Effect of Ni-Coated Carbon Nanotubes Additions on the Eutectic Sn-0.7Cu Lead-Free Composite Solder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Ni-Coated Nanotubes
2.2. Preparation of Nanotube Loaded Composite Solder
2.3. Melting Point Test
2.4. Solder Wettability Test
2.5. Microstructural Characterization
3. Results and Discussion
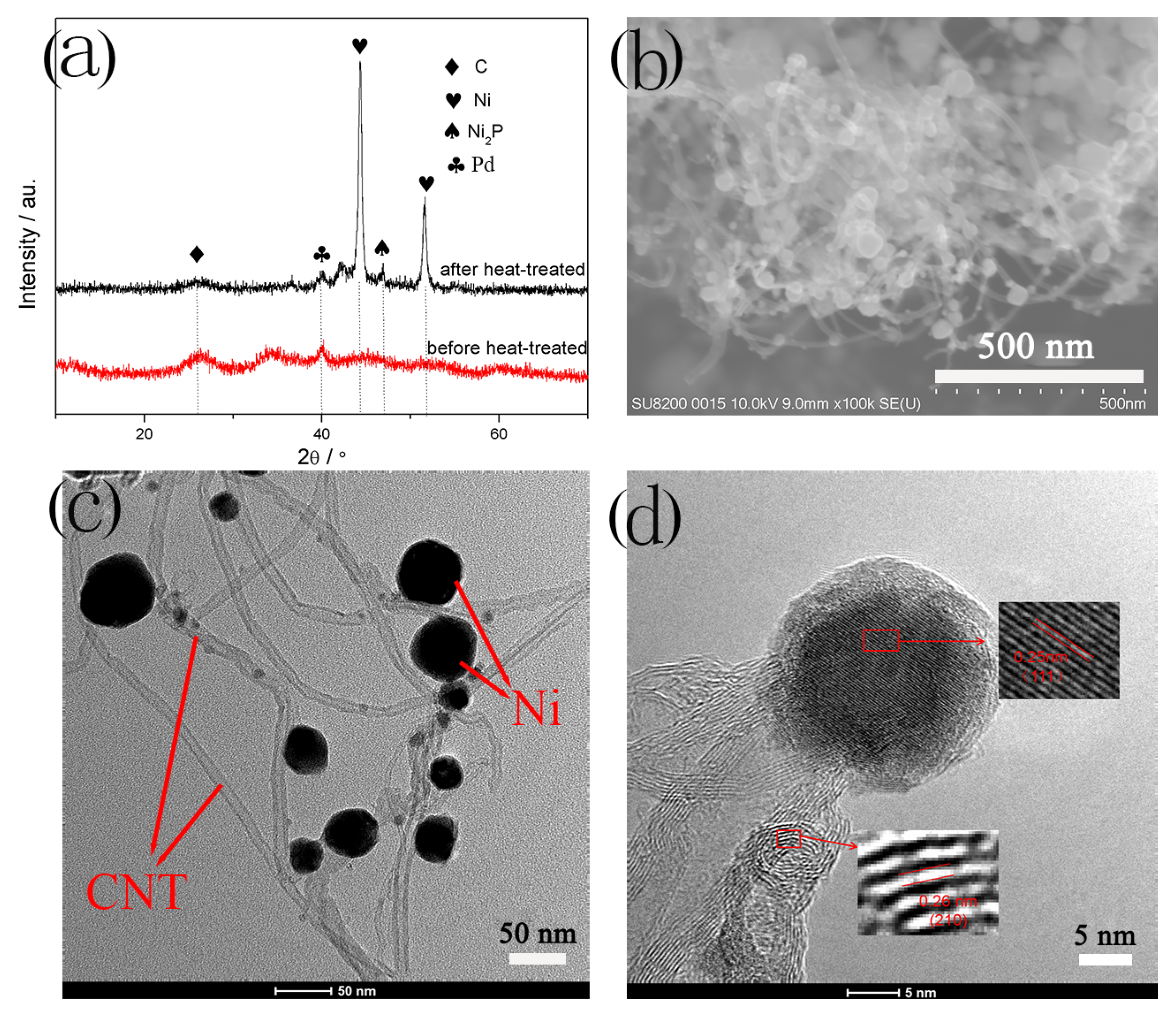
3.1. Ni-Coated Multi-Walled Carbon Nanotubes
3.2. Solder Microstructure Analysis
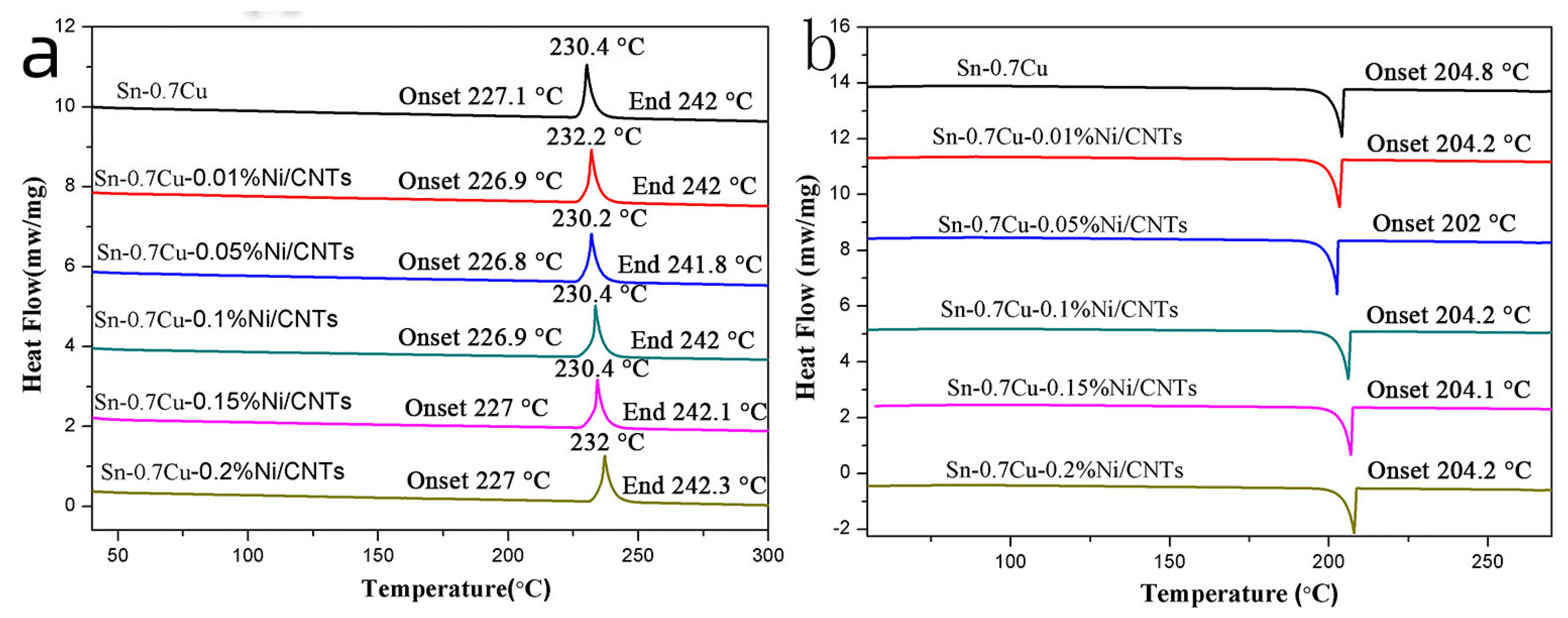
3.3. Solder Melting Point
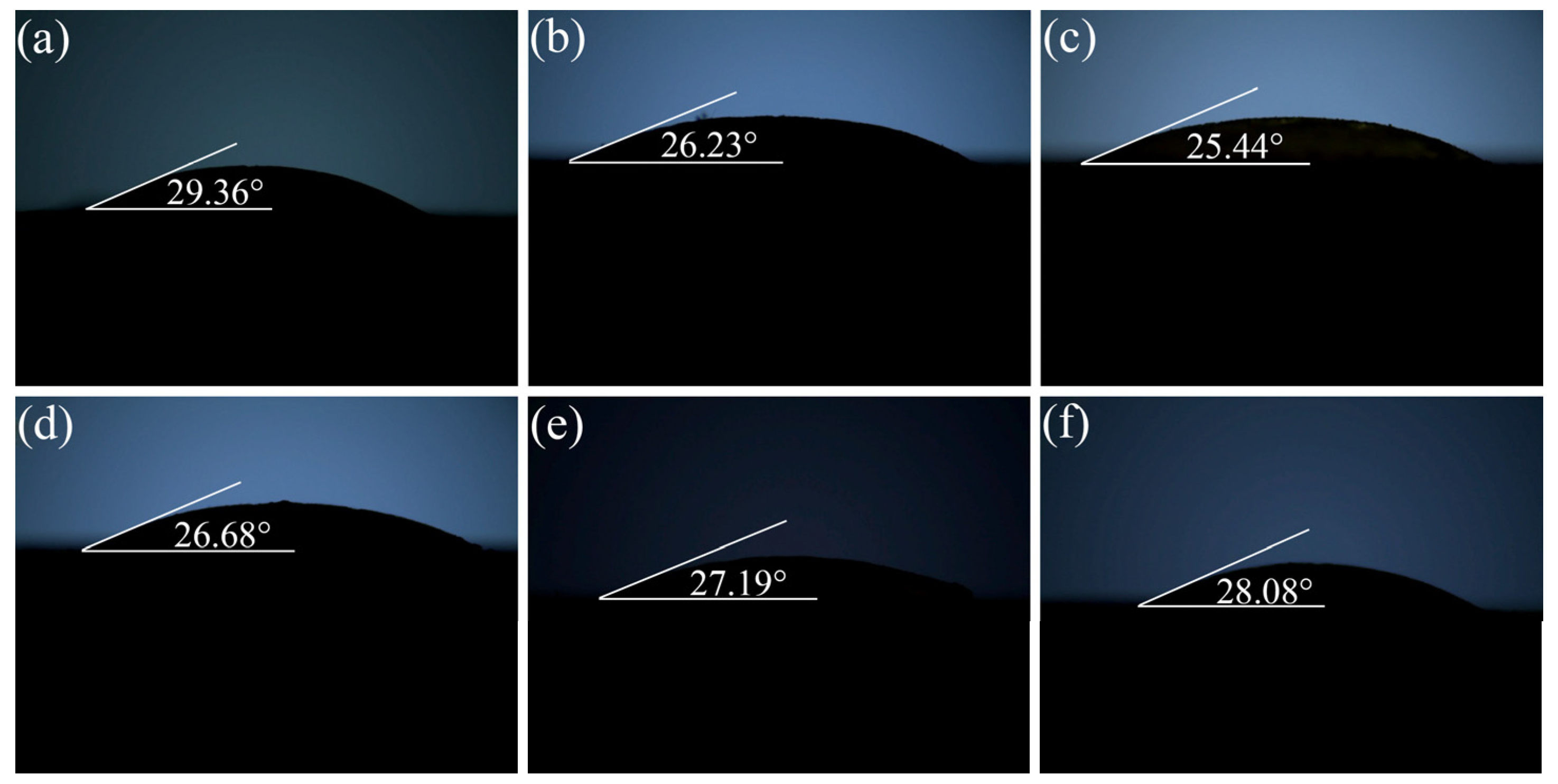
3.4. Solder Wettability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, Q.; Bailey, H.J.; Armstrong, W.D. Aging effects on microstructure and tensile property of Sn3.9Ag0.6Cu solder alloy. J. Electron. Packag. 2004, 126, 208–212. [Google Scholar] [CrossRef]
- Nguyen, V.L.; Kim, S.H.; Jeong, J.W.; Lim, T.-S.; Yang, D.-Y.; Kim, K.B.; Kim, Y.J.; Lee, J.H.; Kim, Y.-J.; Yang, S. Microstructure and mechanical behavior of low-melting point Bi-Sn-In solder joints. Electron. Mater. Lett. 2017, 13, 420–426. [Google Scholar] [CrossRef]
- Dele-Afolabi, T.T.; Azmah Hanim, M.A.; Norkhairunnisa, M.; Yusoff, H.M.; Suraya, M.T. Growth kinetics of intermetallic layer in lead-free Sn–5Sb solder reinforced with multi-walled carbon nanotubes. J. Mater. Sci. Mater. Electron. 2015, 26, 8249–8259. [Google Scholar] [CrossRef]
