Effect of Local Fluid Disturbance Induced by Weld Reinforcement Height on the Corrosion of a Low Alloy Steel Weld
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Specimens
2.2. RDE Apparatus and Testing Conditions
2.3. Electrochemical Measurements
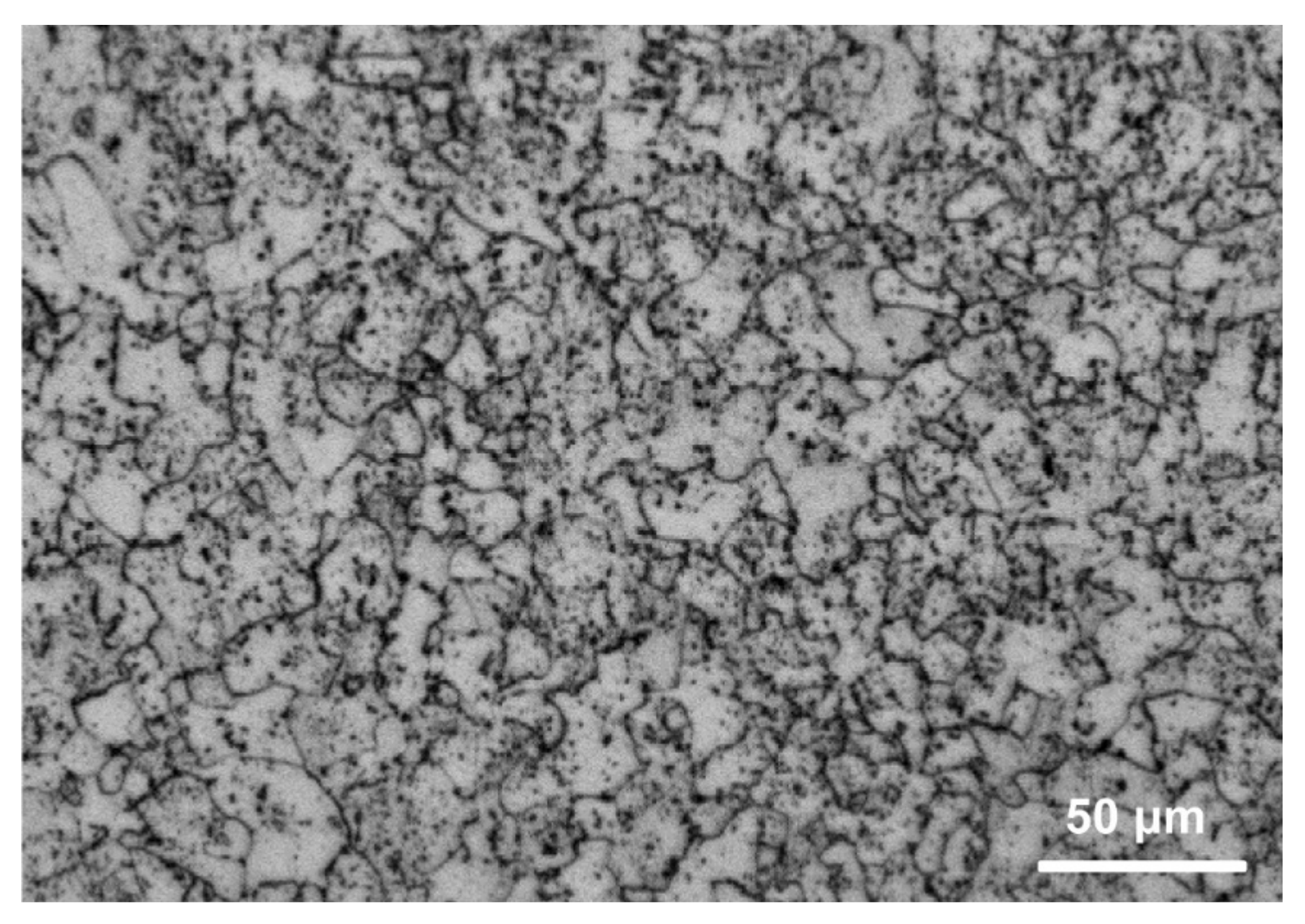
2.4. Characterization of Corrosion Products and Corrosion Morphologies
