Effect of Indium on the Properties of Mg-Zn-Based Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- (1)
- Indium enhances the properties of Mg-20Zn alloys—both the corrosion and the hardness;
- (2)
- Samples with the addition of indium and milled for 36 h exhibit the best combination of properties;
- (3)
- All samples containing indium offer a better corrosion resistance than the unmodified Mg-20Zn alloy.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of Magnesium-Based Biomaterials and Their Applications. J. Magnes. Alloys 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Amukarimi, S.; Mozafari, M. Biodegradable Magnesium-based Biomaterials: An Overview of Challenges and Opportunities. MedComm 2021, 2, 123–144. [Google Scholar] [CrossRef]
- Mohamad Rodzi, S.N.H.; Zuhailawati, H.; Dhindaw, B.K. Mechanical and Degradation Behaviour of Biodegradable Magnesium–Zinc/Hydroxyapatite Composite with Different Powder Mixing Techniques. J. Magnes. Alloys 2019, 7, 566–576. [Google Scholar] [CrossRef]
- Salama, M.; Vaz, M.F.; Colaço, R.; Santos, C.; Carmezim, M. Biodegradable Iron and Porous Iron: Mechanical Properties, Degradation Behaviour, Manufacturing Routes and Biomedical Applications. J. Funct. Biomater. 2022, 13, 72. [Google Scholar] [CrossRef]
- Rabeeh, V.P.M.; Hanas, T. Progress in Manufacturing and Processing of Degradable Fe-Based Implants: A Review. Prog. Biomater. 2022, 11, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Ullah, S.; Raza, M.R.; Abbas, N.; Ali, A. Recent Developments in Zn-Based Biodegradable Materials for Biomedical Applications. J. Funct. Biomater. 2022, 14, 1. [Google Scholar] [CrossRef]
- Nečas, D.; Marek, I.; Pinc, J.; Vojtěch, D.; Kubásek, J. Advanced Zinc–Magnesium Alloys Prepared by Mechanical Alloying and Spark Plasma Sintering. Materials 2022, 15, 5272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, W.; Liu, J.; Wang, L.; Tang, Y.; Wang, K. A Review on Magnesium Alloys for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 953344. [Google Scholar] [CrossRef]
- Virtanen, S. Biodegradable Mg and Mg Alloys: Corrosion and Biocompatibility. Mater. Sci. Eng. B 2011, 176, 1600–1608. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, K. Corrosion and Protection of Magnesium Alloys: Recent Advances and Future Perspectives. Coatings 2023, 13, 1533. [Google Scholar] [CrossRef]
- Brar, H.S.; Platt, M.O.; Sarntinoranont, M.; Martin, P.I.; Manuel, M.V. Magnesium as a Biodegradable and Bioabsorbable Material for Medical Implants. JOM 2009, 61, 31–34. [Google Scholar] [CrossRef]
- Salleh, E.M.; Hussain, Z. Effect of Zn Content on Biodegradable Mg Alloy Synthesized via Mechanical Alloying for Biomedical Application; Springer: Berlin/Heidelberg, Germany, 2023; ISBN 9789811995088. [Google Scholar]
- Yuan, W.; Xia, D.; Wu, S.; Zheng, Y.; Guan, Z.; Rau, J.V. A Review on Current Research Status of the Surface Modification of Zn-Based Biodegradable Metals. Bioact. Mater. 2022, 7, 192–216. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh Bagha, P.; Khaleghpanah, S.; Sheibani, S.; Khakbiz, M.; Zakeri, A. Characterization of Nanostructured Biodegradable Zn-Mn Alloy Synthesized by Mechanical Alloying. J. Alloys Compd. 2018, 735, 1319–1327. [Google Scholar] [CrossRef]
- Hernández-Escobar, D.; Champagne, S.; Yilmazer, H.; Dikici, B.; Boehlert, C.J.; Hermawan, H. Current Status and Perspectives of Zinc-Based Absorbable Alloys for Biomedical Applications. Acta Biomater. 2019, 97. [Google Scholar] [CrossRef] [PubMed]
- Mostaed, E.; Sikora-Jasinska, M.; Mostaed, A.; Loffredo, S.; Demir, A.G.; Previtali, B.; Mantovani, D.; Beanland, R.; Vedani, M. Novel Zn-Based Alloys for Biodegradable Stent Applications: Design, Development and in Vitro Degradation. J. Mech. Behav. Biomed. Mater. 2016, 60, 581–602. [Google Scholar] [CrossRef]
- Yandong, Y.; Shuzhen, K.; Teng, P.; Jie, L.; Caixia, L. Effects of Mn Addition on the Microstructure and Mechanical Properties of As-Cast and Heat-Treated Mg-Zn-Ca Bio-Magnesium Alloy. Metallogr. Microstruct. Anal. 2015, 4, 381–391. [Google Scholar] [CrossRef]
- Guleryuz, L.F.; Ipek, R.; Aritman, I. Effect of Ca Additions on the Mechanical Properties of Mg and in Vitro Degradation Behavior of Mg-XCa Alloys Produced by Powder Metallurgy. In Proceedings of the World PM 2016 Congress and Exhibition, Hamburg, Germany, 9–13 October 2016. [Google Scholar]
