The Effect of Fatigue Damage on the Corrosion Fatigue Crack Growth Mechanism in A7N01P-T4 Aluminum Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
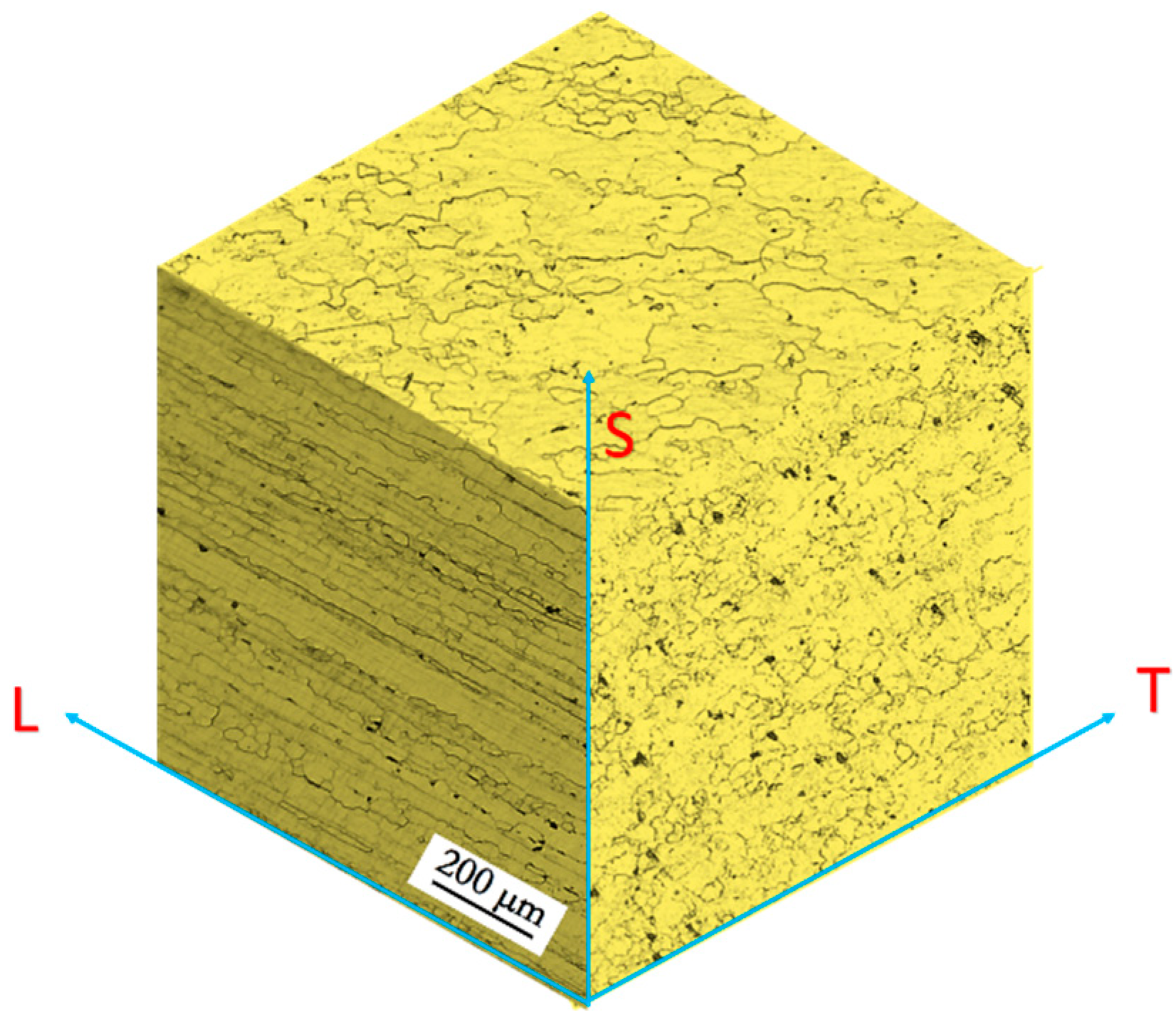
2.2. Microstructure
2.3. Electrotesting
2.4. Corrosion Fatigue Crack Propagation
3. Results
3.1. Microstructure
3.2. Electrochemical Analysis