2.5. CFD Simulation
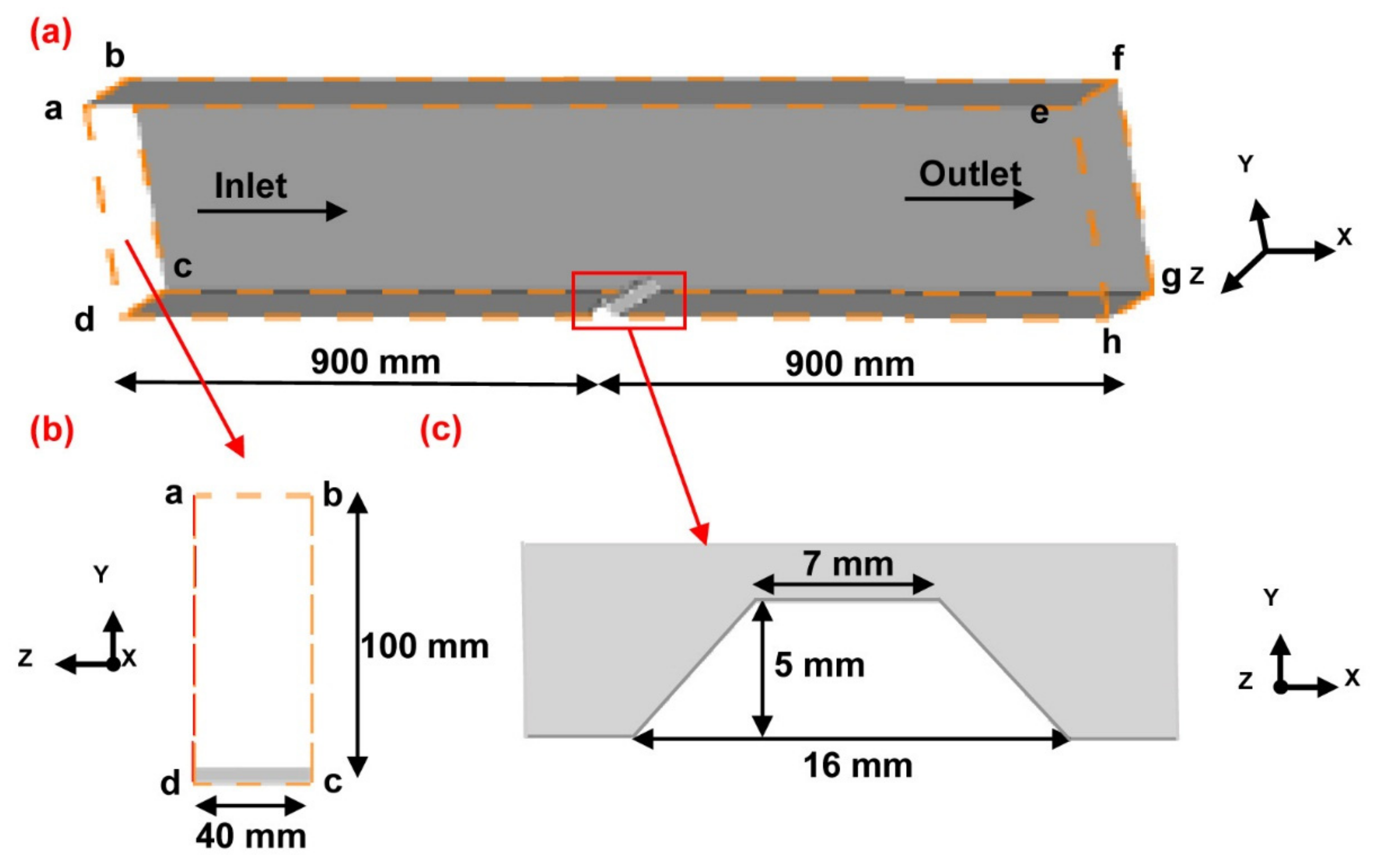
2.5.1. Geometric Model
2.5.2. Fluid Flow Model
2.5.3. Boundary Conditions
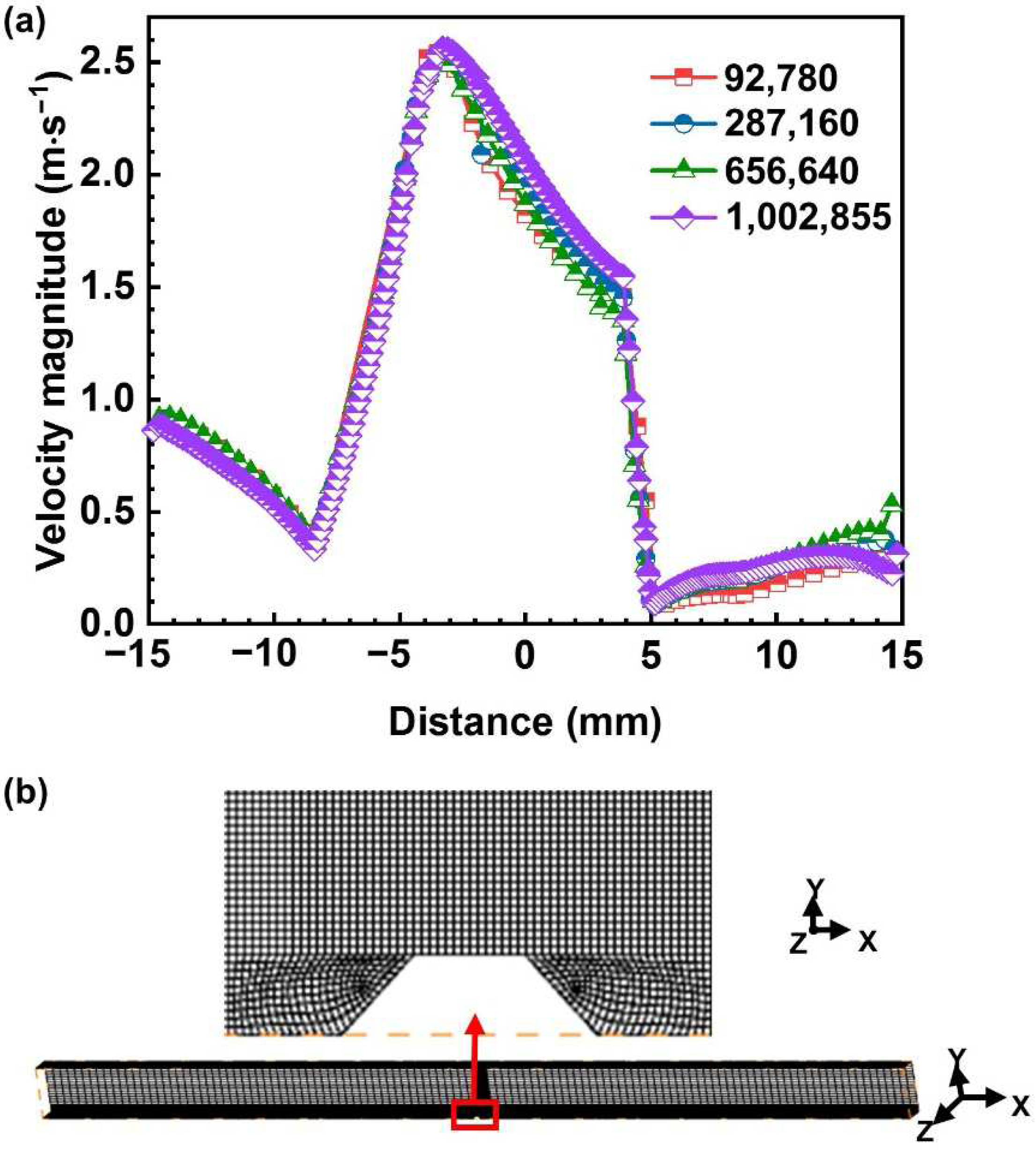
2.5.4. CFD Model Mesh
3. Results
3.1. Electrochemical Measurements Analysis
3.1.1. EIS Analysis
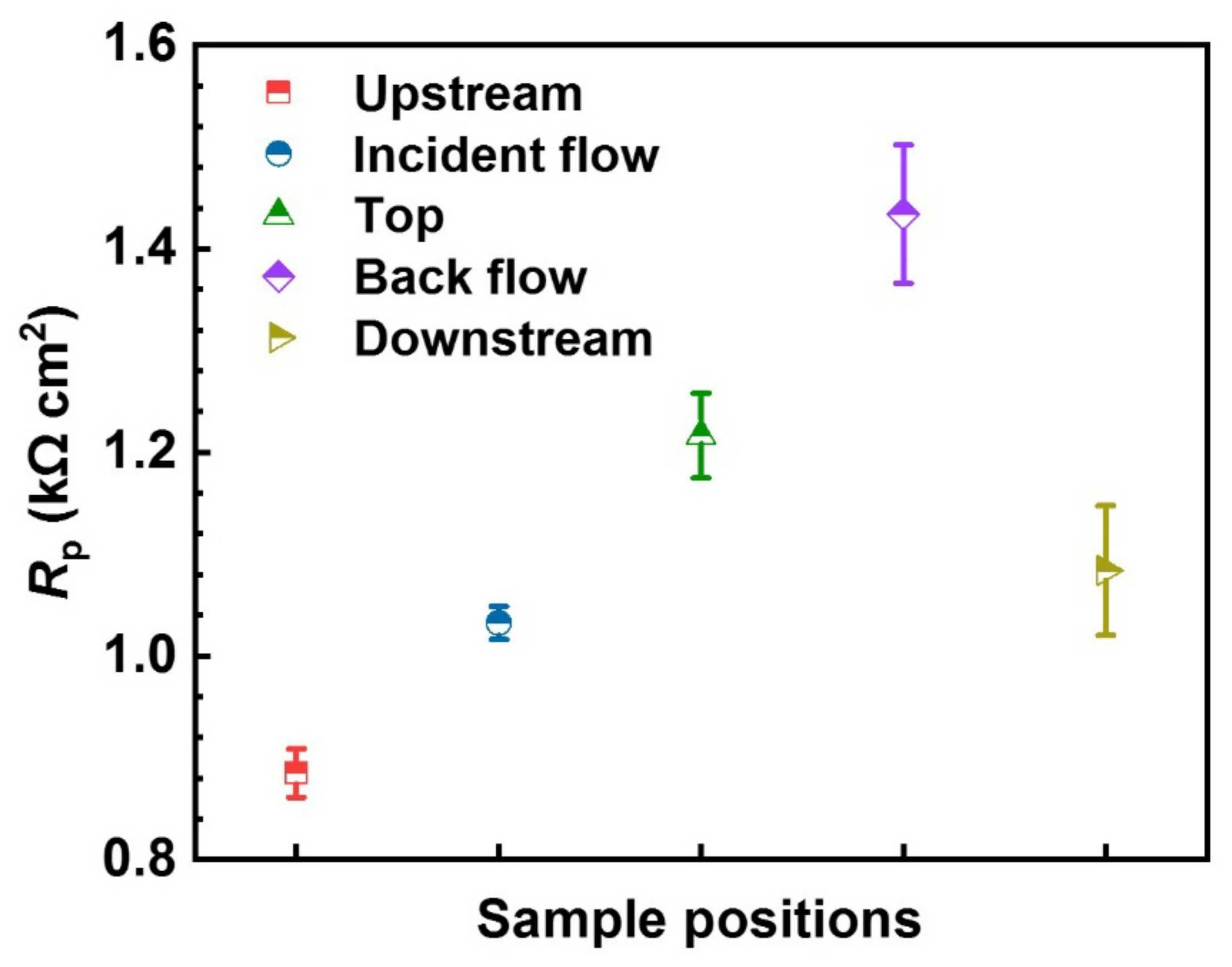
3.1.2. LPR Analysis