- Salahshoor, M.; Guo, Y. Biodegradable Orthopedic Magnesium-Calcium (MgCa) Alloys, Processing, and Corrosion Performance. Materials 2012, 5, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Verissimo, N.C.; Brito, C.; Afonso, C.R.; Spinelli, J.E.; Cheung, N.; Garcia, A. Microstructure Characterization of a Directionally Solidified Mg-12wt.%Zn Alloy: Equiaxed Dendrites, Eutectic Mixture and Type/Morphology of Intermetallics. Mater. Chem. Phys. 2018, 204, 105–131. [Google Scholar] [CrossRef]
- Jiang, P.; Blawert, C.; Zheludkevich, M.L. The Corrosion Performance and Mechanical Properties of Mg-Zn Based Alloys—A Review. Corros. Mater. Degrad. 2020, 1, 92–158. [Google Scholar] [CrossRef]
- Becerra, A.; Pekguleryuz, M. Effects of Zinc, Lithium, and Indium on the Grain Size of Magnesium. J. Mater. Res. 2009, 24, 1722–1729. [Google Scholar] [CrossRef]
- Zengin, H.; Turen, Y.; Ahlatci, H.; Sun, Y. Effect of Indium Addition on Microstructure and Mechanical Properties of AS-CAST and Hot-Rolled AM60 Magnesium Alloy. In Proceedings of the METAL 2017-26th International Conference on Metallurgy and Materials, Conference Proceedings, Brno, Czech Republic, 24–26 May 2017. [Google Scholar]
- Wang, X.W.; Wang, W.; Chen, W.; Chen, D.M. Effects of Indium on Corrosion Behavior of Mg-Al-Cu Alloy. Mater. Charact. 2021, 177, 111157. [Google Scholar] [CrossRef]
- Bao, T.; Hou, L.; Sun, J.; Liu, X.; Du, H.; Wei, H.; Wei, Y. Effect of Indium on the Negative Difference Effect for Magnesium Alloy. J. Electrochem. Soc. 2021, 168, 031515. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying: A Critical Review. Mater. Res. Lett. 2022, 10, 619–647. [Google Scholar] [CrossRef]
- Raducanu, D.; Cojocaru, V.D.; Nocivin, A.; Hendea, R.E.; Ivanescu, S.; Stanciu, D.; Trisca-Rusu, C.; Serban, N.; Drob, S.I.; Campian, R.S. Microstructure Evolution during Mechanical Alloying of a Biodegradable Magnesium Alloy. Crystals 2022, 12, 1641. [Google Scholar] [CrossRef]
- Gonzaga, S.R.; Sedano, A.; Colín, J.M.; Torres-Islas, A.; Molina-Ocampo, A. Production of Biodegradable Mg-Based Alloys by Mechanical Alloying. Dig. J. Nanomater. Biostruct. 2021, 16, 1537–1545. [Google Scholar] [CrossRef]
- Lesz, S.; Karolus, M.; Gabryś, A.; Kremzer, M. Influence of Milling Time on Phase Composition and Product Structure of Mg-Zn-Ca-Ag Alloys Obtained by Mechanical Synthesis. Materials 2022, 15, 7333. [Google Scholar] [CrossRef]
- Lesz, S.; Karolus, M.; Gabryś, A.; Hrapkowicz, B.; Walke, W.; Pakieła, W.; Gołombek, K.; Popis, J.; Palček, P. Characteristics of Mg-Based Sintered Alloy with Au Addition. Materials 2023, 16, 1915. [Google Scholar] [CrossRef]
- Salleh, E.M.; Ramakrishnan, S.; Hussain, Z. Synthesis of Biodegradable Mg-Zn Alloy by Mechanical Alloying: Effect of Milling Time. Procedia Chem. 2016, 19, 525–530. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Yang, M.; Yang, Y.; Wang, D.; Liu, Z.; Shuai, C. Laser-Sintered Mg-Zn Supersaturated Solid Solution with High Corrosion Resistance. Micromachines 2021, 12, 1368. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, X.; Qiao, Y.; Wang, X.; Lin, Q. Preparation of Medical Mg–Zn Alloys and the Effect of Different Zinc Contents on the Alloy. J. Mater. Sci. Mater Med. 2022, 33, 9. [Google Scholar] [CrossRef]
- Youssef, K.M.; Wang, Y.B.; Liao, X.Z.; Mathaudhu, S.N.; Kecskés, L.J.; Zhu, Y.T.; Koch, C.C. High Hardness in a Nanocrystalline Mg97Y2Zn1 Alloy. Mater. Sci. Eng. A 2011, 528, 7494–7499. [Google Scholar] [CrossRef]
- Hwang, S.; Nishimura, C.; McCormick, P.G. Mechanical Milling of Magnesium Powder. Mater. Sci. Eng. A 2001, 318, 22–33. [Google Scholar] [CrossRef]
- Kowalski, K. Effects of Mechanical Alloying Conditions on the Properties of Mg-Based Nanomaterials. Inżynieria Mater. 2015, 1, 23–26. [Google Scholar] [CrossRef]
- Kowalski, K.; Nowak, M.; Jakubowicz, J.; Jurczyk, M. The Effects of Hydroxyapatite Addition on the Properties of the Mechanically Alloyed and Sintered Mg-RE-Zr Alloy. J. Mater. Eng. Perform. 2016, 25, 4469–4477. [Google Scholar] [CrossRef]
- PN-EN ISO 6507-1:2018-05; Metallic materials- Vickers hardness test- Part 1: Test method. PKN: Warsaw, Poland, 2018.
