Abstract
Isothermal solidification process of pure metal Al was studied by molecular dynamics (MD) simulation using EAM potential. The effects of different cooling rates on the isothermal solidification process of metallic Al were studied. Al was first subjected to a rapid cooling process, and then it was annealing under isothermal conditions. The mean first-passage times (MFPT) method and Johnson-Mehl-Avrami (JMA) law were used to qualify the solidification kinetic processing, and the nucleation rate, critical nucleus size, Avrami exponent and growth exponent of grains were calculated. Results show that the nucleation rate and critical size decrease as the cooling rate increases. Also, an increase in the cooling rate leads to the increase of grain growth rate. At all investigated cooling rates, nucleation and growth processes are in the typical three-dimensional growth mode.
1. Introduction
The solidification process of pure metal includes the nucleation and growth processes of crystals or grains. The solidification structures of materials significantly influence their mechanical and physical properties [1,2,3]. If larger cooling rates can be achieved before crystal nucleation occurs, different crystalline forms of the metal may be produced [4]. It is of great significance to study the nucleation process and solidification mechanism of melts to understand the solidification laws of metals and control different solidification structures [5,6,7,8].
According to the classical nucleation theory (CNT) [9], there are energy and structural fluctuations in the supercooled melt; when the size of a crystal nucleus reaches or exceeds the critical nucleus size, it will grow spontaneously. In general, as the degree of supercooling increases, the nucleation rate increases and the critical nucleation radius decreases. Neilson et al. [10] used CNT to predict the homogeneous nucleation rate of Li2O · 2SiO2 glasses. The predicted theoretical nucleation rates are quite different from the observed values under different temperatures. Several factors limit the experimental study of metal solidification nucleation processes. First, homogeneous nucleation only occurs under ideal conditions, and it is difficult to achieve homogeneous nucleation under experimental conditions. Second, the quantification of the free energy of the solid–liquid interface and its anisotropy is difficult [11]. In addition, the homogeneous nucleation of metal melts starts from the interior of supercooled melts, and it is difficult to directly observe the nucleation process experimentally due to the opacity of metals [12].
Since the study of homogeneous nucleation cannot be carried out under experimental conditions, computation is increasingly becoming an effective method for studying the solidification process of metals. Currently, microscale and mesoscale models, including density functional theory (DFT) [13], Monte Carlo (MC) models [14,15], cellular automata (CA) models [16,17], and phase-field (PF) models [18,19,20,21], have been successfully used to reveal the nature of microstructural evolution. Studies on DFT electronic structure calculations are limited to a few hundred atoms. MC models, CA models, and PF models are limited to microscale simulations. MD simulations bridge the gap between electronic and microscale computational studies on melt solidification nucleation. Using MD simulation, the nucleation process of metal solidification can be observed intuitively, which can help us better understand the cluster evolution mechanism during metal solidification. At the same time, we can obtain some important parameters and information that cannot be obtained by traditional methods. There have been reports on the use of MD simulations on clusters during nucleation [22], nucleation rates [23,24], and nucleation barriers [25]. Shibuta et al. [26] used MD simulations to study the solidification nucleation process at the submicron scale in pure metallic Fe, successfully linking empirical explanations in metallurgy to the atomic behavior of nucleation and solidification. They confirmed that there is a threshold thickness dividing the growth mode between two- and three-dimensional growths. Hou [27] used large-scale MD to study the effects of cooling rate on the solidification process of Al liquid, and found that there are many different disordered structures in the liquid, among which the icosahedral-like coordination structure (ICO) is the main one. It was found that the melt tends to form an amorphous structure with ICO characteristics at a faster cooling rate, while the melt tends to form a face-centered cubic (FCC) crystal structure at a slower cooling rate. In addition, at a moderate cooling rate, both FCC and hexagonal closed pack (HCP) structures exist. It was also found that the cooling rate during the quenching process has effects on the formation of amorphous, nanocrystalline, and single crystals, and the formation mechanism of nanocrystals was discovered by the method of tracking the nanoclusters. However, the effects of the cooling rate on the nucleation rate and critical size have not been studied. Mahata et al. [12] studied the homogeneous nucleation of Al melts with million-atom MD simulations. Initially, isothermal crystal nucleation from undercooled melt was studied at different constant temperatures, and later, superheated Al melt was quenched with different cooling rates. They calculated nucleation rate and critical size in quenching and isothermal solidification processes. Unfortunately, the nucleation rate was obtained by observing directly, and the critical size was calculated by direct measurement, which may have large errors.
This study adopts the process of rapid cooling from 2000 to 600 K first and then isothermal solidification to study the effects of the cooling rate on the nucleation and growth during the isothermal solidification process of metal Al. The MFPT method was used to calculate the nucleation rate and critical size, and the JMA method was used to calculate the Avrami exponent for the nucleation and growth mode. The growth exponent was also determined.
