Abstract
The results of this research on the electrochemical behavior of Cu24Zn5Al alloy in a 0.1 mol/dm3 sodium sulfate (Na2SO4) solution containing 1-phenyl-5-mercaptotetrazole (PMT) are presented in this paper. The influence of PMT concentration, chloride ion concentration, and pre-treatment were examined. The influence of pre-treatment was studied in terms of the effect of the immersion time of the electrode in the appropriate inhibitor solution. After selecting the optimal immersion time, its effect on the behavior of the Cu24Zn5Al alloy was tested in a 0.1 mol/dm3 solution of sodium sulfate in the presence of different concentrations of chloride ions. Research shown that with the increase of PMT concentration, the anodic current density around the corrosion potential decreases, indicating that PMT behaves as a corrosion inhibitor for Cu24Zn5Al alloy.
1. Introduction
CuZnAl is one of the first shape memory alloys (SMAs) based on copper, which has found its commercial application [1]; for example, the devices of fire-preventing systems in underground pit, the damper for reducing vibration [2], fluid fitting system [3], automotive industry [4], various applications in engineering fields [5,6,7]. The primary benefit of CuZnAl alloys is that they can be produced using conventional metallurgical methods from relatively low-cost metals, which makes them more affordable compared to commercial shape memory alloys (SMAs) [8,9,10,11]. The transformation temperature of the binary alloy is typically too elevated for practical applications, and a third element (Zn) is usually incorporated to create a useful alloy [12]. Also, elevating the concentration of zinc enhances the corrosion resistance of the alloys. CuAlNi-based SMAs have also shown some advantages, but their use in biomedicine is problematic due to the fact that nickel is an allergen and due to the tendency of these alloys towards pitting corrosion [13].
Due to the application of SMA alloys in the fluid fitting system [3], an investigation of the corrosion behavior of SMA alloys in different environments is necessary.
The behavior of copper-based alloys in sodium sulfate solutions in the presence of halides has been the subject of investigation by some researchers. Majed et al. [14] investigated the behavior of brass in a sodium sulfate solution, with and without various halides. It was observed that the corrosion potential shifts toward lower values in a sodium sulfate solution with the presence of halide ions, compared to the corrosion potential of brass in a sodium sulfate solution. Also, it was found that the presence of iodide ions had the greatest influence on increasing the rate of brass corrosion.
Vrsalovic et al. [15] investigated the behavior of the CuAlNi shape memory alloy in sodium chloride solutions. The corrosion potential shift towards more negative values, with increasing chloride ion concentration, was observed. No pitting corrosion was identified on the electrode surface as shown by the results obtained using scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM/EDS) in a 0.1 wt.% sodium chloride (NaCl) solution. However, it was identified in NaCl solutions of concentrations higher than 0.1 wt.%. EDS analysis showed the presence of copper oxide on the electrode surface, with a small proportion of aluminum in the form of aluminum oxide. Many researchers have investigated the possibility of inhibiting the corrosion of copper and copper alloys. Inhibition of the corrosion process using some organic compounds containing N and/or S atoms has been particularly studied and explained by the fact that a bond with transition metals, such as copper, is easily formed through these atoms [16,17,18,19]. The effectiveness of inhibition can be ascribed to the adsorption process, i.e., the formation of a thin film of complexes on the copper surface, which was represented as an inhibitor–copper bond [20,21,22,23]. For these reasons, a large number of copper and copper alloy corrosion inhibitors from the group of azoles [16,17,18], amines [19,20,21,22,23], amino acids [24,25,26], and others have been investigated. The copper atom has an unoccupied d orbital, allowing it to form coordinate bonds with atoms that donate electrons [27].
Due to the application of CuZnAl alloys in the fluid fitting system [3], as well as various applications in engineering fields where the CuZnAl alloy can be exposed to various corrosion environments, it is necessary to test the effectiveness of the application of various corrosion inhibitors. In their research, Ye et al. [28] showed that the coordinative compound formed on the surface of copper in the presence of 1-phenyl-5-mercaptotetrazole (PMT) more efficiently inhibited the corrosion process of copper compared to the films formed in the presence of tetrazole (TTA), benzotriazole (BTA), hydroxy benzotriazole (HBTA), 2-mercaptobenzotriazole (MBT), 2-mercaptobenzimidazole (MBI), 2-aminopyrimidine (2-AP), imidazole (IBM), and chromate. In the presence of PMT, a film was formed on the surface of copper that can be best described as a composite structure Cu-PMT/Cu2O/Cu. By measuring the weight loss in a solution of 1 mmol/dm3 sulfuric acid (H2SO4) in the presence of 5-mercapto-1-phenyltetrazole and without the presence of an inhibitor, it was found that the weight loss was reduced in the solution with the inhibitor. This can be related to the strong chemical bond of 5-mercapto-1-phenyltetrazole to the surface of copper [29].
The electron donating nitrogen atom and the properties of the -SH group have an important role in the process of adsorption [1]. The PMT molecule probably adsorbs via the -S or the coordinative bond of the -N atom from the tetrazole. Szocs et al. [30] evaluated the inhibitory properties of PMT in acidic sulfate solutions, in which PMT exhibited excellent inhibitory characteristics.
The aim of this study is to evaluate the corrosion behavior of the copper alloy Cu24Zn5Al in blank 0.1 mol/dm3 sodium sulfate, and in the presence of PMT as an inhibitor. Additionally, the electrochemical behavior of the copper alloy was investigated in 0.1 mol/dm3 sodium sulfate in the presence of different concentrations of chloride.
2. Materials and Methods
2.1. Materials
For the experimental investigations, a Cu24Zn5Al electrode was used as the working electrode, which was obtained by sealing a Cu24Zn5Al wire in a methyl methacrylate-based mixture. The electrode surface area was 0.5 cm2. The chemical composition of the electrode was determined by measuring with an optical emission spectrometer (Spectro 20, Boschstrasse 10, Kleve, Germany) and is shown in Table 1.

Table 1.
Chemical composition of the investigated alloy (wt.%).
All solutions were prepared from chemicals of analytical purity. The test solution was a 0.1 mol/dm3 sodium sulfate solution.
The influence of the inhibitor on the corrosion behavior of the copper alloy was investigated in solutions of different concentrations of 1-phenyl-5-mercaptotetrazole (PMT) as follows: 2.5 × 10−3, 1 × 10−3, 5 × 10−4, 1 × 10−4, and 1 × 10−5 mol/dm3 of PMT in a 0.1 mol/dm3 sodium sulfate solution.
The impact of chloride ions on the corrosion behavior of the copper alloy was investigated in a 0.1 mol/dm3 sodium sulfate solution with the following concentrations of Cl−: 5 × 10−2, 1 × 10−2, 1 × 10−3, and 1 × 10−4 mol/dm3.
To investigate the effect of electrode immersion time in the inhibitor solution, a PMT solution with a concentration of 0.017 mol/dm3 was used, which was prepared by dissolving 3.0298 g of PMT in 1 dm3 of distilled water.
2.2. Methods
The samples of the investigated alloy were polished with alumina (Al2O3) with a coarseness of 1 μm, washed with distilled water, dried, and immersed in the appropriate solutions.
The electrochemical measurements were performed using a potentiostat that was directly connected to a computer via an AD card, with a three-electrode cell. The saturated calomel electrode (SCE) was used as the reference electrode, while a platinum electrode was utilized as the auxiliary electrode.
The open circuit potential was determined for 30 min, after which anodic polarization curves were recorded from the open circuit potential to approximately 1.0 V vs. SCE. The measurements were performed at a scan rate of 1 mV/s. Cyclic voltammograms were recorded from the corrosion potential to 1.0 V vs. SCE as well as from −1.0 V to 1.0 V vs. SCE, with scan rates of 10 mV/s. All measurements were performed at room temperature.
The electrochemical characteristics of Cu24Zn5Al alloy in a sodium sulfate solution with the addition of the investigated inhibitor, 1-phenyl-5-mercaptotetrazole, and chloride ions, were tested as follows:
- (A)
- Potentiodynamic polarization measurements (linear and cyclic voltammetry) were conducted in a 0.1 mol/dm3 sodium sulfate solution without and with the addition of various concentrations of the inhibitor (2.5 × 10−3 mol/dm3, 1 × 10−3 mol/dm3, 5 × 10−4 mol/dm3, 1 × 10−4 mol/dm3, 1 × 10−5 mol/dm3), as well as in 0.1 mol/dm3 sodium sulfate solutions containing various concentrations of chloride ions (5 × 10−2 mol/dm3, 1 × 10−2 mol/dm3, 1 × 10−3 mol/dm3, 1 × 10−4 mol/dm3).
- (B)
- The testing of the effect of the immersion time of the Cu24Zn5Al alloy in the 0.017 mol/dm3 PMT solution was conducted by allowing the Cu24Zn5Al electrode to stand in the solution for 15 min, 60 min, and 240 min, then rinsing with distilled water and immediately subjecting it to potentiodynamic polarization measurements (linear and cyclic voltammetry) in a 0.1 mol/dm3 sodium sulfate solution. After obtaining the optimal exposure time, the effect of chloride ions was examined by rinsing the Cu24Zn5Al electrode that was immersed for 60 min (optimal time) in the 0.017 mol/dm3 PMT solution with distilled water and immediately subjecting it to potentiodynamic polarization measurements (linear and cyclic voltammetry) in a 0.1 mol/dm3 sodium sulfate solution containing different concentrations of chloride ions (5 × 10−2 mol/dm3, 1 × 10−2 mol/dm3, 1 × 10−3 mol/dm3, 1 × 10−4 mol/dm3).
All tests were performed in duplicate.
