Abstract
Implementing mixed metal oxide (MMO) anodes in zinc electrowinning is highly desired due to the considerable reduction in electrical energy consumption. However, the presence of manganese in the electrolyte is a major obstacle for implementing MMO anodes in the zinc cell houses. In this work, we explore the possibility of using plant off-gas, containing SO2, to remove manganese. A SO2/air gas mixture with different SO2 and O2 concentrations was therefore used for the oxidative precipitation of manganese. It was shown that the manganese oxidation reaction is highly pH-dependent. Calcium hydroxide was used to control the pH during the process. Different operating parameters, i.e., pH, SO2/air ratio, reaction time, and effect of cobalt as a reaction catalyst, were investigated. Optimal conditions for manganese removal were reported. Under the optimal conditions, the manganese concentration decreased from 1 g L−1 to less than 1 mg L−1 within 30 min. Precipitates were characterized using EDS, XRF, and XPS techniques and showed coprecipitation of manganese, zinc, gypsum, and cobalt.
1. Introduction
Energy consumption is one of the main issues in the conventional zinc electrowinning process where lead-based electrodes are used as the anode and aluminium plates as the cathode. Zinc electrowinning cell houses consume approximately 80% of the total electrical energy in zinc plants [1,2]. The high cell voltage of around 3.5 V with a zinc current efficiency of 90% results in considerable energy consumption of about (3200 kWh t−1) [3]. A significant part of the total cell voltage (1.2 V) is related to the anode voltage, where oxygen evolution reaction (OER) overvoltage accounts for around 0.8 V [3]. In the past few decades, great efforts have been made to minimize the OER overvoltage. It has been reported that the electrocatalytic properties of anode materials can play an essential role in determining the OER overvoltage [4,5,6]. Thus, the more electrocatalytic an anode is implemented, the more energy saving that can be obtained.
Various anode materials, such as alloyed lead-based anodes, composite anodes, and mixed metal oxide (MMO) anodes have been introduced in recent decades. Compared to lead-based anodes, MMO anodes are well known for having superior electrocatalytic performance toward reducing OER overvoltage [7,8]. MMO anodes typically consist of a coated titanium substrate with catalytic and conductive layers of various mixed metal oxides such as RuO2, IrO2, TiO2, and TaO2, which are usually fabricated through thermal decomposition methods [8,9].
Although MMO anodes have already been applied in copper electrowinning plants, their implementation in electrolytic zinc plants is more challenging due to the presence of manganese ions in the zinc electrolyte solution. Typically, manganese comes from two different sources in electrolytic zinc plants. The first source is the manganese in the zinc concentrate, as an inherent ore impurity. The second one is associated with the addition of manganese, in the form of KMnO4, as an oxidant to eliminate iron in the upstream leaching stage [10,11]. No extra effort is made to remove manganese from the electrolyte in the subsequent purification steps. In the current practice in which lead-based anodes are used, a minimum level of manganese ions is deliberately kept in the electrolyte to protect anodes against corrosion. Manganese ions are oxidized on the lead anode, forming an in situ manganese dioxide coating, which is periodically cleaned from the anode surface. For MMO anodes, such a protection is not necessary. On the other hand, manganese dioxide deposition on the surface of the MMO anode may deactivate the electrocatalytic sites, resulting in them losing their efficiency [12,13]. Moreover, cleaning and removing manganese dioxide from these anodes is accompanied by a detachment of expensive electrocatalytic oxide coatings [13]. Therefore, for implementing MMO anodes in the zinc electrowinning process, the first challenge is to remove manganese ions from the solution before they enter the electrolysis cell.
For controlling and removing manganese from aqueous solutions, several methods have been suggested, i.e., ion exchange, solvent extraction, electrooxidation, adsorption, and oxidative precipitation processes. Ion exchange is widely applied for water softening to remove manganese at very low levels, and it is not economically feasible to eliminate manganese at high concentrations (1 g L−1) [14,15]. Solvent extraction is not applicable in the zinc industry due to the coprecipitation of zinc with manganese [11,12]. Electrooxidation of manganese is not economically attractive for removing manganese at concentrations lower than 30 g L−1 [14,16], while the typical concentration of zinc in the electrolysis hall is about 50 g L −1 and that in upstream solutions is around 150 g L−1. The adsorption method with a functionalized composite adsorbent has limitations in acidic pH regions for detecting and removing metals ions [17,18]. The oxidative precipitation technique in the presence of strong oxidants, based on oxidizing soluble manganese ions (Mn2+) to their higher oxidation states (Mn3+, Mn4+), could be an interesting alternative, provided that the cost of oxidants not to be prohibitive.
Many oxidizing agents for the oxidative precipitation process have been proposed including ozone [19], SO2/O2 or SO2/air gas mixtures [10,20,21], Caro’s acid (H2SO5) [22], and potassium permanganate (KMnO4) [23]. The SO2/air gas mixture, in comparison with the other oxidants, is relatively cheaper [10,20]. This parameter becomes particularly important when large amounts of liquor (typically several cubic meters per hour) need to be treated and SO2 gas is available from the off-gas of the roasting facility inside zinc plants. Therefore, the oxidative precipitation process, specially using a SO2/air gas mixture as an oxidant, can be a suitable method for manganese removal from a zinc-rich solution.
Several researchers have explored the elimination of manganese as an impurity from zinc, cobalt, and nickel leach liquors through the oxidative precipitation method. Most research studies have focused on the selective oxidative precipitation of manganese from leach liquors by a SO2/O2 gas mixture [20,21,24]. Menard et al. [25] showed that the kinetics of the manganese removal process highly depend on the SO2 flow rate followed by the SO2/O2 ratio. Furthermore, they pointed out that the optimal SO2/O2 ratio should be selected based on the reactor design to obtain fast and maximum manganese elimination [25]. It has also been shown that manganese precipitation is favored at high pH values [25]. Although this technique seems to be efficient, the cost related to O2 may still be considered as an obstacle.
One possible avenue to reducing the processing cost is to directly use the off-gas from roasting furnaces, provided that it efficiently removes manganese. Roasting off-gas, abundantly available in zinc plants, contains (7–9)% SO2, but its oxygen level is low (around 10%), due to the oxidation of ZnS in the furnace. The off-gas also contains a large amount of nitrogen (about 80%) which makes the SO2 and O2 very diluted, compared to the SO2/O2 reactant. Therefore, the concentration of SO2 and O2 as well as the SO2/O2 ratio in the off-gas is well below the concentrations and the ratios used in most of the published results in the literature. To the best of our knowledge, detailed information on manganese removal by such a diluted SO2/air mixture from zinc electrolytes is scarce. Bello-Teodoro et al. [22] used a SO2/air mixture to precipitate manganese from a solution containing 0.085 ML−1 (4.6 g L−1) manganese. This level of manganese is in the range of the application targeted in the present work. The authors showed that the manganese removal rate increased by increasing the pH from 4 to 6, but even at pH 6, only 60% of the manganese was removed after 150 min of reaction [22]. The thermodynamic aspects of manganese oxidation are well known, and the stability of different manganese phases can be predicted from Pourbaix diagrams, indicating a positive effect of pH on manganese oxide stability. However, the pH of the zinc electrolyte cannot be increased beyond 4 since it results in excessive precipitation of zinc along with manganese, given that the concentration of zinc in the electrolyte is near the saturation point. Too much loss of zinc is not acceptable since it results in the loss of process productivity. Mulaudzi and Mahlangu [20] performed remarkable research on this matter and used a SO2/air gas mixture to precipitate manganese from solutions containing 6.5 g L−1 cobalt and 2 g L−1 manganese. They showed that manganese removal was quite low at pH values up to 3.5, while they reached almost full manganese removal at pH 4 [20]. The same results cannot be achieved in removing manganese from zinc electrolyte since, as we will demonstrate in the present contribution, the presence of cobalt in the solutions used by Mulaudzi et al. [20] may have played a key role in their success.
This work aimed to explore the possibility of removing manganese from zinc electrolyte by using a gas mixture with a composition close to that of roasting off-gas. We also aimed at finding the optimal conditions under which manganese could be efficiently removed without excessive zinc loss. The first step in the process development was to determine the level up to which manganese needed to be removed. Our preliminary results showed that the level of manganese needed to be reduced to less than 100 mg L−1 (ideally to less than 50 mg L−1) in order for the commercially available MMO anodes to be used in the cell house without deactivation. To optimize the manganese removal process, we first assessed the efficiency of a diluted SO2/O2 gas mixture by using a SO2/air mixture with different SO2/air ratios. The SO2 concentration range was chosen in a way that it would be possible to obtain from off-gas in an eventual industrial deployment. The effects of three parameters were investigated, i.e., solution pH, SO2/air ratio, and reaction time. As the manganese removal efficiency was not high enough, we then proposed using cobalt as a catalyst to enhance the reaction and to remove the manganese in a reasonable time frame, knowing that cobalt is an undesirable impurity, already present in the impurified zinc solution. Table S1 (Supplementary Information) presents chronological description of the experiments were conducted in this study.
2. Materials and Methods
2.1. Materials and Reagents
Two different synthetic zinc-rich solutions were employed as the feeding solution. The main differences between the two solutions were related to the presence of cobalt ions. All solutions were prepared with demineralized water. All chemicals were of analytical grade and used as purchased without further purification. Sulfuric acid with a purity of (95–98)% was purchased from Fisherbrand and nitric acid with a purity of 70% was obtained from Anachemia.
Manganese sulfate pentahydrate (MnSO4·5H2O) and cobalt sulfate heptahydrate (CoSO4·7H2O) were used as sources of manganese and cobalt, respectively, and obtained from Laboratoire MAT INC and Sigma-Aldrich Company Ltd., USA. Ca(OH)2 was used as a neutralizing agent to adjust the solution pH and was purchased from PTI Process Chemicals, Inc. Activated zinc oxide was used as the source of zinc to prepare the feeding solutions.
