Corrosion Behaviour Modelling Using Artificial Neural Networks: A Case Study in Biogas Environment
Abstract
1. Introduction
2. Materials and Methods
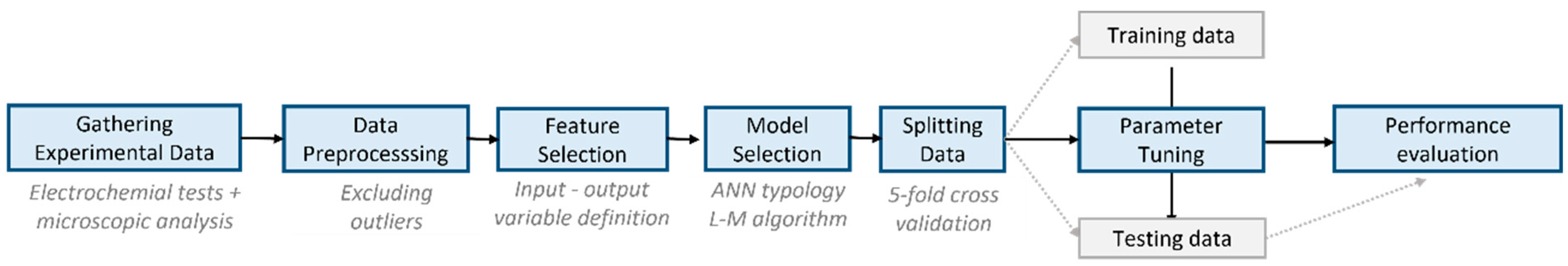
2.1. Methodology
2.2. Classification Performance of ANN Models
- -
- Fail classifier: 0.5 ≤ Accuracy < 0.6;
- -
- Poor Classifier: 0.6 ≤ Accuracy < 0.7;
- -
- Fair Classifier: 0.7 ≤ Accuracy < 0.8;
- -
- Good Classifier: 0.8 ≤ Accuracy < 0.9;
- -
- Excellent Classifier: 0.9 ≤ Accuracy ≤ 1.0.
3. Results and Discussion
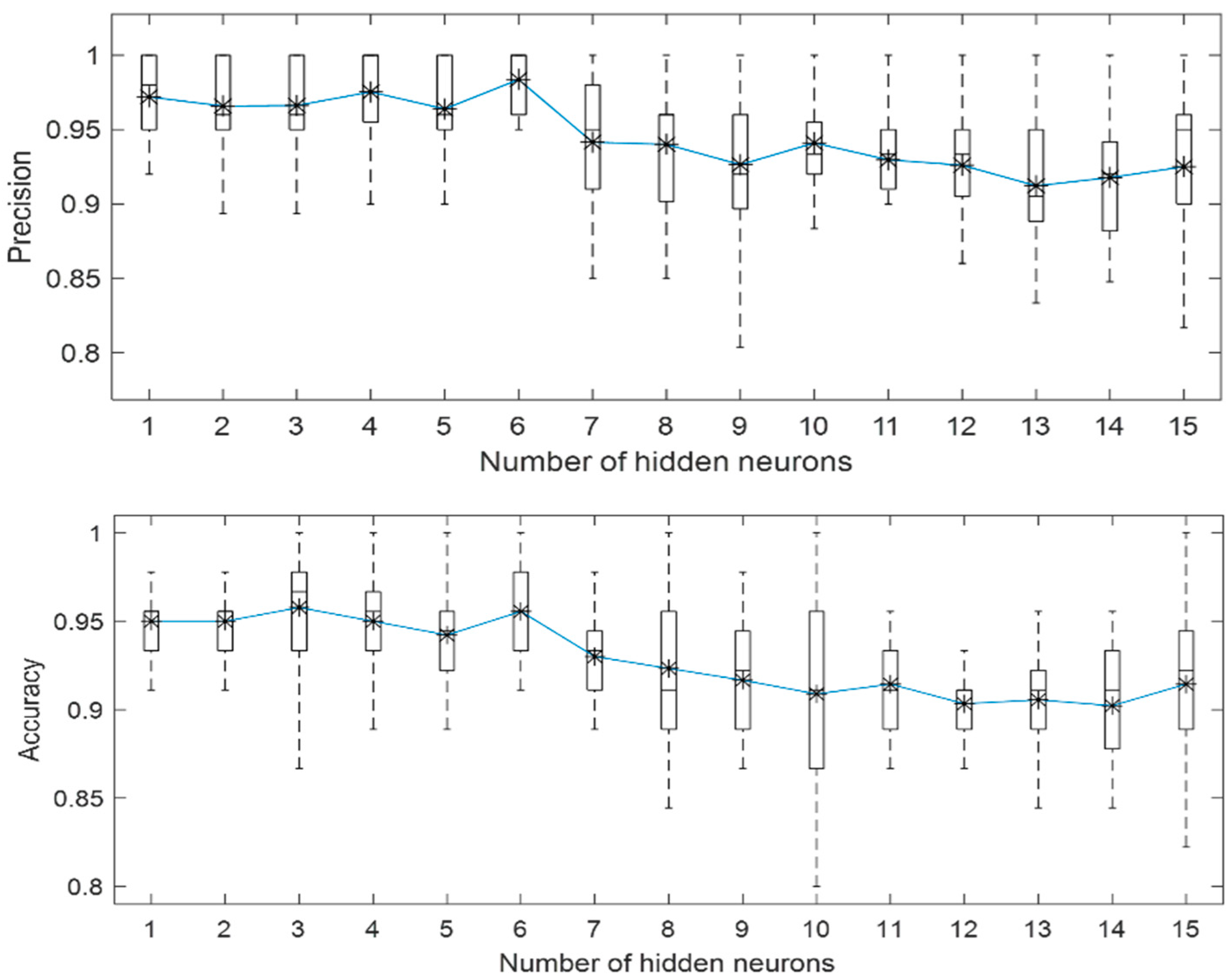
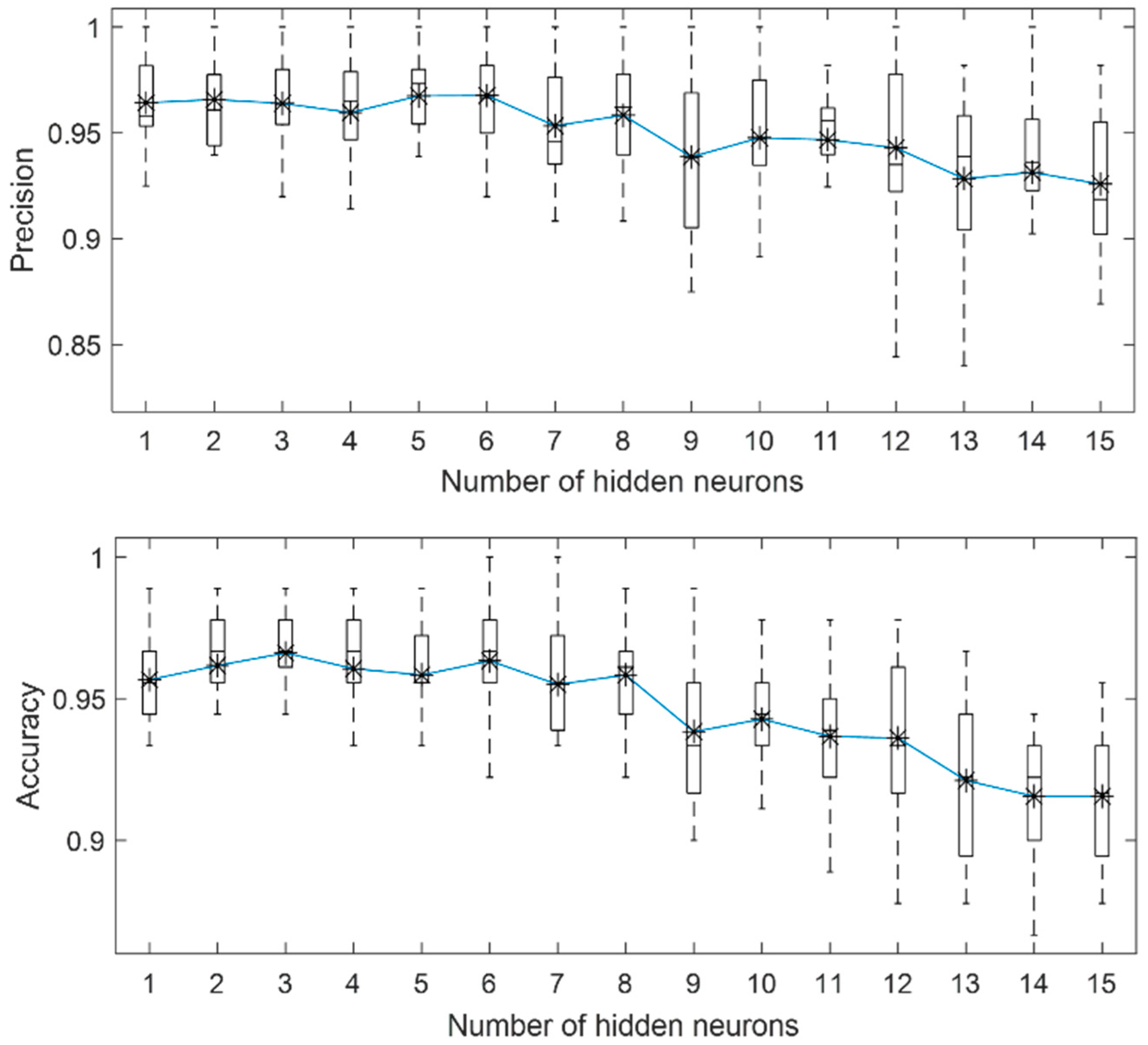
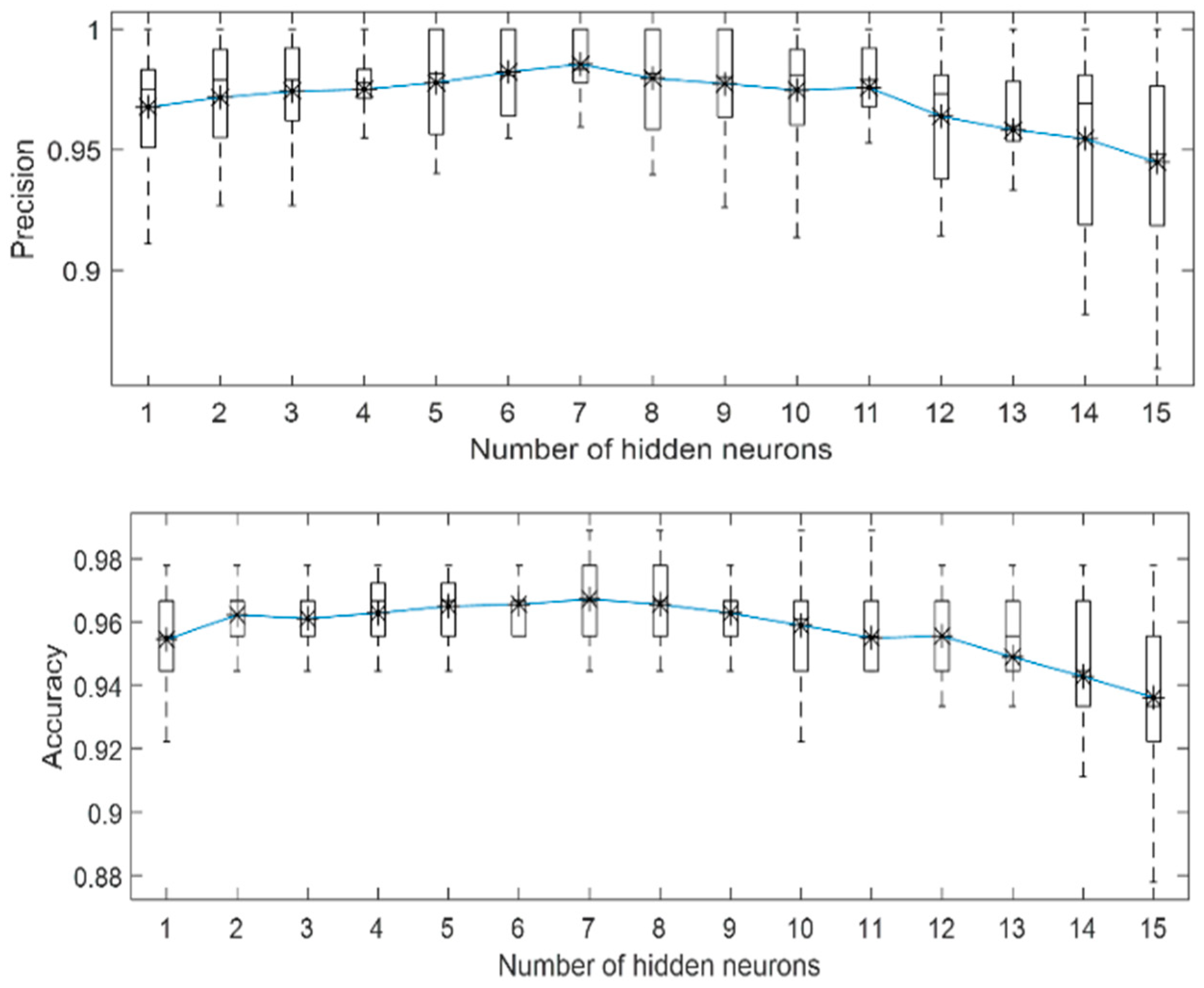
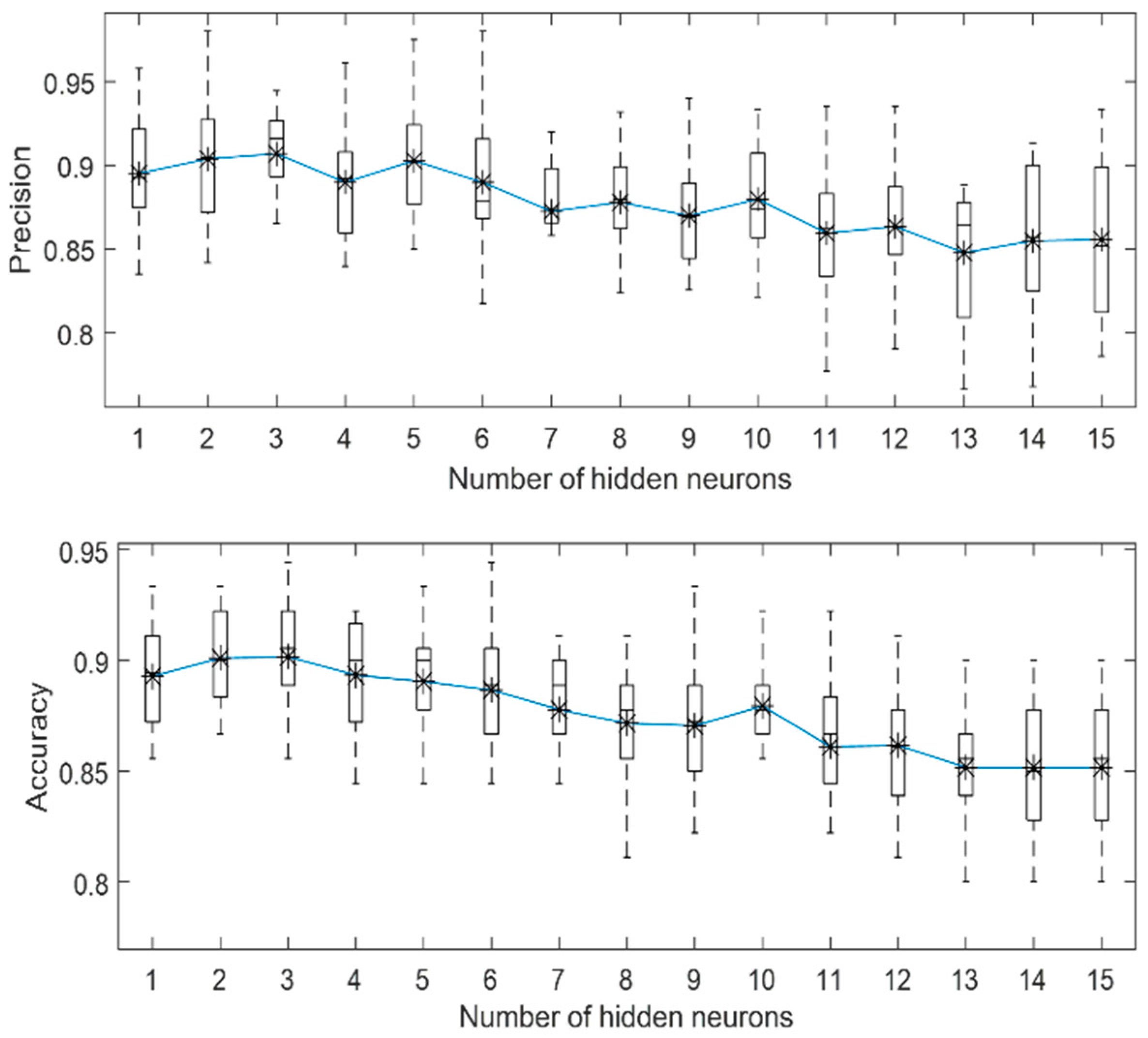
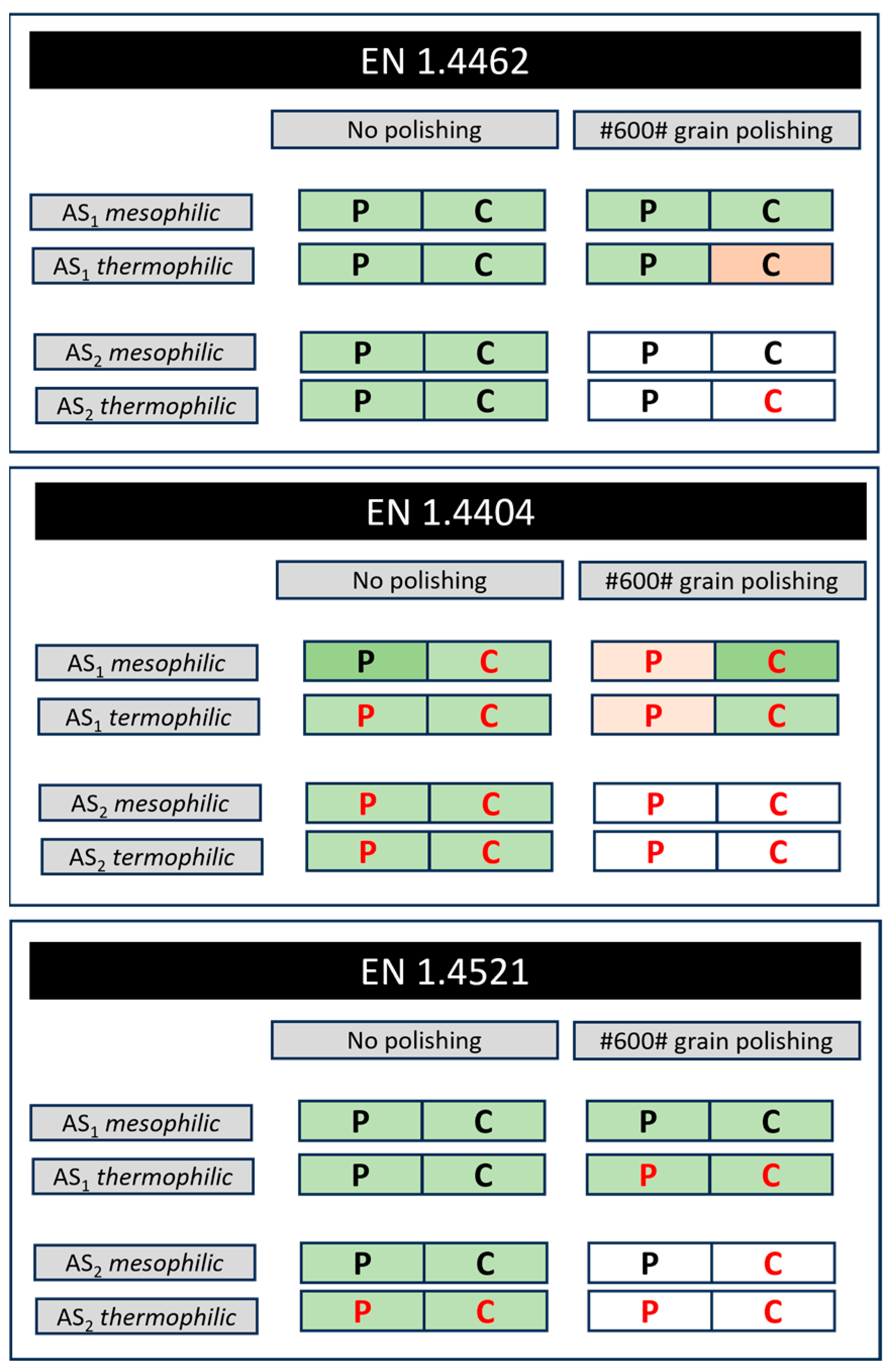
3.1. Localized Corrosion Behaviour Modelling: Results from OBJECTIVE 1
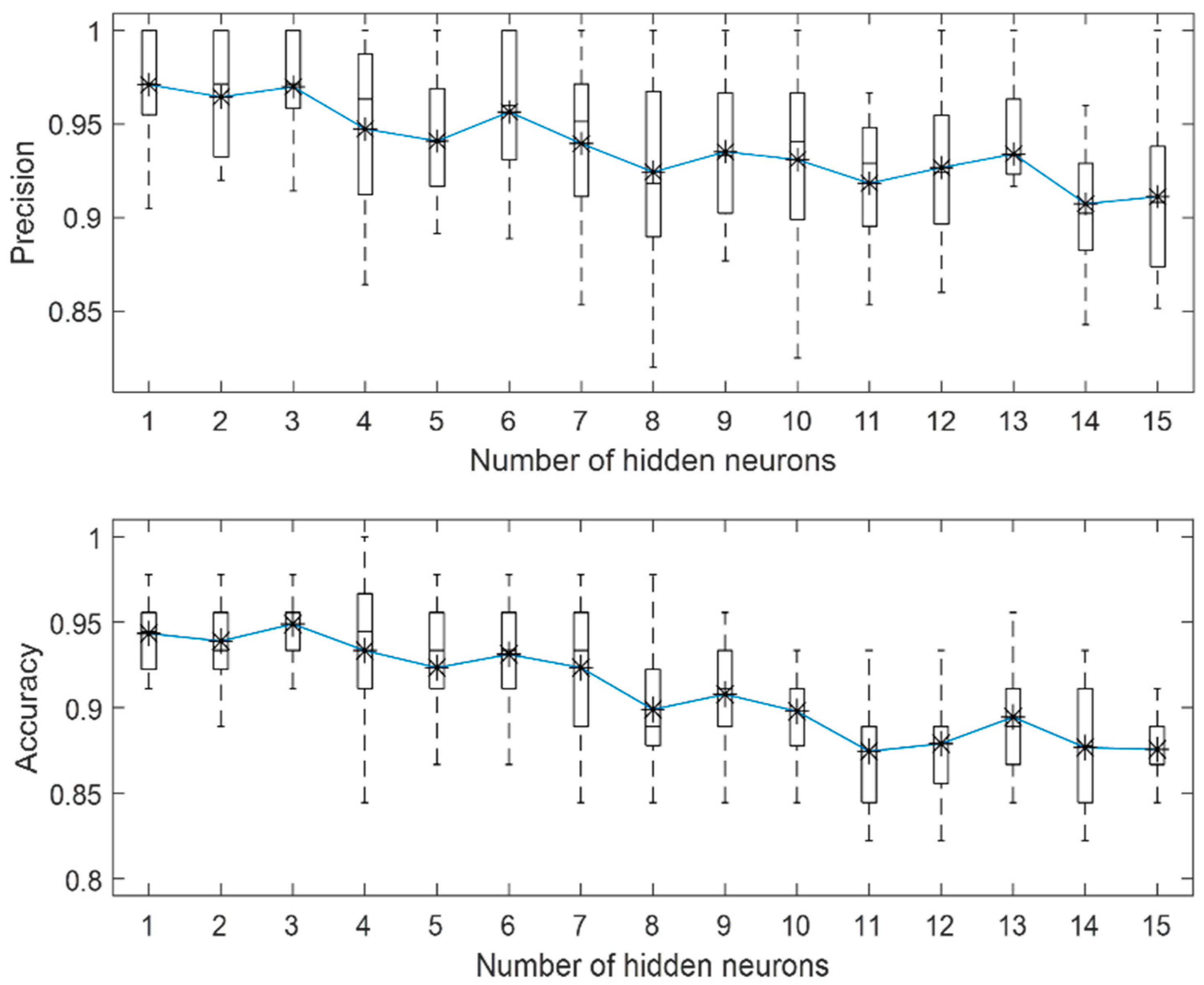
3.2. Influence of PREN on Corrosion Modelling of Stainless Steel in Biogas Production
3.3. Influence of Breakdown Potential on Corrosion Modelling of Stainless Steel in Biogas Production
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cesaro, A.; Belgiorno, V. Combined Biogas and Bioethanol Production: Opportunities and Challenges for Industrial Application. Energies 2015, 8, 8121–8144. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Buhain, J. Bioenergy and biofuels: History, status, and perspective. Renew. Sustain. Energy Rev. 2015, 42, 712–725. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kawasaki, Y.; Oshita, K.; Takaoka, M.; Minami, D.; Inoue, G.; Tanaka, T. Economic assessment of biogas purification systems for removal of both H2S and siloxane from biogas. Renew. Energy 2021, 168, 119–130. [Google Scholar] [CrossRef]
- Koch, G.H.; Brongers, M.P.H.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. Corrosion Costs and Preventive Strategies in the United States; FHWA: Washingt, DC, USA, 2001.