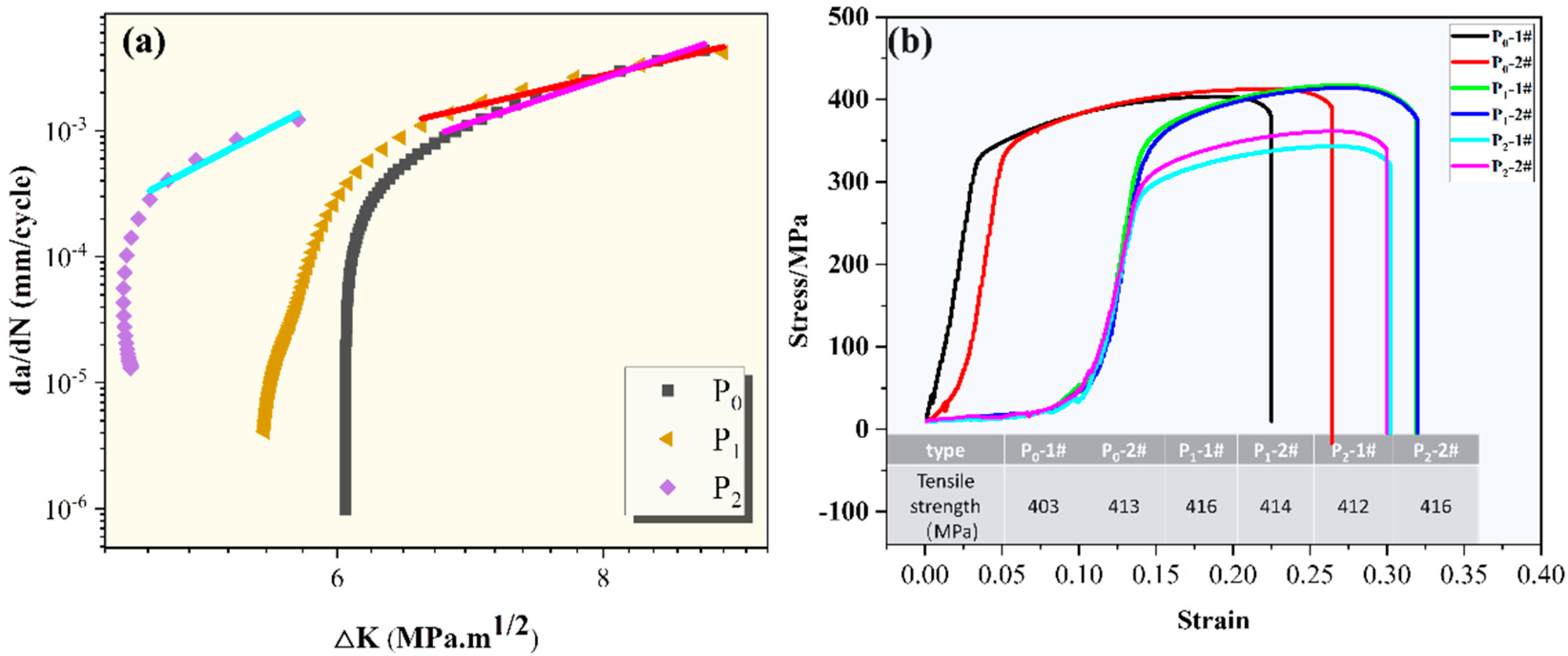
3.3. Corrosion Fatigue Crack Growth Rate Curve
3.3.1. Fracture Morphology of Corrosion Fatigue Crack Growth
3.3.2. Path Analysis of Corrosion Fatigue Crack Growth
4. Discussion
4.1. The Effect of Fatigue Damage on Microstructure
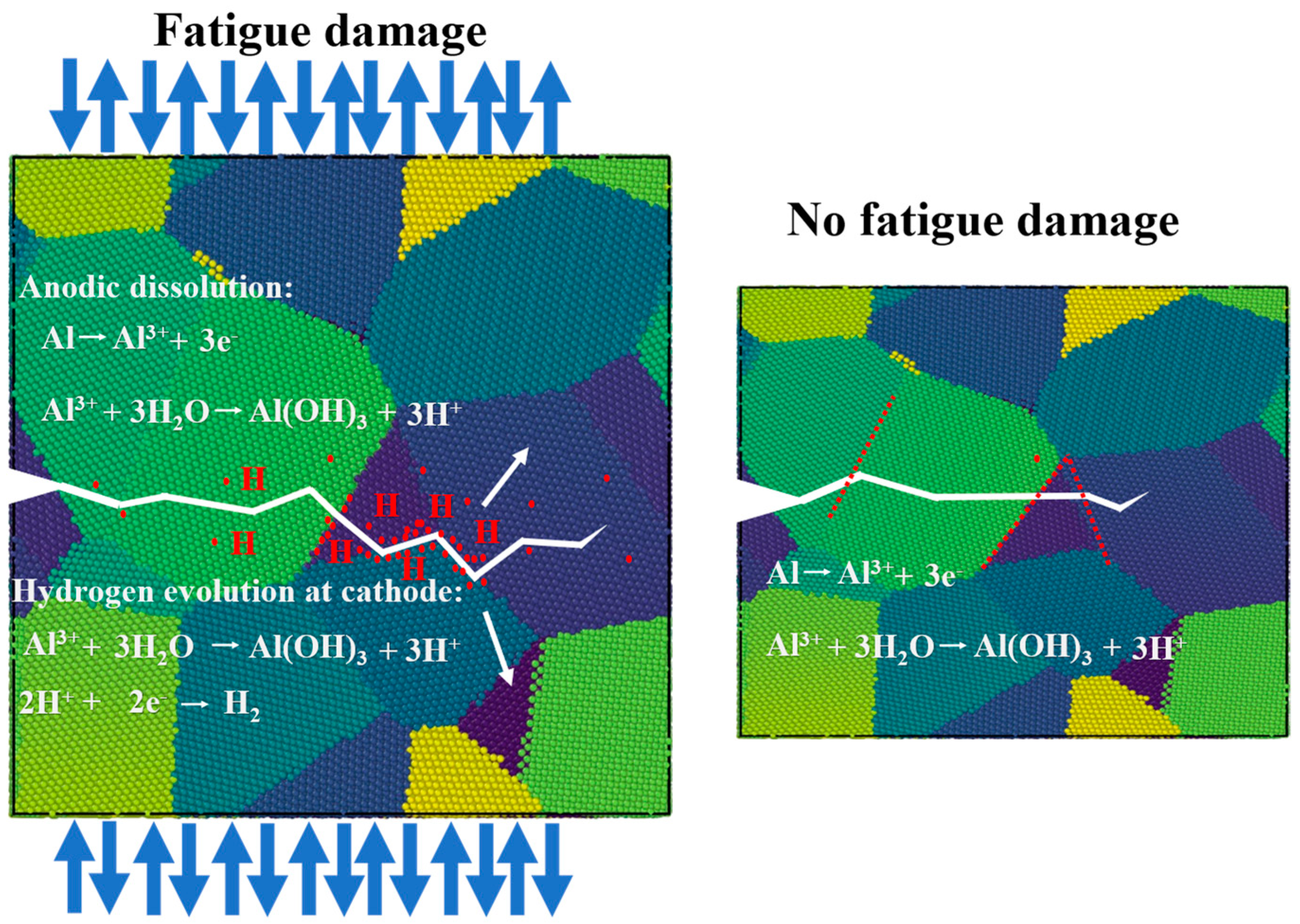
4.2. Fatigue Damage Promotes Stress Corrosion Cracking
5. Conclusions
- (1)
- Material corrosion resistance decreases following fatigue damage. The corrosion fatigue crack growth rate of fatigue-damaged specimens increases as the fatigue damage degree also increases, and the corrosion fatigue crack growth rate of fatigue-damaged specimen P2 (loading stress 70 MPa) is fastest.
