Sn-0.7Cu-10Bi Solder Modification Strategy by Cr Addition
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sn-0.7Cu-10Bi-xCr Composite Solders
2.2. Soldering of Sn-0.7Cu-10Bi-xCr Composite Solders
2.3. Melting Point Test
2.4. Wetting Test
3. Results and Discussion
3.1. Melting Point of Composite Solders
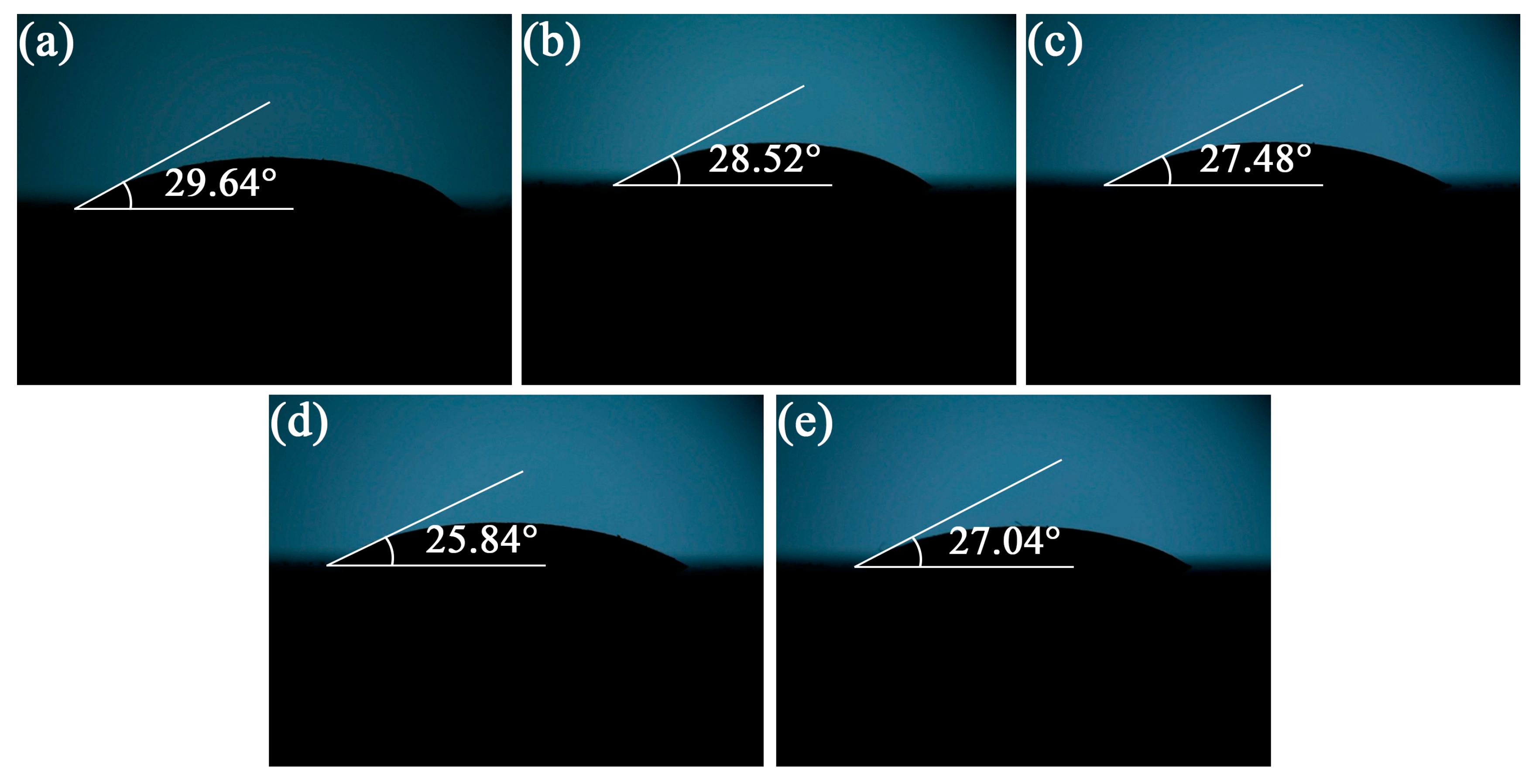
3.2. Wettability of Composite Solders
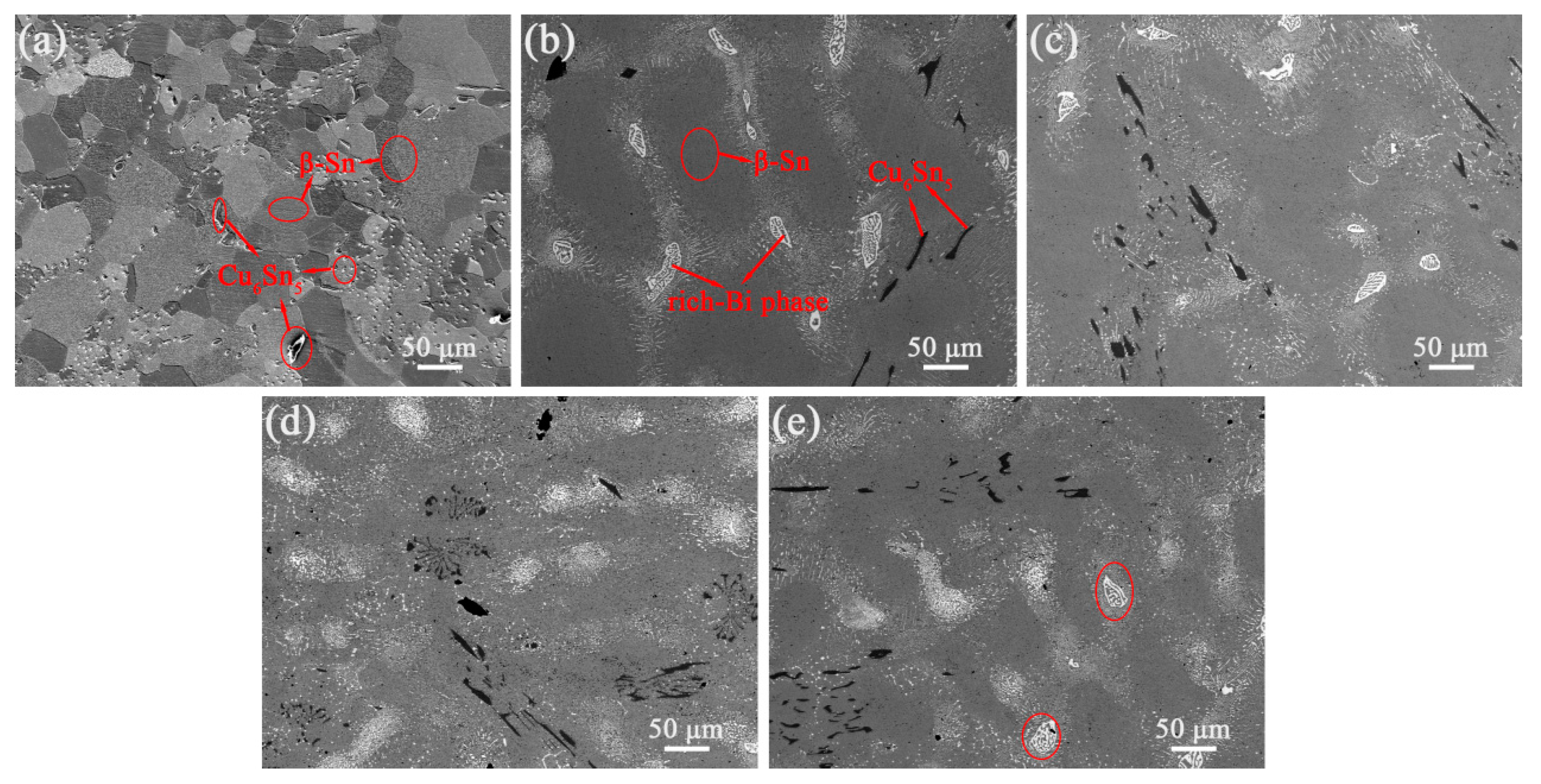
3.3. Microstructure of Composite Solders
3.4. Soldering Interface of Composite Solders
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, H.; Wei, F.; Lin, C.; Shu, T.; Sui, Y.; Qi, J.; Zhang, X. Thermal stability of intermetallic compounds at Sn-0.7Cu-10Bi-xNi/Co interface during reflows. Mater. Lett. 2019, 254, 69–72. [Google Scholar] [CrossRef]
- Nobari, A.H.; Maalekian, M.; Seelig, K.; Pekguleryuz, M. Effect of Ag, Ni and Bi Additions on Solderability of Lead-Free Solders. J. Electron. Mater. 2017, 46, 4076–4084. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, L.; Liu, Z.Q.; Sun, L.; Long, W.M.; He, P.; Xiong, M.Y.; Zhao, M. Reliability issues of lead-free solder joints in electronic devices. Sci. Technol. Adv. Mater. 2019, 20, 876–901. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, L.; Liu, Z.Q.; Xiong, M.Y.; Sun, L. Structure and properties of Sn-Cu lead-free solders in electronics packaging. Sci. Technol. Adv. Mater. 2019, 20, 421–444. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wei, F.; Lin, C.; Shu, T.; Sui, Y.; Qi, J. Growth and evolution kinetics of intermetallic compounds in Sn-0.7Cu-10Bi-0.15Co/Cu interface. Mater. Res. Express. 2019, 6, 0965d2. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, F.; Song, Z. Transient Soldering Reaction Mechanisms of SnCu Solder on CuNi Nano Conducting Layer and Fracture Behavior of the Joint Interfaces. J. Electron. Mater. 