- Bo, T.; Zhu, X.; Zhang, L.; Tao, Y.; He, X.; Li, D.; Yan, Z. A new upgraded biogas production process: Coupling microbial electrolysis cell and anaerobic digestion in single-chamber, barrel-shape stainless steel reactor. Electrochem. Commun. 2014, 45, 67–70. [Google Scholar] [CrossRef]
- Ruiz, D.; Miguel, G.S.; Corona, B.; Gaitero, A.; Domínguez, A. Environmental and economic analysis of power generation in a thermophilic biogas plant. Sci. Total. Environ. 2018, 633, 1418–1428. [Google Scholar] [CrossRef]
- Bao, L.; Li, K.; Zheng, J.; Zhang, Y.; Zhan, K.; Yang, Z.; Zhao, B.; Ji, V. Surface characteristics and stress corrosion behavior of AA 7075-T6 aluminum alloys after different shot peening processes. Surf. Coat. Technol. 2022, 440, 128481. [Google Scholar] [CrossRef]
- Shekari, E.; Khan, F.; Ahmed, S. Economic risk analysis of pitting corrosion in process facilities. Int. J. Press. Vessel. Pip. 2017, 157, 51–62. [Google Scholar] [CrossRef]
- NACE International. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study 2012; NACE International: Houston, TX, USA, 2012. [Google Scholar]
- Chen, Z.; Gao, L.; Koleva, D.A. Evaluating the stray current corrosion of steel rebar in different layouts. Measurement 2022, 196, 111217. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Z.; Behnamian, Y.; Gao, Z.; Wu, Z.; Qin, Z.; Xia, D.-H. Measuring atmospheric corrosion with electrochemical noise: A review of contemporary methods. Measurement 2019, 138, 54–79. [Google Scholar] [CrossRef]
- Sanni, O.; Adeleke, O.; Ukoba, K.; Ren, J.; Jen, T.-C. Application of machine learning models to investigate the performance of stainless steel type 904 with agricultural waste. J. Mater. Res. Technol. 2022, 20, 4487–4499. [Google Scholar] [CrossRef]