2.2. Methods
A schematic diagram of the Mn removal set-up is shown in Figure 1. Air (99.9%) and sulfur dioxide gas (99.98%) were provided by PRAXAIR (Mississauga, ON, Canada). Two different mass flow controllers were used to control the flow rate of gases. Both air and SO2 mass flow controllers were provided by POLYCONTROLS (models SLA5850S1BBC6C2A1 and 58505, respectively) (Brossard, QC, Canada). A BROOKS instrument model 0254 (POLYCONTROLS, Brossard, QC, Canada). was used as a power supplier and set point controller for the air and SO2 mass flow controllers. SO2 and air were mixed through a T-conjunction and introduced to the reactor by a single 316-stainless steel sparger.
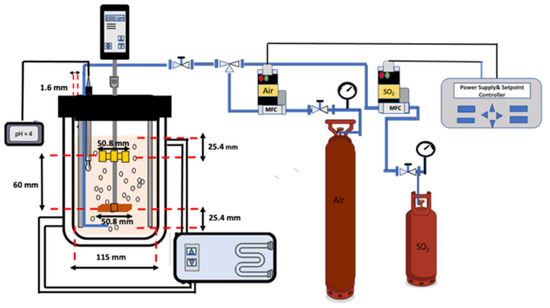
Figure 1.
Schematic representation of the experimental set-up.
The reactor was made of a 3000 mL water-jacketed glass beaker with four 316 stainless steel baffles, symmetrically mounted near the wall of the cell. A removable lid with several openings was used to hold the temperature probe, pH electrode, sampling, stirrer shaft, and SO2/air gas mixture sparger. Agitation was provided by a Caframo mixer motor model BDC 3030 (Caframo Lab Solutions, Georgian Bluffs, ON, Canada) with two different 316 stainless steel impellers, six blades, and a diameter of 50.8 mm. A Rushton impeller and a 45° upward pitched blade were provided by INDCO models #6BR-3 and #ATI-3, respectively (INDCO Inc., New Albany, OH, USA).
The lower impeller (45° upward pitched blade) was suspended 25.4 mm from the bottom of the reactor. The distance between the two impellers was 60 mm. To keep the temperature constant at (80 ± 0.5) °Ca water bath IsotempTM model 6200 H7 by Fisherbrand (Waltham, MA, USA) was connected to the jacketed glass beaker for circulating hot water. A Cole-Parmer combination pH electrode and Ag/AgCl refillable reference electrode were accommodated into the reactor for continuous monitoring of the solution pH. The pH signals were processed by an OAKTON pH meter model 700 (Quebec, QC, Canada).
The jacketed glass beaker was filled with 1300 mL of the synthetic zinc-rich solution containing 150 g L−1 zinc, 1 g L−1 manganese, and various cobalt concentrations, ranging between (0–70) mg L−1, depending on the test conditions. Time was allowed until the solution temperature reached (80 ± 0.5) °C. The pH was adjusted with some drops of sulfuric acid to reach a pH around 4. The sparger was inserted into the reactor to inject the SO2 and air gas mixture. The solution was agitated at a rotation speed of 2000 rpm under atmospheric pressure. Calcium hydroxide in powder form was added to the solution throughout the experiments to neutralize the produced sulfuric acid during the test and to keep the pH constant at around 4. Samples were taken periodically. The pH, temperature, and weight of the consumed calcium hydroxide powder were recorded during the experiments. The process also allowed visual manganese dioxide precipitation by a colourless to brownish change.
2.3. Analysis and Characterization
The manganese concentration was measured using a microwave plasma-atomic emission spectrometer (MP-AES) (Model 4200; Agilent Technologies, Santa Clara, CA, USA). An amount of 10 mL of aqueous samples were taken during the test and filtered through 0.2 μm pore size wool glass. Then, 1 mL of the transparent filtered solution was diluted 100 times with HNO3 5 vol.% and was kept in the sealed tube to prevent contamination. The morphology of precipitates was examined by field emission gun-scanning electron microscopy (FEG-SEM, FEI Company; Model: Inspect F50, Hillsboro, OR, USA). The elemental analysis of sediments was determined by energy dispersive X-ray spectrometry (EDS) using an Edax Ametek model Octane Super-A coupled with SEM. The working voltage for this analysis was 15 kV, with a working distance of 10 mm and magnification of 3 kx. The SEM analyses were performed in backscattered electron (BSE) modes.
To prepare a solid phase sample, the precipitates were allowed to settle spontaneously down at the bottom of the cell for 24 h without using a clarifying agent. Next, the sediments were washed several times with hot distilled water (80 °C) to remove any zinc ions from the sediments and were filtered by filter paper provided by Whatman with a 125 mm diameter. Finally, the obtained particles were placed in an oven at 70 °C for 24 h. The oven was operated without any protective atmosphere. X-ray photoelectron spectroscopy (XPS) analyses were carried out by a PHI 5600-ci spectrometer (Physical Electronics, Eden Prairie, MN, USA). A monochromatic aluminum X-ray source (1486.6 eV, 300 W) was used to record the survey spectra (1400.0 eV) with a pass energy of 187.85 eV at a high vacuumed atmosphere (). High-resolution (HR) spectra were obtained using an achromatic magnesium X-ray source (1253.6 eV, 300 W) with a pass energy of 5.85 eV. No charge neutralization was applied for the survey and HR spectra. The analyzed area was 0.5 mm2. Moreover, HR curve fittings were determined using the least squares method, Gauss–Lorentz functions, and a Shirley background subtraction.
The chemical composition of precipitates was analyzed using an Epsilon1 X-ray fluorescence spectrometer manufactured by Malvern Panalytical, Malvern, UK). An Ag X-ray tube was used to acquire XRF spectra at different voltages ranging from 0 kV to 50 kV in the air atmosphere. In addition, a silicon drift X-ray detector with a 10 mm2 active area was used, and spectra were analyzed by Omnian software (version 1.7.F, Malvern Panalytical Ltd., Malvern, UK).
3. Theory (Reaction Mechanisms)
The primary mechanism of manganese oxidation with a SO2/air gas mixture has arisen from the radical chain mechanism. It consists of the oxidation of various sulfur (IV) species () in the presence of trace amounts of transition metals (Mn3+, Co3+, Fe3+) as the catalyst. These oxidation reactions lead to the formation of some radicals, as strong oxidizing agents such as , which are known as the main ones responsible for oxidizing manganese ions [26,27]. The radical chain mechanism in this system consists of several stages, as detailed below:
3.1. Initiation
It has been reported that the first stage of this mechanism starts with dissolving SO2 gas in the solution, followed by the formation of different sulfur species, namely bisulfite and sulfite as a function of pH, according to the Equations (1) and (2) [26].
3.2. Manganese-Sulfite Complex Formation
Generally, two reaction paths have been proposed to form manganese-sulfite complex (Mn). The first path, Equation (3), is based on the reaction between and manganese ions [26], and the second path is related to the reaction of ions in forming this complex, according to Equation (4) [28,29].
3.3. Peroxymonosulfate Radical Generation
Peroxymonosulfate radical (originates from the decomposition of Mn complex, resulting in sulfite radical formation (Equation (5)). Afterwards, sulfite radical converts to peroxymonosulfate radical by reacting with dissolved O2 in the solution (Equation (6)) [27].
It is worth mentioning that the formation of requires constant O2 supply. Otherwise, dithionate formation takes place in the absence of O2, based on Equation (7). It has been known as a radical scavenger which is detrimental to the manganese oxidation process [26,28].
3.4. Regeneration of Mn3+ as a Catalyst
It has been postulated that the Mn3+ ions are recovered by a reaction between and Mn2+, as shown in Equation (8). According to this reaction, Mn2+ is oxidized, resulting in the formation of Mn3+ and hydrogen peroxymonosulfate anion (), which is one of the strong oxidants in this system [28].
Since ions are consumed to produce the manganese-sulfite complex in a sufficient SO2/O2 gas mixture (Equation (3)), this stage facilitates the continuation of the reaction by regenerating ions.
3.5. Manganese Dioxide Precipitation
This stage can be considered as the last stage in the radical chain mechanism. According to Equation (9), ions are oxidized to MnO2 precipitates by reacting with and water molecules [27]. The other products of this reaction are sulfate ions and H+ ions, representing one of the main reasons for the pH drop in this process.
When the manganese concentration reaches near zero, only sulfuric acid is produced, as shown in Equations (10) and (11) [29]. This is the reason why it is critical to stop SO2/air injection at the right time to prevent a pH drop.
The main challenge associated with applying the SO2/air gas mixture in this approach is the considerably less O2 content of the mixture (21% of O2 in the air) compared to that of the SO2/O2 gas mixture. It indeed results in slower oxidation kinetics. In this case, the hypotheses of utilizing a suitable catalyst can be considered as a promising approach for increasing the rate of the reactions by activating peroxymonosulfate species.
Recent studies have shown that transition metals such as (Co2+, Mn2+, Fe2+, Ni2+, Cu2+) are excellent candidates for activating peroxymonosulfate species (, ) and forming sulfate ( and hydroxyl radicals [30,31,32]. These two radicals are well known as the main ones responsible for removing impurities due to their remarkable redox potential. Moreover, sulfate radical is much more efficient than hydroxyl radical in the degradation of pollutants because of its longer half-life (30–40) s compared to 20 ns for hydroxyl radicals, excellent mass transfer, and reactivity [30].