- Zhong, X.L.; Gupta, M. Development of lead-free Sn–0.7Cu/Al2O3nanocomposite solders with superior strength. J. Phys. D Appl. Phys. 2008, 41, 095403. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L.; Chan, Y.C. Microstructure, elastic modulus and shear strength of alumina (Al2O3) nanoparticles-doped tin–silver–copper (Sn–Ag–Cu) solders on copper (Cu) and gold/nickel (Au/Ni)-plated Cu substrates. J. Mater. Sci. Mater. Electron. 2015, 26, 7039–7048. [Google Scholar] [CrossRef]
- Tsao, L.C.; Huang, C.H.; Chung, C.H.; Chen, R.S. Influence of TiO2 nanoparticles addition on the microstructural and mechanical properties of Sn0.7Cu nano-composite solder. Mater. Sci. Eng. A 2012, 545, 194–200. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Dai, J.; Jing, Y.; Ge, J.; Zhang, N. Microstructure, interfacial IMC and mechanical properties of Sn–0.7Cu–xAl (x = 0–0.075) lead-free solder alloy. Mater. Des. 2015, 67, 209–216. [Google Scholar] [CrossRef]
- Tsao, L.C.; Chang, S.Y. Effects of Nano-TiO2 additions on thermal analysis, microstructure and tensile properties of Sn3.5Ag0.25Cu solder. Mater. Des. 2010, 31, 990–993. [Google Scholar] [CrossRef]
- Gain, A.K.; Chan, Y.C.; Yung, W.K.C. Microstructure, thermal analysis and hardness of a Sn–Ag–Cu–1wt% nano-TiO2 composite solder on flexible ball grid array substrates. Microelectron. Reliab. 2011, 51, 975–984. [Google Scholar] [CrossRef]
- Sun, R.; Sui, Y.; Qi, J.; Wei, F.; He, Y.; Chen, X.; Meng, Q.; Sun, Z. Influence of SnO2 Nanoparticles Addition on Microstructure, Thermal Analysis, and Interfacial IMC Growth of Sn1.0Ag0.7Cu Solder. J. Electron. Mater. 2017, 46, 4197–4205. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Fawzy, A.; Mansour, S.F.; Younis, M.J. Novel SiC nanoparticles-containing Sn–1.0Ag–0.5Cu solder with good drop impact performance. Mater. Sci. Eng. A 2013, 578, 62–71. [Google Scholar] [CrossRef]