2. Methods
Simulation Details
MD simulations were performed using the Lammps (Large-Scale Atomic/Molecular Massively Parallel Simulator) software (Sandia National Laboratories, Livermore, CA, USA). Previous studies have shown that when the scale of simulation reaches millions of atoms, the effects of simulation size on various nucleation parameters are convergent, and simulation results are more accurate [28]. In this work, all simulations were conducted with a box of 293 Å × 251 Å × 251 Å containing 1,008,000 atoms in FCC lattice. The EAM potential developed by Mendelev et al. [29] was used in this study. This potential predicts the melting point temperature of Al to be 926 K, which is close to the experimental value. Periodic boundary conditions were applied in all three dimensions, and the integration time step was set to 5 fs. The constant pressure–temperature ensemble (NPT) was applied. Temperature was controlled with a Noose–Hoover thermostat, and pressure via volume scaling. Since time and space scales are finite in MD simulations and the nucleation of metal melts is a random event, independent runs were required to obtain statistically meaningful results. In this work, each simulation was repeated 50 times.
Initially a perfect Al single crystal was heated from solid states at 300 K into a liquid state at 2000 K. The liquid was subsequently cooled with different rates down to different temperatures and then kept at a constant temperature for a period of time for nucleation and growth. By changing the total number of quenching steps, four different cooling rates (2.0 × 1010 K/s, 2.0 × 1011 K/s, 2.0 × 1012 K/s, 2.0 × 1013 K/s) were obtained. The final cooling temperatures were selected in the range of 0.6 and 0.75 with 0.05 intervals, which are 695 K (0.75 ), 648 K (0.7 ), 600 K (0.65 ), and 556 K (0.6 ). All simulations were performed at a pressure of 0 MPa.
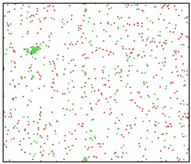
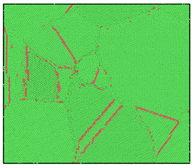
The microstructural evolution during solidification was observed using the visualization software OVITO [30]. The adaptive common neighbor analysis (CNA) method was used to determine the local environment of atoms [31]. CNA determines the atomic type by calculating the topology of the first-nearest neighbors of each atom. Comparing the calculated values with known characteristics of standard crystals can distinguish the local atomistic crystal structures. Atoms that do not identify as FCC structures, HCP structures, BCC structures, or any other crystal type in OVITO are identified as ICO liquid or ICO solid atoms. As shown in Figure 1a, the atoms in the 2000 K Al melt are all identified to be an ICO liquid structure. Its radial distribution function (RDF) is shown in Figure 1b, and there is no long-range peak in the curve, which proves that Al was completely melted at 2000 K.
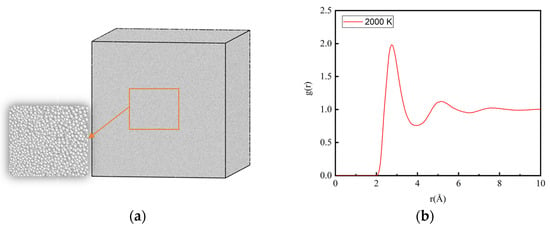
Figure 1.
Al melt model at 2000 K and its RDF curve. (a) MD model. The left inset is the magnified view of the center part of Al melt. (b) RDF curve.
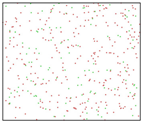
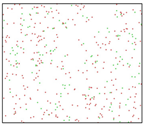
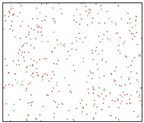
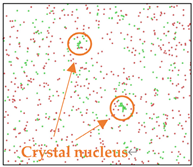
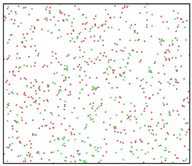
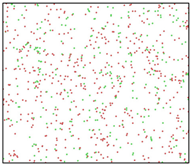
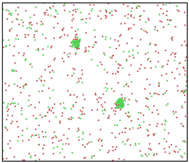
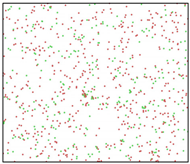
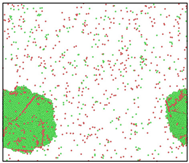
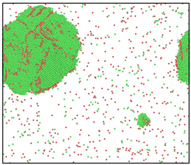
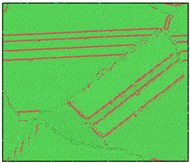
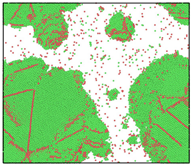
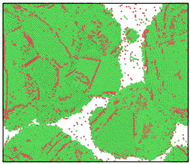
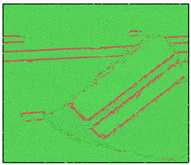
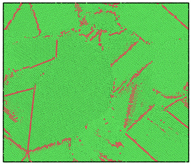
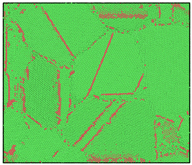
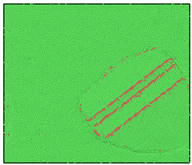
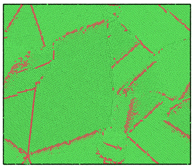
After the Al melt was cooled at different cooling rates from 2000 K, supercooled melts at different temperatures were obtained, whose three-dimensional structure snapshots are shown in Figure 2. Atoms marked in green are FCC atoms, and atoms marked in red are HCP atoms. It can be seen from Figure 2a–d that under a cooling rate of 2.0 × 1010 K/s, the nucleation of the aluminum melt occurs when it is cooled to 600 K. The reason is that the cooling rate is slow and the nucleation temperature is high, and only when the cooling rate is fast enough will the amorphous state be maintained under a certain degree of supercooling. It can be seen from Figure 2e that nucleation also occurs when the aluminum melt is cooled to 556 K at a cooling rate of 2.0 × 1011 K/s. It can be seen from Figure 2i that under a cooling rate of 2.0 × 1012 K/s, when the Al melt is cooled to 556 K, there are a few crystal nuclei existing. These nuclei will become heterogeneous nucleation sites in the isothermal process, which will affect the calculation of the nucleation rate and critical size. As shown in Figure 2m–p, no nucleation occurs when the Al melt is cooled to four temperatures at a cooling rate of 2.0 × 1013 K/s.