- (2)
- The existence of secondary cracks in the sample without fatigue damage reduces crack tip stress concentration while delaying crack propagation. High magnification SEM of the fatigue-damaged specimen demonstrates that the shedding of the second phase at the grain boundary is promoted by H, and voids are formed.
- (3)
- The EBSD results demonstrate that the undamaged samples are mainly transgranular, while the fatigue-damaged samples are alternately intergranular and transgranular to a certain extent. This is caused by the anodic dissolution mechanism and hydrogen embrittlement, indicating that fatigue damage facilitates the promotion of stress corrosion cracking.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cavalcante, T.R.F.; Pereira, G.S.; Koga, G.Y.; Bolfarini, C.; Bose Filho, W.W.; Avila, J.A. Fatigue crack propagation of aeronautic AA7050-T7451 and AA2050-T84 aluminum alloys in air and saline environments. Int. J. Fatigue 2022, 154, 106519. [Google Scholar] [CrossRef]
- Huan, Z.; Poulami, C.; Dirk, P.; Tilmann, H.; Binhan, S.; Hung, W.C.; Baptiste, G.; Dierk, R. Hydrogen trapping and embrittlement in high-strength Al alloys. Nature 2022, 602, 437–441. [Google Scholar]
- An, J.; Chen, J.; Gou, G. (v19preprint40) Research on Corrosion Fatigue Crack Propagation Behavior of Welded Joints of A7N01P-T4 Aluminum Alloys. J. Corros. Sci. Eng. 2016, 19, 1. [Google Scholar]
- Qi, X.; Song, R.; Qi, W.; Jin, J.; Wang, C.; Li, H.; Sun, B. Correspondence between Susceptibility to SCC of 7050 Aluminum Alloy and Passive Film-induced Stress at Various pH Values. J. Cent. South Univ. 2017, 32, 173–178. [Google Scholar] [CrossRef]
- Lu, W.; Ma, C.; Gou, G.; Fu, Z.; Sun, W.; Che, X.; Chen, H.; Gao, W. Corrosion fatigue crack propagation behavior of A7N01P-T4 aluminum alloy welded joints from high-speed train underframe after 1.8 million km operation. Mater. Corros. 2021, 72, 879–887. [Google Scholar] [CrossRef]
- Arena, F.; Frusteri, F.; Mondello, N.; Giordano, N.; Parmaliana, A. Interaction pathway of chloride ions with ?-Al2O3: Surface acidity and thermal stability of the Cl/?-Al2O3 system. J. Chem. Soc. Faraday Trans. 1992, 88, 3353–3356. [Google Scholar] [CrossRef]
- Costa, D.; Ribeiro, T.; Cornette, P.; Marcus, P. DFT Modeling of Corrosion Inhibition by Organic Molecules: Carboxylates as Inhibitors of Aluminum Corrosion. J. Phys. Chem. C Nanomater. Interfaces 2016, 120, 28607–28616. [Google Scholar] [CrossRef]
- de Hass, M.; De Hosson, J.T.M. Grain boundary segregation and precipitation in aluminium alloys. Scr. Mater 2001, 44, 281–286. [Google Scholar] [CrossRef]
- Shah, S.R.; Pryce, I.L.; John, T.S.; Greer, J.M. Effect of Environmental Corrosion on Fatigue Crack Growth Morphology: A Detailed Investigation of the Boundary between Fatigue and Corrosion Control Regimes. In Advanced Materials Research; Trans Tech Publications Ltd.: Baech, Switzerland, 2014; Volume 891. [Google Scholar]
- Holroyd, N.; Scamans, G.M. Crack Propagation During Sustained-Load Cracking of Al–Zn–Mg–Cu Aluminum Alloys Exposed to Moist Air or Distilled Water. Metall. Mater. Trans. A 2011, 42, 3979–3998. [Google Scholar] [CrossRef]