- Kumar, K.M.; Kripesh, V.; Tay, A.A.O. Single-wall carbon nanotube (SWCNT) functionalized Sn–Ag–Cu lead-free composite solders. J. Alloy. Compd. 2008, 450, 229–237. [Google Scholar] [CrossRef]
- Nai, S.M.L.; Wei, J.; Gupta, M. Improving the performance of lead-free solder reinforced with multi-walled carbon nanotubes. Mater. Sci. Eng. A 2006, 423, 166–169. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubes of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Shao, H.; Chen, B.; Li, B.; Tang, S.; Li, Z. Influence of dispersants on the properties of CNTs reinforced cement-based materials. Constr. Build. Mater. 2017, 131, 186–194. [Google Scholar] [CrossRef]
- Svatoš, V.; Sun, W.; Kalousek, R.; Gablech, I.; Pekárek, J.; Neužil, P. Single Measurement Determination of Mechanical, Electrical, and Surface Properties of a Single Carbon Nanotube via Force Microscopy. Sens. Actuators A Phys. 2018, 271, 217–222. [Google Scholar] [CrossRef]
- Esawi, A.M.K.; El Borady, M.A. Carbon nanotube-reinforced aluminium strips. Compos. Sci. Technol. 2008, 68, 486–492. [Google Scholar] [CrossRef]
- Guerret-Piécourt, C.; Bouar, Y.; Lolseau, A.; Pascard, H. Relation between metal electronic structure and morphology of metal compounds inside carbon nanotubes. Nature 1994, 372, 761–765. [Google Scholar] [CrossRef]
- Prem Kumar, T.; Ramesh, R.; Lin, Y.Y.; Fey, G.T.-K. Tin-filled carbon nanotubes as insertion anode materials for lithium-ion batteries. Electrochem. Commun. 2004, 6, 520–525. [Google Scholar] [CrossRef]