3. Results and Discussion
3.1. Electrochemical Measurements
Influence of the Concentration of PMT
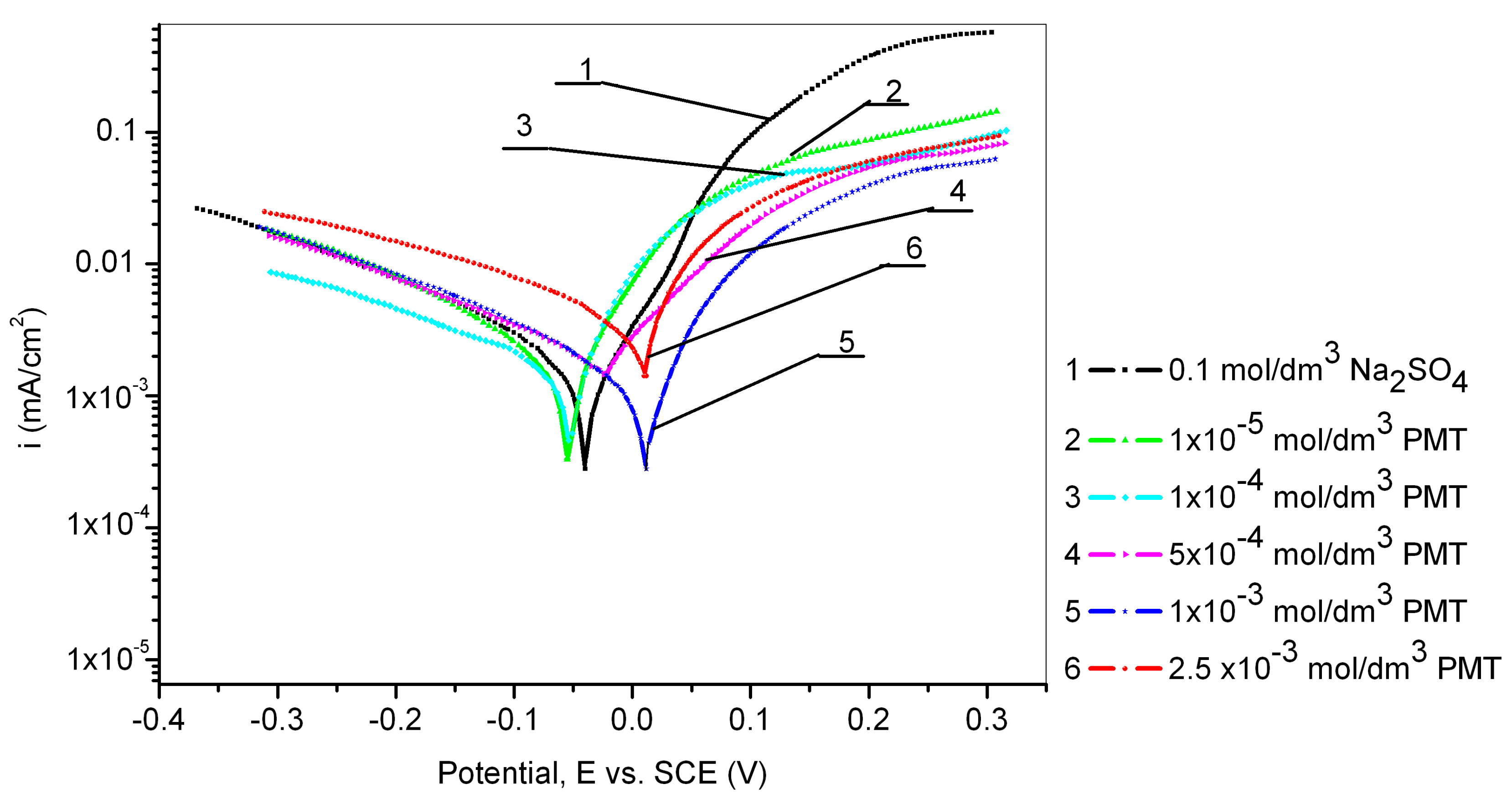
The electrochemical behavior of the Cu24Zn5Al alloy was investigated in a sodium sulfate solution with different concentrations of PMT (1 × 10−5, 1 × 10−4, 5 × 10−4, 1 × 10−3, and 2.5 × 10−3 mol/dm3). The obtained results are shown in Figure 1 and Table 2.
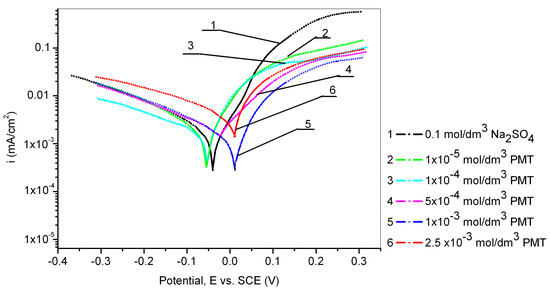
Figure 1.
Potentiodynamic polarization curves of the Cu24Zn5Al alloy in 0.1 mol/dm3 sodium sulfate solution without and with the addition of various concentrations of PMT (scan rate 1 mV/s).

Table 2.
Corrosion parameters of the Cu24Zn5Al alloy in 0.1 mol/dm3 Na2SO4 without and with the addition of various concentrations of PMT.
From Figure 1, it can be observed that the corrosion potential shifts towards positive values with the increase of PMT concentration in the solution [31]. Additionally, all current densities, obtained by the polarization of the Cu24Zn5Al alloy in the solution of sodium sulfate in the presence of different concentrations of PMT inhibitor, have lower values (curves 2, 3, 4, 5, and 6) than the current density obtained by polarization of the Cu24Zn5Al alloy in the solution of sodium sulfate without an inhibitor (curve 1) [1,31,32,33].
This fact indicates that the tested PMT has an inhibitory effect in the vicinity of the corrosion potential [31].
From Table 2, it can be seen that the OCP established on the Cu24Zn5Al alloy in the sodium sulfate solution in the presence of different concentrations of the PMT inhibitor depends on the concentration of the inhibitor. The obtained OCP of the Cu24Zn5Al alloy with PMT concentrations of 5 × 10−4 mol/dm3, 1 × 10−3 mol/dm3, and 2.5 × 10−3 mol/dm3 was more positive than the OCP of the alloy in the blank solution. In the presence of lower PMT concentrations, the OCP values were close to the OCP in the blank solution (Table 2). By comparing these values, it can be said that there is a shift in the OCP in the positive direction, which is consistent with the literature [24]. However, the OCP shift was less than 85 mV, indicating that PMT behaved as a mixed type [1,31,34].
The mechanism of inhibition relies on the creation of a polymer film consisting of Cu-PMT [28,35]. Through the dissolution of the relatively more electronegative zinc and aluminum, the copper content on the surface of the alloy increases, enabling the inhibitor to form a protective film that strongly attaches to the surface by reacting with Cu+ ions [29,36].
The inhibition efficiency (IE) is calculated according to the following equation [1,37]:
where the symbols are:
- jcorr—corrosion current density in the sodium sulfate solution without inhibitor (μA/cm2);
- jcorr(inh)—corrosion current density in the sodium sulfate solution with PMT (μA/cm2).
The electrochemical corrosion parameters of Cu24Zn5Al alloy, such as open circuit potential (OCP), corrosion potential (Ecorr), corrosion current density (jcorr), cathodic and anodic Tafel slopes (βc and βa), inhibition efficiency (IE), are summarized in Table 2. The values for Ecorr, jcorr, βc, and βa were obtained based on the polarization curves shown in Figure 1.
The highest efficiency of corrosion inhibition for the Cu24Zn5Al alloy in 0.1 mol/dm3 sodium sulfate solution was achieved at a PMT concentration of 1 × 10−3 mol/dm3 [29,30]. Further, increase in the PMT concentration led to a diminution in the inhibition efficiency, which can be explained by the fact that an increase in the inhibitor concentration led to a faster formation of the protective layer on the alloy surface, causing the layer to become more porous and less compact, resulting in a reduced protective effect [29,30].