2020, 49, 3383–3390. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Liu, Z.; Jiu, Y.; Zhang, S.; Geng, J.; Chen, X.; Wu, S.; He, P.; Long, W. Corrosion behavior of Sn-based lead-free solder alloys: A review. J. Mater. Sci. Mater. Electron. 2020, 31, 9076–9090. [Google Scholar] [CrossRef]
- Lu, G.; Lin, B.; Gao, Z.; Li, Y.; Wei, F. Effect of Ni-MOF Derivatives on the Electrochemical Corrosion Behavior of Sn-0.7Cu Solders. Metals 2022, 12, 1172. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Z.; Hu, A.; Li, M. Formation of SnAg solder bump by multilayer electroplating. Microelectron. Eng. 2013, 106, 33–37. [Google Scholar] [CrossRef]
- Kunwar, A.; Ma, H.; Ma, H.; Sun, J.; Zhao, N.; Huang, M. On the increase of intermetallic compound’s thickness at the cold side in liquid Sn and SnAg solders under thermal gradient. Mater. Lett. 2016, 172, 211–215. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, J.; Shang, J.; Song, X. Highly solderability of FeP film in contact with SnAgCu solder. J. Alloys Compd. 2020, 818, 152900. [Google Scholar] [CrossRef]
- Alghoul, T.; Wentlent, L.; Sivasubramony, R.; Greene, C.; Thompson, P.; Borgesen, P. Effects of Thermal Cycling on Creep of SnAgCu Solder Joints. IEEE Trans. Compon. Packag. Manuf. Technol. 2019, 9, 888–894. [Google Scholar] [CrossRef]
- Gancarz, T. The effect of aging temperature on the phenomena occurring at the interface of solder SnZn with Na on Cu substrate. Mater. Lett. 2016, 171, 187–190. [Google Scholar] [CrossRef]
- Ren, G.; Wilding, I.J.; Collins, M.N. Alloying influences on low melt temperature SnZn and SnBi solder alloys for electronic interconnections. J. Alloys Compd. 2016, 665, 251–260. [Google Scholar] [CrossRef]
- Lee, K.-O.; Morris, J.W.; Hua, F. Martensitic Transformation in Sn-Rich SnIn Solder Joints. J. Electron. Mater. 2011, 41, 336–351. [Google Scholar] [CrossRef]