3.2. Surface Characterization Analysis
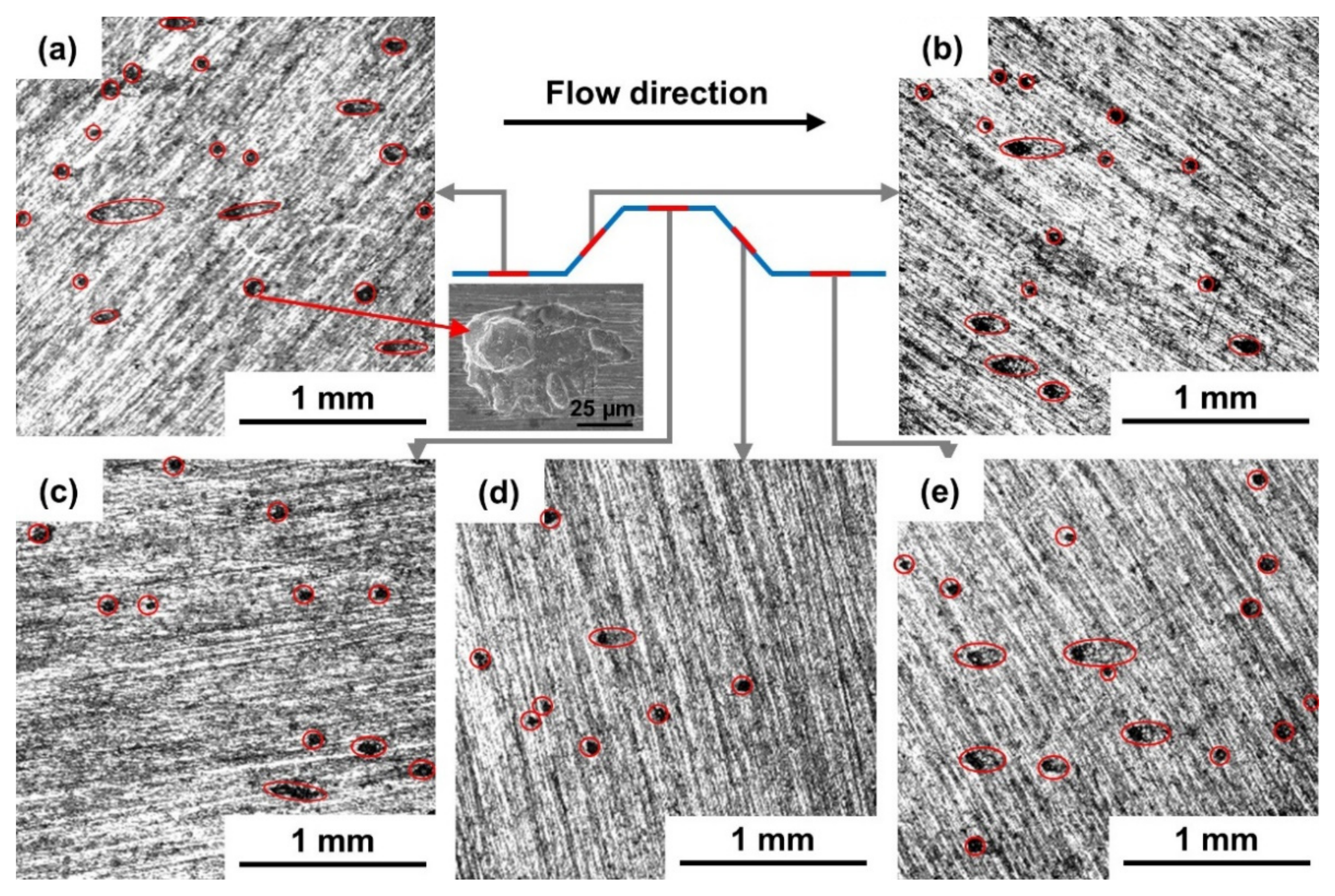
3.2.1. Corrosion Morphologies before Removing Corrosion Products
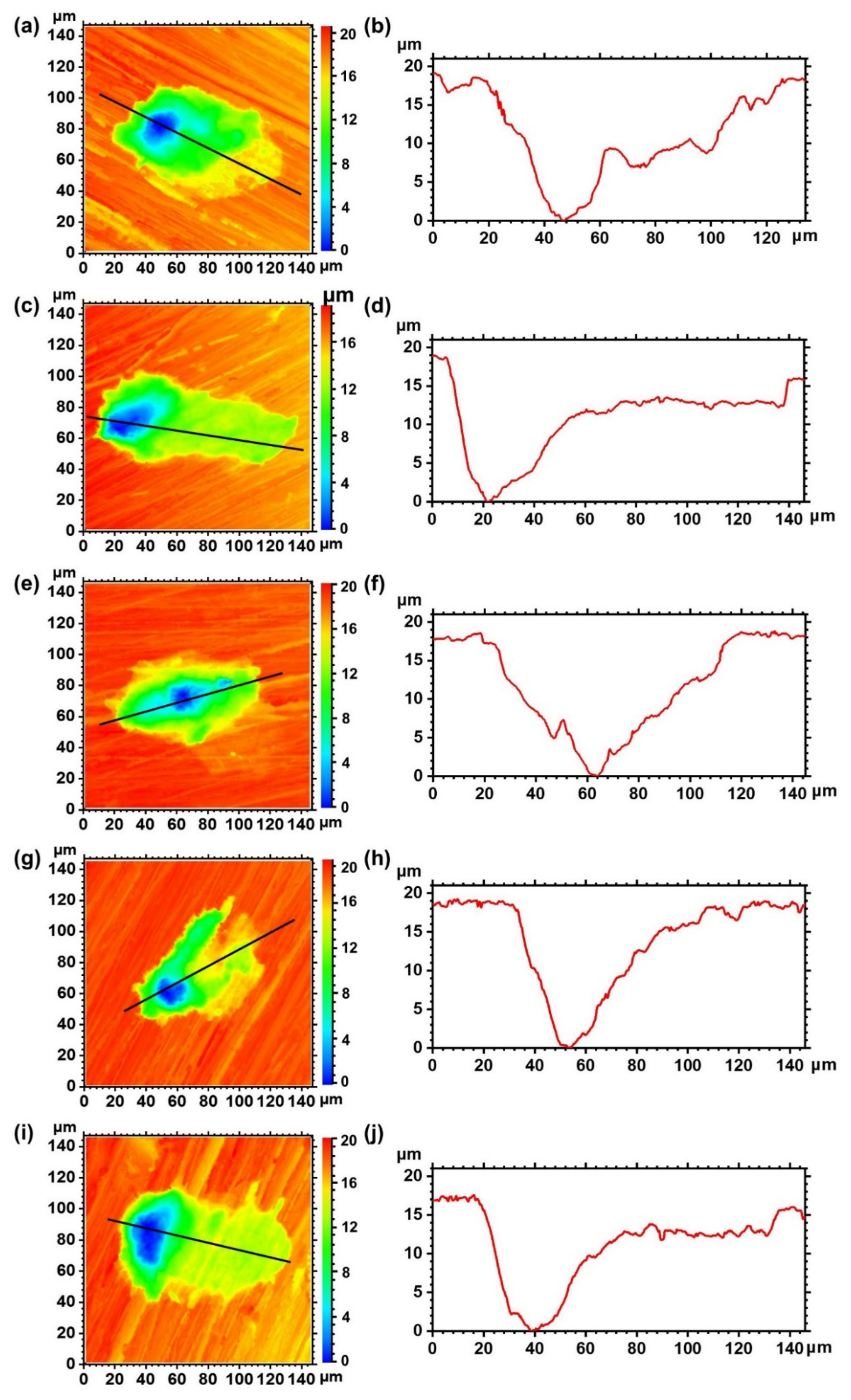
3.2.2. Corrosion Morphologies after Removing Corrosion Products
4. Discussion
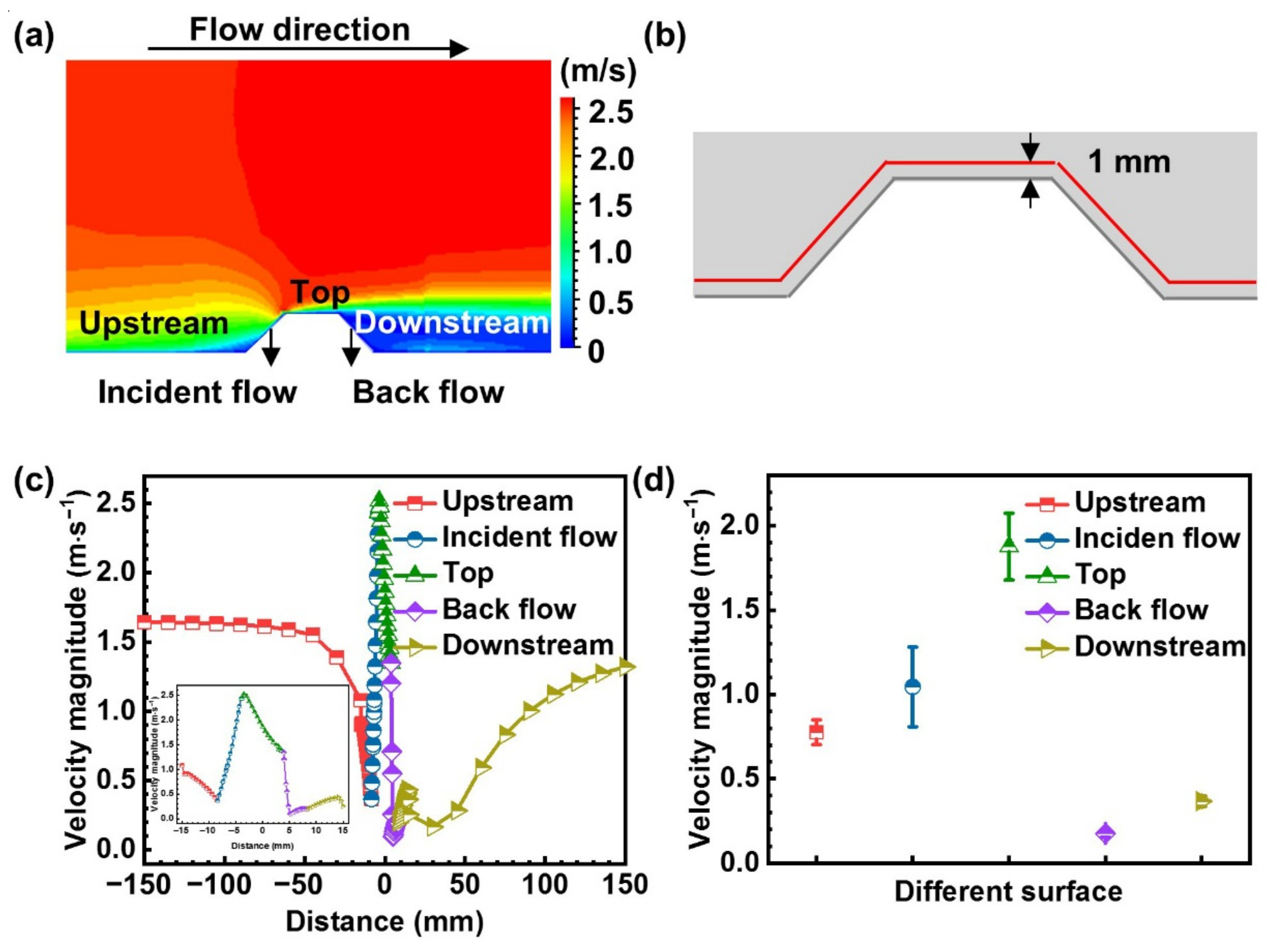
4.1. Mass Transfer Effect