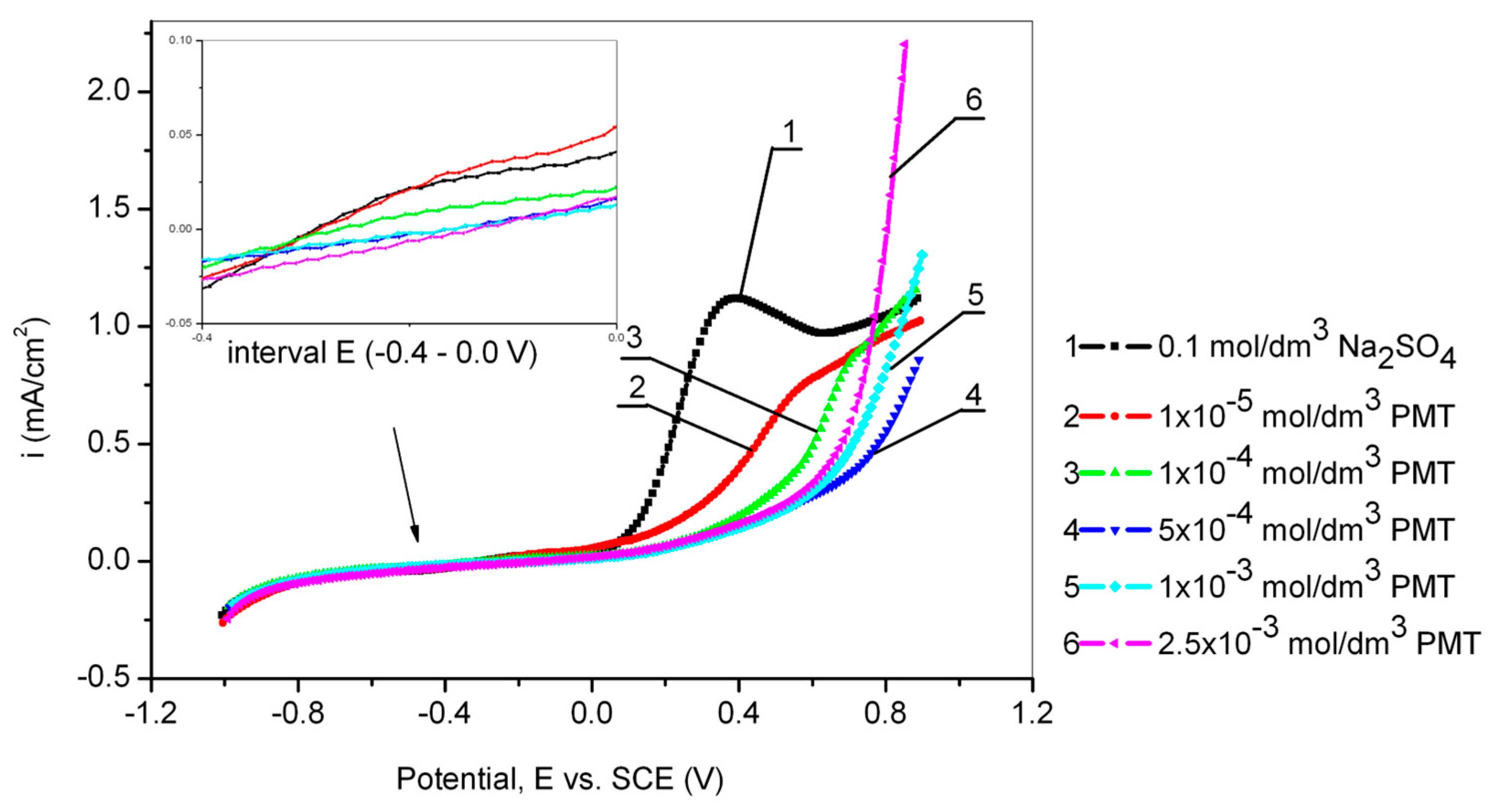
Figure 2 shows the results obtained by cyclic voltammetry from −1 V to 1 V vs. SCE for Cu24Zn5Al alloy in 0.1 mol/dm3 sodium sulfate solution in the presence of different concentrations of the PMT inhibitor (scan rate 10 mV/s).
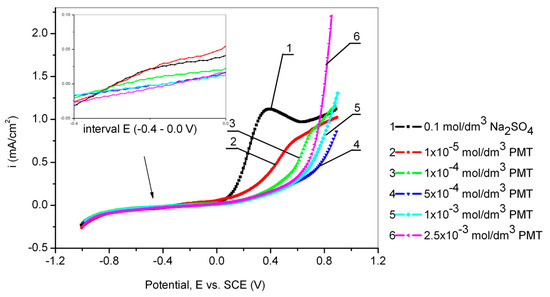
Figure 2.
Anodic curves of the Cu24Zn5Al alloy in 0.1 mol/dm3 sodium sulfate solution without and with the addition of various concentrations of PMT obtained by cyclic voltammetry (scan rate 10 mV/s).
The results obtained by cyclic voltammetry from OCP to 1 V of Cu24Zn5Al alloy in a 0.1 mol/dm3 sodium sulfate solution in the presence of different concentrations of PMT inhibitor (scan rate 10 mV/s) are shown in Figure 3.
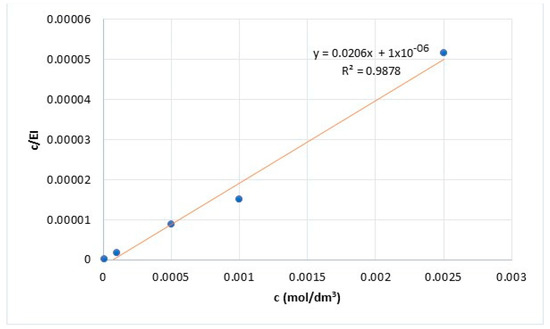
Figure 3.
Langmuir adsorption isotherm model of PMT.
On some polarization curves (curves 1 and 2) in Figure 2, a peak originating from the formation of Cu2O can be observed [38]. This peak is not present on the polarization curves 5 and 6 [38,39], due to the relatively rapid formation of a stable protective film of Cu-PMT, which completely prevents the formation of Cu2O. The creation of the Cu2O protective film in a neutral environment has been confirmed by other authors as well [40,41].
A slight increase in the corrosion current can also be observed in the polarization curves obtained from solutions containing inhibitors at potentials more positive than 0.4 V, compared to the increase in corrosion current obtained in the blank solution, as seen in Figure 2. This can be explained by the disruption of the compactness of the Cu-PMT protective film, which results in its damage [29]. At even higher potentials, greater than 0.5 V, the curve becomes steeper, which concurs with the research of Ye et al. [28], where it was found that complete destruction of the Cu-PMT protective film occurs at potentials greater than 0.6 V. At a potential of 0.8 V, there is a sudden increase in current density, indicating the onset of PMT oxidation in the solution [42,43]. Also, Figure 1 shows that the lowest current density value was obtained at a PMT concentration of 1 × 10−3 mol/dm3. Mihit et al. [44], conducted polarization measurements of brass in 0.2 mol/dm3 HNO3 with and without the addition of PMT and concluded that the anodic current density decreased with increasing PMT concentration. The optimal inhibitor concentration was 1 × 10−3 mol/dm3. At the lowest PMT concentration and up to a potential of 0.3 V, the current density was the highest compared to the current densities in the polarization curves recorded in solutions with higher inhibitor concentrations, indicating that the formation of a protective film on the alloy surface in sodium sulfate solution at neutral pH was relatively slow and thin [28,45,46].
3.2. Adsorption Isotherm
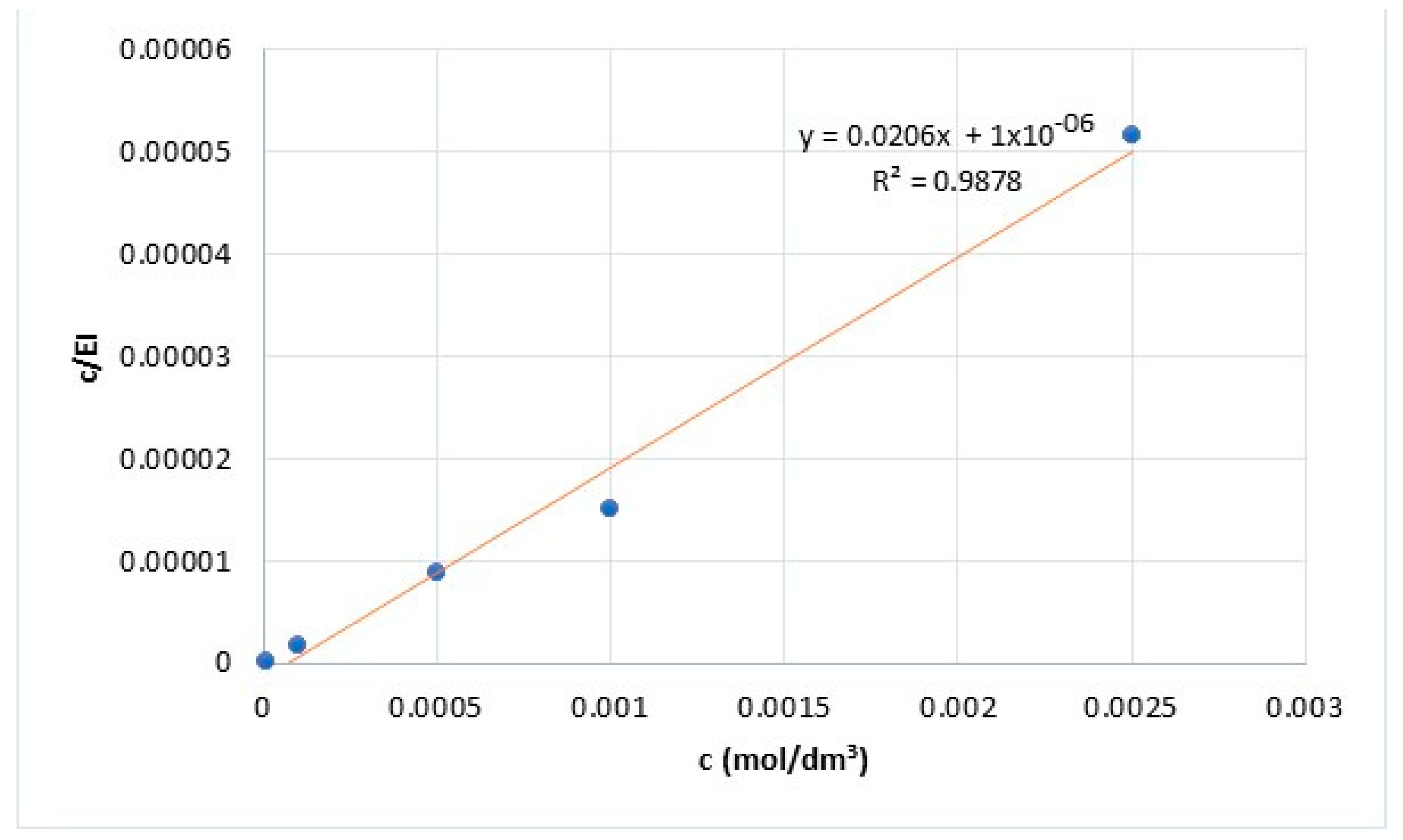
Potentiodynamic polarization measurements provided results for the determination of adsorption mechanisms. The adsorption isotherm that best fits the obtained data was determined. Research results showed that PMT adsorption on the surface of Cu24Zn5Al alloy can best be described by the Langmuir adsorption isotherm [47]. The Langmuir adsorption isotherm was formulated using the following relationships [1,38,44]:
where they are:
- K—adsorptive equilibrium constant;
- ΔG—the Gibbs free energy of adsorption (J/mol);
- c—the concentration of PMT (mol/dm3);
- IE—the inhibition efficiency (%).