- Hao, J.-Z.; Xu, S.-A.; Xu, J.-J.; Cao, H.-L.; Miao, H. Modeling and optimization of the corrosion resistance of Cr-free and Cr-based chemical conversion coatings on nickel foil by artificial neural network and response surface method. Mater. Today Commun. 2023, 36, 106858. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Akbarzadeh, K.; Ramezanzadeh, M.; Naderi, R.; Mahdavian, M.; Olivier, M.-G. Corrosion resistance enhancement of a sol-gel coating by incorporation of modified carbon nanotubes: Artificial neural network (ANN) modeling and experimental explorations. Prog. Org. Coat. 2023, 174, 107296. [Google Scholar] [CrossRef]
- Moses, A.; Chen, D.; Wan, P.; Wang, S. Prediction of electrochemical corrosion behavior of magnesium alloy using machine learning methods. Mater. Today Commun. 2023, 37, 107285. [Google Scholar] [CrossRef]
- Kumari, P.; Halim, S.Z.; Kwon, J.S.-I.; Quddus, N. An integrated risk prediction model for corrosion-induced pipeline incidents using artificial neural network and Bayesian analysis. Process Saf. Environ. Prot. 2022, 167, 34–44. [Google Scholar] [CrossRef]
- Woldesellasse, H.; Tesfamariam, S. Data augmentation using conditional generative adversarial network (cGAN): Application for prediction of corrosion pit depth and testing using neural network. J. Pipeline Sci. Eng. 2023, 3, 100091. [Google Scholar] [CrossRef]
- Duan, Y.; Ma, L.; Qi, H.; Li, R.; Li, P. Developed constitutive models, processing maps and microstructural evolution of Pb-Mg-10Al-0.5B alloy. Mater. Charact. 2017, 129, 353–366. [Google Scholar] [CrossRef]
- Pintos, S.; Queipo, N.V.; de Rincón, O.T.; Rincón, A.; Morcillo, M. Artificial neural network modeling of atmospheric corrosion in the MICAT project. Corros. Sci. 2000, 42, 35–52. [Google Scholar] [CrossRef]
- Díaz, V.; López, C.; Rivero, S. Low carbon steel corrosion damage prediction in rural and urban environments. Rev. De Met. 2003, 39, 188–193. [Google Scholar] [CrossRef]