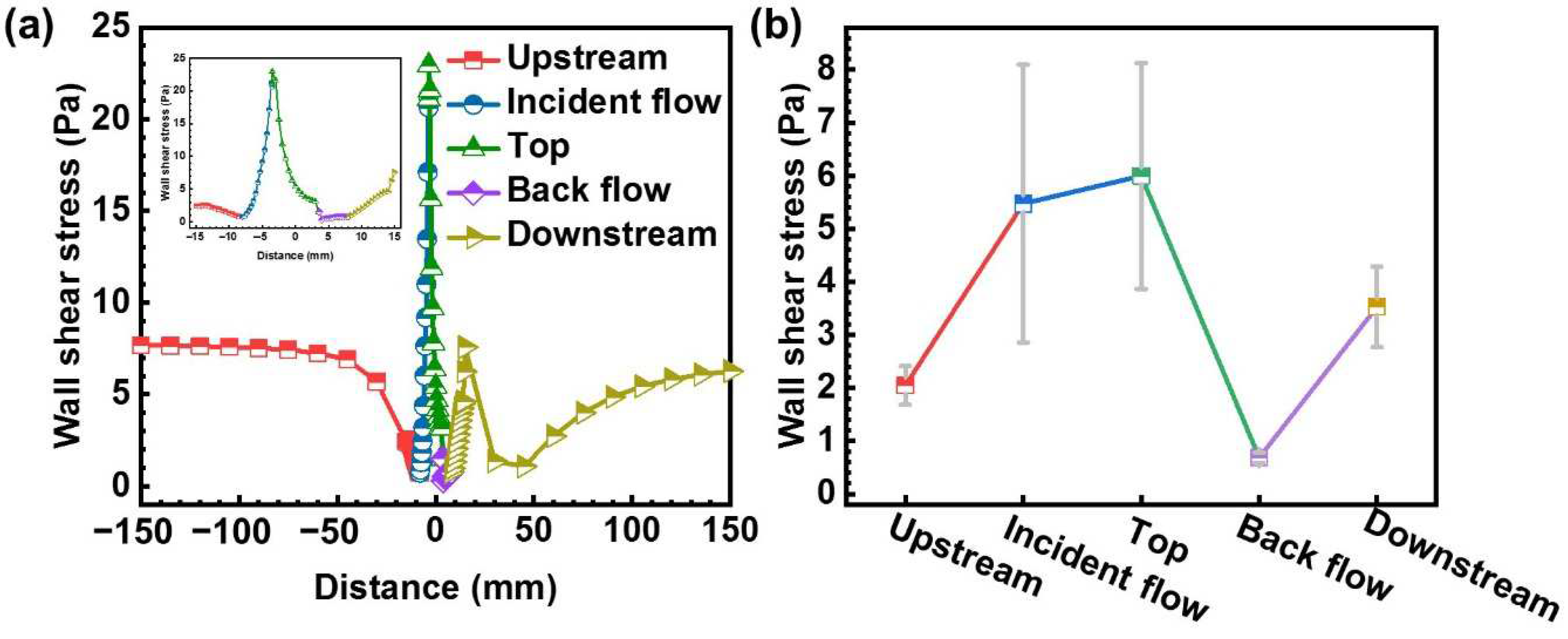
4.2. Shear Stress Effect
4.3. Formation and Development of Pitting under Different Flow Conditions
5. Conclusions
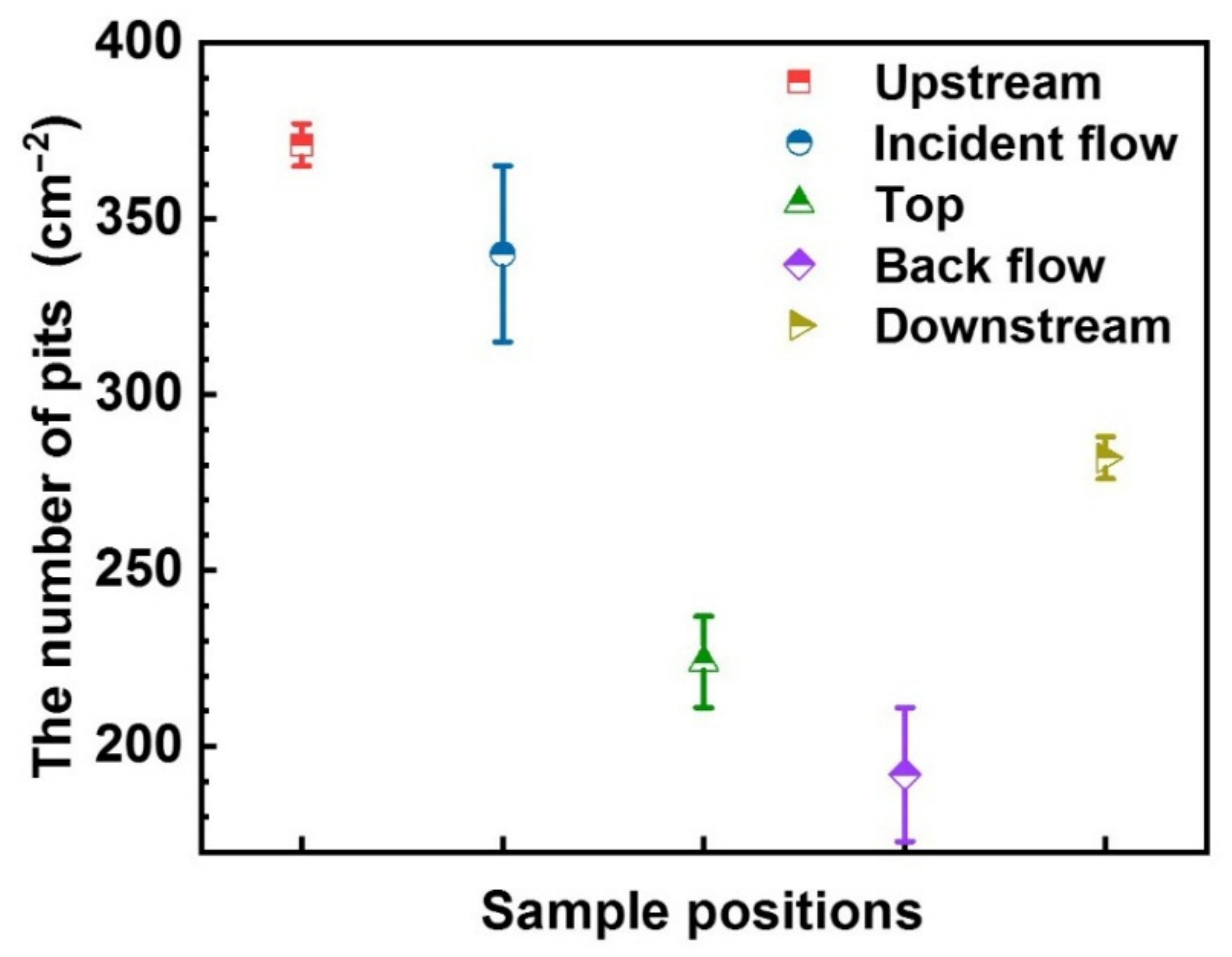
- Pitting corrosion prevails on all the surfaces around the WRH. The number of pits is consistent with the 1/Rp in the decline sequence of the upstream surface, incident flow surface, downstream surface, top surface, and backflow surface.
- Local hydrodynamics affect the number of pits on the surfaces instead of the maximum pit depth and the profile.
- On the incident flow surface and the top surface of WRH, high WSS inhibits the pitting, while for the other surfaces, the corrosion is almost unaffected by WSS.
