S. Reactive air brazing of the YSZ/AISI 310s couples using a novel Ag-Nb2O5 sealant. Ceram. Int. 2020, 46, 5168–5174. [Google Scholar]
- Weil, K.S.; Coyle, C.A.; Darsell, J.T.; Xia, G.G.; Hardy, J.S. Effects of thermal cycling and thermal aging on the hermeticity and strength of silver-copper oxide air-brazed seals. J. Power Sources 2005, 152, 97–104. [Google Scholar]
- Schilm, J.; Rost, A.; Poenicke, A.; Kusnezoff, M.; Michaelis, A. Ceramic integration technologies for solid oxide fuel cells. Int. J. Appl. Ceram. Technol. 2012, 9, 688–699. [Google Scholar]
- Ponicke, A.; Schilm, J.; Kusnezoff, M.; Michaelis, A. Aging behavior of reactive air brazed seals for SOFC. Fuel Cells 2015, 15, 735–741. [Google Scholar]
- Si, X.; Cao, J.; Ritucci, I.; Talic, B.; Feng, J.; Kiebach, R. Enhancing the long-term stability of Ag-based seals for solid oxide fuel/electrolysis applications by simple interconnect aluminization. Int. J. Hydrogen Energy 2019, 44, 3063–3074. [Google Scholar]
- Wang, N.; Wang, D.P.; Yang, Z.W.; Wang, Y. Interfacial microstructure and mechanical properties of zirconia ceramic and niobium joints vacuum brazed with two Ag-based active filler metals. Ceram. Int. 2016, 42, 12815–12824. [Google Scholar]
- Zhang, X.; Xu, P.; Zhang, M.; Liu, G.; Xu, Z.; Yang, J.; Shao, H.; Qiao, G. Improving the wettability of Ag/ZrB2 system by Ti, Zr, and Hf addition: An insight from first-principle calculations. Appl. Surf. Sci. 2020, 517, 146201–146209. [Google Scholar]
- Guo, X.; Si, X.; Li, C.; Zhao, S.; Liu, Y.; Wang, Z.; He, Z.; Wang, X.; Cao, J. Active brazing of C/C composites and single crystal Ni-based superalloy: Interfacial microstructure and formation mechanism. J. Alloys Compd. 2021, 886, 161183–161196. [Google Scholar]
- Kobsiriphat, W.; Barnett, S. Ag–Cu–Ti braze materials for sealing SOFCs. J. Fuel Cell Sci. Technol. 2008, 5, 011002. [Google Scholar]
- Ahn, B. Recent advances in brazing fillers for joining of dissimilar materials. Metals 2021, 11, 1037–1060. [Google Scholar]
- Wang, Y.; Wei, Y.; Zheng, X.; Yang, Z. Interfacial structure and mechanical properties of ZrO2 ceramic and GH4169 alloy joint brazed with Ag-TiH2 composite filler. Mater. Charact. 2023, 197, 112696–112706. [Google Scholar]
- Tang, L.; Yao, D.; Xia, Y.; Liang, H.; Zeng, Y.P. Effect of interfacial microstructure evolution on the peeling strength and fracture of silicon nitride/oxygen-free copper foil joints brazed with Ag-Cu-TiH2 filler. J. Eur. Ceram. Soc. 2023, 43, 4374–4385. [Google Scholar]
- Hudycz, M. Titanium metallization coating deposited on AlN ceramics substrate by means friction surfacing process. Weld. Technol. Rev. 2020, 92, 35–44. [Google Scholar]
- Huang, L.W.; Shiue, R.K.; Liu, C.K.; Cheng, Y.N.; Lee, R.Y.; Tsay, L.W. Vacuum brazing of metalized YSZ and Crofer alloy using 72Ag-28Cu filler foil. Materials 2022, 15, 939–950. [Google Scholar] [PubMed]
- Asghari, M.; Beidokhti, B.; Beidokhti, S.M. Effect of the metallization method on properties of hybrid zirconia/Ti–6Al–4V joints. Mater. Chem. Phys. 2023, 296, 127212–127216. [Google Scholar]
- Villars, P. Ag-Ge-Si Phase Diagram. In Handbook of Ternary Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1995; pp. 2444–2446. [Google Scholar]
- Huang, L.W.; Liu, C.K.; Cheng, Y.N.; Lee, R.Y. Brazing Material Composition and Manufacturing Method Thereof. Taiwan Patent No. I634220, 1 September 2018. [Google Scholar]
- Mannan, S. Plant Commissioning and Inspection. In Lees’ Loss Prevention in the Process Industries, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2012; Chapter 19; p. 1794. [Google Scholar]
- Cassel, B.; Menard, K. Coefficient of Thermal Expansion Measurement Using the TMA 4000. In PerkinElmer Technical Manual; PerkinElmer: Shelton, CT, USA, 2013. [Google Scholar]
- Data sheet of Ticusil®, Morgan Advanced Materials, USA. Available online: https://www.morganbrazealloys.com/media/6985/wesgo_ticusil_technical-data-sheet-2018.pdf (accessed on 6 November 2023).