- Díaz, V.; López, C. Discovering key meteorological variables in atmospheric corrosion through an artificial neural network model. Corros. Sci. 2007, 49, 949–962. [Google Scholar] [CrossRef]
- Silva, R.; Guerreiro, J.; Loula, A. A study of pipe interacting corrosion defects using the FEM and neural networks. Adv. Eng. Softw. 2007, 38, 868–875. [Google Scholar] [CrossRef]
- Kenny, E.D.; Paredes, R.S.C.; de Lacerda, L.A.; Sica, Y.C.; de Souza, G.P.; Lázaris, J. Artificial neural network corrosion modeling for metals in an equatorial climate. Corros. Sci. 2009, 51, 2266–2278. [Google Scholar] [CrossRef]
- Halama, M.; Kreislova, K.; Van Lysebettens, J. Prediction of Atmospheric Corrosion of Carbon Steel Using Artificial Neural Network Model in Local Geographical Regions. Corrosion 2011, 67, 065004-1–065004-6. [Google Scholar] [CrossRef]
- Lin, H.-T.; Lo, C.-M.; Lin, M.-D. Application of Artificial Neural Networks on Predicting Corrosion Rates of Carbon Steel in Taiwan Industrial Zones. Adv. Intell. Syst. Res. 2017, 132, 278–282. [Google Scholar] [CrossRef][Green Version]
- Tran, N.-L.; Nguyen, T.-H.; Phan, V.-T.; Nguyen, D.-D. A Machine Learning-Based Model for Predicting Atmospheric Corrosion Rate of Carbon Steel. Adv. Mater. Sci. Eng. 2021, 2021, 6967550. [Google Scholar] [CrossRef]
- Kim, H.-S.; Park, S.-J.; Seo, S.-M.; Yoo, Y.-S.; Jeong, H.-W.; Jang, H. Regression analysis of high-temperature oxidation of Ni-based superalloys using artificial neural network. Corros. Sci. 2021, 180, 109207. [Google Scholar] [CrossRef]
- Zhu, Y.; Macdonald, D.D.; Qiu, J.; Urquidi-Macdonald, M. Corrosion of rebar in concrete. Part III: Artificial Neural Network analysis of chloride threshold data. Corros. Sci. 2021, 185, 109438. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Xin, G.; Wang, Y.; Xu, S.; Fan, M. Novel method for prediction of corrosion current density of gas pipeline steel under stray current interference based on hybrid LWQPSO-NN model. Measurement 2022, 200, 111592. [Google Scholar] [CrossRef]