Equation (2) can be represented as follows:
The adsorption constant K can be expressed by the following equation:
where the symbols are:
- R—the universal gas constant;
- T—the thermodynamic temperature (293 K).
Based on Equation (2) and Figure 3 the adsorption constant K is calculated. Further, the Gibbs free energy of PMT adsorption in the sodium sulfate solution is calculated based on Equation (6). The calculated value of the Gibbs free energy of PMT adsorption in the sodium sulfate solution was ΔG = −43.44 kJ/mol. The obtained value of the ΔG indicates spontaneous PMT adsorption on the surface of the Cu24Zn5Al alloy. Also, based on the value of the Gibbs free energy, it can be concluded that the chemisorption of the inhibitor occurs on the surface of the Cu24Zn5Al alloy in a neutral Na2SO4 solution [1,48,49,50].
3.3. The Effect of Chloride Ion Concentration
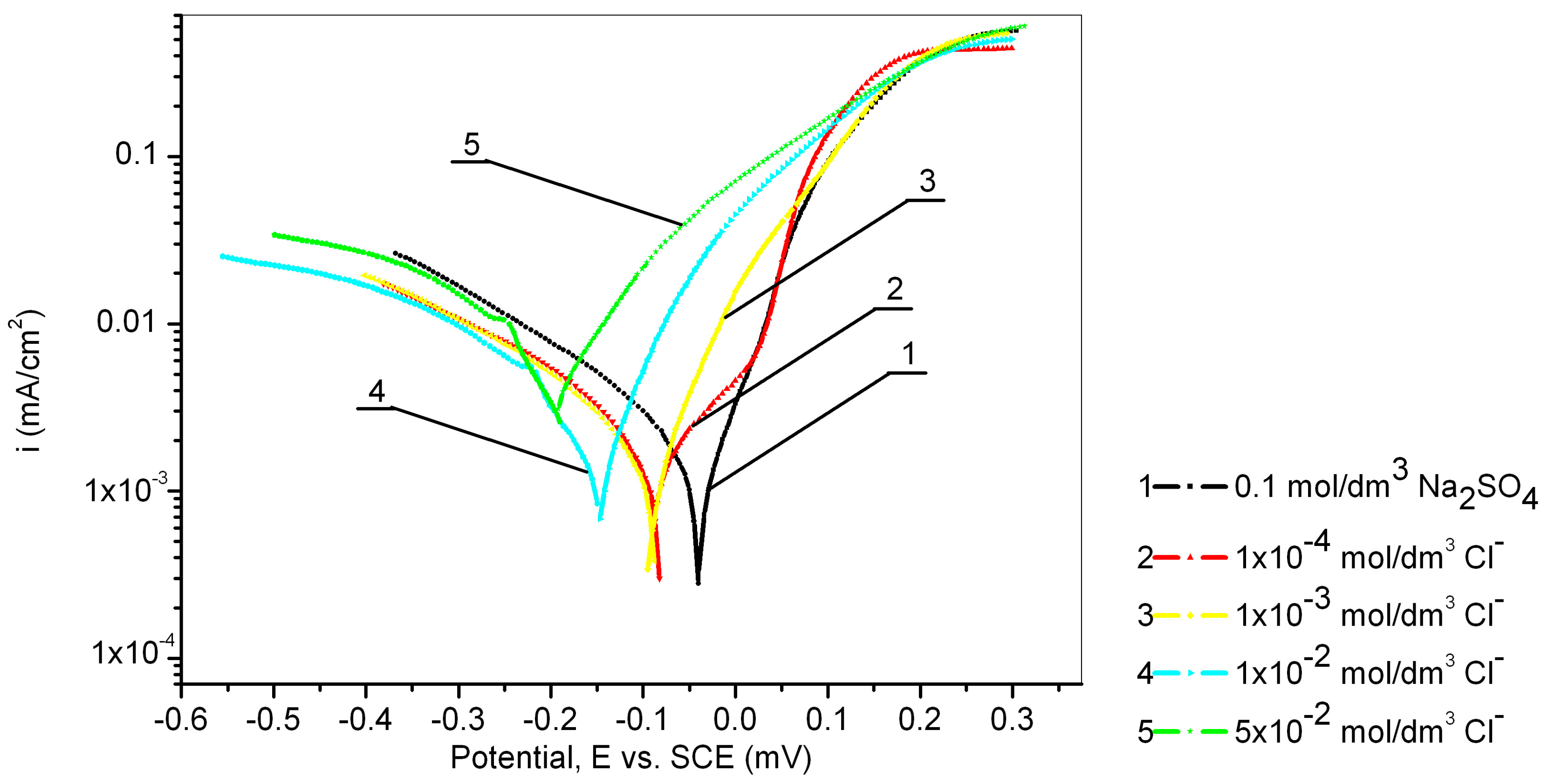
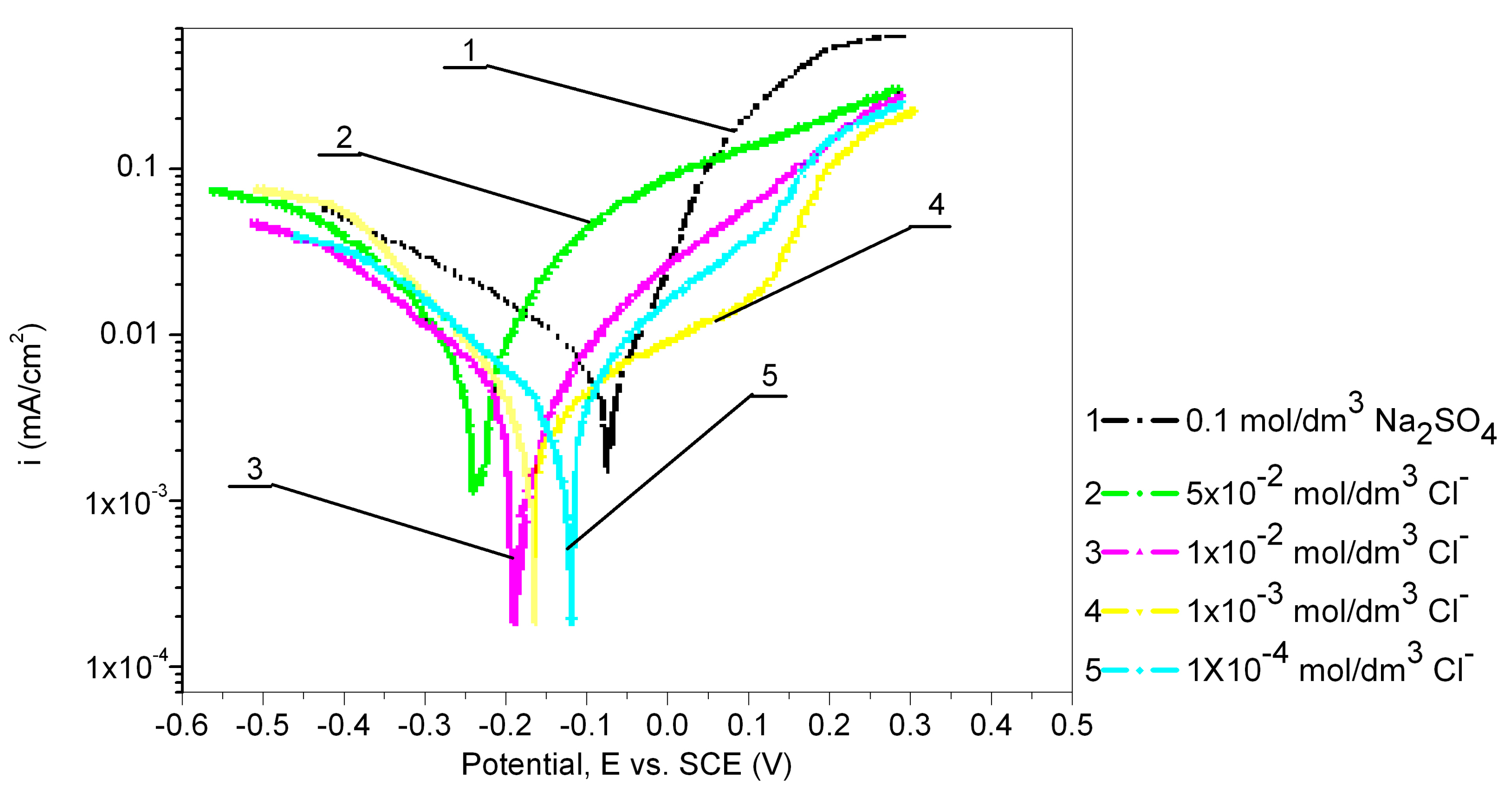
The electrochemical behavior of the Cu24Zn5Al alloy was investigated in a 0.1 mol/dm3 sodium sulfate solution containing various concentrations of chloride ions (1 × 10−4, 1 × 10−3, 1 × 10−2, and 5 × 10−2 mol/dm3), and the obtained results are presented in Figure 4 and Table 3. Figure 4 shows that all recorded anodic currents of the Cu24Zn5Al alloy in solutions containing chloride ions are higher than the anodic current observed in sodium sulfate solutions without Cl− ions.