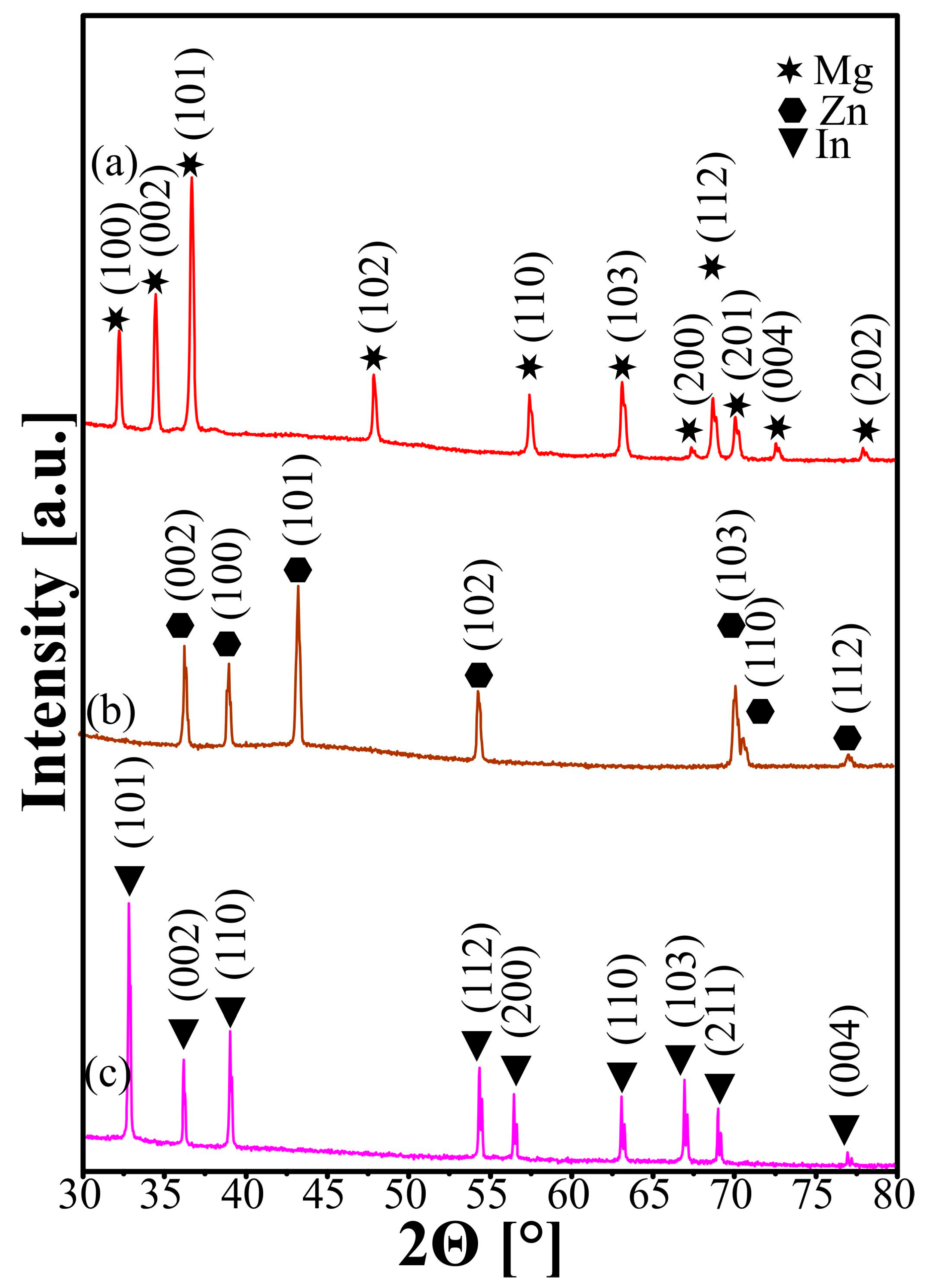
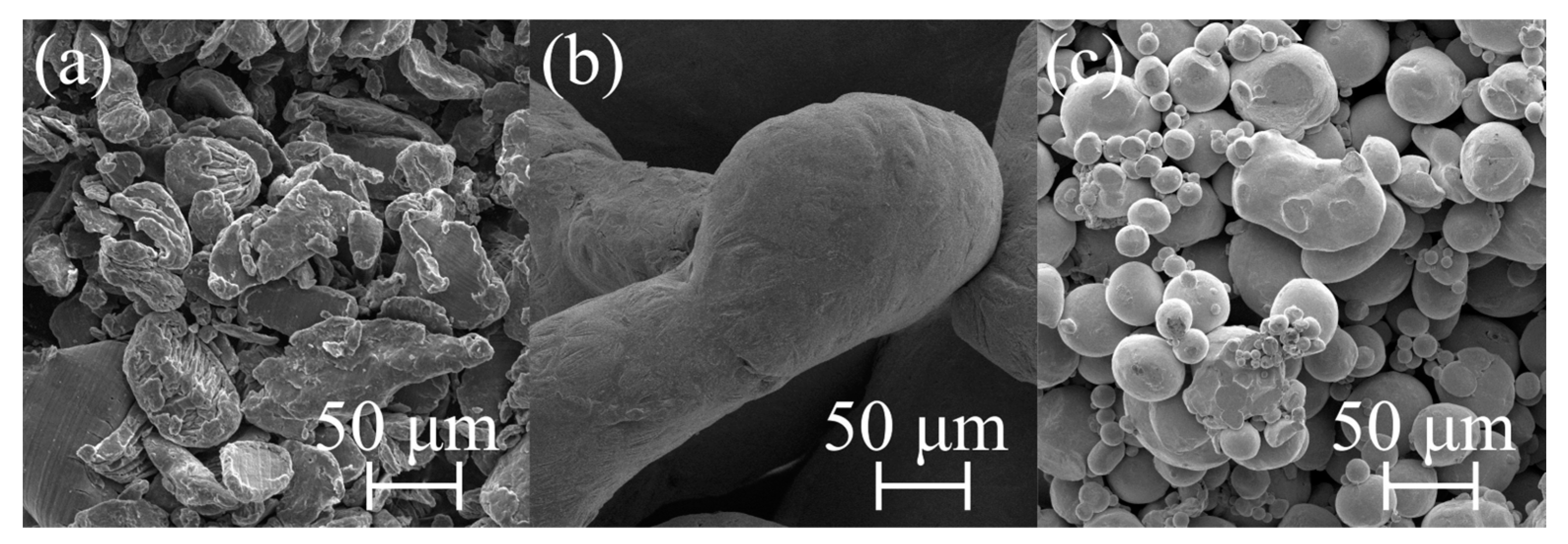
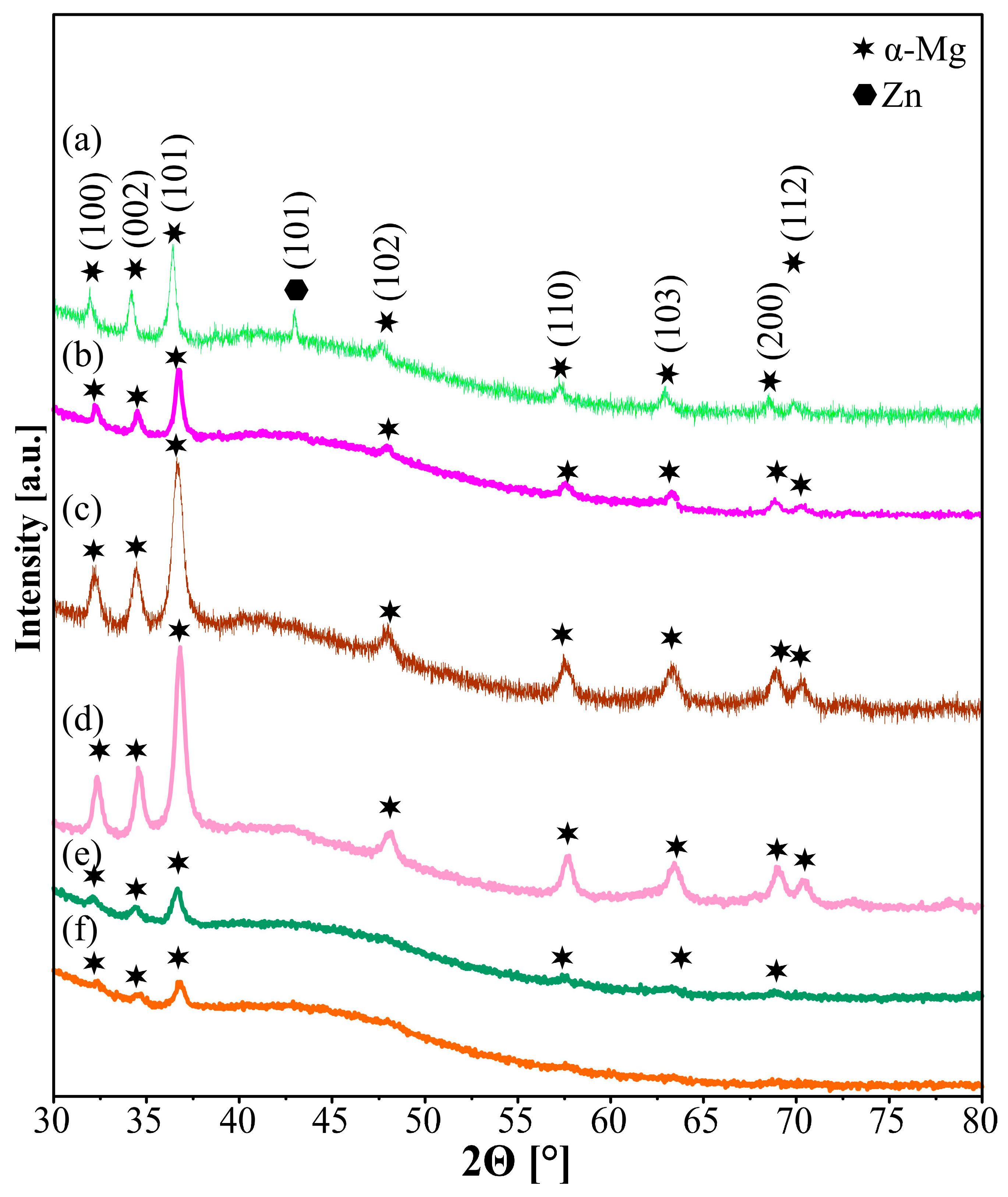
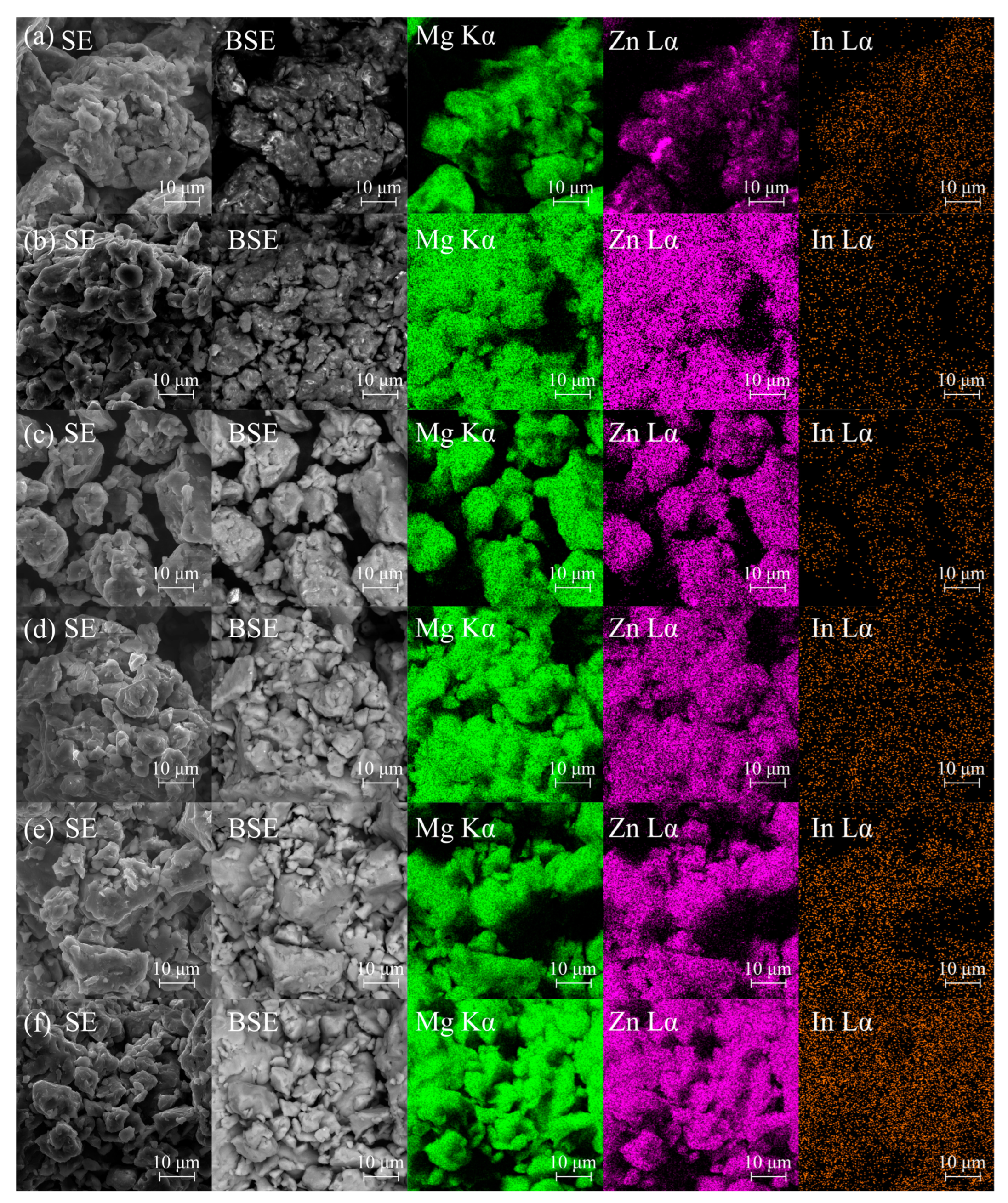
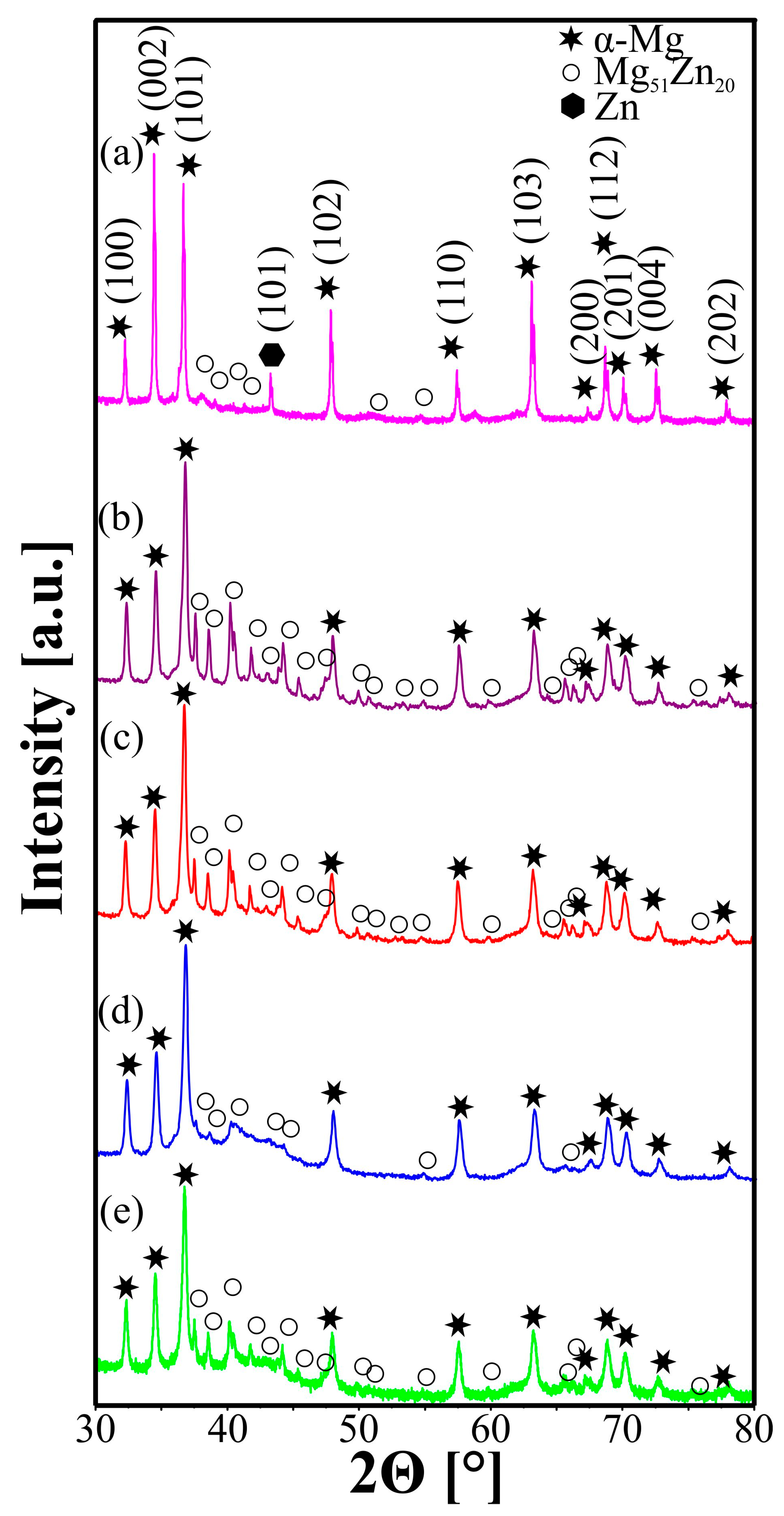
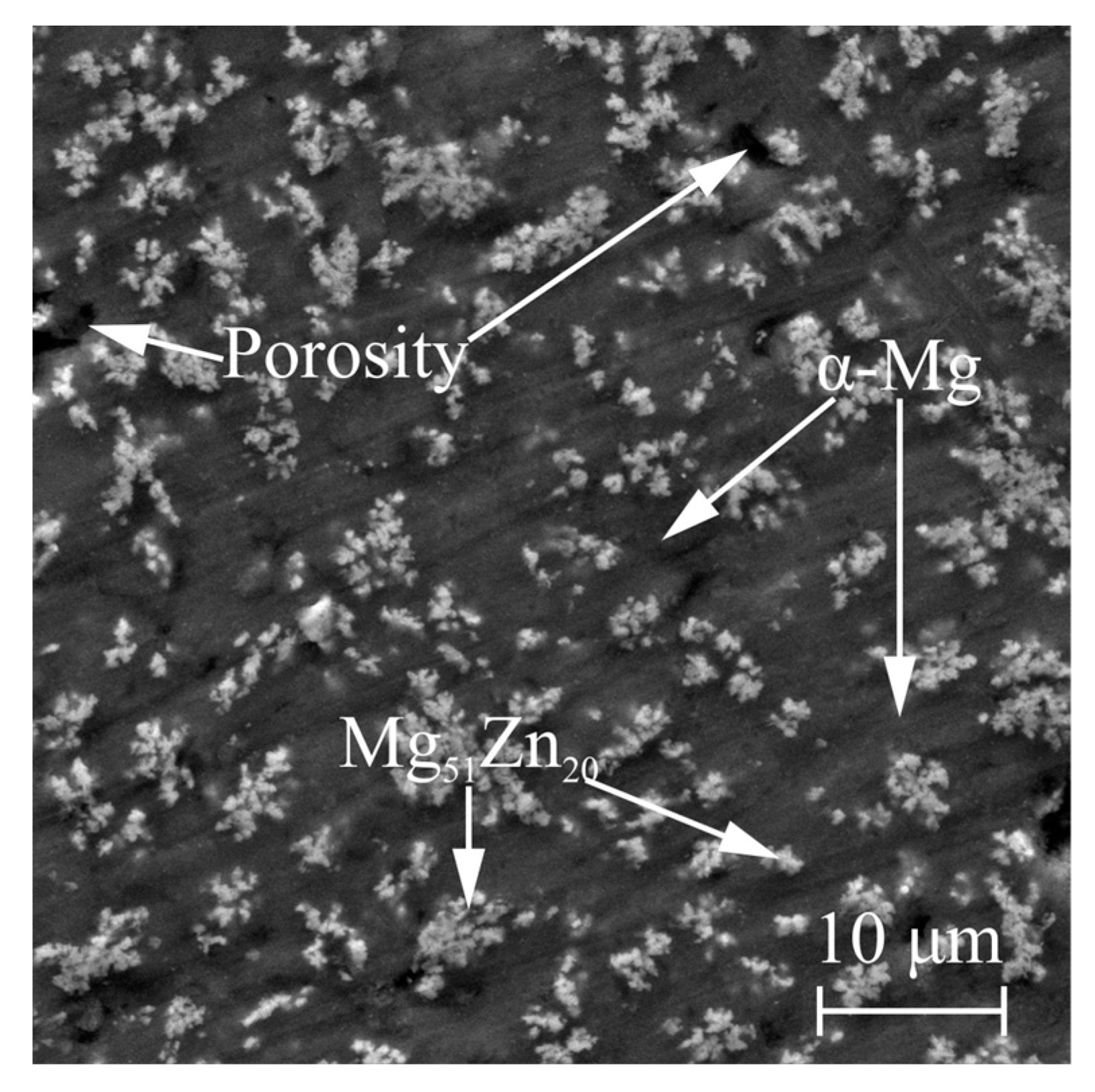

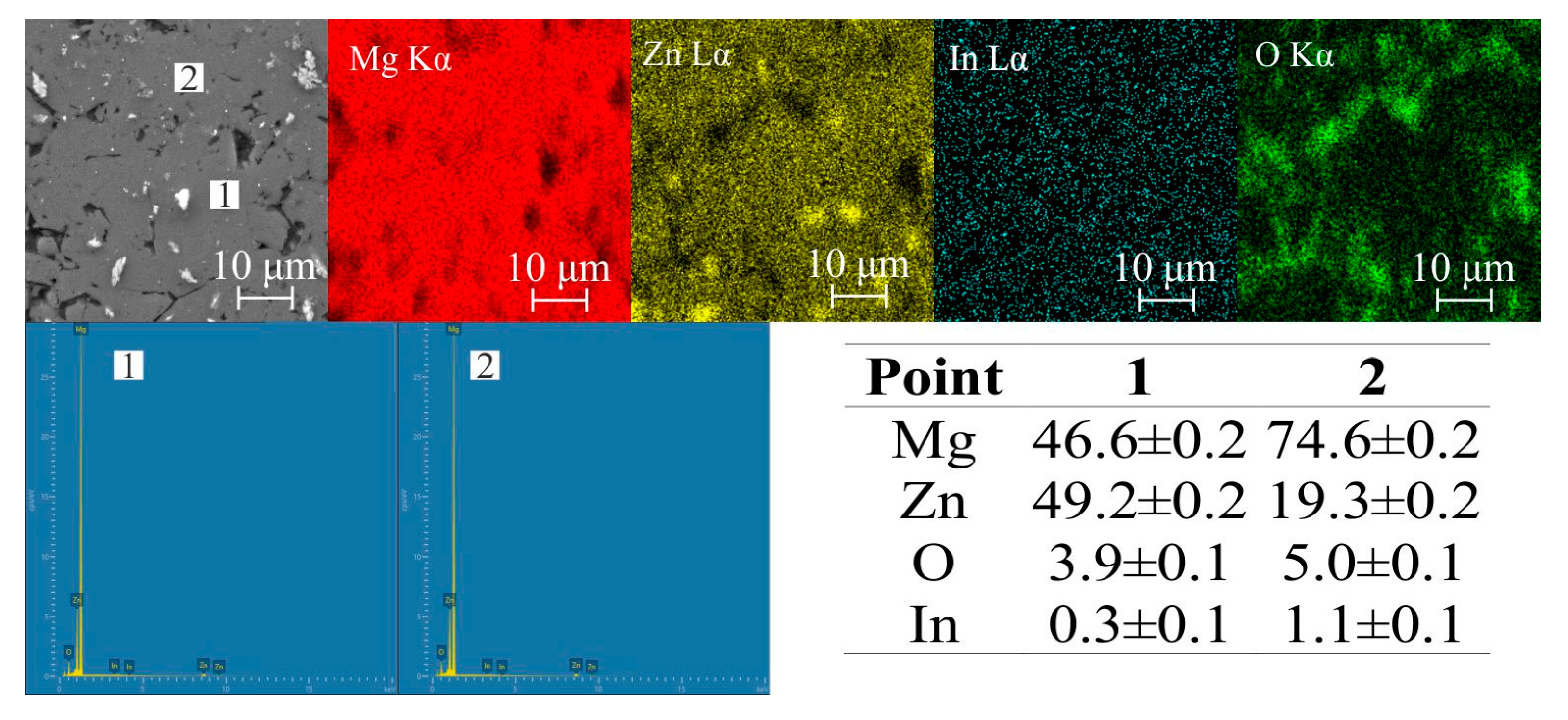
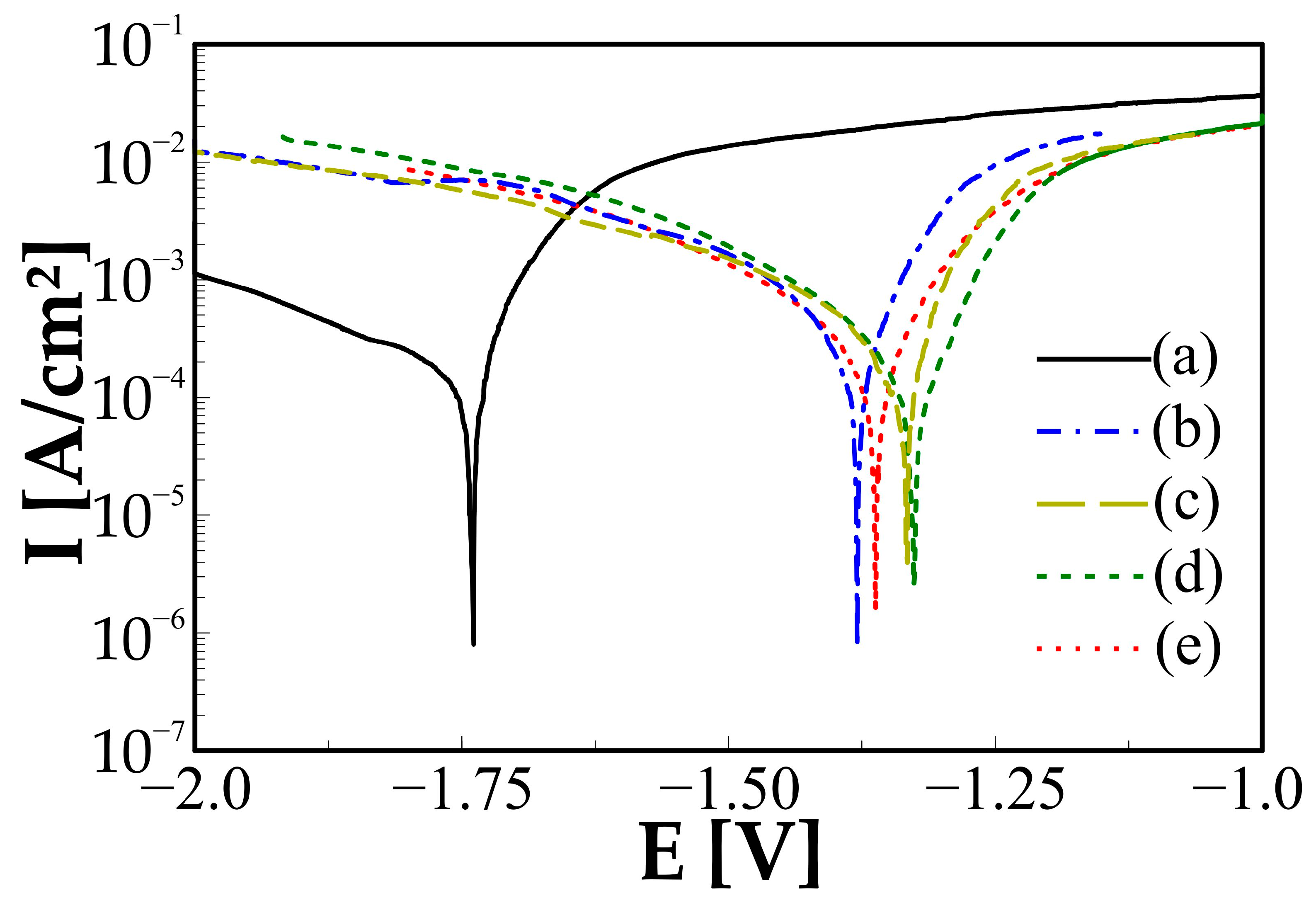
| Sample | Mg-20Zn | Mg-20Zn-1In | |||
|---|---|---|---|---|---|