- Zhang, F.; Li, J.; Wang, T.; Huang, C.; Ji, F.; Shan, L.; Zhang, G.; Sun, R.; Wong, C.P. Fluorinated graphene/polyimide nanocomposites for advanced electronic packaging applications. J. Appl. Polym. Sci. 2020, 138, 49801. [Google Scholar] [CrossRef]
- Chantaramanee, S.; Wisutmethangoon, S.; Sikong, L.; Plookphol, T. Development of a lead-free composite solder from Sn–Ag–Cu and Ag-coated carbon nanotubes. J. Mater. Sci. Mater. Electron. 2013, 24, 3707–3715. [Google Scholar] [CrossRef]
- Song, H.-Y.; Zha, X.-W. Mechanical properties of nickel-coated single-walled carbon nanotubes and their embedded gold matrix composites. Phys. Lett. A 2010, 374, 1068–1072. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, G.; Zheng, R.; Zhao, Y.; Liang, C. Influence of synthesis process on preparation and properties of Ni/CNT catalyst. Diam. Relat. Mater. 2006, 15, 15–21. [Google Scholar] [CrossRef]
- Kong, F.Z.; Zhang, X.B.; Xiong, W.Q.; Liu, F.; Huang, W.Z.; Sun, Y.L.; Tu, J.P.; Chen, X.W. Continuous Ni-layer on multiwall carbon nanotubes by an electroless plating method. Surf. Coat. Technol. 2002, 155, 33–36. [Google Scholar] [CrossRef]
- Wang, F.; Arai, S.; Endo, M. Preparation of nickel–carbon nanofiber composites by a pulse-reverse electrodeposition process. Electrochem. Commun. 2005, 7, 674–678. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Lun, N.; Wen, S. Synthesis of gold nano-catalysts supported on carbon nanotubes by using electroless plating technique. Mater. Chem. Phys. 2006, 97, 351–356. [Google Scholar] [CrossRef]