- Young, G.A.; Scully, J.R. The effects of test temperature, temper, and alloyed copper on the hydrogen-controlled crack growth rate of an Al–Zn–Mg–(Cu) alloy. Metall. Mater. Trans. A 2002, 33, 1167–1181. [Google Scholar] [CrossRef]
- Borchers, T.E.; McAllister, D.P.; Zhang, W. Macroscopic Segregation and Stress Corrosion Cracking in 7xxx Series Aluminum Alloy Arc Welds. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2015, 46, 1827–1833. [Google Scholar] [CrossRef]
- Horner, D.A.; Connolly, B.J.; Zhou, S.; Crocker, L.; Turnbull, A. Novel images of the evolution of stress corrosion cracks from corrosion pits. Corros. Sci. 2011, 53, 3466–3485. [Google Scholar] [CrossRef]
- Shen, L.; Chen, H.; Xu, L.D.; Che, X.L.; Chen, Y. Stress corrosion cracking and corrosion fatigue cracking behavior of A7N01P-T4 aluminum alloy. Mater. Corros. 2018, 69, 207–214. [Google Scholar] [CrossRef]
- Xiao, F.; An, J.; Chen, H.; Li, P.; Gao, W. x-The Effect of Microstructure on the Corrosion Fatigue Property of A7N01P-T4 Aluminum Alloy Welding Joints. Corrosion 2018, 74, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yuan, S.; Kong, D.; Zhao, G.; Zhang, C. Influence of aging treatment on the microstructure, mechanical properties and anisotropy of hot extruded Al–Mg–Si plate. Mater Des. 2019, 182, 107999. [Google Scholar] [CrossRef]
- Liu, X.S.; Zhang, L.; Wang, L.S.; Wu, S.H.; Fang, H.Y. Fatigue behavior and life prediction of A7N01 aluminium alloy welded joint. Trans. Nonferrous Met. Soc. China 2012, 22, 2930–2936. [Google Scholar] [CrossRef]
- Song, W.; Wang, P.; Wan, D.; Qian, G.; Correia, J.; Berto, F. Fatigue crack growth behavior of Ni–Cr–Mo–V steel welded joints considering strength mismatch effect. Int. J. Fatigue 2021, 151, 106389. [Google Scholar] [CrossRef]
- Hornbogen, E.; Gahr, K.Z. Microstructure and fatigue crack growth in a γ–Fe–Ni–Al alloy. Acta Metall. 1976, 24, 581–592. [Google Scholar] [CrossRef]
- Aboura, Y.; Garner, A.J.; Euesden, R.; Barrett, Z.; Engel, C.; Holroyd, N.J.H.; Prangnell, P.B.; Burnett, T.L. Understanding the environmentally assisted cracking (EAC) initiation and propagation of new generation 7xxx alloys using slow strain rate testing. Corros. Sci. 2022, 199, 110161. [Google Scholar] [CrossRef]
- Onofrio, G.; Osinkolu, G.A.; Marchionni, M. Effects of loading waveform on fatigue crack growth of Udimet 720 Li superalloy. Int. J. Fatigue 2004, 26, 203–209. [Google Scholar] [CrossRef]
- GB/T 12160-2002. Calibration of Extensometers for Uniaxial Tests; Standardization Administration of the People’s Republic of China: Beijing, China, 2002.
- GB/T 20120.2-2006. Corrosion of Metals and Alloys—Corrosion Fatigue Test—Part 2: Crack Propagation Test on Pre-Cracked Specimens; Standardization Administration of the People’s Republic of China: Beijing, China, 2006.
- GB/T 6398-2017. Metallic Materials-Fatigue Test-Fatigue Crack Growth Method; Standardization Administration of the People’s Republic of China: Beijing, China, 2017.