- Li, Q.; Wang, D.; Zhao, M.; Yang, M.; Tang, J.; Zhou, K. Modeling the corrosion rate of carbon steel in carbonated mixtures of MDEA-based solutions using artificial neural network. Process Saf. Environ. Prot. 2021, 147, 300–310. [Google Scholar] [CrossRef]
- Soomro, A.A.; Mokhtar, A.A.; Kurnia, J.C.; Lashari, N.; Lu, H.; Sambo, C. Integrity assessment of corroded oil and gas pipelines using machine learning: A systematic review. Eng. Fail. Anal. 2022, 131, 105810. [Google Scholar] [CrossRef]
- Yang, J.; Li, R.; Chen, L.; Hu, Y.; Dou, Z. Research on equipment corrosion diagnosis method and prediction model driven by data. Process Saf. Environ. Prot. 2022, 158, 418–431. [Google Scholar] [CrossRef]
- Entezari, A.; Aslani, A.; Zahedi, R.; Noorollahi, Y. Artificial intelligence and machine learning in energy systems: A bibliographic perspective. Energy Strat. Rev. 2023, 45, 101017. [Google Scholar] [CrossRef]
- Akhshik, M.; Bilton, A.; Tjong, J.; Singh, C.V.; Faruk, O.; Sain, M. Prediction of greenhouse gas emissions reductions via machine learning algorithms: Toward an artificial intelligence-based life cycle assessment for automotive lightweighting. Sustain. Mater. Technol. 2022, 31, e00370. [Google Scholar] [CrossRef]
- Kaab, A.; Sharifi, M.; Mobli, H.; Nabavi-Pelesaraei, A.; Chau, K.-W. Combined life cycle assessment and artificial intelligence for prediction of output energy and environmental impacts of sugarcane production. Sci. Total. Environ. 2019, 664, 1005–1019. [Google Scholar] [CrossRef]
- Ryan, M.P.; Williams, D.E.; Chater, R.J.; Hutton, B.M.; McPhail, D.S. Why stainless steel corrodes. Nature 2002, 415, 770–774. [Google Scholar] [CrossRef]