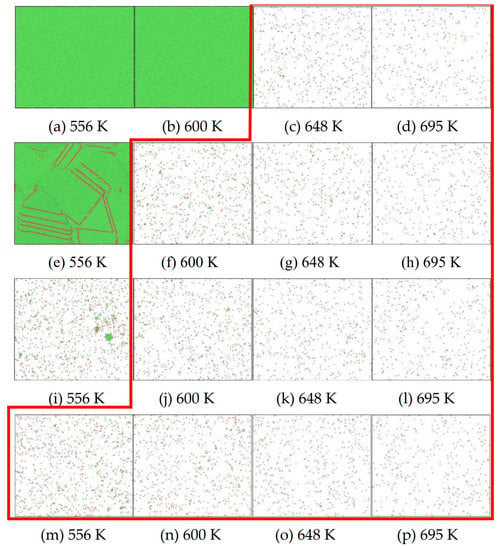
Figure 2.
Three-dimensional structure snapshot of Al melt after rapid cooling to different temperatures at different cooling rates. (a–d) 2.0 × 1010 K/s, (e–h) 2.0 × 1011 K/s, (i–l) 2.0 × 1012 K/s, (m–p) 2.0 × 1013 K/s.
In summary, after cooling to 695 and 648 K at cooling rates of 2.0 × 1010 K/s, 2.0 × 1011 K/s, 2.0 × 1012 K/s, and 2.0 × 1013 K/s; to 600 K at cooling rates of 2.0 × 1011 K/s, 2.0 × 1012 K/s, and 2.0 × 1013 K/s; and to 556 K at a cooling rate of 2.0 × 1013 K/s, relatively pure supercooled liquid melts can be obtained, excluding heterogeneous nucleation points, as shown in the red wireframe in Figure 2, whose results were used in the isothermal solidification process.
To facilitate the observation of nucleation and growth during the isothermal solidification process, the time of the isothermal process was set as 1000 ps. Three-dimensional structure snapshots of Al melts isothermally heated for 1000 ps at different temperatures after being cooled at different cooling rates are shown in Table 1. Supercooled liquids cooled to 695 K at all cooling rates and to 648 K at 2.0 × 1010 K/s, 2.0 × 1011 K/s, and 2.0 × 1012 K/s cooling rates cannot solidify within 1000 ps, whose processes were excluded from this study. The cooling rates and isothermal temperature selected for the simulation are shown in Table 2.

Table 1.
Three-dimensional structure snapshot of Al melts isothermally heated for 1000 ps at different temperatures after being cooled at different cooling rates, where T is the annealing temperature.

Table 2.
Cooling rates and temperature of isothermal solidification used in the simulation.
3. Results and Discussion
3.1. Microstructure Evolution
The potential energy of the Al melt was calculated to understand the energy fluctuations during the nucleation process. Figure 3a shows the local potential energy for Al melt with an isothermal time of 25 ps at a 2.0 × 1011 K/s cooling rate. It can be found that the potential energies of atoms or clusters are different everywhere, and the blue aggregated area between the white lines is darker and larger. The area between the white lines was selected for potential energy calculation. The average value of potential energy was calculated every 1 nm from left to right along the white line, as shown in Figure 3b. It can be seen that the potential energy curve fluctuates irregularly with time, proving that there are energy fluctuations inside the supercooled Al melt, and the energy fluctuations around the nucleation region are the largest, which is consistent with the results based on CNT [9].
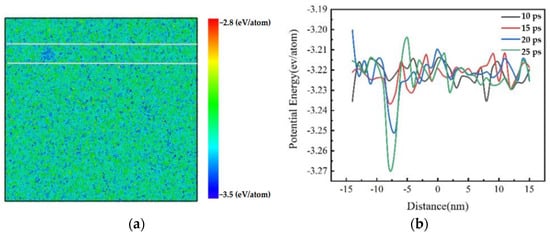
Figure 3.
Potential energy of Al melt at a cooling rate of 2.0 × 1011 K/s for 25 ps. (a) Potential energy map; (b) potential energy change diagram.