| MA time [h] | 48 | 12 | 24 | 36 | 48 |
| BPR | 20:1 | 20:1 | |||
| Compacting pressure [MPa] | 600 | 600 | |||
| Sintering conditions | 300 °C/1 h/vacuum | 300 °C/1 h/vacuum | |||
| Sample | Mg-20Zn | Mg-20Zn-1In | |||
|---|---|---|---|---|---|
| MA time [h] | 48 | 12 | 24 | 36 | 48 |
| a [nm] | 3.20788 | 3.20563 | 3.20306 | 3.20341 | 3.20045 |
| c [nm] | 5.20883 | 5.20527 | 5.20099 | 5.20302 | 5.19716 |
| HV0.3 | 52 ± 3 | 134 ± 7 | 140 ± 7 | 143 ± 3 | 145 ± 3 |
| ρth [g/cm3] | 2.047 | 2.0653 | |||
| ρ [g/cm3] | 1.99 ± 0.03 | 1.99 ± 0.02 | 1.99 ± 0.06 | 1.92 ± 0.02 | 1.96 ± 0.01 |
| Porosity [%] | 2.55 ± 0.02 | 3.43 ± 0.02 | 5.84 ± 0.02 | 7.02 ± 0.03 | 5.10 ± 0.04 |
| Sample | Ic [µA/cm2] | Ec [V] |
|---|---|---|
| Mg-20Zn | 293.7 ± 1.3 | −1.74 ± 0.05 |