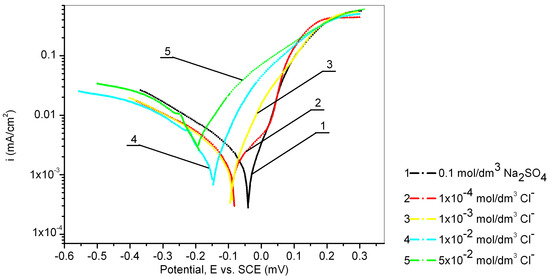
Figure 4.
Potentiodynamic polarization curves of Cu24Zn5Al alloy in 0.1 mol/dm3 sodium sulfate solution with the addition of various concentration of chloride ions (scan rate 1 mV/s).

Table 3.
Corrosion parameters of Cu24Zn5Al alloy in 0.1 mol/dm3 Na2SO4 in the presence of various concentrations of Cl− ions.
It was also observed that the OCP shifts in the negative direction with the increasing concentration of chloride ions.
The observed behavior of the Cu24Zn5Al alloy in 0.1 mol/dm3 Na2SO4 containing chloride ions can be explained through Reactions (7)–(19) that occur at the cathode and anode.
The cathodic reaction represents the reduction of dissolved oxygen according to the following reaction [1,37,50,51,52,53]:
The main anodic reaction of Cu24Zn5Al alloy in 0.1 mol/dm3 Na2SO4 containing chloride ions can be represented by the following equations [1,38,51,52,53,54,55,56]:
With prolonged immersion time in the solution containing dissolved oxygen copper, from the surface of the Cu24Zn5Al alloy, it oxidizes according to the following reactions [50,51,52,53,54,55]:
At the same time, the dezincification process takes place according to the following equation [50,51,54]:
As well as the oxidation of zinc which can lead to passivation by the formation of a zinc oxide layer on the surface of the alloy according to the following equation [50,51,54]:
Due to the presence of aluminum as an alloy element, additional passivation occurs due to the dissolution of aluminum and the formation of an aluminum oxide layer on the surface of the alloy according to the following equations [50,51,53,54,55]:
The aluminum oxide film can protect the surface of the alloy from severe corrosion, but it also dissolves in chloride solutions, according to the following equations [51]:
or
The anodic reactions according to Equations (9), (13), (15)–(17) or (18), and (19) result in the release of hydrogen ions, which causes local acidity and further promotes surface dezincation [36] and dealumination reactions, leading to a potential shift in the negative direction and increased corrosion currents compared to the solution without chloride ions present [32].
According to this, the surface becomes enriched in copper content and Equations (8) and (9) become dominant.
The electrochemical corrosion parameters of Cu24Zn5Al alloy in a 0.1 mol/dm3 sodium sulfate solution containing various concentrations of Cl− ions are presented in Table 3. The values of Ecorr, jcorr, βc, and βa were obtained based on the polarization curves shown in Figure 4.
Based on the results presented in Table 3, it can be concluded that the presence of chloride ions at concentrations of 1 × 10−4 mol/dm3, 1 × 10−3 mol/dm3, and 1 × 10−2 mol/dm3 has an inhibitory effect, as the corrosion current obtained in these solutions is lower than that obtained in the 0.1 mol/dm3 sodium sulfate solution, while the concentration of chloride ions of 5 × 10−2 mol/dm3 has a significant influence on increasing the corrosion current of the Cu24Zn5Al alloy. The inhibitory effect of the present chloride ions has been confirmed by other authors as well [35,56,57].
3.4. The Influence of the Immersion Time of Cu24Zn5Al Alloy in a Solution of 0.017 mol/dm3 PMT
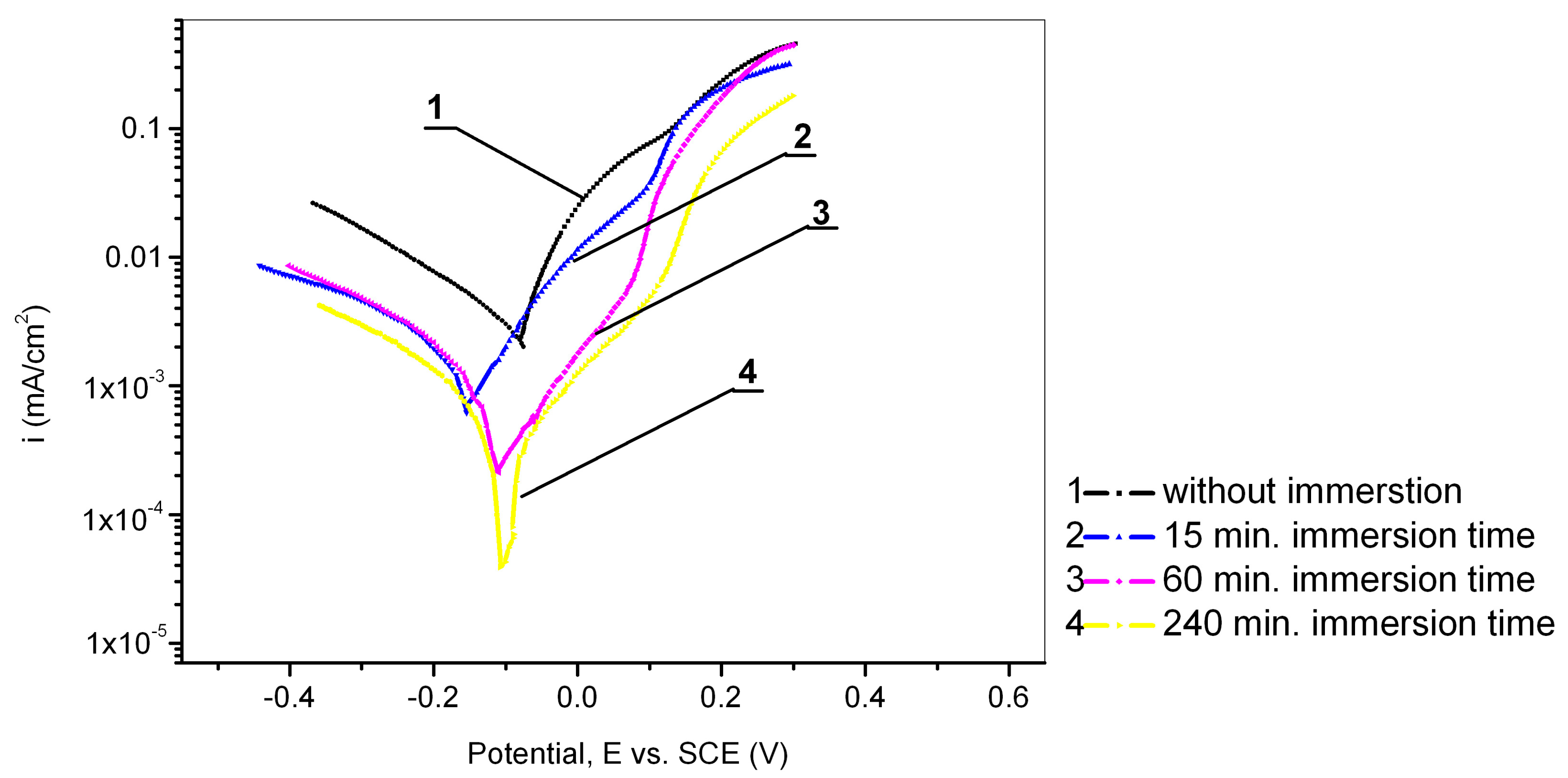
The influence of the immersion time was investigated by immersing the Cu24Zn5Al electrode in a 0.017 mol/dm3 PMT solution for a specific time (15 min, 60 min, and 240 min). Afterwards, the electrode was taken out, washed with distilled water and alcohol, and moved into an electrochemical cell where open circuit potential (OCP) was determined, followed by polarization testing in a sodium sulfate solution with a concentration of 0.1 mol/dm3. The results of these experiments are shown in Figure 5 and Table 4. Based on the recorded OCP values, it can be said that the OCPs after immersion in the inhibitor solution for 15 min, 60 min, and 240 min (−0.035 V, −0.045 V, and −0.035 V) were close to the OCP value of the electrode obtained without the pretreatment (−0.030 V). This confirms the assertion that a Cu2O film was formed on the surface of the copper alloy in a neutral medium [41], which has a similar role as the film formed during immersion in the PMT solution [58]. From Figure 6, it can be seen that the lowest current densities were obtained when the Cu24Zn5Al alloy was immersed in the inhibitor solution for 240 min. By analyzing the results shown in Figure 1 and Figure 5, it can be concluded that the current densities in the case when the electrode was pretreated in the inhibitor solution for a period of 15 min do not differ significantly from the current densities when the Cu24Zn5Al alloy was in the solution of PMT. This also supports the claim that the Cu-PMT protective layer forms more slowly in neutral sodium sulfate solutions. However, the inhibition efficiency was higher with immersion times of 60 min and 240 min compared to the maximum inhibition efficiency value recorded in solutions with the inhibitor present. This observation supports the fact that the thickness of the Cu-PMT protective film formed on the surface of the alloy increases over time [30,50,59].
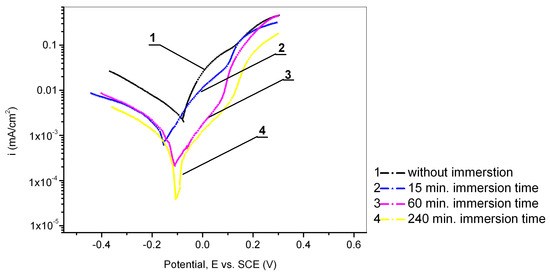
Figure 5.