- Schwarzenböck, E.; Ollivier, E.; Garner, A.; Cassell, A.; Hack, T.; Barrett, Z.; Engel, C.; Burnett, T.L.; Holroyd, N.J.H.; Robson, J.D.; et al. Environmental cracking performance of new generation thick plate 7000-T7x series alloys in humid air. Corros. Sci. 2020, 171, 108701. [Google Scholar] [CrossRef]
- Williams, J.C.; Starke, E.A. Progress in structural materials for aerospace systems. Acta Mater 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Baggerly, R.G. Hydrogen-assisted stress cracking of high-strength wheel bolts. Eng. Fail. Anal 1996, 3, 231–240. [Google Scholar] [CrossRef]
- Arafin, M.A.; Szpunar, J.A. A new understanding of intergranular stress corrosion cracking resistance of pipeline steel through grain boundary character and crystallographic texture studies. Corros. Sci. 2009, 51, 119–128. [Google Scholar] [CrossRef]
- Rong, W. A fracture model of corrosion fatigue crack propagation of aluminum alloys based on the material elements fracture ahead of a crack tip. Int. J. Fatigue 2008, 30, 1376–1386. [Google Scholar]
- Wang, D.; Zhang, W.; Huang, S.; Yi, Y.; He, H. Effect of three-dimensional deformation at different temperatures on microstructure, strength, fracture toughness and corrosion resistance of 7A85 aluminum alloy. J. Alloys Compd. 2022, 928, 167200. [Google Scholar] [CrossRef]
- Chemin, A.E.A.; Saconi, F.; Bose Filho, W.W.; Spinelli, D.; Ruchert, C.O.F.T. Effect of saline corrosion environment on fatigue crack growth of 7475-T7351 aluminum alloy under TWIST flight loading. Eng. Fract. Mech. 2015, 141, 274–290. [Google Scholar] [CrossRef]
- Rao, A.C.U.; Vasu, V.; Govindaraju, M.; Srinadh, K.V.S. Stress corrosion cracking behaviour of 7xxx aluminum alloys: A literature review. Trans. Nonferrous Met. Soc. China 2016, 26, 1447–1471. [Google Scholar] [CrossRef]
- Knight, S.P.; Birbilis, N.; Muddle, B.C.; Trueman, A.R.; Lynch, S.P. Correlations between intergranular stress corrosion cracking, grain-boundary microchemistry, and grain-boundary electrochemistry for Al–Zn–Mg–Cu alloys. Corros. Sci. 2010, 52, 4073–4080. [Google Scholar] [CrossRef]