- Sedriks, A.J. Corrosion of Stainless Steel; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Vargel, C. Crevice Corrosion. In Corrosion of Aluminium; Elsevier: Amsterdam, The Netherlands, 2020; pp. 267–271. [Google Scholar] [CrossRef]
- Blackwood, D.J.; Lim, C.S.; Teo, S.L.; Hu, X.; Pang, J. Macrofouling induced localized corrosion of stainless steel in Singapore seawater. Corros. Sci. 2017, 129, 152–160. [Google Scholar] [CrossRef]
- Mele, C.; Bozzini, B. Localised corrosion processes of austenitic stainless steel bipolar plates for polymer electrolyte membrane fuel cells. J. Power Sources 2010, 195, 3590–3596. [Google Scholar] [CrossRef]
- Jiménez-Come, M.J.; Martín, M.d.l.L.; Matres, V.; Baladés, J.D.M. The use of artificial neural networks for modelling pitting corrosion behaviour of EN 1.4404 stainless steel in marine environment: Data analysis and new developments. Corros. Rev. 2020, 38, 339–353. [Google Scholar] [CrossRef]
- Jiménez-Come, M.J.; Turias, I.J.; Ruiz-Aguilar, J.J. A two-stage model based on artificial neural networks to determine pitting corrosion status of 316L stainless steel. Corros. Rev. 2016, 34, 113–125. [Google Scholar] [CrossRef]
- ASTM International. Annual Book of ASTM Standards; American Society for Testing & Materials: West Conshohocken, PA, USA, 2004. [Google Scholar]
- Deng, B.; Jiang, Y.; Gong, J.; Zhong, C.; Gao, J.; Li, J. Critical pitting and repassivation temperatures for duplex stainless steel in chloride solutions. Electrochim. Acta 2008, 53, 5220–5225. [Google Scholar] [CrossRef]
- White, H. Learning in Artificial Neural Networks: A Statistical Perspective. Neural Comput. 1989, 1, 425–464. [Google Scholar] [CrossRef]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Lippmann, R. An introduction to computing with neural nets. IEEE Assp Mag. 1987, 4, 4–22. [Google Scholar] [CrossRef]