Potentiodynamic polarization curves of Cu24Zn5Al alloy in 0.1 mol/dm3 Na2SO4 solution: 1—without pretreatment, and with different immersion time of electrode in 0.017 mol/dm3 PMT: 2—15 min, 3—60 min, 4—240 min.

Table 4.
Electrochemical parameters of Cu24Zn5Al alloy in 0.1 mol/dm3 Na2SO4 without and after pretreatment in a 0.017 mol/dm3 PMT solution.
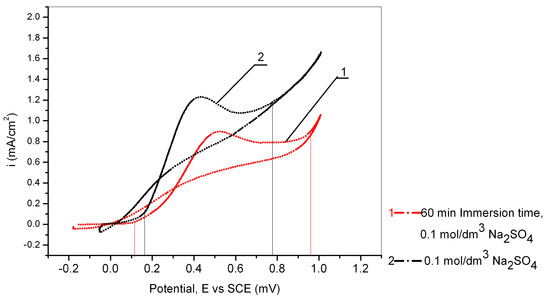
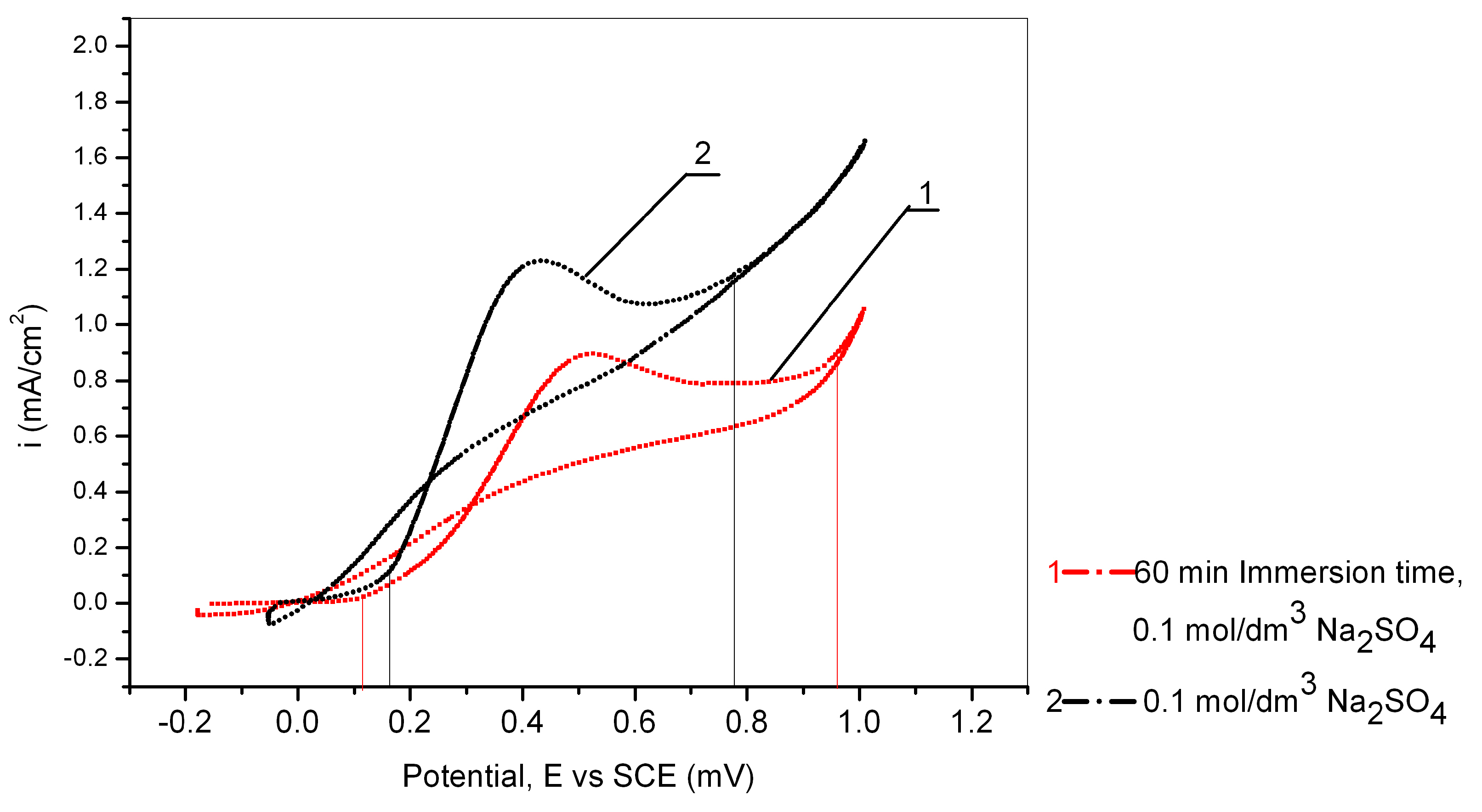
Figure 6.
Cyclic voltametric curves of Cu24Zn5Al without pretreatment and with pretreatment of 60 min in 0.017 mol/dm3 PMT solution (scan rate 10 mV/s).
The OCP of Cu24Zn5Al alloy after 15 min, 60 min, and 240 min of immersion in the solution of sodium sulfate varies from −0.045 V to −0.035 V, which is close to the OCP value of Cu24Zn5Al alloy without immersion in the sodium sulfate solution, −0.030 V. The OCP of the Cu-PMT protective film is similar to the OCP of Cu24Zn5Al alloy in the sodium sulfate solution.
The inhibition efficiency (IE) is calculated according to the following equation:
where they are:
- jcorr—corrosion current density obtained in sodium sulfate solution without pretreatment (μA/cm2);
- jcorr(immersion)—current density obtained in the sodium sulfate solution after pretreatment in the 0.017 mol/dm3 PMT solution (μA/cm2).
Electrochemical corrosion parameters of Cu24Zn5Al alloy such as open circuit potential (OCP), corrosion potential (Ecorr), corrosion current density (jcorr), cathodic and anodic Tafel slopes (βc and βa), and inhibition efficiency (IE) are also shown in Table 4. The values for Ecorr, jcorr, βc, and βa were obtained based on the polarization curves shown in Figure 5.
The effect of Cu24Zn5Al alloy immersing in a solution of 0.017 mol/dm3 PMT can also be observed from Figure 6, which shows the results obtained by cyclic voltammetry from a potential of −1 V to 1 V (vs. SCE).
Figure 6 clearly shows a shift of the peak originating from the formation of Cu2O towards more positive potentials, as well as a decrease in anodic current densities obtained by recording the behavior of the Cu24Zn5Al alloy that had previously been allowed to stand for 60 min in 0.017 mol/dm3 PMT solution. This clearly shows the formation of a protective Cu-PMT film on the surface of the alloy during its standing in a PMT solution with a concentration of 0.017 mol/dm3 [58].
The inhibition efficiency (IE) is calculated according to the following equation:
where they are:
- —the area provided by integrated curve obtained in the sodium sulfate solution without pretreatment (mV mA/cm2);
- —the area provided by integrated curve obtained in the sodium sulfate solution after pretreatment in the 0.017 mol/dm3 PMT solution (μA/cm2).
The values for area under each peak are provided by integrating the curves in Figure 6 in range of potential from 0 mV to 1 mV and from 0 mV to 0.3 mV. Results are shown in Table 5.

Table 5.
The inhibition efficiency (IE) of Cu24Zn5Al without pretreatment and with pretreatment of 60 min in 0.017 mol/dm3 PMT solution in different range of potential.
We obtained the inhibition efficiency (IE) by integrating the curves in Figure 6 in range of potential from 0 mV to 0.3 mV confirm data for inhibition efficiency (IE) given in Table 4 and Figure 5.
The Effect of Chloride Ion Concentration
The electrochemical behavior of the Cu24Zn5Al alloy was tested in a 0.1 mol/dm3 sodium sulfate solution containing various concentrations of chloride ions (1 × 10−4, 1 × 10−3, 1 × 10−2, and 5 × 10−2 mol/dm3), and after 60 min of immersing in a 0.017 mol/dm3 PMT solution. The results obtained are shown in Figure 7 and Table 5. As seen in Figure 7, an increase in the concentration of chloride ions leads to a shift in the potential of Cu24Zn5Al in the negative direction [32,60] after 60 min of immersion in a 0.017 mol/dm3 PMT solution. The current density is higher in all solutions containing chloride ions in comparison to the current density in the sodium sulfate solution measured under the same conditions, but significantly lower than the current densities obtained by polarization in solutions with chloride ions of the same concentration, without 60 min of immersion in a 0.017 mol/dm3 PMT solution. The results obtained in this way indicate the influence and importance of the process of immersing the alloy in the inhibitor solution.
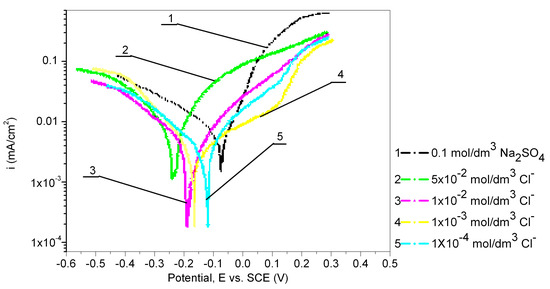
Figure 7.
Potentiodynamic polarization curves of Cu24Zn5Al alloy in 0.1 mol/dm3 Na2SO4 in the presence of various chloride ions concentrations, after 60 min pretreatment in 0.017 mol/dm3 PMT (scan rate 1 mV/s).