- Flow velocity and mass transfer are found to be not the only parameters determining the corrosion rate of local places of WRH. They should be cautiously considered when predicting corrosion in the presence of corrosion products.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, X.; Zheng, Y.; Ke, W. Effect of flow velocity and entrained sand on inhibition performances of two inhibitors for CO2 corrosion of N80 steel in 3% NaCl solution. Corros. Sci. 2005, 47, 2636–2658. [Google Scholar] [CrossRef]
- Ortega-Toledo, D.; Gonzalez-Rodriguez, J.; Casales, M.; Martinez, L.; Martinez-Villafañe, A. CO2 corrosion inhibition of X-120 pipeline steel by a modified imidazoline under flow conditions. Corros. Sci. 2011, 53, 3780–3787. [Google Scholar] [CrossRef]
- Zhang, G.; Zeng, L.; Huang, H.; Guo, X. A study of flow accelerated corrosion at elbow of carbon steel pipeline by array electrode and computational fluid dynamics simulation. Corros. Sci. 2013, 77, 334–341. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y. Critical flow velocity phenomenon in erosion-corrosion of pipelines: Determination methods, mechanisms and applications. J. Pipeline Sci. Eng. 2021, 1, 63–73. [Google Scholar] [CrossRef]
- Kim, S.-T.; Lee, I.-S.; Kim, J.-S.; Jang, S.-H.; Park, Y.-S.; Kim, K.-T.; Kim, Y.-S. Investigation of the localized corrosion associated with phase transformation of tube-to-tube sheet welds of hyper duplex stainless steel in acidified chloride environments. Corros. Sci. 2012, 64, 164–173. [Google Scholar] [CrossRef]
- Wu, W.; Hu, S.; Shen, J. Microstructure, mechanical properties and corrosion behavior of laser welded dissimilar joints between ferritic stainless steel and carbon steel. Mater. Des. 2015, 65, 855–861. [Google Scholar] [CrossRef]
- Okonkwo, B.O.; Ming, H.; Zhang, Z.; Wang, J.; Rahimi, E.; Hosseinpour, S.; Davoodi, A. Microscale investigation of the correlation between microstructure and galvanic corrosion of low alloy steel A508 and its welded 309/308L stainless steel overlayer. Corros. Sci. 2019, 154, 49–60. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Han, Y.; Zhao, L.; Jing, H. Insights into the intergranular corrosion of overlay welded joints of X65-Inconel 625 clad pipe and its relationship to damage penetration. Corros. Sci. 2019, 160, 108169. [Google Scholar] [CrossRef]
- Rizk, T.; Thompson, G.; Dawson, J. Mass transfer enhancement associated with sudden flow expansion. Corros. Sci. 1996, 38, 1801–1814. [Google Scholar] [CrossRef]
- Owen, J.; Godfrey, J.; Ma, W.; de Boer, G.; Al-Khateeb, M.; Thompson, H.; Neville, A.; Ramsey, C.; Barker, R. An experimental and numerical investigation of CO2 corrosion in a rapid expansion pipe geometry. Corros. Sci. 2020, 165, 108362. [Google Scholar] [CrossRef]
- Zhong, X.; Shang, T.; Zhang, C.; Hu, J.; Zhang, Z.; Zhang, Q.; Yuan, X.; Hou, D.; Zeng, D.; Shi, T. In situ study of flow accelerated corrosion and its mitigation at different locations of a gradual contraction of N80 steel. J. Alloys Compd. 2020, 824, 153947. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, D.; Yang, L.; Wang, Z.; Cheng, F.; Zhang, M.; Jin, Y.; Zhu, S. Development mechanism of local corrosion pit in X80 pipeline steel under flow conditions. Tribol. Int. 2020, 146, 106145. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, L.; Tan, Z.; Xing, S.; Bai, S.; Wei, E.; Tang, X.; Jin, Y. Corrosion behavior of X65 steel at different depths of pitting defects under local flow conditions. Exp. Therm. Fluid Sci. 2021, 124, 110333. [Google Scholar] [CrossRef]
- Ahmed, W.H.; Bello, M.M.; El Nakla, M.; Al Sarkhi, A. Flow and mass transfer downstream of an orifice under flow accelerated corrosion conditions. Nucl. Eng. Des. 2012, 252, 52–67. [Google Scholar] [CrossRef]
- Yamagata, T.; Ito, A.; Sato, Y.; Fujisawa, N. Experimental and numerical studies on mass transfer characteristics behind an orifice in a circular pipe for application to pipe-wall thinning. Exp. Therm. Fluid Sci. 2014, 52, 239–247. [Google Scholar] [CrossRef]
- Zheng, Y.; Kim, J.; Kim, I. A Study on Flow-accelerated corrosion of SA106 Gr.C weldment. J. Korean Weld. Soc. 2001, 19, 334–341. [Google Scholar]
- Barker, R.; Hu, X.; Neville, A. The influence of high shear and sand impingement on preferential weld corrosion of carbon steel pipework in CO2-saturated environments. Tribol. Int. 2013, 68, 17–25. [Google Scholar] [CrossRef]
- Shirinzadeh-Dastgiri, M.; Mohammadi, J.; Behnamian, Y.; Eghlimi, A.; Mostafaei, A. Metallurgical investigations and corrosion behavior of failed weld joint in AISI 1518 low carbon steel pipeline. Eng. Failure Anal. 2015, 53, 78–96. [Google Scholar] [CrossRef]
- Zeng, L.; Shuang, S.; Guo, X.; Zhang, G. Erosion-corrosion of stainless steel at different locations of a 90° elbow. Corros. Sci. 2016, 111, 72–83. [Google Scholar] [CrossRef]
- Owen, J.; Ducker, E.; Huggan, M.; Ramsey, C.; Neville, A.; Barker, R. Design of an elbow for integrated gravimetric, electrochemical and acoustic emission measurements in erosion-corrosion pipe flow environments. Wear 2019, 428–429, 76–84. [Google Scholar] [CrossRef]
- Xu, Y.; Tan, M. Visualising the dynamic processes of flow accelerated corrosion and erosion corrosion using an electrochemically integrated electrode array. Corros. Sci. 2018, 139, 438–443. [Google Scholar] [CrossRef]
- Xu, Y.; Tan, M. Probing the initiation and propagation processes of flow accelerated corrosion and erosion corrosion under simulated turbulent flow conditions. Corros. Sci. 2019, 151, 163–174. [Google Scholar] [CrossRef]
- Tomarov, G.V.; Lovchev, V.N.; Gutsev, D.F.; Shipkov, A.A.; Golubeva, T.N.; Greblov, P.N. Problems concerned with local erosion-corrosion of welded connections in the pipelines of a power unit at a nuclear power station. Therm. Eng. 2012, 59, 628–636. [Google Scholar] [CrossRef]
- Hosseini, F.; Isfahani, A.M.; Emami, M.D.; Mohseni, E. The effect of excessive penetration of welding on sand erosion pattern due to high speed gas-solid flows in elbows and reducers. Eng. Fail. Anal. 2022, 131, 105902. [Google Scholar] [CrossRef]
- Satoh, T.; Shao, Y.; Cook, W.G.; Lister, D.H.; Uchida, S. Flow-assisted corrosion of carbon steel under neutral water conditions. Corrosion 2007, 63, 770–780. [Google Scholar] [CrossRef]
- Fujiwara, K.; Domae, M.; Yoneda, K.; Inada, F. Model of physico-chemical effect on flow accelerated corrosion in power plant. Corros. Sci. 2011, 53, 3526–3533. [Google Scholar] [CrossRef]
- Wang, J.R.; Shirazi, S.A. A CFD based correlation for mass transfer coefficient in elbows. Int. J. Heat Mass Transf. 2001, 44, 1817–1822. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, Y. Electrochemical characterization and computational fluid dynamics simulation of flow-accelerated corrosion of X65 steel in a CO2-saturated oilfield formation water. Corros. Sci. 2010, 52, 2716–2724. [Google Scholar] [CrossRef]
- Ige, O.; Umoru, L. Effects of shear stress on the erosion-corrosion behaviour of X-65 carbon steel: A combined mass-loss and profilometry study. Tribol. Int. 2016, 94, 155–164. [Google Scholar] [CrossRef]
- Li, W.; Pots, B.; Brown, B.; Kee, K.E.; Nesic, S. A direct measurement of wall shear stress in multiphase flow-Is it an important parameter in CO2 corrosion of carbon steel pipelines? Corros. Sci. 2016, 110, 35–45. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, S.; Ling, X. Creep life prediction of small punch creep testing specimens for service-exposed Cr5Mo using the theta-projection method. Eng. Failure Anal. 2017, 72, 58–66. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, H.; Zheng, Y.; Guo, X. Effect of groove microstructure on slurry erosion in the liquid-solid two-phase flow. Wear 2021, 466-467, 203561. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, H.; Guo, X.; Zheng, Y. Effect of groove depth on the slurry erosion of V-shaped grooved surfaces. Wear 2022, 488-489, 204133. [Google Scholar] [CrossRef]
- EN ISO 5817:2014; Welding-Fusion-Weld Joints in Steel, Nickel, Titanium and Their Alloys (Beam Welding Excluded)-Quality Levels for Imperfectins. ISO: Brussels, Belgium, 2014.