- Pan, S.; Dong, S.; Xu, M. Electrochemical origin for mitigated pitting initiation in AA7075 alloy with TiB2 nanoparticles. Appl. Surf. Sci. 2022, 601, 154275. [Google Scholar] [CrossRef]
- Kayani, S.H.; Park, S.; Euh, K.; Seol, J.B.; Kim, J.G.; Sung, H. Dislocation-aided electrochemical behavior of precipitates in stress corrosion cracking of Al–Zn–Mg–Cu alloys. Mater Charact. 2022, 190, 112019. [Google Scholar] [CrossRef]
- Krishnan, M.A.; Raja, V.S. Development of high strength AA 7010 aluminum alloy resistant to environmentally assisted cracking. Corros. Sci. 2016, 109, 94–100. [Google Scholar] [CrossRef]
- Li, X.D.; Wang, X.S.; Ren, H.H.; Chen, Y.L.; Mu, Z.T. Effect of prior corrosion state on the fatigue small cracking behaviour of 6151-T6 aluminum alloy. Corros. Sci. 2012, 55, 26–33. [Google Scholar] [CrossRef]
- Lin, C.K.; Yang, S.T. Corrosion fatigue behavior of 7050 aluminum alloys in different tempers. Eng. Fract. Mech. 1998, 59, 779–795. [Google Scholar] [CrossRef]
- Wang, L.; Hui, L.; Zhou, S.; Xu, L.; He, B. Effect of corrosive environment on fatigue property and crack propagation behavior of Al 2024 friction stir weld. Trans. Nonferrous Met. Soc. China 2016, 26, 2830–2837. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, J.; Yi, D.; Wang, B. Investigation of high-cycle fatigue and fatigue crack propagation characteristic in 5083-O aluminum alloy. Int. J. Fatigue 2019, 126, 357–368. [Google Scholar] [CrossRef]
- Bai, L.Y.; Shao, F.; Ma, Q.N.; Xu, Q.; Hu, J.X.; Hou, Y.N. Mechanism of corrosion fatigue fracture of friction stir welding joints of 7075 aluminium alloy in 3.5% NaCl solution. J. Cent. South Univ. 2022, 29, 1015–1028. [Google Scholar] [CrossRef]
- Landes, J.D.; Wei, R.P. Correlation Between Sustained-Load and Fatigue Crack Growth in High-Strength Steels. Mater. Res. Stand 1969, 9, 25–46. [Google Scholar]