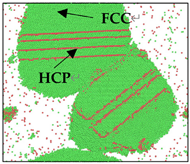
The Al melt cooled to 600 K by different cooling rates (2.0 × 1011 K/s, 2.0 × 1012 K/s, 2.0 × 1013 K/s) was subjected to the isothermal process. The microstructure changes with time, as shown in Table 3 (atoms identified by OVITO as ICO structures and BCC structures have been deleted).

Table 3.
Nucleation and growth during the isothermal solidification process after cooling with different cooling rates.
It can be seen that the FCC atoms serve as the matrix, and the HCP atoms exist in the form of grain boundaries, twin boundaries, and stacking faults inside the FCC atoms. The formation energy between FCC and HCP Al is much smaller than that between FCC and BCC Al. During the solidification, there will be random fluctuations of energy, but this energy fluctuation is small, which can lead to the formation of HCP stacking faults in the system, but is not enough to promote the formation of BCC, so BCC atoms are not considered [32].
Nucleation occurs in Al melts in the isothermal process, as shown in Table 3, but the time of nucleation and the final grain size are different. In the case of the 2.0 × 1011 K/s cooling rate, two clusters exist in Al melt at 10 ps. The clusters continue to grow at 20 ps, and then grow into two larger clusters at 100 ps. Their atoms are mainly FCC atoms, and some HCP atoms exist in the form of stacking faults. As time increases, the two nuclei gradually grow up and engulf each other. When the time is between 150 and 500 ps, the liquid phase in the system exhausted, and some HCP atoms ablated and replaced by FCC atoms, the grains gradually grow up. In the case of 2.0 × 1012 K/s, there is only one cluster at 100 ps, and the number of clusters increases at 150 ps. At 250 ps, the whole system is occupied by crystalline atoms, and the stacking faults and twins of the HCP structure are distributed on the matrix of the FCC structure. Between 250 and 500 ps, some HCP atoms are replaced by FCC atoms, and grains continue to grow. In the case of 2.0 × 1013 K/s, there is only one cluster at 20 ps, two clusters at 100 ps. The liquid atoms are mostly occupied by crystalline atoms at 250 ps. Part of the HCP atoms is replaced by FCC atoms, and the grains grow gradually.
3.2. Influence of Cooling Rate on Nucleation Parameters
According to CNT, the nucleation process is an activation process, which needs to overcome and cross the free energy barrier through energy fluctuations so that the system can be transformed from one state to another.
It is difficult to directly obtain the nucleation rate and critical size from MD [33]. In this study, MFPT [33,34,35,36] is used to analyze the kinetics of the nucleation, which allows simple and straightforward modeling of various parameters in the nucleation process using purely kinetic methods. In the MFPT method, only the number of atoms in the largest cluster in a certain time and the average time of its appearance need to be counted. The number of atoms in the largest cluster is obtained from the cluster analysis in OVITO. Two atoms belong to the same nucleus if they are within the nearest-neighbor distance of each other, which is identified from the RDF [37]. Figure 4 shows the evolution of the number of atoms contained in the largest cluster during the isothermal process at three different cooling rates. The data in each figure are the results of 50 simulations.
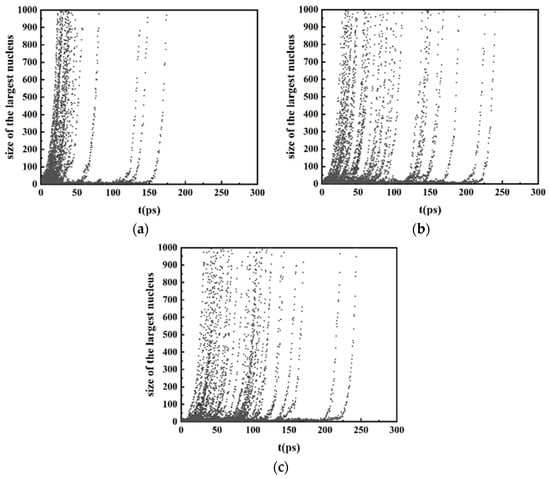
Figure 4.
Evolution of the number of atoms in the largest cluster during the isothermal solidification process with different cooling rates repeatedly for 50 simulations. (a) 2.0 × 1011 K/s, (b) 2.0 × 1012 K/s, (c) 2.0 × 1013 K/s.
Figure 5 shows the MFPT curves of the largest cluster during the isothermal solidification process with different cooling rates, the abscissa is the number of atoms in the largest cluster, and the ordinate is the average time. The black point is the average time of the largest cluster appearing in 50 simulations, which is obtained by first interpolating the data and then averaging the time at which the largest clusters appear. The blue curve is the result obtained after fitting according to the MFPT Equation (1) [36].
Here, is related to the steady-state transition rate as , where is the system volume under consideration, is the size of the largest nucleus, denotes the local curvature at the top of the free energy barrier, and . can be obtained statistically from N independent runs. is the critical size. By fitting, the blue MFPT curve and and were obtained. The curve is S-shaped. The first half of the curve has a good fit with Equation (1), but it does not form a clear plateau in the latter stage, which is consistent with the simulation results of Eisenstein et al., possibly due to the inhibited growth of nuclei and the slower growth rate in the later stage [36].