- Zeng, L.; Zhang, G.; Guo, X. Erosion-corrosion at different locations of X65 carbon steel elbow. Corros. Sci. 2014, 85, 318–330. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, G.; Guo, X.; Chai, C. Inhibition effect of thioureidoimidazoline inhibitor for the flow accelerated corrosion of an elbow. Corros. Sci. 2015, 90, 202–215. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Qiao, Y.; Zheng, Y. Synergistic effects of deposits and sulfate reducing bacteria on the corrosion of carbon steel. Corros. Sci. 2022, 199, 110210. [Google Scholar] [CrossRef]
- Zheng, K.; Hu, H.; Chen, F.; Zheng, Y. Failure analysis of the blackwater regulating valve in the coal chemical industry. Eng. Failure Anal. 2021, 125, 105442. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, Y. On the fundamentals of electrochemical corrosion of X65 steel in CO2-containing formation water in the presence of acetic acid in petroleum production. Corros. Sci. 2009, 51, 87–94. [Google Scholar] [CrossRef]
- Tang, Y.B.; Shen, X.W.; Liu, Z.H.; Qiao, Y.X.; Yang, L.L.; Lu, D.H.; Zou, J.S.; Xu, J. Corrosion Behaviors of Selective Laser Melted Inconel 718 Alloy in NaOH Solution. Acta Metall. Sin. 2022, 58, 324–333. [Google Scholar]
- Zhang, G.; Cheng, Y. Micro-electrochemical characterization of corrosion of welded X70 pipeline steel in near-neutral pH solution. Corros. Sci. 2009, 51, 1714–1724. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, H.; Zheng, Y.; Ke, W.; Qiao, Y. Comparison of the corrosion behavior of pure titanium and its alloys in fluoride-containing sulfuric acid. Corros. Sci. 2016, 103, 50–65. [Google Scholar] [CrossRef]
- Pang, L.; Wang, Z.; Zheng, Y.; Lai, X.; Han, X. On the localised corrosion of carbon steel induced by the in-situ local damage of porous corrosion products. J. Mater. Sci. Technol. 2020, 54, 95–104. [Google Scholar] [CrossRef]
- Wharton, J.; Wood, R. Influence of flow conditions on the corrosion of AISI 304L stainless steel. Wear 2004, 256, 525–536. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Q.; Zhou, Q.; Gao, S.; Bin Wang, B.; Wang, X.; Huang, Y. Flow accelerated corrosion and erosion-corrosion behavior of marine carbon steel in natural seawater. NPJ Mater. Degrad. 2021, 5, 1–3. [Google Scholar] [CrossRef]
- Zheng, R.; Zhao, X.; Dong, L.; Liu, G.; Huang, Y.; Xu, Y. On the cavitation erosion-corrosion of pipeline steel at different locations of Venturi pipe. Eng. Fail. Anal. 2022, 138, 106333. [Google Scholar] [CrossRef]
- Efird, K.D. Jet Impingement Testing for Flow Accelerated Corrosion; NACE International: Orlando, FL, USA, 2000. [Google Scholar]
- Gulbrandsen, E.; Grana, A. Testing of carbon dioxide corrosion inhibitor performance at high flow velocities in jet impingement geometry. Effects of Mass Transfer and Flow Forces. Corrosion 2007, 63, 1009–1020. [Google Scholar] [CrossRef]
- Halvorsen, A.; Sontvedt, T. CO2 Corrosion Model for Carbon Steel Including Wall Shear Stress Model for Multiphase Flow and Limits for Production Rate to Avoid Mesa Attack; NACE International: San Antonio, TX, USA, 1999. [Google Scholar]
- Nesic, S.; Solvi, G.T.; Enerhaug, J. Comparison of the rotating cylinder and pipe flow tests for flow-sensitive carbon dioxide corrosion. Corrosion 2012, 51, 773–786. [Google Scholar] [CrossRef]
- Poulson, B. Advances in understanding hydrodynamic effects on corrosion. Corros. Sci. 1993, 35, 655–665. [Google Scholar] [CrossRef]