- Zhao, B.; Yadian, B.L.; Li, Z.J.; Liu, P.; Zhang, Y.F. Improvement on wettability between carbon nanotubes and Sn. Surf. Eng. 2013, 25, 31–35. [Google Scholar] [CrossRef]
- Han, Y.D.; Nai, S.M.L.; Jing, H.Y.; Xu, L.Y.; Tan, C.M.; Wei, J. Development of a Sn–Ag–Cu solder reinforced with Ni-coated carbon nanotubes. J. Mater. Sci. Mater. Electron. 2010, 22, 315–322. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Z.; Xu, L.; Shao, Z. Preparation and Characterization of Highly Aligned Carbon Nanotubes/Polyacrylonitrile Composite Nanofibers. Polymers 2017, 9, 1. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Lu, G.; Ji, Z.; Wei, F.; Yao, C.; Wang, J. Effect of Ni-Coated Carbon Nanotubes Additions on the Eutectic Sn-0.7Cu Lead-Free Composite Solder. Metals 2022, 12, 1196. https://doi.org/10.3390/met12071196
Liu X, Lu G, Ji Z, Wei F, Yao C, Wang J. Effect of Ni-Coated Carbon Nanotubes Additions on the Eutectic Sn-0.7Cu Lead-Free Composite Solder. Metals. 2022; 12(7):1196. https://doi.org/10.3390/met12071196
Chicago/Turabian StyleLiu, Xin, Guoge Lu, Zhe Ji, Fuxiang Wei, Chuandang Yao, and Jiajian Wang. 2022. "Effect of Ni-Coated Carbon Nanotubes Additions on the Eutectic Sn-0.7Cu Lead-Free Composite Solder" Metals 12, no. 7: 1196. https://doi.org/10.3390/met12071196
APA StyleLiu, X., Lu, G., Ji, Z., Wei, F., Yao, C., & Wang, J. (2022). Effect of Ni-Coated Carbon Nanotubes Additions on the Eutectic Sn-0.7Cu Lead-Free Composite Solder. Metals, 12(7), 1196. https://doi.org/10.3390/met12071196