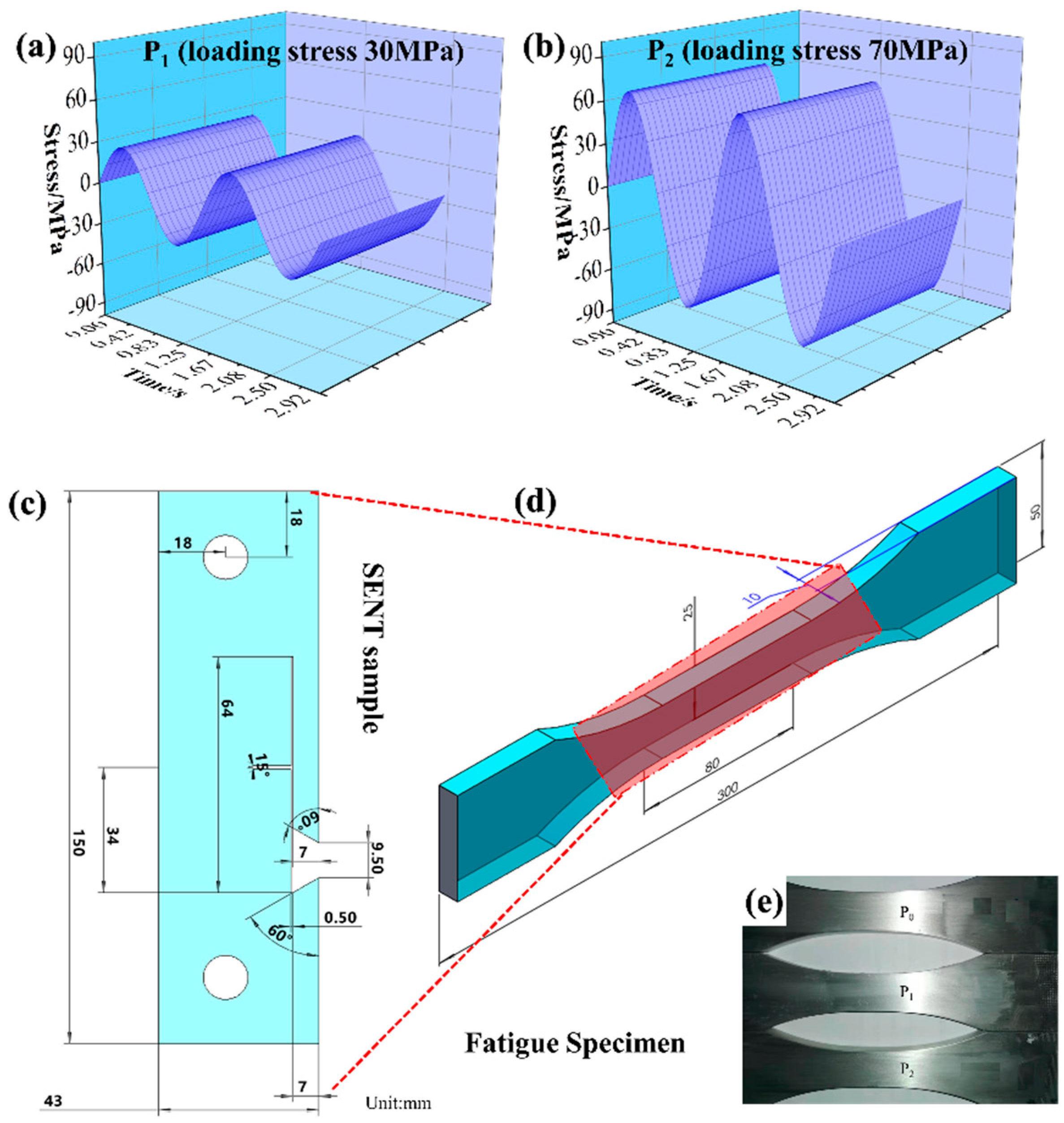




| Sample Type | Test Temperature °C | Loading Stress △σ/MPa | Fa/ Dynamic Load | Fb/ Static Load | Frequency f/Hz | Number of Cycles N/Cycle |
|---|---|---|---|---|---|---|
| P0 | 25 °C | 0 | 0 | 0 | 0 | 0 |
| P1 | 25 °C | 30 | 3.768 | 4.605 | 103.8 | 107 |
| P2 | 25 °C | 70 | 8.66 | 10.58 | 105.9 | 107 |
| Electrode | Ecorr/V | Icorr/A•cm2 |
|---|---|---|
| P0 | −1.23 | 7.80 × 10−5 |
| P1 | −1.26 | 7.24 × 10−5 |
| P2 | −1.24 | 6.91 × 10−5 |
| Sample Type | Rs/(Ω•cm2) | CPE-T/(μF•cm2) | CPE-P | Rp/(Ω•cm2) |
|---|---|---|---|---|
| P0 | 3.543 | 6.47 × 10−6 | 0.827 | 1.76 × 105 |
| P1 | 3.23 | 1.13 × 10−5 | 0.823 | 3.15 × 104 |
| P2 | 0.475 | 9.31 × 10−6 | 0.853 | 8.91 × 104 |
| Degree of Fatigue Damage | Constant C | Constant m | Paris Formula |
|---|---|---|---|
| P0 (non-fatigue damage) | 7.09 × 10−8 | 5.69 | da/dN = 7.09 × 10−8(ΔK)5.69 |
| P1 (σ = 30 MPa) | 6.45 × 10−7 | 4.02 | da/dN =6.45 × 10−7(ΔK)4.02 |
| P2 (σ = 70 MPa) | 2.92 × 10−10 | 8.78 | da/dN =2.92 × 10−10(ΔK)8.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Lu, W.; Gou, G.; Dian, L.; Zhu, Z.; Jin, J. The Effect of Fatigue Damage on the Corrosion Fatigue Crack Growth Mechanism in A7N01P-T4 Aluminum Alloy. Metals 2023, 13, 104. https://doi.org/10.3390/met13010104
Chen W, Lu W, Gou G, Dian L, Zhu Z, Jin J. The Effect of Fatigue Damage on the Corrosion Fatigue Crack Growth Mechanism in A7N01P-T4 Aluminum Alloy. Metals. 2023; 13(1):104. https://doi.org/10.3390/met13010104
Chicago/Turabian StyleChen, Wenjing, Wei Lu, Guoqing Gou, Liwen Dian, Zhongyin Zhu, and Junjun Jin. 2023. "The Effect of Fatigue Damage on the Corrosion Fatigue Crack Growth Mechanism in A7N01P-T4 Aluminum Alloy" Metals 13, no. 1: 104. https://doi.org/10.3390/met13010104
APA StyleChen, W., Lu, W., Gou, G., Dian, L., Zhu, Z., & Jin, J. (2023). The Effect of Fatigue Damage on the Corrosion Fatigue Crack Growth Mechanism in A7N01P-T4 Aluminum Alloy. Metals, 13(1), 104. https://doi.org/10.3390/met13010104