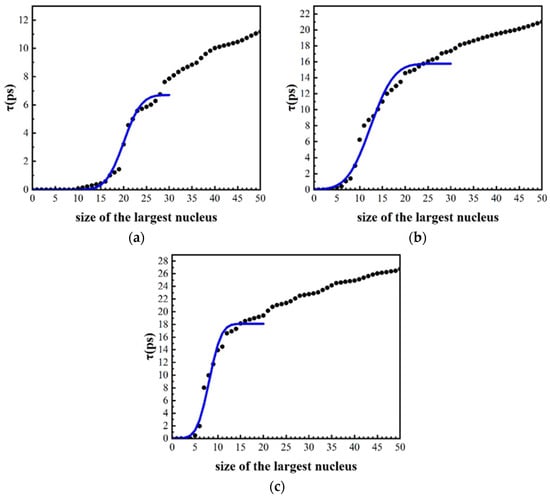
Figure 5.
MFPT curves during the isothermal solidification process with different cooling rates. (a) 2.0 × 1011 K/s, (b) 2.0 × 1012 K/s, (c) 2.0 × 1013 K/s.
According to the number of atoms in the critical nucleus obtained by MFPT, the critical size is calculated through the equivalent volume method. By combining the observation of the microstructure with the classical nucleation theory, during the nucleation process, the nucleus tends to form a spherical shape to reduce the surface area and, thus, the surface energy for nucleation. The atom volume is the ratio of the total volume of the system to the number of atoms. The volume of the critical size is the total of all atoms’ volume. Assuming that the critical nucleus is spherical, the approximate radius of the critical nucleus can be calculated. The radius of Al atoms is calculated to be 0.161 nm, which is consistent with the theoretical radius of Al atoms [38].
Nucleation rate and critical size during the isothermal process with different cooling rates are listed in Table 4. It can be found that with the increase in cooling rate, the nucleation rate decreases, and the critical size decreases slightly.

Table 4.
Critical size n*, nucleation rate , and nucleus radius size r* during the isothermal solidification process with different cooling rates.
3.3. Influence of Cooling Rate on Crystal Growth
To study the effects of different cooling rates on the crystallization kinetics. The JMA method is used to quantify phase transition kinetics [36,39,40,41]. It describes the volume fraction of the daughter phase as a function of time ,
where is a prefactor and is the characteristic growth or Avrami exponent. The volume fraction of crystallites within a liquid is computed from the evolution curves of the atomic volume during the isothermal solidification process, as shown in Figure 6:
where and are the maximum and minimum plateau values on the curves in Figure 6, representing complete melting and solidification states, respectively.
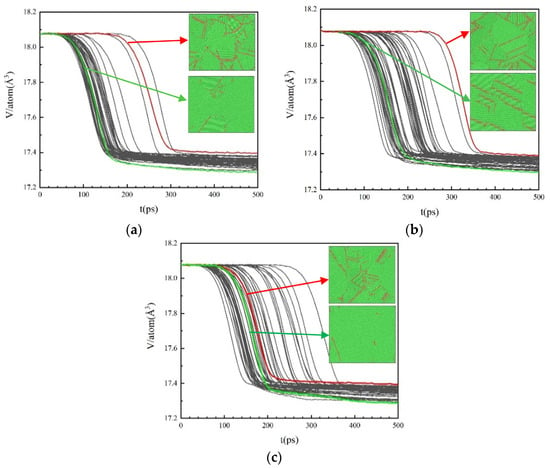
Figure 6.
The evolution curves of the atomic volume during the isothermal solidification process with different cooling rates for 50 times. (a) 2.0 × 1011 K/s, (b) 2.0 × 1012 K/s, (c) 2.0 × 1013 K/s.
Figure 6 is the evolution curves of the atomic volume during the isothermal solidification process after cooling at different cooling rates for 50 times. The red and green curves represent the minimum and maximum volume changes in 50 simulations, respectively. The microstructures at the end of crystallization for both cases are shown in the inset of Figure 6. It can be found that when the volume change is small, the proportion of HCP atoms in the system is high, and the grains with different orientations are more. In the case of a large volume change, the proportion of HCP atoms is low, and the grain orientation is relatively similar.
Substituting calculated from Equation (3) in both cases into Equation (2), the relationship between and is obtained, as shown in Figure 7. The blue curve represents the case with a large volume change, and the red curve represents the case with a small volume change. The slope of the curve is the Avrami exponent , which is indicated in the figure. In Figure 7, the whole process can be divided into three stages. The first stage is too dispersive and, thus, not fitted. In the second stage, becomes larger, with the main growth stage of grains, and is between ~4 and ~15, indicating that nucleation and growth processes are a three-dimensional growth mode [26]. In the subsequent third stage, growth becomes slow again, mainly due to the depletion of the parent phase during growth [36].