The degree of corrosion current density reduction (%) is calculated according to the following equations:
where they are:
- Sjcorr—the degree of corrosion current reduction (%);
- —corrosion current density obtained in sodium sulfate solution (μA/cm2);
- jcorr(immersion)—current density obtained in the sodium sulfate solution without and with the presence of different concentrations of chloride ions, after the immersion time of 60 min in a solution of 0.017 mol/dm3 PMT (μA/cm2).
And
where they are:
- —the degree of corrosion current reduction (%);
- —corrosion current density obtained in sodium sulfatase solution with different concentrations of chloride ions (μA/cm2);
- —current density obtained in sodium sulfate solution with different concentrations of chloride ions, and after the immersion time of 60 min in 0.017 mol/dm3 PMT solution (μA/cm2).
Electrochemical corrosion parameters of the Cu24Zn5Al alloy, such as open circuit potential (OCP), corrosion potential (Ecorr), corrosion current density (jcorr), cathodic and anodic Tafel slopes (βc and βa), and corrosion current reduction efficiency (S) are presented in Table 6. The values for Ecorr, jcorr, βc, and βa were obtained based on the polarization curves shown in Figure 7.

Table 6.
Electrochemical corrosion parameters of the Cu24Zn5Al alloy in 0.1 mol/dm3 Na2SO4 without and with the presence of chloride ions, without and after 60 min immersion time in 0.017 mol/dm3 PMT.
Based on the results presented in Table 6, it can be concluded that by immersing the Cu24Zn5Al alloy in a 0.017 mol/dm3 PMT solution, there is an additional influence on the reduction of the corrosion current density compared to the results presented in Table 3, which shows the electrochemical corrosion parameters of the Cu24Zn5Al alloy in a 0.1 mol/dm3 sodium sulfate solution with varying concentrations of chloride ions, without prior immersing.
4. Conclusions
Polarization measurements have shown that with the increase of the PMT concentration, the anodic current density around the corrosion potential decreases, indicating that PMT behaves as a corrosion inhibitor for the Cu24Zn5Al alloy.
The investigation that involved immersing the alloy in a 0.017 mol/dm3 inhibitor solution suggests the formation of a protective layer (Cu-PMT).
The presence of chloride ions in the tested solutions has an impact on the corrosion behavior of the Cu24Zn5Al alloy, causing a negative shift in the corrosion potential and an increase in the current density as the concentration of chloride ions rises.
The investigation that involved immersing the alloy in a 0.017 mol/dm3 inhibitor solution and testing it in sodium sulfate solutions with different concentrations of chloride indicates the formation of a protective layer (Cu-PMT), as the current densities measured in chloride solutions with prior immersion of the alloy in the inhibitor solution are lower than those measured in chloride solutions of the same concentrations without immersion in the inhibitor solution.
Author Contributions
Conceptualization, V.G. and M.M.A.; methodology, M.M.A.; validation, Ž.Z.T., M.B.P.M. and M.B.R.; investigation, V.G.; resources, V.G.; data curation, M.B.P.M. and M.B.R.; writing—V.G.; visualization, Ž.Z.T.; supervision, M.M.A.; project administration, M.B.P.M. and M.B.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Ministry of Science and Technological Development and Innovations of the Republic of Serbia, Grant No. 451-03-47/2023-01/200052.
Data Availability Statement
Data availability is not applicable to this article as no new data were created or analysed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A.R. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Liu, H.X.; Si, N.C.; Xu, G.F. Influence of process factors on shape memory effect of CuZnAl alloys. Trans. Nonferrous Met. Soc. China 2006, 16, 1402–1409. [Google Scholar] [CrossRef]
- Guerioune, M.; Amiour, Y.; Bounour, W.; Guellati, O.; Benaldjia, A.; Amara, A.; Chakri, N.E.; Ali-Rachedi, M.; Vrel, D. SHS of Shape Memory CuZnAl Alloys. Int. J. Self-Propagating High-Temp. Synth. 2008, 17, 41–48. [Google Scholar] [CrossRef]
- Jani, J.M.; Leary, M.; Subic, A.; Gibson, M.A. A review of shape memory alloy research, applications and opportunities. Mater. Des. 2014, 56, 1078–1113. [Google Scholar] [CrossRef]
- De Filippo, B.; Brotzu, A.; Natali, S. Corrosion Behavior of Cu-Zn-Al Shape Memory Alloy in Controlled Environments. AIP Conf. Proc. 2020, 2257, 020013. [Google Scholar] [CrossRef]
- Cubela, D. Shape Memory Alloys. Masinstvo 2002, 2, 83–92. [Google Scholar]
- Huang, W. Shape Memory Alloys and their Application to Actuators for Deployable Structures. Ph.D. Dissertation, Department of Engineering University of Cambridge, Cambridge, MA, USA, 1998. [Google Scholar]
- Subramanian, A. Fatigue Behavior of Copper Zinc Aluminum Shape Memory Alloys. Master’s Thesis, The Faculty of Graduate Studies, University of Manitoba, Winnipeg, MB, Canada, 1998. [Google Scholar]
- Agnihotri, R.; Bhardwaj, S. Synthesis and Characterization of Cuznal Based Shape Memory Alloys and to Optimize Behavior on Different Properties by Varying Weight Percentage. Int. J. Mater. Sci. Eng. 2016, 4, 229–234. [Google Scholar]
- Wang, Y.; Jing, T.; Peng, H.; He, W.; Yan, J.; Wang, S.; Li, N.; Wen, Y. Re-examination of martensitic stabilization in Cu-based shape memory alloys Part I. Identification of occurrence stage and degree of martensitic stabilization in CuZnAl alloys. J. Alloys Compd. 2022, 913, 165276. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Okotete, E.A.; Oluwafemi, A.; Inyang, U. Assessment of the mechanical behaviour of thermally aged B and Fe modified CuZnAl shape memory alloys. Rev. De Metal. 2019, 55, 1–8. [Google Scholar] [CrossRef]
- Sampath, V. Improvement of Shape-Memory Characteristics and Mechanical Properties of Copper-Zinc-Aluminum Shape-Memory Alloy with Low Aluminum Content by Grain Refinement. Mater. Manuf. Process. 2006, 21, 789–795. [Google Scholar] [CrossRef]
- Sathish, S.; Mallik, U.S.; Raju, T.N. Corrosion Behavior of Cu-Zn-Ni Shape Memory Alloys. J. Miner. Mater. Charact. Eng. 2013, 1, 49–54. [Google Scholar] [CrossRef]
- Majed, R.A.; Elia, S.M.; Fadhel, Z.; Mohamed, D. Effect of Halogen Ions on the Corrosion of Brass in Na2SO4 Solution. Eng. Technol. J. 2010, 28, 1386–1395. [Google Scholar]
- Vrsalović, L.; Ivanić, I.; Čudina, D.; Lokas, L.; Kožuh, S.; Gojić, M. The Influence of Chloride Ion Concentration on the Corrosion Behavior of the Cualni Alloy. Tech. Bull. 2017, 11, 67–72. [Google Scholar]
- Qafsaou, W.; Blanc, C.; Peabeare, N.; Srhiri, A.; Mankowski, G. Study of different triazole derivative inhibitors to protect copper against pitting corrosion. J. Appl. Electrochem. 2000, 8, 959–966. [Google Scholar] [CrossRef]
- Lalitha, A.; Ramesh, S.; Rajeswari, S. Surface protection of copper in acid medium by azoles and surfactants. Electrochim. Acta 2005, 51, 47–55. [Google Scholar] [CrossRef]
- Gomma, G.K. Effect of azole compounds on corrosion of copper in acid medium. Mater. Chem. Phys. 1998, 56, 27–34. [Google Scholar] [CrossRef]
- Warraky, A.A.E. The effect of sulphide ions on the corrosion inhibition of copper in acidic chloride solutions. Anti-Corros. Methods Mater. 2003, 50, 40–46. [Google Scholar] [CrossRef]
- Ehteshamzade, M.; Shahrabi, T.; Hosseini, M.G. Inhibition of copper corrosion by self-assembled films of new Schiff bases and their modification with alkanethiols in aqueous medium. Appl. Surf. Sci. 2006, 252, 2949–2959. [Google Scholar] [CrossRef]
- Ma, H.; Chen, S.; Niu, L.; Zhao, S.; Li, S.; Li, D. Inhibition of copper corrosion by several Schiff bases in aerated halide solutions. Appl. Electrochem. 2002, 32, 65–72. [Google Scholar] [CrossRef]
- Sherif, E.M.; Park, S.M. Inhibition of copper corrosion in acidic pickling solutions by N-phenyl-1,4-phenylenediamine. Electrochim. Acta 2006, 51, 4665–4673. [Google Scholar] [CrossRef]
- Lisac, E.S.; Brnada, A.; Mance, A.D. Secondary amines as copper corrosion inhibitors in acid media. Corros. Sci. 2000, 42, 243–257. [Google Scholar] [CrossRef]