Among the transition metal catalysts, we targeted cobalt since it was present in the impure zinc electrolysis solution. It is worth mentioning that in the current zinc extraction process, considerable effort was made to remove cobalt impurities from the zinc solution by a cementation process at the last purification stage [33]. Anipsitakis and Dionysiou [34] demonstrated the high efficiency of cobalt ions in activating peroxymonosulfate (PMS) and generating sulfate radicals according to the following reaction:
Moreover, they proved that Co/PMS coupling is the best combination for degrading organic pollutants due to its high reactivity among other transition metals. To the best of our knowledge, this is the first study investigating the catalytic effect of cobalt ions for manganese removal from a zinc-rich solution by using off-gas from a roasting plant in this technology. Therefore, we selected cobalt as a catalyst to take advantage of its presence in the zinc purification solution.
4. Results
4.1. Manganese Removal without pH Adjustment
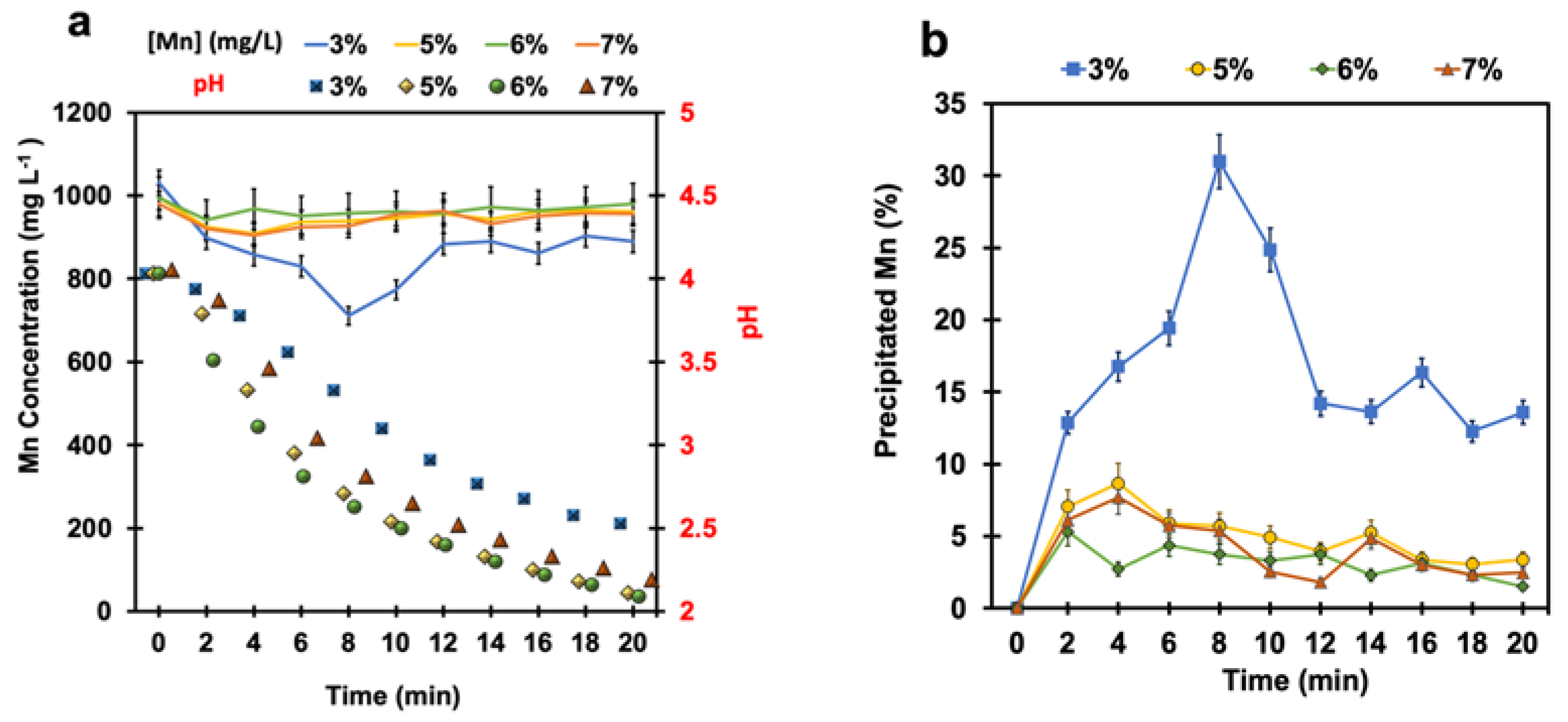
Sulfuric acid is generated upon introducing SO2 gas into a reactor, resulting in a pH drop. Therefore, a series of tests were performed without controlling the pH to investigate the sensitivity of manganese oxidation reaction to the pH. Several tests were carried out at different SO2/air ratios of 3%, 5%, 6%, and 7% to reveal the importance of pH variation in the manganese removal process. The initial pH was adjusted at (4 ± 0.04) by the addition of few drops of sulfuric acid to the synthetic solution containing 150 g L−1 zinc. Menard et al. [14] reported that, along with manganese removal, zinc is also removed in the form of zinc hydroxide precipitate at 80 °C and pH 4.3. This reaction is indeed an undesirable side reaction since ideally, no zinc should be removed from the solution. Although manganese oxidation with a SO2/air gas mixture is favoured at higher pH levels, to reduce zinc loss, the pH must be below 4.3 [25]. Thus, the initial pH was set at (4 ± 0.04) in all experiments in this work.
Figure 2a shows the monotonic pH drop upon injecting the SO2/air gas mixture into the reactor; the pH started to drop from 4 at the beginning of the test to around 2 after 20 min of testing in all various SO2/air ratios. The formation of sulfuric acid was the main reason for the pH fall. When pH values were between 3 and 4, manganese concentration was reduced and reached its lowest level of 800 mg L−1 at a SO2/air ratio of 3%. By contrast, at the pH values less than 3, not only was there no longer manganese oxidation, but also, the reduction of manganese dioxide occurred in the system. In other words, the oxidized manganese came back into the solution and its concentration increased to around 970 mg L−1 for all SO2/air ratios. Corresponding exponential equations were fit for each manganese concentration curve (Figure S1, Supplementary Information). As shown in Figure 2b, the maximum amount of precipitated manganese reached 31% at a SO2/air ratio of 3% within 8 min. At the other SO2/air ratios (i.e., 5%, 6%, and 7%), the pH drop was faster due to the injection of more SO2 and, consequently, the formation of more sulfuric acid in the solution. As a result, the total precipitated manganese after 20 min was only 2% at the higher SO2/air ratios. These results are consistent with the previous studies of Zhang et al. [24]. They determined that the oxidation of manganese with SO2/O2 is strongly pH-dependent, with virtually no manganese oxidation below pH 3 [24]. The oxidation of manganese and the reduction of manganese dioxide precipitates were also visually observed by the change in colour of the solution during the experiments. As shown in Figure 3, the solution colour was transparent before starting the test. In contrast, during the process, the colour of the solution turned into light brown, when the pH was above 3, and manganese oxidation happened. Then, it became transparent at the end of the test (after 20 min) when the pH declined to 2.58, confirming the reduction of all manganese dioxide precipitates. It is well known that oxygen solubility is reduced by decreasing the pH. Therefore, the main cause of the reducing condition could correspond to a considerable reduction in dissolved oxygen in the solution. Furthermore, Brandt et al. [35] showed that dithionate () species, known as radical scavengers, are created at the pH range between 2 and 3, whereas at pH 4, there is no detectable dithionate [35]. In this case, the formation of dithionate could have been the likely reason for the creation of reducing conditions.

Figure 2.
(a) The manganese concentration (black colour) and pH variation (red colour), and (b) the percentage of manganese precipitation vs. time at different SO2/air ratios under uncontrolled pH conditions (air flow rate = (500 ± 10) mL min−1, SO2 flow rate varied from 15 mL min−1 to 35 mL min−1, T = (80 ± 0.5) °C, agitation speed = 2000 rpm, initial [Mn] ≈ 1 g L−1).

Figure 3.
The colour change of the solution as a function of pH at different time intervals (SO2/air ratio = 0.03, air flow rate = (500 ± 10) mL min−1, SO2 flow rate = (15 ± 2) mL min−1, T = (80 ± 0.5) °C, agitation speed = 2000 rpm, initial [Mn] ≈ 1 g L−1).