- Data sheet of Cusil-ABA®, Morgan Advanced Materials, USA. Available online: https://www.morganbrazealloys.com/media/6941/wesgo_cusil-aba_technical-data-sheet-2018.pdf (accessed on 6 November 2023).
- Data sheet of Gold-ABA®, Morgan Advanced Materials, USA. Available online: https://www.morganbrazealloys.com/media/6949/wesgo_gold-aba_technical-data-sheet-2018.pdf (accessed on 6 November 2023).
- Massalski, T.B. Ag-Si Phase Diagram. In Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1992; pp. 92–94. [Google Scholar]
- Massalski, T.B. Ag-Ge Phase Diagram. In Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1992; pp. 39–41. [Google Scholar]
- Massalski, T.B. Ge-Si Phase Diagram. In Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1992; pp. 2000–2001. [Google Scholar]
- Massalski, T.B. Cr-Si Phase Diagram. In Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1992; pp. 1333–1335. [Google Scholar]
- Massalski, T.B. Fe-Si Phase Diagram. In Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1992; pp. 1771–1772. [Google Scholar]
- Sofie, S.W.; Gannon, P.; Gorokhovsky, V. Silver–chromium oxide interactions in SOFC environments. J. Power Sources 2009, 191, 465–472. [Google Scholar]
- Zhou, L.; Mason, J.H.; Li, W.; Liu, X. Comprehensive review of chromium deposition and poisoning of solid oxide fuel cells (SOFCs) cathode materials. Renew. Sustain. Energy Rev. 2020, 134, 110320–110342. [Google Scholar]



| Element/wt % | Ag | Ge | Si | Other Minor Elements |
|---|---|---|---|---|
| Average composition | 95.13 | 2.35 | 2.48 | ≤0.04 |
| Nominal composition | 95.00 | 2.50 | 2.50 | --- |
| Temperature Range (°C) | CTE (ppm/K) |
|---|---|
| 33–200 | 16.6 |
| 200–400 | 20.0 |
| 400–560 | 21.3 |
| 560–777 | 22.7 |
| Element/at% | Ag | Ge | Si | Possible Phase |
|---|---|---|---|---|
| A1 | 97.8 | 2.2 | 0.0 | Ag-rich |
| B1 | 97.4 | 2.5 | 0.1 | Ag-rich |
| C1 | 20.0 | 9.6 | 70.4 | Si-rich |
| D1 | 16.5 | 9.4 | 74.1 | Si-rich |
| Element/at% | Ag | Cu | Cr | Fe | Ge | O | Si | Ti | Y | Zr | Possible Phase |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A2 | 96.6 | 0.9 | 0.0 | 0.0 | 1.7 | 0.4 | 0.3 | 0.0 | 0.0 | 0.0 | Ag-rich |
| B2 | 0.2 | 73.2 | 0.0 | 0.0 | 2.3 | 5.6 | 18.6 | 0.0 | 0.0 | 0.0 | Cu/Si-rich |
| C2 | 1.9 | 74.4 | 0.0 | 0.0 | 2.2 | 3.0 | 18.5 | 0.0 | 0.0 | 0.0 | Cu/Si-rich |