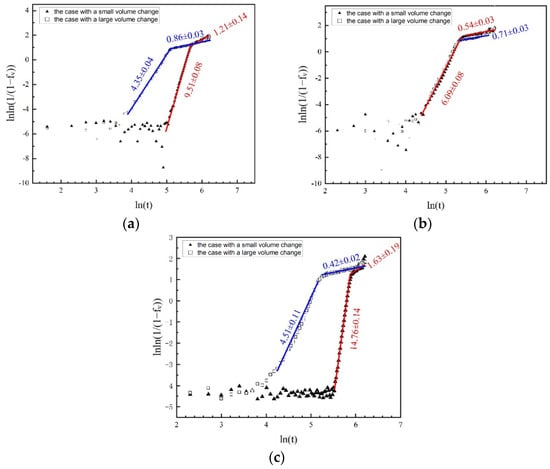
Figure 7.
Grain growth kinetics curve of the isothermal solidification process with different cooling rates. (a) 2.0 × 1011 K/s, (b) 2.0 × 1012 K/s, (c) 2.0 × 1013 K/s.
When the cluster size reaches the critical size, the clusters start to grow continuously. For the normal grain growth, the observed grain growth kinetics can be described as [42,43,44].
Here, is the grain size at time , is the constant, and is the growth exponent. Taking the logarithm of both sides of Equation (4), we obtain
The crystal growth rate can be obtained by a straight line fitted to a logarithmic curve, where is the slope and is the intercept. The growth rate of grains is related to the value of . The larger the , the faster the grain growth rate. In this work, the average grain diameter was obtained by calculating the equivalent radius of the largest cluster in a single simulation and then averaging 50 repeated simulations.
Figure 8 shows the grain growth kinetics curve of the isothermal solidification process with different cooling rates. The grain growth exponents are calculated to be 1.08, 1.46, and 1.65. With the increase in cooling rate, the growth exponent of grains increases and grain growth accelerates.
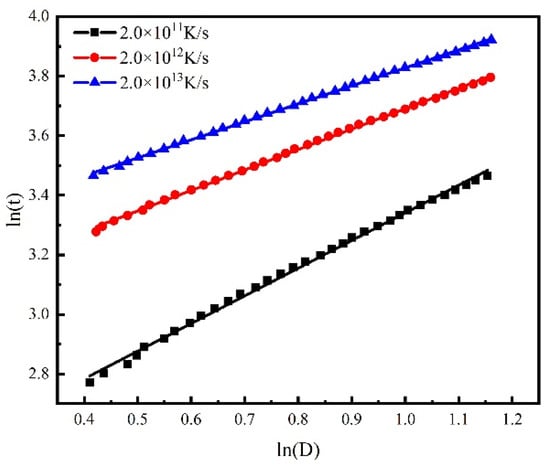
Figure 8.
Grain growth kinetics curve of the isothermal solidification process with different cooling rates.
4. Conclusions
In this study, the solidification process of metal Al was studied by using MD simulation. Conclusions are drawn as follows.
(1) The nucleus in the supercooled Al melt tends to grow into a spherical shape. As the Al is completely solidified, there are mainly two types of atomic structures existing, FCC and HCP. HCP atoms mainly exists in the form of several local defects, such as grain boundaries, subgrain boundaries, twin boundaries, and stacking faults of FCC atoms.
(2) The nucleation parameters are estimated by the MFPT method. With the increase in cooling rate, the nucleation rate decreases and the critical size decreases accordingly.
(3) In the second grain growth stage, the Avrami exponent η is in the range of 4 and 15, indicating that nucleation and growth processes are in the typical three-dimensional growth mode. The first decreases and then increases with the increase in the cooling rate, indicating that the grain growth mode in the isothermal stage was affected by the cooling rate. With the increase in the cooling rate, the grain growth exponent increases, indicating that the grain growth rate is accelerated in the isothermal stage.
(4) This study is of fundamental significance for further understanding the solidification process. The MFPT method can be used with other different situations, such as alloys, proteins, or liquids, which has practical significance.