4.2. Manganese Removal with pH Adjustment
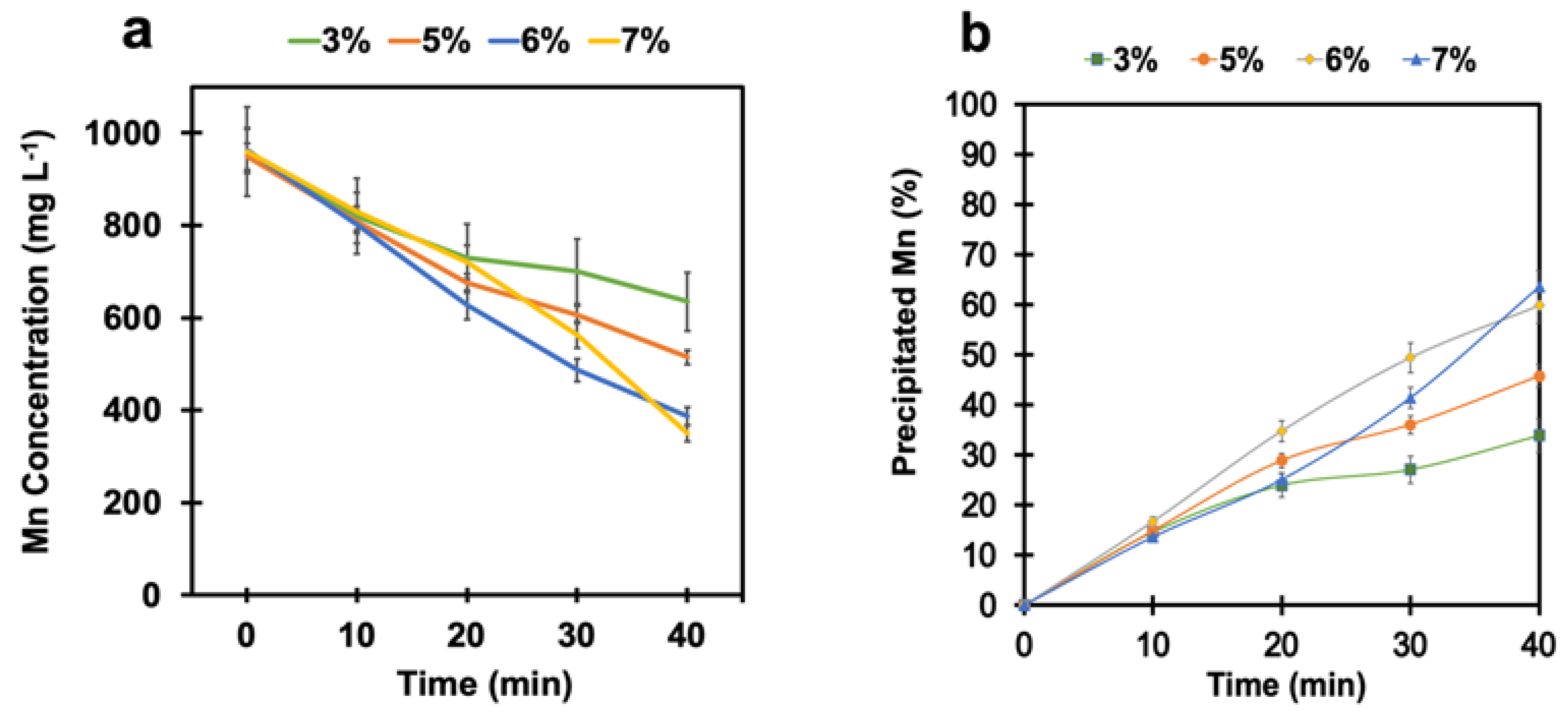
To adjust the pH and keep it constant at around 4 during the reaction, calcium hydroxide (Ca(OH)2) in powder form was continuously added for the whole test duration. Several tests were performed at different SO2/air ratios of 3%, 5%, 6%, and 7% under pH-controlled conditions to examine its effect on the manganese removal process. Figure 4a shows that manganese oxidation was significantly promoted in this system when the pH was kept constant at around 4. As a result, manganese was monotonically removed from the solution. The final manganese concentration after 40 min was reduced from 1 g L−1 to 387 mg L−1 and 350 mg L−1 at the SO2/air ratios of 6% and 7%, respectively. To predict the manganese removal trend, corresponding exponential equations were fit for each curve (Figure S2, Supplementary Information). As shown in Figure 4b, the percentages of the precipitated manganese at the SO2/air ratios of 6% and 7% were estimated to be 60% and 64%, respectively. Based on these observations, it can be concluded that controlling the pH by using a neutralizing agent is essential for improving the efficiency of the manganese removal process, and calcium hydroxide could be a suitable agent to keep the pH constant at around 4.
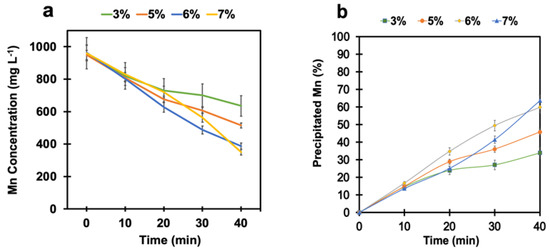
Figure 4.
(a) Manganese concentration and (b) the percentage of precipitated manganese vs. time at different SO2/air ratios under pH-controlled conditions (air flow rate = (500 ± 10) mL min−1, SO2 flow rate varied from 15 mL min−1 to 35 mL min−1, T = (80 ± 0.5) °C, agitation speed = 2000 rpm, initial [Mn] ≈ 1 g L−1).
4.3. Effect of Time
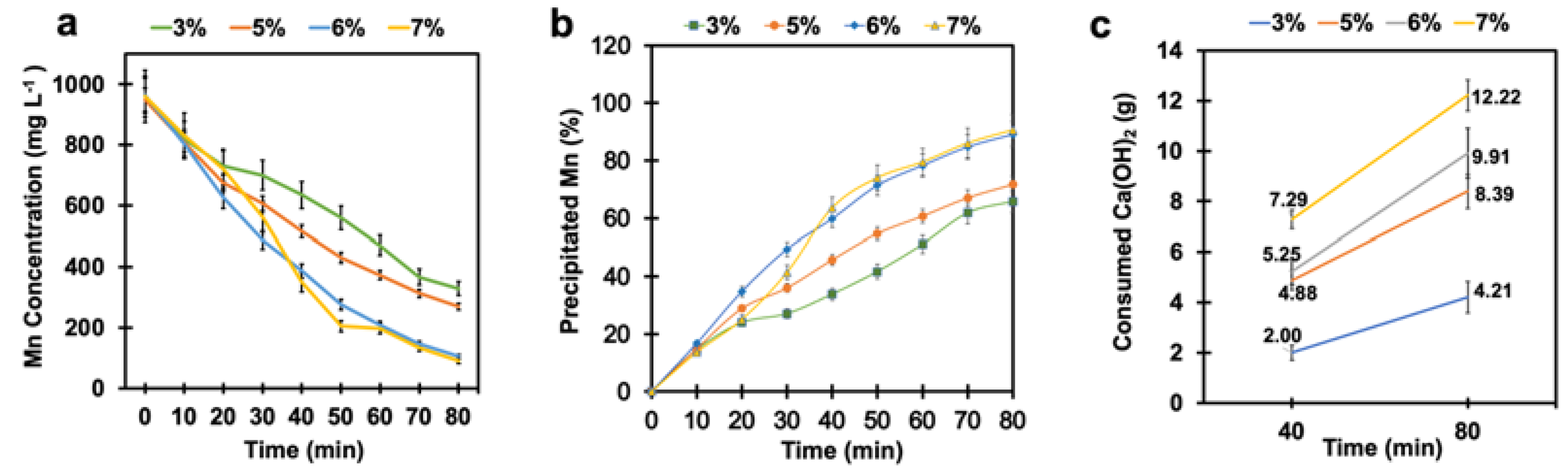
A series of tests were conducted to study the effect of time on the manganese removal process. In this module, the test duration was doubled. It can be observed from Figure 5a that much more manganese was removed from the solution by increasing the time of the process. The final manganese concentration after 80 min of reaction decreased from 1 g L−1 to 105 mg L−1 and 90 mg L−1 at the SO2/air ratios of 6% and 7%, respectively. According to Figure 5b, the total precipitated manganese was 89% and 91% at the SO2/air ratios of 6% and 7% after 80 min, respectively.

Figure 5.
(a) The manganese concentration, (b) the percentage of precipitated manganese, and (c) calcium hydroxide consumption vs. time at different SO2/air ratios under pH-controlled conditions and prolonged tests (air flow rate = (500 ± 10) mL min−1, SO2 flow rate varied from 15 mL min−1 to 35 mL min−1, T = (80 ± 0) °C, agitation speed = 2000 rpm, initial [Mn] ≈ 1 g L−1).
It can also be seen that there was no significant difference between the final manganese concentration when the SO2/air ratio changed from 6% to 7%. The results also demonstrate that the kinetics of the manganese oxidation reaction decreased with the progression of the test. Figure S3 (Supplementary Information) displays the corresponding mathematical equations for each curve. This phenomenon was evident between the first 40 min and the second 40 min. Manganese concentrations more than halved at both the 6% and 7% SO2/air ratios in the first 40 min, while the reduction of manganese concentration was only 25% for both ratios in the second 40 min. This could be associated with the influence of manganese ion concentration in the solution on the kinetics of the oxidation reaction.
The effects of the SO2/air ratios and time on calcium hydroxide consumption were also assessed. Figure 5c shows the total calcium hydroxide consumption as a function of reaction time and SO2/air ratio. There was an over twofold increase in calcium hydroxide consumption (from 2 g to 5.25 g) when the SO2/air ratio increased from 3% to 6% within the first 40 min. Furthermore, calcium hydroxide consumption increased by about 39%, with increasing SO2/air ratios from 6% to 7% after 40 min. Calcium hydroxide was consumed 88% more by increasing the time from 40 min to 80 min at the SO2/air ratio of 6%. Although the prolonged tests resulted in much more manganese removal, this increased calcium hydroxide consumption considerably. As a result, the more calcium hydroxide used, the more gypsum () produced as a by-product (Equation (13)), requiring further treatment for recycling. Considering the rate of manganese removal reaction and calcium hydroxide consumption, we concluded that the SO2/air ratio of 6% was the optimal ratio because it resulted both in less calcium hydroxide consumption and in maximum manganese elimination simultaneously.