- Liu, J.-C.; Wang, Z.-H.; Xie, J.-Y.; Ma, J.-S.; Shi, Q.-Y.; Zhang, G.; Suganuma, K. Effects of intermetallic-forming element additions on microstructure and corrosion behavior of Sn–Zn solder alloys. Corros. Sci. 2016, 112, 150–159. [Google Scholar] [CrossRef]
- Qu, D.; Li, C.; Bao, L.; Kong, Z.; Duan, Y. Structural, electronic, and elastic properties of orthorhombic, hexagonal, and cubic Cu3Sn intermetallic compounds in Sn–Cu lead-free solder. J. Phys. Chem. Solids 2020, 138, 109253. [Google Scholar] [CrossRef]
- Chukka, R.N.; Telu, S.; NRMR, B.; Chen, L. A novel method of reducing melting temperatures in SnAg and SnCu solder alloys. J. Mater. Sci. Mater. Electron. 2011, 22, 281–285. [Google Scholar] [CrossRef]
- Yan, Y.-F.; Zhu, J.-H.; Chen, F.-X.; He, J.-G.; Yang, D.-X. Creep behavior on Ag particle reinforced SnCu based composite solder joints. Trans. Nonferrous Met. Soc. China 2006, 16, 1116–1120. [Google Scholar] [CrossRef]
- Chen, G.; Wu, F.; Liu, C.; Silberschmidt, V.V.; Chan, Y.C. Microstructures and properties of new Sn–Ag–Cu lead-free solder reinforced with Ni-coated graphene nanosheets. J. Alloys Compd. 2016, 656, 500–509. [Google Scholar] [CrossRef]
- Xiong, M.-Y.; Zhang, L. Interface reaction and intermetallic compound growth behavior of Sn-Ag-Cu lead-free solder joints on different substrates in electronic packaging. J. Mater. Sci. 2018, 54, 1741–1768. [Google Scholar] [CrossRef]
- Roman, K.; Robert, A.; Maros, M.; Michal, C. Comparison study of SAC405 and SAC405 + 0.1% Al lead free solders. Solder. Surf. Mt. Technol. 2013, 25, 175–183. [Google Scholar]