| Mg-20Zn-1In -12 h MA | 211.6 ± 3.2 | −1.38 ± 0.04 |
| Mg-20Zn-1In -24 h MA | 178.9 ± 2.8 | −1.33 ± 0.07 |
| Mg-20Zn-1In -36 h MA | 133.6 ± 7.4 | −1.33 ± 0.03 |
| Mg-20Zn-1In -48 h MA | 176.4 ± 4.5 | −1.36 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalski, K.; Kozlowski, M.; Lukaszkiewicz, N.; Kobus, M.; Bielecki, J.; Jurczyk, M. Effect of Indium on the Properties of Mg-Zn-Based Alloys. Metals 2023, 13, 1786. https://doi.org/10.3390/met13101786
Kowalski K, Kozlowski M, Lukaszkiewicz N, Kobus M, Bielecki J, Jurczyk M. Effect of Indium on the Properties of Mg-Zn-Based Alloys. Metals. 2023; 13(10):1786. https://doi.org/10.3390/met13101786
Chicago/Turabian StyleKowalski, Kamil, Mikolaj Kozlowski, Natalia Lukaszkiewicz, Mateusz Kobus, Jakub Bielecki, and Mieczyslaw Jurczyk. 2023. "Effect of Indium on the Properties of Mg-Zn-Based Alloys" Metals 13, no. 10: 1786. https://doi.org/10.3390/met13101786
APA StyleKowalski, K., Kozlowski, M., Lukaszkiewicz, N., Kobus, M., Bielecki, J., & Jurczyk, M. (2023). Effect of Indium on the Properties of Mg-Zn-Based Alloys. Metals, 13(10), 1786. https://doi.org/10.3390/met13101786