- Lourakis, M.I.A. A brief description of the Levenberg-Marquardt algorithm implemented by levmar. Found. Res. Technol. 2005, 11, 1–6. [Google Scholar]
- David, W.H.; Stanley, L. Applied Survival Analysis; Elsevier Science Inc.: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Fawcett, T. An Introduction to ROC analysis. Pattern Recogn. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
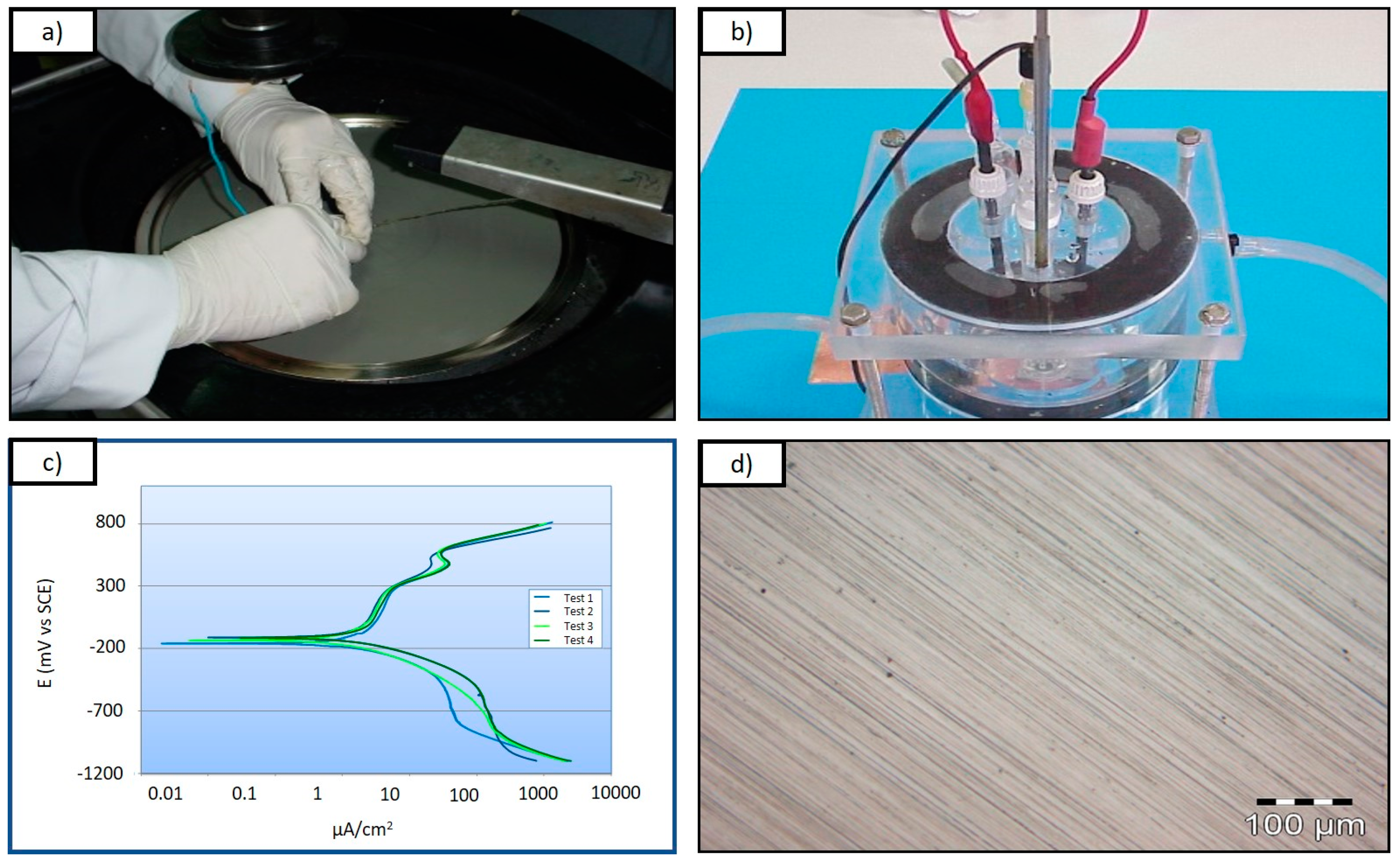



| Stainless Steel Grade | Cr (%) | Mo (%) | N (%) | Mn (%) | Ti (%) | Nb (%) |
|---|---|---|---|---|---|---|
| EN 1.4404 | 16.71 | 2.018 | 0.038 | 1.291 | 0.016 | 0.016 |
| EN 1.4462 | 22.892 | 3.279 | 0.1619 | 1.397 | 0.034 | 0.011 |
| EN 1.4482 | 19.94 | 0.223 | 0.1374 | 4.016 | 0.025 | 0.006 |
| EN 1.4003 | 11.45 | 0.01 | 0.0115 | 0.547 | 0.015 | 0.006 |
| EN 1.4571 | 16.801 | 2.066 | 0.0134 | 1.578 | 0.301 | 0.008 |
| EN 1.4509 | 18.492 | 0.051 | 0.0235 | 0.43 | 0.148 | 0.393 |
| EN 1.4521 | 18.555 | 1.999 | 0.024 | 0.507 | 0.127 | 0.416 |
| EN 1.4318 | 17.744 | 0.089 | 0.144 | 1.258 | 0.002 | 0.007 |
| Reagent | g/L | AS 1 | AS 2 |
|---|---|---|---|
| Sodium Sulphate, Na2SO3 | 12.67 | X | X |
| Ammonium Chloride, NH4Cl | 69.81 | X | X |
| Ammonium carbonate, (NH4)2CO3 | 15.13 | X | X |
| Sodium Acetate Trihydrate, NaCH3COOH·3H2O | 17.06 | X | X |
| Hydrogen chloride, HCl, from FeCl2 desulfuration | 7.31 | -- | X |
| pH range | 8.2–8.5 | 6.6–7.2 |
| Target Status | ||
|---|---|---|
| Positive (Corrosion Pattern) | Negative (No-Corrosion Pattern) | |
| Classification results | ||
| Positive | TP (True Positive) | FP (False Positive) |
| Negative | FN (False Negative) | TN (True Negative) |
| Identity | Corrosion Type | Input Neurons | Output Neurons: |
|---|---|---|---|
| Model 1 | Pitting | 10 units: %Cr, %Mo, %N, %Mn, %Ti, %Nb, Eb, T, AS, SF | 2 units: 1: corrosion pattern 0: no-corrosion pattern |