- Kang, Y.; Choi, J.-J.; Kim, D.-G.; Shim, H.-W. The Effect of Bi and Zn Additives on Sn-Ag-Cu Lead-Free Solder Alloys for Ag Reduction. Metals 2022, 12, 1245. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, Z.-C.; Gao, L.-Y.; Liu, Z.-Q. Effect of Bi addition on the shear strength and failure mechanism of low-Ag lead-free solder joints. J. Mater. Sci. Mater. Electron. 2021, 32, 2172–2186. [Google Scholar] [CrossRef]
- Huang, H.; Chen, B.; Hu, X.; Jiang, X.; Li, Q.; Che, Y.; Zu, S.; Liu, D. Research on Bi contents addition into Sn–Cu-based lead-free solder alloy. J. Mater. Sci. Mater. Electron. 2022, 33, 15586–15603. [Google Scholar] [CrossRef]
- Rashidi, R.; Naffakh-Moosavy, H. Metallurgical, physical, mechanical and oxidation behavior of lead-free chromium dissolved Sn–Cu–Bi solders. J. Mater. Res. Technol. 2021, 13, 1805–1825. [Google Scholar] [CrossRef]
- Song, Q.; Yang, W.; Li, Y.; Mao, J.; Qin, W.; Zhan, Y. Interfacial reaction and mechanical properties of Sn58Bi-XCr solder joints under isothermal aging conditions. Vacuum 2021, 194, 110559. [Google Scholar] [CrossRef]
- Son, J.; Yu, D.-Y.; Kim, M.-S.; Ko, Y.-H.; Byun, D.-J.; Bang, J. Nucleation and Morphology of Cu6Sn5 Intermetallic at the Interface between Molten Sn-0.7Cu-0.2Cr Solder and Cu Substrate. Metals 2021, 11, 210. [Google Scholar] [CrossRef]
- Bang, J.; Yu, D.-Y.; Yang, M.; Ko, Y.-H.; Yoon, J.-W.; Nishikawa, H.; Lee, C.-W. Improvement in Thermomechanical Reliability of Low Cost Sn-Based BGA Interconnects by Cr Addition. Metals 2018, 8, 586. [Google Scholar] [CrossRef]
- Ju, Y.; Yamauchi, H.; Oshima, H.; Tanaka, K. Reflow of lead-free solder by microwave heating. Mater. Struct. Integr. 2014, 8, 32. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, L.; Gao, L.-L.; Song, X.-G.; He, P. Recent advances on SnBi low-temperature solder for electronic interconnections. J. Mater. Sci. Mater. Electron. 2021, 32, 22731–22759. [Google Scholar] [CrossRef]