| D2 | 0.4 | 0.3 | 0.0 | 0.0 | 15.9 | 0.4 | 82.9 | 0.1 | 0.0 | 0.0 | Si-rich |
| E2 | 1.8 | 0.2 | 0.0 | 0.0 | 9.2 | 0.2 | 87.5 | 1.1 | 0.0 | 0.0 | Si-rich |
| F2 | 2.3 | 1.4 | 0.1 | 0.2 | 1.4 | 32.7 | 33.1 | 26.2 | 0.1 | 2.6 | Si/Ti-rich oxide |
| G2 | 0.1 | 0.1 | 16.8 | 47.2 | 0.1 | 0.0 | 34.9 | 0.0 | 0.0 | 0.0 | Fe-Cr s. s. alloyed with Si |
| Element/at% | Ag | Cu | Cr | Fe | Ge | O | Si | Ti | Y | Zr | Possible Phase |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A3 | 97.4 | 1.0 | 0.0 | 0.1 | 0.6 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | Ag-rich |
| B3 | 0.7 | 44.6 | 0.1 | 0.2 | 4.6 | 48.5 | 0.8 | 0.1 | 0.0 | 0.0 | CuO |
| C3 | 3.1 | 2.4 | 0.0 | 0.1 | 1.6 | 65.6 | 26.5 | 0.5 | 0.0 | 0.0 | SiO2 |
| D3 | 3.9 | 0.1 | 0.0 | 0.0 | 0.3 | 68.2 | 26.2 | 1.2 | 0.0 | 0.0 | SiO2 |
| E3 | 4.9 | 3.1 | 0.2 | 0.5 | 0.7 | 52.2 | 12.1 | 24.8 | 0.0 | 1.5 | Si/Ti-rich oxide |
| F3 | 27.9 | 7.2 | 21.5 | 0.9 | 0.4 | 41.0 | 0.3 | 0.0 | 0.0 | 0.0 | AgCrO2 |
| G3 | 0.1 | 0.1 | 8.2 | 68.0 | 1.9 | 0.1 | 21.0 | 0.0 | 0.0 | 0.0 | Fe-Cr s. s. alloyed with Si |
| H3 | 0.9 | 0.4 | 41.2 | 3.3 | 0.4 | 51.9 | 0.6 | 0.0 | 0.0 | 0.0 | Cr-rich oxide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.-W.; Shiue, R.-K.; Liu, C.-K. Microstructural Evolution and Gas-Tight Properties of Yttria-Stabilized Zirconia/Crofer 22H Stainless Steel Brazed Joints with the Ag-Ge-Si Filler for Use in Solid-Oxide Fuel Cells. Metals 2023, 13, 1866. https://doi.org/10.3390/met13111866
Huang L-W, Shiue R-K, Liu C-K. Microstructural Evolution and Gas-Tight Properties of Yttria-Stabilized Zirconia/Crofer 22H Stainless Steel Brazed Joints with the Ag-Ge-Si Filler for Use in Solid-Oxide Fuel Cells. Metals. 2023; 13(11):1866. https://doi.org/10.3390/met13111866
Chicago/Turabian StyleHuang, Liang-Wei, Ren-Kae Shiue, and Chien-Kuo Liu. 2023. "Microstructural Evolution and Gas-Tight Properties of Yttria-Stabilized Zirconia/Crofer 22H Stainless Steel Brazed Joints with the Ag-Ge-Si Filler for Use in Solid-Oxide Fuel Cells" Metals 13, no. 11: 1866. https://doi.org/10.3390/met13111866
APA StyleHuang, L.-W., Shiue, R.-K., & Liu, C.-K. (2023). Microstructural Evolution and Gas-Tight Properties of Yttria-Stabilized Zirconia/Crofer 22H Stainless Steel Brazed Joints with the Ag-Ge-Si Filler for Use in Solid-Oxide Fuel Cells. Metals, 13(11), 1866. https://doi.org/10.3390/met13111866