| Model 2 | Crevice | 10 units: %Cr, %Mo, %N, %Mn, %Ti, %Nb, Eb, T, AS, SF | 2 units: 1: corrosion pattern 0: no-corrosion pattern |
| Model 3 | Pitting and Crevice | 11 units: %Cr, %Mo, %N, %Mn, %Ti, %Nb, Eb, T, AS, SF, corrosion type (pitting or crevice) | 2 units: 1: corrosion pattern 0: no-corrosion pattern |
| Identity | Corrosion Type | Input Neurons | Output Neurons: |
|---|---|---|---|
| Model 4 | Pitting and Crevice | 6 units: PREN, Eb, T, AS, SF, corrosion type (pitting or crevice) | 2 units: 1: corrosion pattern 0: no-corrosion pattern |
| Identity | Corrosion Type | Input Neurons | Output Neurons: |
|---|---|---|---|
| Model 5 | Pitting and Crevice | 10 units: %Cr, %Mo, %N, %Mn, %Ti, %Nb, T, AS, SF, corrosion type (pitting or crevice) | 2 units: 1: corrosion pattern 0: no-corrosion pattern |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Come, M.J.; González Gallero, F.J.; Álvarez Gómez, P.; Mena Baladés, J.D. Corrosion Behaviour Modelling Using Artificial Neural Networks: A Case Study in Biogas Environment. Metals 2023, 13, 1811. https://doi.org/10.3390/met13111811
Jiménez-Come MJ, González Gallero FJ, Álvarez Gómez P, Mena Baladés JD. Corrosion Behaviour Modelling Using Artificial Neural Networks: A Case Study in Biogas Environment. Metals. 2023; 13(11):1811. https://doi.org/10.3390/met13111811
Chicago/Turabian StyleJiménez-Come, María Jesús, Francisco Javier González Gallero, Pascual Álvarez Gómez, and Jesús Daniel Mena Baladés. 2023. "Corrosion Behaviour Modelling Using Artificial Neural Networks: A Case Study in Biogas Environment" Metals 13, no. 11: 1811. https://doi.org/10.3390/met13111811
APA StyleJiménez-Come, M. J., González Gallero, F. J., Álvarez Gómez, P., & Mena Baladés, J. D. (2023). Corrosion Behaviour Modelling Using Artificial Neural Networks: A Case Study in Biogas Environment. Metals, 13(11), 1811. https://doi.org/10.3390/met13111811