- Soares, D.; Leitão, H.; Lau, C.S.; Teixeira, J.C.; Ribas, L.; Alves, R.; Teixeira, S.; Cerqueira, M.F.; Macedo, F. Effect of the Soldering Atmosphere on the Wettability Between Sn4.0Ag0.5Cu (in wt.%) Lead-Free Solder Paste and Various Substrates. J. Mater. Eng. Perform. 2018, 27, 5011–5017. [Google Scholar] [CrossRef]
- Qu, M.; Cao, T.; Cui, Y.; Liu, F.; Jiao, Z. Effect of nano-ZnO particles on wettability, interfacial morphology and growth kinetics of Sn–3.0Ag–0.5Cu–xZnO composite solder. J. Mater. Sci. Mater. Electron. 2019, 30, 19214–19226. [Google Scholar] [CrossRef]
- Li, Z.H.; Tang, Y.; Guo, Q.W.; Li, G.Y. A diffusion model and growth kinetics of interfacial intermetallic compounds in Sn-0.3Ag-0.7Cu and Sn-0.3Ag-0.7Cu-0.5CeO2 solder joints. J. Alloys Compd. 2020, 818, 152893. [Google Scholar] [CrossRef]
- He, M.; Acoff, V.L. Effect of Bi on the Interfacial Reaction between Sn-3.7Ag-xBi Solders and Cu. J. Electron. Mater. 2007, 37, 288–299. [Google Scholar] [CrossRef]
- Chen, X.; Hu, A.; Li, M.; Mao, D. Effect of a trace of Cr on intermetallic compound layer for tin–zinc lead-free solder joint during aging. J. Alloys Compd. 2009, 470, 429–433. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, P.; Lu, Z.; Zhang, X. Sn-0.7Cu-10Bi Solder Modification Strategy by Cr Addition. Metals 2022, 12, 1768. https://doi.org/10.3390/met12101768
Han P, Lu Z, Zhang X. Sn-0.7Cu-10Bi Solder Modification Strategy by Cr Addition. Metals. 2022; 12(10):1768. https://doi.org/10.3390/met12101768
Chicago/Turabian StyleHan, Pin, Zhenpo Lu, and Xuping Zhang. 2022. "Sn-0.7Cu-10Bi Solder Modification Strategy by Cr Addition" Metals 12, no. 10: 1768. https://doi.org/10.3390/met12101768
APA StyleHan, P., Lu, Z., & Zhang, X. (2022). Sn-0.7Cu-10Bi Solder Modification Strategy by Cr Addition. Metals, 12(10), 1768. https://doi.org/10.3390/met12101768