4.4. Effect of Cobalt on Manganese Removal
4.4.1. Cobalt as Catalyst
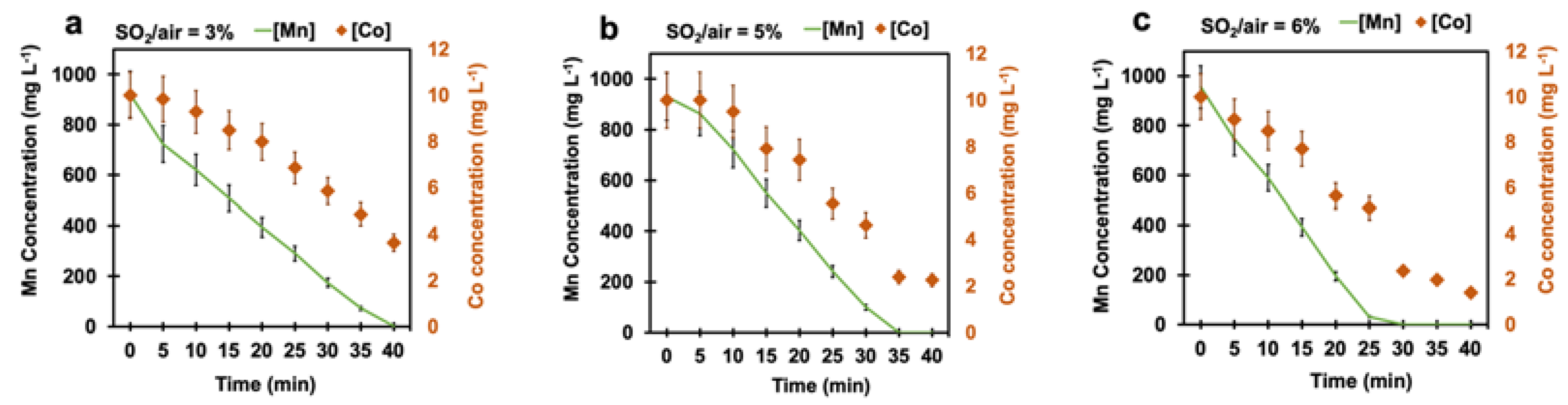
According to the results so far, 100 mg L−1 was the minimum manganese concentration obtained after 80 min at a SO2/air ratio of 6%. This concentration was still too high for employing MMO anodes, where our target was less than 50 mg L−1. Therefore, we explored the possibility of using cobalt as a catalyst to accelerate the reaction. We used 10 mg L−1 cobalt in the synthetic solution since this concentration is close to the cobalt concentration in the plant solution before the cementation stage. Figure 6a–c show the manganese concentration of the solution with 10 mg L−1 cobalt at different SO2/air ratios of 3%, 5%, and 6%. The presence of 10 mg L−1 cobalt ions in the solution drastically accelerated the manganese oxidation reaction rate. Manganese concentration in the solution decreased from 1 g L−1 to less than 1 mg L−1, after 40 min at a SO2/air ratio of 3%. By increasing the SO2/air ratio, the manganese removal time further decreased and reached 35 min and 30 min, respectively, for the SO2/air ratios of 5% and 6% (Figure 6b,c). This remarkable effect of cobalt is attributable to its effect on the activation of peroxymonosulfate and, consequently, on the generation of sulfate radicals (Equation (12)). Although several transition metals can have such an effect, according to a systematic study performed by Anipsitakis et al. [36], cobalt and Ru are the best metals for the activation of peroxymonosulfates.

Figure 6.
Manganese and cobalt concentrations at different SO2/air ratios under pH-controlled conditions: (a) 3%, (b) 5%, and (c) 6%. The left y-axis is related to manganese concentration (black colour), and the right y-axis is associated with cobalt concentration (orange colour) (air flow rate = (500 ± 10) mL min−1, SO2 flow rate varied from 15 mL min−1 to 30 mL min−1, T = (80 ± 0.5) °C, agitation speed = 2000 rpm, initial [Mn] ≈ 1 g L−1, initial [Co] = 10 mg L−1).
Interestingly, cobalt ions were also removed along with manganese. The cobalt concentration was reduced from 10 mg L−1 to 4 mg L−1, 2 mg L−1 and 1 mg L−1 at the SO2/air ratios of 3%, 5%, and 6%, respectively (Figure 6a–c). The cobalt removal in this process could be attributed to the ability of manganese oxide, like birnessite, to trap trace metal cations such as Co2+, Zn2+, Ni2+, and Cu2+ due to its lamellar and negatively charged structure [37,38].
The negative charge of manganese dioxide is created due to structural features such as edge shared MnO6 octahedra, layer vacancies, and substitution of Mn4+ by lower valance cations (e.g., Mn3+, Co3+, Ni3+). This negative charge is compensated by uptaking the surrounding cations of metals in various ways including adsorption, coprecipitation, and oxidation [37,39,40].
4.4.2. Cobalt Removal
To prove the role of manganese dioxide precipitates as cobalt scavengers, one test was performed in a solution without any manganese ions at the SO2/air ratio of 6% under the same conditions. The results, as shown in Figure S4 (Supplementary Information), revealed that in the absence of manganese ions in the solution, there was no cobalt removal, and the cobalt concentration in the solution remained constant at the initial concentration of about 10 mg L−1 during 30 min of operation. This observation opens a new horizon for further investigation of the effect of manganese dioxide precipitates in removing other elements such as (Ni, Cd, Cu, and Zn) in a zinc purification solution.
4.4.3. Effect of Cobalt Concentration on Manganese Removal
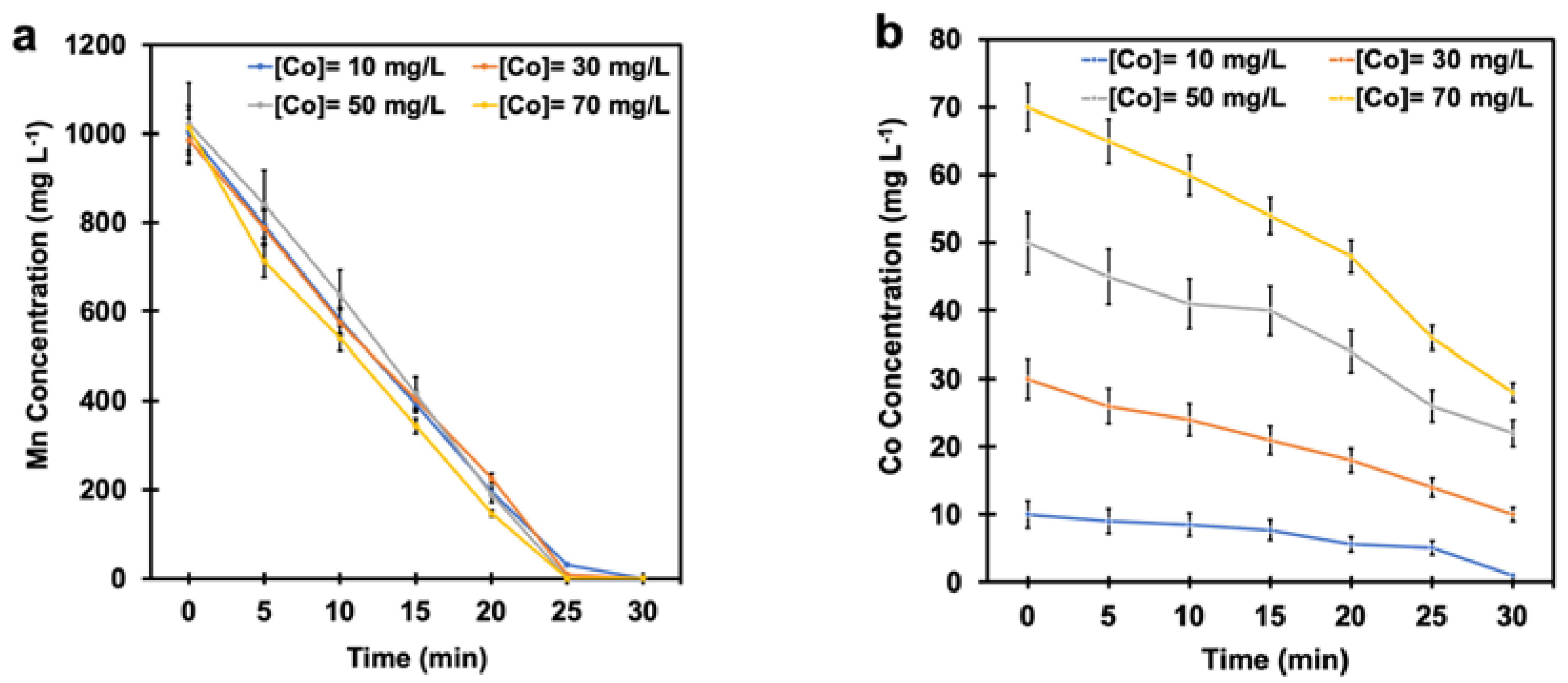
Figure 7a,b show the effect of various cobalt concentrations (10 mg L−1, 30 mg L−1, 50 mg L−1, and 70 mg L−1) on manganese and cobalt removal at the SO2/air ratio of 6% under pH-controlled conditions. According to Figure 7a, manganese ions were removed entirely at all different cobalt concentrations. The manganese oxidation reaction was slightly accelerated (5 min earlier) at the cobalt concentrations of 50 mg L−1 and 70 mg L−1. This observation suggests that 10 mg L−1 Co is sufficient to remove manganese; beyond this concentration, no more beneficial effect is achieved. The results, as shown in Figure 7b, also revealed that the cobalt concentration decreased from the initial concentrations of 10 mg L−1, 30 mg L−1, 50 mg L−1, and 70 mg L−1 to 1 mg L−1, 10 mg L−1, 22 mg L−1, and 28 mg L−1, respectively. These observations suggest that cobalt catalyzes the manganese oxidation reaction, resulting in precipitation of manganese dioxide while manganese dioxide, in turn, helps in removing cobalt from the solution.

Figure 7.
(a) The manganese concentration and (b) the cobalt concentration under pH-controlled conditions at a SO2/air ratio of 6% with various cobalt concentrations air flow rate = (500 ± 10) mL min−1, SO2 flow rate = (30 ± 2) mL min−1, T = (80 ± 0.5) °C, agitation speed = 2000 rpm, initial [Mn] ≈ 1 g L−1).