Author Contributions
Conceptualization, X.C. and Z.W.; methodology, W.F., W.J. and D.L.; software, W.J.; validation, X.C. and Z.W.; formal analysis, W.J. and Y.W.; investigation, W.F.; resources, W.F. and W.J.; data curation, W.J.; writing—original draft preparation, X.C. and W.F.; writing—review and editing, X.C. and X.H.; visualization, W.F.; supervision, X.C. and D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors thank the support of the State Key Laboratory of Advanced Metals and Materials and the National Supercomputing Guangzhou Center/Guangzhou Supercomputing Center.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pinomaa, T.; Lindroos, M.; Walbrühl, M.; Provatas, N.; Laukkanen, A. The significance of spatial length scales and solute segregation in strengthening rapid solidification microstructures of 316L stainless steel. Acta Mater. 2020, 184, 1–16. [Google Scholar] [CrossRef]
- Liu, S.C.; Jie, J.C.; Guo, Z.K.; Yin, G.M.; Wang, T.M.; Li, T.J. Solidification microstructure evolution and its corresponding mechanism of metastable immiscible Cu80Fe20 alloy with different cooling conditions. J. Alloys Compd. 2018, 742, 99–106. [Google Scholar] [CrossRef]
- He, J.; Zhao, J.Z.; Ratke, L. Solidification microstructure and dynamics of metastable phase transformation in undercooled liquid Cu–Fe alloys. Acta Mater. 2006, 54, 1749–1757. [Google Scholar] [CrossRef]
- Xu, X.L.; Tang, C.; Wang, H.F.; An, Y.K.; Zhao, Y.H. Microstructure evolution and grain refinement mechanism of rapidly solidified single-phase copper based alloys. J. Mater. Sci. Technol. 2022, 128, 160–179. [Google Scholar] [CrossRef]
- Grisell, D.L.; Ralf, D.; Jutta, R. Atomistic insight into the non-classical nucleation mechanism during solidification in Ni. J. Chem. Phys. 2017, 146, 154702. [Google Scholar]
- Zheng, L.; Guo, Q.Z.; Dominik, D.; Zhou, L.; Cheng, B.X. The Effect of Process Parameters in Interdendritic-Melt Solidification Control Technique on the Microstructure and Properties of a Ni-Base Superalloy. Mater. Sci. Forum 2016, 879, 1129–1134. [Google Scholar] [CrossRef]
- Mi, G.F.; Dong, C.F.; Li, C.Y.; Wang, H.Y. Microstructures Development in Al-5Fe and Al-5Fe-3Y Alloys Solidified at Different Cooling Rate. Adv. Mater. Res. 2011, 189–193, 2462–2466. [Google Scholar] [CrossRef]
- Ren, W.M.; Chen, Z.Y.; Xiang, Z.L.; Chai, L.H. Microstructure and Properties of Rapidly Solidified Al-Zn-Mg-Cu Alloy. Mater. Sci. Forum 2020, 993, 203–207. [Google Scholar] [CrossRef]
- Vitalij, I.K. Classical Nucleation Theory. In Nucleation Theory; Lecture Notes in Physics; Springer: Dordrecht, the Netherlands, 2013; Volume 860, pp. 17–41. [Google Scholar]
- Neilson, G.F.; Weinberg, M.C. A test of classical nucleation theory: Crystal nucleation of lithium disilicate glass. J. Non-Cryst. Solids 1979, 34, 137–147. [Google Scholar] [CrossRef]
- Yan, R.; Ma, S.D.; Sun, W.Z.; Jing, T.; Dong, H.B. The solid–liquid interface free energy of Al: A comparison between molecular dynamics calculations and experimental measurements. Comput. Mater. Sci. 2020, 184, 109910. [Google Scholar] [CrossRef]
- Mahata, A.; Zaeem, M.A.; Baskes, M.I. Understanding homogeneous nucleation in solidification of aluminum by molecular dynamics simulations. Model. Simul. Mater. Sci. Eng. 2018, 26, 025007. [Google Scholar] [CrossRef]
- Jin, Y.M.M.; Khachaturyan, A.G. Atomic density function theory and modeling of microstructure evolution at the atomic scale. J. Appl. Phys. 2006, 100, 013519. [Google Scholar] [CrossRef]
- Grest, G.S.; Srolovitz, D.J.; Anderson, M.P. Computer simulation of grain growth—IV. Anisotropic grain boundary energies. Acta Metall. 1985, 33, 509–520. [Google Scholar] [CrossRef]
- Huang, C.M.; Joanne, C.L.; Patnaik, B.S.V.; Jayaganthan, R. Monte Carlo simulation of grain growth in polycrystalline materials. Appl. Surf. Sci. 2006, 252, 3997–4002. [Google Scholar] [CrossRef]
- Rappaz, M.; Gandin, C.-A. Probabilistic modelling of microstructure formation in solidification processes. Acta Metall. Et Mater. 1993, 41, 345–360. [Google Scholar] [CrossRef]
- Gandin, C.-A.; Rappaz, M. A Coupled Finite Element-cellular Automaton Model for the Prediction of Dendritic Grain Structures in Solidification Process. Acta Metall. Et Mater. 1994, 42, 2233–2246. [Google Scholar] [CrossRef]
- Zuo, X.J.; Coutinho, Y.; Chatterjee, S.; Moelans, N. Phase field simulations of FCC to BCC phase transformation in (Al)CrFeNi medium entropy alloys. Mater. Theory 2022, 6, 12. [Google Scholar] [CrossRef]
- Boldrini, J.L.; Caretta, B.M.C.; Fernández-Cara, E. Some optimal control problems for a two-phase field model of solidification. Rev. Matemática Complut. 2009, 23, 49. [Google Scholar] [CrossRef]