- Matos, J.B.; Pereira, L.P.; Agostinho, S.M.L.; Barcia, O.E.; Cordeiro, G.G.O.; D’Elia, E. Effect of cysteine on the anodic dissolution of copper in sulfuric acid medium. J. Electroanal. Chem. 2004, 570, 91–94. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, L.; Zhou, G. Inhibition of copper corrosion in aerated hydrochloric acid solution by amino-acid Compounds. J. Appl. Electrochem. 2005, 35, 1081–1085. [Google Scholar] [CrossRef]
- Scendo, M. The effect of purine on the corrosion of copper in chloride solutions. Corros. Sci. 2007, 49, 373–390. [Google Scholar] [CrossRef]
- Subramanian, R.; Lakshminarayanan, V. Effect of adsorption of some azoles on copper passivation in alkaline medium. Corros. Sci. 2002, 44, 535–554. [Google Scholar] [CrossRef]
- Ye, X.R.; Xin, X.Q.; Zhu, J.J.; Xue, Z.L. Coordination compound films of 1-phenyl-5-mercaptotetrazole on copper surface. Appl. Surf. Sci. 1998, 135, 307–317. [Google Scholar] [CrossRef]
- Szocs, E.; Vastag, G.; Shaban, A.; Kalman, E. Electrochemical behavior of an inhibitor film formed on copper surface. Corros. Sci. 2005, 47, 893–908. [Google Scholar] [CrossRef]
- Szocs, E.; Vastag, G.; Shaban, A.; Konczos, G.; Kalman, E. Investigation of copper corrosion inhibition by STM and EQCM techniques. J. Appl. Electrochem. 1999, 29, 1339–1345. [Google Scholar] [CrossRef]
- Pi, J.; Chen, M.; Chen, T.; Wang, Q.; Cheng, S.; Fu, C. Corrosion inhibition effect of 1-phenyl-5-mercaptotetrazole on nickel-aluminum bronze in seawater: A combined experimental and theoretical study. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131354. [Google Scholar] [CrossRef]
- Altaf, F. Effect of Inhibitors on the Corrosion of Copper and its Alloys by Using Electrochemical Impedance Spectroscopy and Cyclic Voltammetry. Ph.D. Thesis, Department of Chemistry, Quaid-i-Azam University, Islamabad, Pakistan, 2013. [Google Scholar]
- Wang, J.L.; Wu, Y.G.; Liu, J. Effectiveness of Corrosion Inhibitors on Bronze and Cast Iron with Prefilming Treatment. Int. J. Electrochem. Sci. 2013, 8, 4631–4640. [Google Scholar] [CrossRef]
- Al-Otaibi, M.S.; Al-Mayouf, A.M.; Khan, M.; Mousa, A.A.; Al-Mazroa, S.A.; Alkhathlan, H.Z. Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media. Arab. J. Chem. 2014, 7, 340–346. [Google Scholar] [CrossRef]
- Hrimla, M.; Bahsis, L.; Laamari, M.R.; Julve, M.; Stiriba, S.E. An Overview on the Performance of 1,2,3-Triazole Derivatives as Corrosion Inhibitors for Metal Surfaces. Int. J. Mol. Sci. 2022, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Mihit, M.; Laarej, K.; El Makarim, H.A.; Bazzi, L.; Salghi, R.; Hammouti, B. Study of the inhibition of the corrosion of copper and zinc in HNO3 solution by electrochemical technique and quantum chemical calculations. Arab. J. Chem. 2010, 3, 55–60. [Google Scholar] [CrossRef]
- Rao, B.V.A.; Reddy, M.N. Formation, characterization and corrosion protection efficiency of self-assembled 1-octadecyl-1H-imidazole films on copper for corrosion protection. Arab. J. Chem. 2017, 10, S3270–S3283. [Google Scholar] [CrossRef]
- Radovanović, M. Influence of Organic Inhibitors on Corrosion Behavior of Brass in Sodium Sulfate Solution. Ph.D. Thesis, University of Belgrade—Technical Faculty Bor, Bor, Serbia, 2012. [Google Scholar]
- Antonijevic, M.M.; Milic, S.M. Electrochemical behavior of Cu24Zn5Al alloy in alkaline medium in the presence of chloride ions and benzotriazole. Mater. Chem. Phys. 2009, 118, 385–391. [Google Scholar] [CrossRef]
- Huković, M.M.; Babić, R.; Paić, I. Copper corrosion at various pH values with and without the inhibitor. J. Appl. Electrochem. 2000, 30, 617–624. [Google Scholar] [CrossRef]
- Benzbiria, N.; Zertoubi, M.; Azzi, M. Oxygen reduction reaction kinetics on pure copper in neutral sodium sulfate solution. SN Appl. Sci. 2020, 2, 2101. [Google Scholar] [CrossRef]
- Gardic, V.; Gupta, V.; Antonijevic, M. Corrosion behaviour of Cu24Zn5Al alloy in a sodium tetraborate solution in the presence of 1-phenyl-5-mercaptotetrazole. Indian J. Chem. Technol. 2014, 21, 350–358. [Google Scholar]
- Kozlica, D.K.; Kokalj, A.; Milosev, I. Corrosion inhibition of copper and aluminium by 2-mercaptobenzimidazole and octylphosphonic acid—Surface pre-treatment and method of film preparation. Electrochim. Acta 2022, 431, 141154. [Google Scholar] [CrossRef]
- Mihit, M.; ElIssami, S.; Bouklah, M.; Bazzi, L.; Hammouti, B.; AitAddi, E.; Salghi, R.; Kertit, S. The inhibited effect of some tetrazolic compounds towards the corrosion of brass in nitric acid solution. Appl. Surf. Sci. 2006, 252, 2389–2395. [Google Scholar] [CrossRef]
- Kozlica, D.K.; Ekar, J.; Kovač, J.; Milošev, I. Roles of Chloride Ions in the Formation of Corrosion Protective Films on Copper. J. Electrochem. Soc. 2021, 168, 031504. [Google Scholar] [CrossRef]
- Kozlica, D.K.; Kokalj, A.; Milosev, I. Synergistic effect of 2-mercaptobenzimidazole and octylphosphonic acid as corrosion inhibitors for copper and aluminium—An electrochemical, XPS, FTIR and DFT study. Corros. Sci. 2021, 182, 109082. [Google Scholar] [CrossRef]
- Kokalj, A. On the use of the Langmuir and other adsorption isotherms in corrosion inhibition. Corros. Sci. 2023, 2017, 111112. [Google Scholar] [CrossRef]
- Yu, P.; Liao, D.M.; Luo, Y.B.; Chen, Z.G. Studies of Benzotriazole and Tolytriazole as Inhibitors for Copper Corrosion in Deionized Water. Corrosion 2003, 59, 314–318. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Tan, B.; Wang, F.; Du, H.; Liu, R.; Han, X.; Zhang, S. Corrosion inhibition effect of benzimidazole and two derivatives on copper in alkaline environments: Experimental and theoretical analyses. J. Mol. Liq. 2023, 390, 122985. [Google Scholar] [CrossRef]
- Yin, M.; Li, Z.; Xiao, Z.; Pang, Y.; Li, Y.; Shen, Z. Corrosion behavior of Cu-Al-Mn-Zn-Zr shape memory alloy in NaCl solution. Trans. Nonferrous Met. Soc. China 2021, 31, 1012–1022. [Google Scholar] [CrossRef]
- Nady, H.M.; El-Rabiei, M.M.; Badawy, W.A. Stability of Some Copper Ternary Alloys in Chloride Solutions Polluted by Sulfide Ions. Chem. Process Eng. Res. 2014, 20, 35–44. [Google Scholar]
- Feng, Y.; Teo, W.K.; Siow, K.S.; Tag, K.L.; Hsieh, A.K. The Corrosion Behaviour of Copper in Neutral Tap Water. Part I: Corrosion Mechanisms. Corros. Sci. 1996, 38, 369–385. [Google Scholar] [CrossRef]
- Gudić, S.; Vrsalović, L.; Radeljić, A.; Oguzie, E.E.; Ivanić, I.; Kožuh, S.; Gojić, M. Comparison of Corrosion Behavior of Copper and Copper Alloys in Aqueous Chloride Solution. Chem. Ind. Chem. Eng. Q. 2021, 27, 383–394. [Google Scholar] [CrossRef]
- Nady, K.; El-Rabiei, M.M.; El-Hafez, G.M.A.; Tribo, J.B. Electrochemical Stability of Cu–10Al–10Zn, Cu–10Al–10Ni, and Cu–10Ni–10Zn Ternary Alloys in Simulated Physiological Solutions. J. Bio Tribo-Corros. 2016, 2, 28. [Google Scholar] [CrossRef]
- Shaik, M.A.; Syed, K.H.; Golla, B.R. Electrochemical behavior of mechanically alloyed hard Cu-Al alloys in marine environment. Corros. Sci. 2019, 153, 249–257. [Google Scholar] [CrossRef]
- Brotzu, A.; Filippo, B.D.; Natali, S.; Zortea, L. Corrosion behavior of Shape Memory Alloy in NaCl environment and deformation recovery maintenance in Cu-Zn-Al system. Frat. Ed Integrità Strutt. 2022, 62, 64–74. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, R.; Qin, Z.; Wu, Z.; Wang, L.; Liu, L.; Lu, W. Evolution of the Corrosion Product Film on Nickel-Aluminum Bronze and Its Corrosion Behavior in 3.5 wt % NaCl Solution. Materials 2019, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Al-Mobarak, N.A.; Khaled, K.F.; Hamed, M.N.H.; Abdel-Azim, K.M. Employing electrochemical frequency modulation for studying corrosion and corrosion inhibition of copper in sodium chloride solutions. Arab. J. Chem. 2011, 4, 185–193. [Google Scholar] [CrossRef]
- Khiatia, Z.; Othmanc, A.A.; Morenoa, M.S.; Bernarda, M.C.; Joireta, S.; Suttera, E.M.M.; Viviera, V. Corrosioninhibitionofcopperinneutralchloridemediabyanovelderivative of 1,2,4-triazole. Corros. Sci. 2011, 53, 3092–3099. [Google Scholar] [CrossRef]
- Yanga, J.; Zenga, y.; Zhua, M.; Liub, L.; Mengc, Y.; Chena, Y.; Maod, S. Study on effect of chloride and temperature on corrosion behavior of CoCrFeMnNi high entropy alloy. Int. J. Electrochem. Sci. 2023, 18, 100132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).