One can observe that the efficiency of cobalt removal depends on manganese concentration. In other words, 1 g L−1 of manganese ions could remove almost all cobalt from a system when the initial concentration of cobalt is 10 mg L−1. At higher cobalt concentrations, there is not enough manganese dioxide to trap the cobalt ions. Therefore, using higher cobalt concentrations not only does not have any further benefit to remove manganese but also requires further purification processes to remove the remaining cobalt as an impurity from the system. In conclusion, 10 mg L−1 of cobalt seems to be the optimal quantity for taking advantage of its catalytic effect in manganese elimination. It is also interesting to note that the cobalt concentration in the zinc purification solution in the plant was in the range of 10 mg L−1 to 15 mg L−1.
4.5. Characterization of the Precipitates
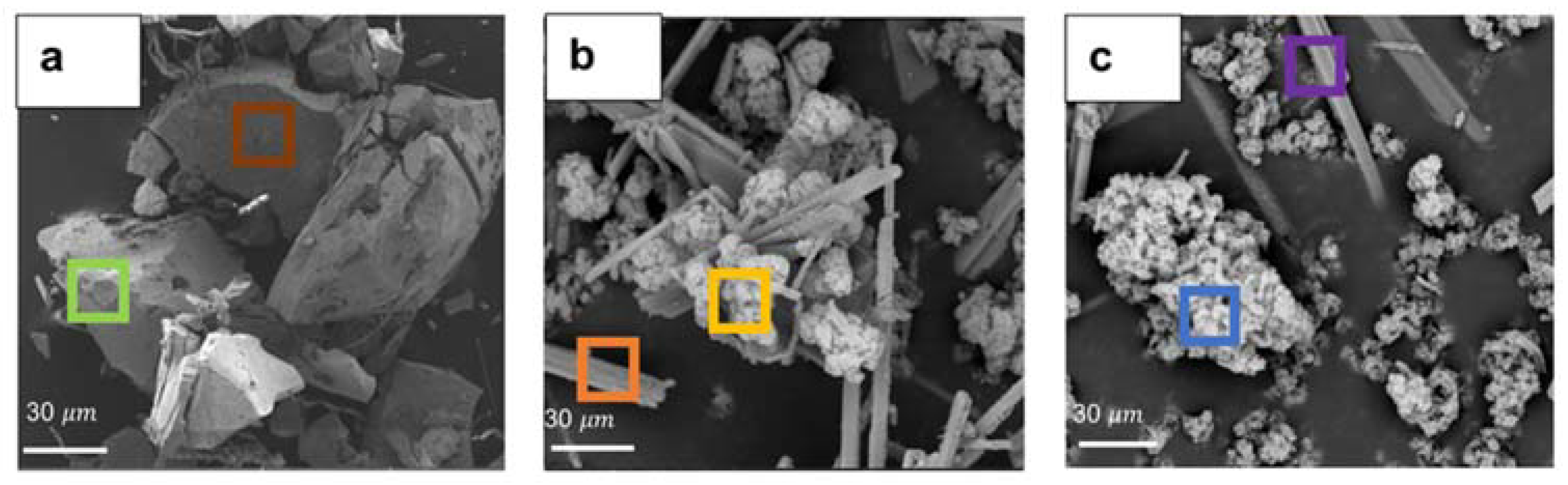
Figure 8a–c and Table 1 show SEM images and corresponding EDS elemental analysis of the precipitates generated under different operating conditions. It was observed that in the absence of an extra agent, i.e., neutralizing agents and cobalt, the morphology of the particles was angular and pebble-like in shape with a relatively uniform size distribution (Figure 8a). According to the corresponding EDS analysis (green and brown frames in Table 1), manganese, zinc, and oxygen were the dominant constituents of these particles, suggesting the formation of manganese dioxide with zinc coprecipitation. According to Figure 8b, the pebble-like shapes were substituted with some cauliflower-like forms among needle-shaped particles after adding calcium hydroxide as a neutralizing agent. XRD diffraction analysis was performed on the precipitates. However, due to the presence of high amounts of gypsum in the precipitates, the XRD peaks of other phases, i.e., MnO2, were considerably suppressed, rendering it difficult to accurately determine the crystalline structure of the precipitates (Figure S5). According to Table 1, the EDS analysis verified that the cauliflower particles, specified with the yellow frame in Figure 8b, were mainly composed of manganese, zinc, and oxygen, while the needle-shaped precipitate (orange frame) was made of calcium, sulfur, and oxygen, indicating manganese dioxide and gypsum precipitates (CaSO4.H2O), respectively. Figure 8c shows the SEM image of the precipitates obtained when we added both calcium hydroxide and cobalt to the solution. The addition of 10 mg L−1 cobalt did not tangibly affect the morphology of particles.
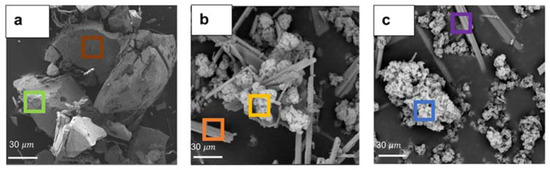
Figure 8.
SEM images of precipitates (a) for uncontrolled pH conditions without cobalt addition, (b) for pH-controlled conditions without cobalt addition, and (c) for both pH-controlled conditions and cobalt addition, obtained at a SO2/air ratio of 6% (air flow rate = (500 ± 10) mL min−1, SO2 flow rate = 30 mL min−1, T = (80 ± 0.5) °C, agitation speed = 2000 rpm, initial [Mn] = 1 g L−1, [Co] = 10 mg L−1). Data were obtained at 15 kV in BSE mode.

Table 1.
EDS elemental analysis of precipitates of the SEM images (a–c). All data are expressed as weight percentages.
Figure 8c depicts the presence of both cauliflower-like and needle-shaped particles. Meanwhile, according to Table 1, the EDS analysis results corresponding to manganese oxide precipitate (cauliflower-shaped specified with the blue frame) verified the cobalt co-precipitation with the manganese dioxide. The gypsum particles were produced by the reaction between calcium hydroxide and sulfuric acid during the process. Furthermore, it was found that the addition of calcium hydroxide caused a remarkable morphological change in the manganese dioxide precipitates compared to the case that was performed without controlling the pH. This can be attributed to the adsorption of calcium cations inside manganese dioxide’s structure, as discussed earlier.
XPS analysis was performed to scrutinize the chemical composition of the precipitates. The collected precipitates under the pH-controlled conditions at a SO2/air ratio of 6% with and without cobalt addition were analyzed. As shown in Table 2, survey spectra of both types of precipitates (with and without cobalt addition) revealed the presence of oxygen, carbon, calcium, zinc, sulfur, and manganese. Oxygen and sulfur were indeed present in the solution while calcium came from a calcium hydroxide additive to control the pH during the test. Manganese and zinc were the main metal ions in the solution precipitated through the experiment. Furthermore, where we had cobalt addition, the existence of cobalt in the precipitates was confirmed, suggesting the coprecipitation of cobalt along with manganese dioxide. Carbon was mainly obtained from atmospheric contamination. Interestingly, in the presence of cobalt ions, the calcium and sulfur contents of the precipitates (in atomic percentages) decreased by 23% and 12.5%, respectively, while the zinc and manganese contents of the precipitates (in atomic percentages) increased by 19% and 9.6%, respectively. These results confirm cobalt’s catalytic effect on the manganese removal process. Furthermore, cobalt led to the shortening of the test duration; therefore, less SO2/air was purged, and consequently, less calcium hydroxide was consumed.

Table 2.
Chemical composition of precipitates extracted from XPS survey spectra.