- Nesic, S.; Postlethwaite, J.; Olsen, S. An electrochemical model for prediction of corrosion of mild steel in aqueous carbon dioxide solutions. Corrosion 1996, 52, 280–294. [Google Scholar] [CrossRef]
- Silverman, D.C. The rotating cylinder electrode for examining velocity-sensitive corrosion-A review. Corrosion 2004, 60, 1003–1023. [Google Scholar] [CrossRef]
- Silverman, C. Rotating cylinder electrode-geometry relationships for prediction of velocity-sensitive corrosion. Corrosion 1988, 44, 42–49. [Google Scholar] [CrossRef]
- Wang, Z.B.; Pang, L.; Zheng, Y.G. A review on under-deposit corrosion of pipelines in oil and gas fields: Testing methods, corrosion mechanisms and mitigation strategies. Corros. Commu. 2022, 7, 70–81. [Google Scholar] [CrossRef]
- Tan, Z.W.; Yang, L.Y.; Zhang, D.L.; Wang, Z.B.; Cheng, F.; Zhang, M.; Jin, Y.H. Development mechanism of internal local corrosion of X80 pipeline steel. J. Mater. Sci. Technol. 2020, 49, 186–201. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Zheng, Y. Interaction between pitting corrosion and critical flow velocity for erosion-corrosion of 304 stainless steel under jet slurry impingement. Corros. Sci. 2019, 158, 108084. [Google Scholar] [CrossRef]
- Zheng, Z.-B.; Long, J.; Guo, Y.; Li, H.; Zheng, K.-H.; Qiao, Y.-X. Corrosion and impact-abrasion-corrosion behaviors of quenching-tempering martensitic Fe-Cr alloy steels. J. Iron Steel Res. Int. 2022, 29, 1853–1863. [Google Scholar] [CrossRef]


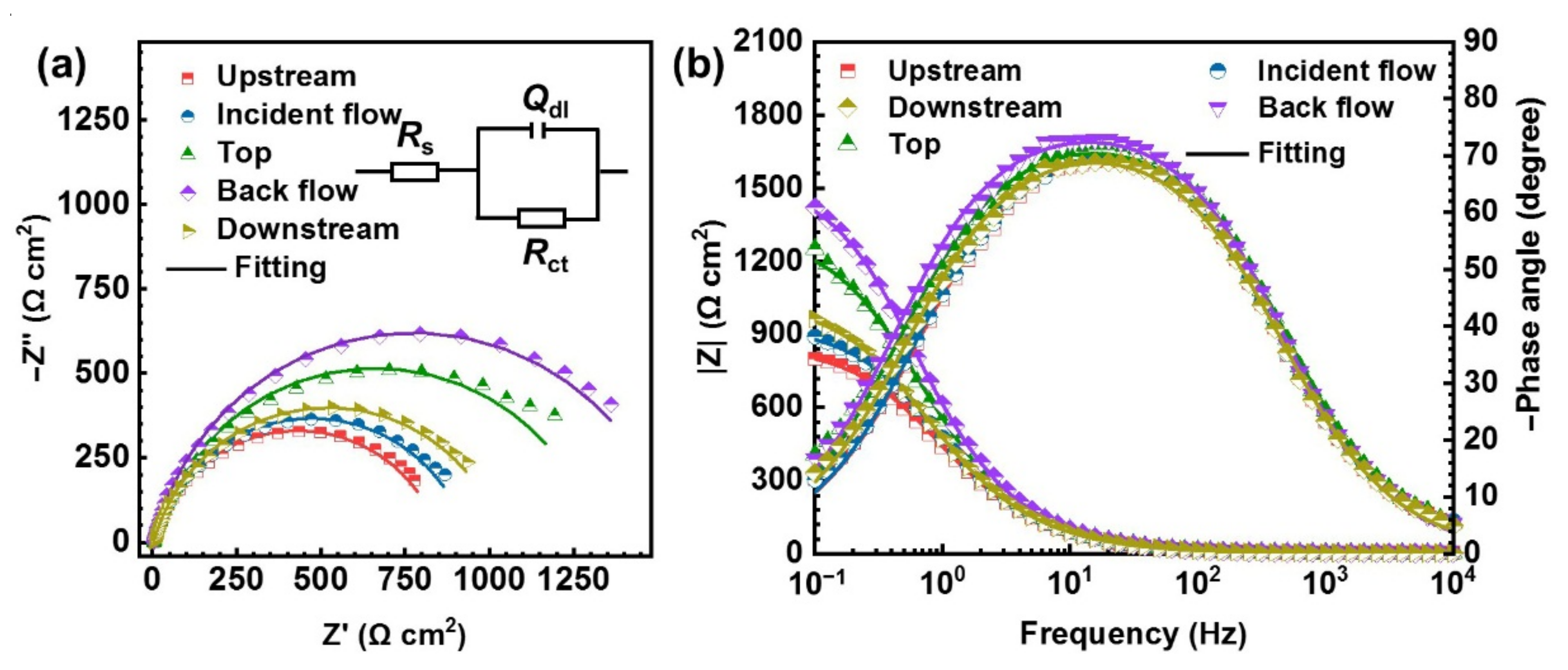



| Positions | Rs (Ω cm2) | Rct (Ω cm2) | Qdl (μF cm−2) | ||
|---|---|---|---|---|---|
| Upstream surface | 3.64 ± 0.21 | 834.55 ± 26.80 | 364.65 ± 8.41 | 0.84 | 4.77 |
| Incident flow surface | 3.63 ± 0.04 | 951.25 ± 22.56 | 322.85 ± 8.98 | 0.85 | 3.03 |
| Top surface | 3.63 ± 0.12 | 1356.00 ± 53.74 | 337.65 ± 39.24 | 0.83 | 6.95 |
| Backflow surface | 3.74 ± 0.16 | 1648.00 ± 152.74 | 294.55 ± 20.86 | 0.86 | 3.98 |
| Downstream surface | 3.63 ± 0.06 | 996.90 ± 55.30 | 365.55 ± 17.61 | 0.84 | 3.74 |
| Positions | Fe (wt.%) | Cr (wt.%) | O (wt.%) |
|---|---|---|---|
| Upstream surface | 81.35 | 4.02 | 11.31 |
| Incident flow surface | 83.20 | 4.10 | 9.64 |
| Top surface | 80.04 | 4.35 | 11.65 |
| Backflow surface | 86.41 | 4.57 | 6.39 |
| Downstream surface | 81.11 | 4.48 | 9.92 |
| Positions | Depth (μm) |
|---|---|
| Upstream surface | 18.56 ± 1.03 |
| Incident flow surface | 18.68 ± 1.91 |
| Top surface | 17.85 ± 1.21 |
| Backflow surface | 18.90 ± 0.40 |
| Downstream surface | 18.67 ± 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, K.; Hu, H.; Wang, Z.; Zheng, Y.; Zhao, L.; Shang, X. Effect of Local Fluid Disturbance Induced by Weld Reinforcement Height on the Corrosion of a Low Alloy Steel Weld. Metals 2023, 13, 103. https://doi.org/10.3390/met13010103
Zheng K, Hu H, Wang Z, Zheng Y, Zhao L, Shang X. Effect of Local Fluid Disturbance Induced by Weld Reinforcement Height on the Corrosion of a Low Alloy Steel Weld. Metals. 2023; 13(1):103. https://doi.org/10.3390/met13010103
Chicago/Turabian StyleZheng, Kexin, Hongxiang Hu, Zhengbin Wang, Yugui Zheng, Liang Zhao, and Xianhe Shang. 2023. "Effect of Local Fluid Disturbance Induced by Weld Reinforcement Height on the Corrosion of a Low Alloy Steel Weld" Metals 13, no. 1: 103. https://doi.org/10.3390/met13010103
APA StyleZheng, K., Hu, H., Wang, Z., Zheng, Y., Zhao, L., & Shang, X. (2023). Effect of Local Fluid Disturbance Induced by Weld Reinforcement Height on the Corrosion of a Low Alloy Steel Weld. Metals, 13(1), 103. https://doi.org/10.3390/met13010103