- Miyoshi, E.; Takaki, T. Validation of a novel higher-order multi-phase-field model for grain-growth simulations using anisotropic grain-boundary properties. Comput. Mater. Sci. 2016, 112, 44–51. [Google Scholar] [CrossRef]
- Miyoshi, E.; Takaki, T. Extended higher-order multi-phase-field model for three-dimensional anisotropic-grain-growth simulations. Comput. Mater. Sci. 2016, 120, 77–83. [Google Scholar] [CrossRef]
- Hou, Z.Y.; Liu, L.X.; Liu, R.S.; Tian, Z.A.; Wang, J.G. Kinetic details of nucleation in supercooled liquid Na: A simulation tracing study. Chem. Phys. Lett. 2010, 491, 172–176. [Google Scholar] [CrossRef]
- Auer, S.; Frenkel, D. Prediction of absolute crystal-nucleation rate in hard-sphere colloids. Nature 2001, 409, 1020–1023. [Google Scholar] [CrossRef] [PubMed]
- Aga, R.S.; Morris, J.R.; Hoyt, J.J.; Mendelev, M. Quantitative Parameter-Free Prediction of Simulated Crystal-Nucleation Times. Phys. Rev. Lett. 2006, 96, 245701. [Google Scholar] [CrossRef]
- Bokeloh, J.; Rozas, R.E.; Horbach, J.; Wilde, G. Nucleation barriers for the liquid-to-crystal transition in Ni: Experiment and simulation. Phys. Rev. Lett. 2011, 107, 145701. [Google Scholar] [CrossRef] [PubMed]
- Shibuta, Y.; Sakane, S.; Takaki, T.; Ohno, M. Submicrometer-scale molecular dynamics simulation of nucleation and solidification from undercooled melt: Linkage between empirical interpretation and atomistic nature. Acta Mater. 2016, 105, 328–337. [Google Scholar] [CrossRef]
- Hou, Z.Y.; Dong, K.J.; Tian, Z.A.; Liu, R.S.; Wang, Z.; Wang, J.G. Cooling rate dependence of solidification for liquid aluminium: A large-scale molecular dynamics simulation study. Phys. Chem. Chem. Phys. 2016, 18, 17461–17469. [Google Scholar] [CrossRef] [PubMed]
- Mahata, A.; Zaeem, M.A. Size effect in molecular dynamics simulation of nucleation process during solidification of pure metals: Investigating modified embedded atom method interatomic potentials. Model. Simul. Mater. Sci. Eng. 2019, 27, 085015. [Google Scholar] [CrossRef]
- Mendelev, M.I.; Kramer, M.J.; Becker, C.A.; Asta, M. Analysis of semi-empirical interatomic potentials appropriate for simulation of crystalline and liquid Al and Cu. Philos. Mag. 2008, 88, 1723–1750. [Google Scholar] [CrossRef]
- Adler, J.; Pine, P. Visualization techniques for modelling carbon allotropes. Comput. Phys. Commun. 2009, 180, 580–582. [Google Scholar] [CrossRef]
- Stukowski, A. Structure identification methods for atomistic simulations of crystalline materials. Model. Simul. Mater. Sci. Eng. 2012, 20, 045021. [Google Scholar] [CrossRef]
- Asadi, E.; Asle Zaeem, M.; Nouranian, S.; Baskes, M.I. Two-phase solid–liquid coexistence of Ni, Cu, and Al by molecular dynamics simulations using the modified embedded-atom method. Acta Mater. 2015, 86, 169–181. [Google Scholar] [CrossRef]
- Jan, W.; Reinhard, S.; David, R. New method to analyze simulations of activated processes. J. Chem. Phys. 2007, 126, 134103. [Google Scholar]
- Anatolii, V.M.; Bulat, N.G. A method for analyzing the non-stationary nucleation and overall transition kinetics: A case of water. J. Chem. Phys. 2014, 140, 024104. [Google Scholar]
- Anatolii, V.M.; Bulat, N.G. Kinetics of the Crystalline Nuclei Growth in Glassy Systems. Phys. Chem. Chem. Phys. 2017, 19, 11340. [Google Scholar]
- Eisenstein, J.C.; Wang, L.; Cai, Y.; Wu, H.A.; Luo, S.N. Crystallization in supercooled liquid Cu: Homogeneous nucleation and growth. J. Chem. Phys. 2015, 142, 064704. [Google Scholar]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Wang, G.Y.; Zheng, K.; Huang, Y.X.; Yu, J.B.; Wu, H.; Chen, X.P.; Tao, L.Q. Investigation of the positive effect of doping Al atom to the adsorption of CO2 on BN nanosheets: A DFT study. Phys. Chem. Chem. Phys. 2020, 22, 9368–9374. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212. [Google Scholar] [CrossRef]
- William, J.; Mehl, R. Reaction kinetics in processes of nucleation and growth. Trans. Metall. Soc. AIME 1939, 135, 416–442. [Google Scholar]
- Cahn, J.W. The kinetics of grain boundary nucleated reactions. Acta Metall. 1956, 4, 449–459. [Google Scholar] [CrossRef]
- Burke, J.E. Some factors affecting rate of grain growth in metals. Aime Trans. 1949, 180, 73–91. [Google Scholar]
- Beck, P.A.; Holzworth, M.L.; Hu, H. Instantaneous rates of grain growth. Phys. Rev. 1948, 73, 526–527. [Google Scholar] [CrossRef]
- Fateme, N.; Sara, K.; Bita, P.; Hamed, M. Recent advances in the kinetics of normal/abnormal grain growth: A review. Arch. Civ. Mech. Eng. 2021, 21, 29. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).