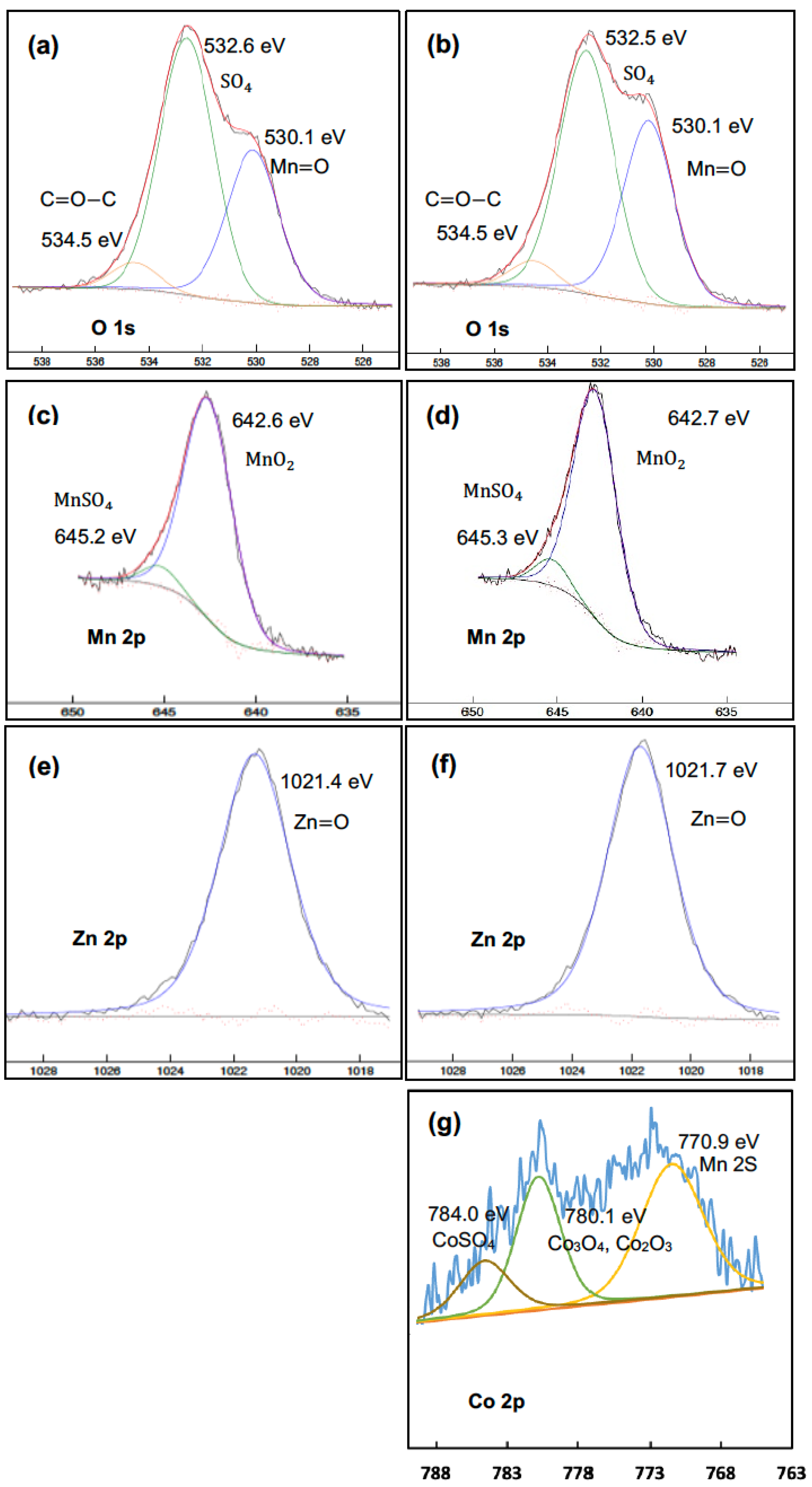
Figure 9a–g display high-resolution (HR) spectra of O 1s, Mn 2p, Zn 2p, and Co 2p for two different types of precipitates. The left-hand side spectra correspond to precipitates obtained without cobalt addition, while the right-hand side corresponds to precipitates where cobalt was added under the same conditions. Peak identification was performed using the handbook of photoelectron spectroscopy [41]. On the left-hand side, the O 1s peak (Figure 9a) reveals three chemical bonds corresponding to Mn=O (530.1 eV), O=S-O (532.6 eV), and C=O-C (534.6 eV). The Mn 2p3 spectrum in Figure 9c corresponds to MnO2 (642.6 eV) and MnSO4 (645.2 eV), indicating the formation of these compounds during the process. The Zn 2p3 peak (Figure 9e) exhibits only a single chemical bond for Zn=O (1021.3 eV). On the right-hand side, the Co 2p spectrum shown in Figure 9g exhibits three peaks at (771, 780, and 784) eV. The peak at 771 eV is associated with Mn 2s due to its overlap with the Co 2p band. The peak at 780 eV corresponds to Co3O4 and Co2O3, and the peak at 784 eV is assigned to CoSO4.

Figure 9.
XPS high-resolution spectra of precipitates obtained under pH-controlled conditions at a SO2/air ratio of 6%. The left-side hand (a,c,e) corresponds to precipitates without cobalt addition, and the right-side hand (b,d,f,g), to precipitates with the addition of cobalt for O 1s, Mn 2p, Zn 2p, and Co 2p, respectively (air flow rate = (500 ± 10) mL min−1, SO2 flow rate = (30 ± 2) mL min−1, T = (80 ± 0.5) °C, agitation speed = 2000 rpm, initial [Mn] = 1 g L−1, [Co] = 10 mg L−1).
The high-resolution spectra of the precipitates obtained with the addition of cobalt were quite similar to those of the precipitates without cobalt addition. The slight differences in the positions of similar peaks in the two series of samples could have arisen from multiple sources, such as coprecipitation (incorporation of another element, i.e., Co, into the structure), hydroxide formation (especially in the case of Mn), or peak deconvolution precision. These results confirm that manganese, zinc, and cobalt ions were oxidized and precipitated as manganese dioxide, zinc, and cobalt oxides during this process, which is consistent with the EDS analysis results.
To confirm the previous results, XRF analysis was also performed to determine the bulk chemical composition of the precipitates collected at a SO2/air ratio of 6%. As summarized in Table 3, in the precipitates with cobalt addition, the concentration of manganese and zinc increased by about 9.5% and 26% compared to the precipitates obtained without cobalt addition. In contrast, less sulfur and calcium were detected in the precipitates obtained with cobalt addition compared to the samples without cobalt, i.e., the sulfur concentration was reduced from 16.7% to 14.7%, and the calcium concentration decreased from 26.1% to 20.9%. This observation confirms the previous results of EDS and XPS, suggesting that the presence of 10 mg L−1 cobalt improves manganese removal efficiency and reduces chemical agent consumption, such as calcium hydroxide. The ratios of S/Ca in the XRF results were 0.63 and 0.7 for two series of samples. These ratios are less than 0.8, which is expected for CaSO4, suggesting that part of the Ca and most of the other elements were in their oxide form.

Table 3.
Chemical composition of precipitates (%) obtained from XRF spectra.
The addition of Ca(OH)2 to control pH results in an appreciable amount of gypsum precipitation which should be removed from the system along with other oxide precipitates. The amount of the precipitated gypsum was approximated using the ratio of Ca/Mn. This approximation indicated that the mass of gypsum would be 6.6 times greater than that of the precipitated MnO2. As the concentration of manganese was known and continuously monitored in the plant, this could help in determining the amount of gypsum to be removed and designing appropriate settling reservoirs and filtering systems. It would most likely be possible to reduce the amount of calcium consumption and consequently the amount of gypsum generation by further optimizing other process parameters, i.e., spargers design.
Finally, the presence of zinc in the precipitates may be considered as a loss of product. The total zinc loss was in the same range as the removed manganese, thus not exceeding the equivalent of 1 g L−1. However, the side benefit of this approach to remove manganese is that cobalt was also removed from the solution simultaneously. In the current practice, the level of cobalt before the purification stage is about 10 mg L−1, which is then removed by cementation operation using zinc dust. It is worth mentioning that typically, (4–5)% of plant zinc product is used to remove Co in the cementation process. Removing cobalt from the solution along with manganese therefore represents a side benefit of this process and may considerably reduce the consumption of zinc dust in the cementation process.
5. Conclusions
We showed that a SO2/air gas mixture alone cannot remove manganese from a solution without controlling the pH. When the pH was kept around 4, a great improvement in the efficiency of manganese elimination was achieved. The final manganese concentration was reduced from 1 g L−1 to 387 mg L−1 after 40 min, which was still too high for the implementation of MMO anodes.
Therefore, we added cobalt to the solution to accelerate the manganese oxidation reaction. We showed that the kinetics of the manganese oxidation reaction could be considerably improved by using 10 mg L−1 cobalt as a catalyst. The manganese concentration was reduced from 1 g L−1 to less than 1 mg L−1 within 30 min, which was an acceptable level for implementing MMO anodes. This finding confirms that a small amount of cobalt can render off-gas as a suitable reactant for the efficient removal of manganese.
We also observed that the sediments consisted mainly of gypsum (CaSO4.2H2O) and manganese dioxides with the coprecipitation of zinc. The XPS results evidenced the absorption of cobalt inside manganese dioxide precipitates. The maximum amount of CaSO4 in the sediment was estimated to be 6.6 times that of the MnO2.
This method seems to be a promising method for removing manganese and cobalt from zinc electrolyte using a SO2/air mixture, available at zinc production plants. The zinc loss during this process is in the same range as the removed manganese. The considerable advantage of this process is that both the SO2/air mixture and cobalt are available in the plant’s off-gas and electrolyte, respectively. More importantly, more than 90% of the cobalt is removed during the process, representing a considerable possible reduction in zinc dust in the purification/cementation step. The implementation of this method however requires technoeconomic analyses to compare the total processing cost vs. the energy gain that MMO anodes may generate.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/met13101675/s1. Figure S1: Manganese concentration (black colour) and pH (red colour) vs. time at different SO2/air ratios under uncontrolled pH conditions (air flow rate = (500 ± 10) mL min–1, SO2 flow rate varied from 15 mL min–1 to 35 mL min–1, T = (80 ± 0.5) °C, agitation speed = 2000 rpm, initial [Mn] ≈ 1 g L–1). Figure S2: Manganese concentration vs. time at different SO2/air ratios under pH-controlled conditions (air flow rate = (500 ± 10) mL min–1, SO2 flow rate varied from 15 mL min–1 to 35 mL min–1, T = (80 ± 0.5) °C, agitation speed = 2000 rpm, initial [Mn] ≈ 1 g L–1). Figure S3: Manganese concentration vs. time at different SO2/air ratios under pH-controlled conditions and prolonged tests without the presence of Co (air flow rate = (500 ± 10) mL min–1, SO2 flow rate varied from 15 mL min–1 to 35 mL min–1, T = (80 ± 0) °C, agitation speed = 2000 rpm, initial [Mn] ≈ 1 g L–1). Figure S4: Cobalt concentration under pH-controlled conditions without manganese in the solution as a function of time at a SO2/air ratio of 6%. The left y-axis is related to cobalt concentration (orange column) and the right y-axis is associated with manganese concentration (green diamonds), which was zero (air flow rate = (500 ± 10) mL min–1, SO2 flow rate = (30 ± 2) mL min–1, T = (80 ± 0.5) °C, agitation speed = 2000 rpm, initial [Co] = 10 mg L–1). Figure S5: XRD diffraction patterns of precipitates obtained under pH-controlled conditions at a SO2/air ratio of 6% without the presence of Co (air flow rate = (500 ± 10) mL min–1, SO2 flow rate = (30 ± 2) mL min–1, T = (80 ± 0.5) °C, agitation speed = 2000 rpm, initial [Mn] = 1 g L–1). Table S1: Chronological description of the experiments.
Author Contributions
M.A.: methodology, investigation, analysis, and writing—original draft, R.M.: investigation and data curation, F.M.: methodology and investigation, E.B.: conceptualization and validation, G.H.: conceptualization and validation, C.B.: conceptualization and validation, H.A.: conceptualization, validation, writing—review and editing, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada (RDCPJ 522069-17), CEZinc, and DeNora.
Data Availability Statement
Please contact the corresponding author for original data.
Acknowledgments
The authors thank the Natural Sciences and Engineering Research Council of Canada (RDCPJ 522069-17), CEZinc, and DeNora for their financial and technical support.
Conflicts of Interest
The authors declare no conflict of interest. Elyse Benguerel from CEZinc and Carl Brown from DeNora participated in the conceptualization and validation of the results as well as in providing technical assistance in the experimental set-up design.
References
- He, S.; Xu, R.; Sun, L.; Fan, Y.; Zhao, Z.; Liu, H.; Lv, H. Electrochemical characteristics of Co3O4-doped β-PbO2 composite anodes used in long-period zinc electrowinning. Hydrometallurgy 2020, 194, 105357. [Google Scholar] [CrossRef]
- Jaimes, R.; Miranda-Hernández, M.; Lartundo-Rojas, L.; González, I. Characterization of anodic deposits formed on Pb–Ag electrodes during electrolysis in mimic zinc electrowinning solutions with different concentrations of Mn(II). Hydrometallurgy 2015, 156, 53–62. [Google Scholar] [CrossRef]
- Sinclair, R.J. The Extractive Metallurgy of Zinc; AusIMM: Carlton, VIC, Australia, 2005. [Google Scholar]
- Wu, F.; Yang, Z.-Q.; Sun, W.; Chen, X.; Qi, H.; Wang, L.-D. Electrochemical Properties of Ti/SnO2-Sb-Ir Electrodes Doped with a Low Iridium Content for the Oxygen Evolution Reaction in an Acidic Environment. Ind. Eng. Chem. Res. 2022. [Google Scholar] [CrossRef]
- Ye, W.; Xu, F.; Jiang, L.; Duan, N.; Li, J.; Ma, Z.; Zhang, F.; Chen, L. Lead release kinetics and film transformation of Pb-MnO2 pre-coated anode in long-term zinc electrowinning. J. Hazard. Mater. 2021, 408, 124931. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; He, Y.; Li, W.; Wang, Z.; Yu, L.; Jiang, Y.; Liu, X.; Kang, J.; Gao, H.; Lin, N. Insight into electrochemical performance of porous FexSiy intermetallic anode for zinc electrowinning. Mater. Des. 2020, 191, 108645. [Google Scholar] [CrossRef]
- Trasatti, S. Electrocatalysis: Understanding the success of DSA®. Electrochim. Acta 2000, 45, 2377–2385. [Google Scholar] [CrossRef]
- Krstić, V.; Pešovski, B. Reviews the research on some dimensionally stable anodes (DSA) based on titanium. Hydrometallurgy 2019, 185, 71–75. [Google Scholar] [CrossRef]
- Ma, D.; Ngo, V.; Raghavan, S.; Sandoval, S. Degradation of Ir-Ta oxide coated Ti anodes in sulfuric acid solutions containing fluoride. Corros. Sci. 2020, 164, 108358. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, C.Y. Manganese metallurgy review. Part III: Manganese control in zinc and copper electrolytes. Hydrometallurgy 2007, 89, 178–188. [Google Scholar] [CrossRef]
- Mahon, M.; Alfantazi, A. Manganese consumption during zinc electrowinning using a dynamic process simulation. Hydrometallurgy 2014, 150, 184–191. [Google Scholar] [CrossRef]
- Zhang, W.; Ghali, E.; Houlachi, G. Review of oxide coated catalytic titanium anodes performance for metal electrowinning. Hydrometallurgy 2017, 169, 456–467. [Google Scholar] [CrossRef]
- de Menezes, D.R. Performance Evaluation of Mixed Metal Oxide Anodes for Zinc Electrowinning. Ph.D. Thesis, Université Laval, Québec, QC, Canada, 2021. [Google Scholar]
- Ménard, V. Controlled Oxidative Precipitation of Manganese from an Industrial Zinc Sulphate Solution Using a Sulphur Dioxide and Oxygen Gas Mixture. Master’s Thesis, McGill University, Montréal, QC, Canada, 2004. [Google Scholar]
- Patil, D.S.; Chavan, S.M.; Oubagaranadin, J.U.K. A review of technologies for manganese removal from wastewaters. J. Environ. Chem. Eng. 2016, 4, 468–487. [Google Scholar] [CrossRef]
- Rethinaraj, J.P.; Visvanathan, S. Preparation and properties of electrolyc manganese dioxide. J. Power Sources 1993, 42, 335–343. [Google Scholar] [CrossRef]
- Awual, M.R. A novel facial composite adsorbent for enhanced copper (II) detection and removal from wastewater. Chem. Eng. J. 2015, 266, 368–375. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Rahman, M.M.; Asiri, A.M. Novel composite material for selective copper (II) detection and removal from aqueous media. J. Mol. Liq. 2019, 283, 772–780. [Google Scholar] [CrossRef]
- Orue, B.P.; Botelho Junior, A.B.; Tenório, J.A.S.; Espinosa, D.C.R.; Baltazar, M.d.P.G. Kinetic study of manganese precipitation of nickel laterite leach based-solution by ozone oxidation. Ozone Sci. Eng. 2021, 43, 324–338. [Google Scholar] [CrossRef]
- Mulaudzi, N.; Mahlangu, T. Oxidative precipitation of Mn (II) from cobalt leach solutions using dilute SO2/air gas mixture. J. South. Afr. Inst. Min. Metall. 2009, 109, 375–382. [Google Scholar]
- Bello-Teodoro, S.; Pérez-Garibay, R.; Uribe-Salas, A. The controlled oxidative precipitation of manganese oxides from Mn(II) leach liquors using SO2/air gas mixtures. Miner. Eng. 2011, 24, 1658–1663. [Google Scholar] [CrossRef]
- Teixeira, L.A.C.; Queiroz, J.P.L.; Marquez-Sarmiento, C. Oxidative precipitation of manganese from dilute waters. Mine Water Environ. 2017, 36, 452–456. [Google Scholar] [CrossRef]
- Saidi, M.; Kadkhodayan, H. Toxic heavy metal removal from sulfide ores using potassium permanganate: Process development and waste management. J. Environ. Manag. 2020, 276, 111354. [Google Scholar] [CrossRef]
- Zhang, W.; Singh, P.; Muir, D. Oxidative precipitation of manganese with SO2/O2 and separation from cobalt and nickel. Hydrometallurgy 2002, 63, 127–135. [Google Scholar] [CrossRef]
- Menard, V.; Demopoulos, G.P. Gas transfer kinetics and redox potential considerations in oxidative precipitation of manganese from an industrial zinc sulphate solution with SO2/O2. Hydrometallurgy 2007, 89, 357–368. [Google Scholar] [CrossRef]
- Brandt, C.; Van Eldik, R. Transition metal-catalyzed oxidation of sulfur (IV) oxides. Atmospheric-relevant processes and mechanisms. Chem. Rev. 1995, 95, 119–190. [Google Scholar] [CrossRef]
- Pérez-Garibay, R.; Bello-Teodoro, S.; Rojas-Montes, J.C. Thermodynamic simulation of the reaction mechanism of Mn2+ oxidation with an SO2/O2 mixture. Hydrometallurgy 2018, 176, 260–265. [Google Scholar] [CrossRef]
- Zhang, W.; Singh, P.; Muir, D. SO2/O2 as an oxidant in hydrometallurgy. Miner. Eng. 2000, 13, 1319–1328. [Google Scholar] [CrossRef]
- Coichev, N.; Van Eldik, R. Kinetics and mechanism of the sulfite-induced autoxidation of cobalt(II) in aqueous azide medium. Inorg. Chem. 1991, 30, 2375–2380. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Z.; Qian, J.; Pan, B. Are Free Radicals the Primary Reactive Species in Co(II)-Mediated Activation of Peroxymonosulfate? New Evidence for the Role of the Co(II)–Peroxymonosulfate Complex. Environ. Sci. Technol. 2021, 55, 6397–6406. [Google Scholar] [CrossRef]
- Hou, J.; He, X.; Zhang, S.; Yu, J.; Feng, M.; Li, X. Recent advances in cobalt-activated sulfate radical-based advanced oxidation processes for water remediation: A review. Sci. Total Environ. 2021, 770, 145311. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.; Van Eldik, R. The formation of dithionate during the iron(III)-catalysed autoxidation of sulfur(IV)-oxides. Atmos. Environ. 1997, 31, 4247–4249. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Environ. Sci. Technol. 2003, 37, 4790–4797. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Li, Q.; Wang, Q.; Yan, X.; Li, B.; Feng, L.; Wu, C.; Qiu, R.; Zhang, H.; Yang, Z.; et al. A review on the transformation of birnessite in the environment: Implication for the stabilization of heavy metals. J. Environ. Sci. 2023, 139, 496–515. [Google Scholar] [CrossRef]
- Hinkle, M.A.G.; Dye, K.G.; Catalano, J.G. Impact of Mn(II)-Manganese Oxide Reactions on Ni and Zn Speciation. Environ. Sci. Technol. 2017, 51, 3187–3196. [Google Scholar] [CrossRef] [PubMed]
- Peña, J.; Bargar, J.R.; Sposito, G. Copper sorption by the edge surfaces of synthetic birnessite nanoparticles. Chem. Geol. 2015, 396, 196–207. [Google Scholar] [CrossRef]
- Xu, T.; Roepke, E.W.; Flynn, E.D.; Rosenfeld, C.E.; Balgooyen, S.; Ginder-Vogel, M.; Schuler, C.J.; Santelli, C.M. Aqueous Co removal by mycogenic Mn oxides from simulated mining wastewaters. Chemosphere 2023, 327, 138467. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).