Evolution and Evaluation of Aesthetic Properties in Weathering Steel Accelerated Patinas: The Role of Lepidocrocite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Color Measurements
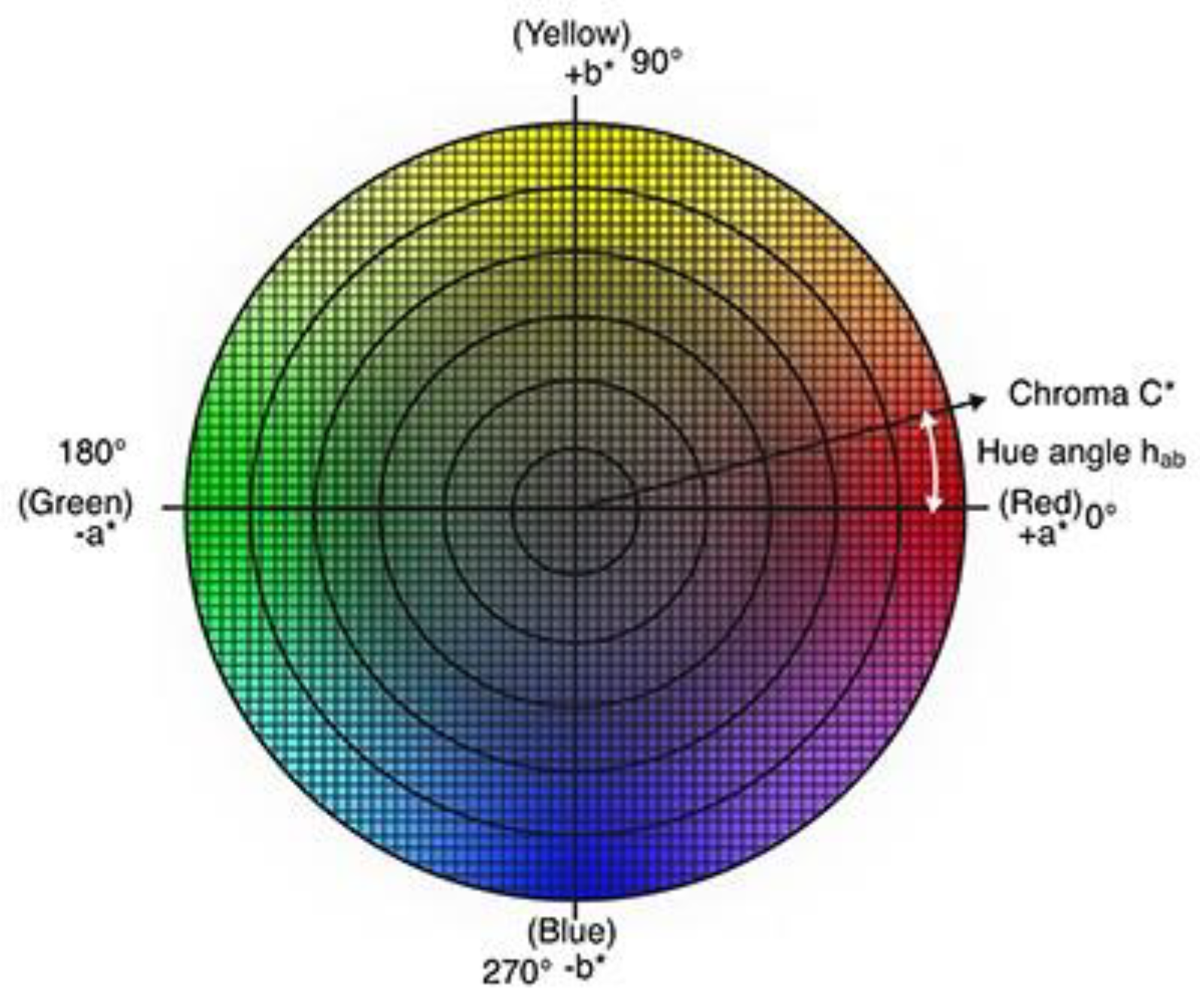
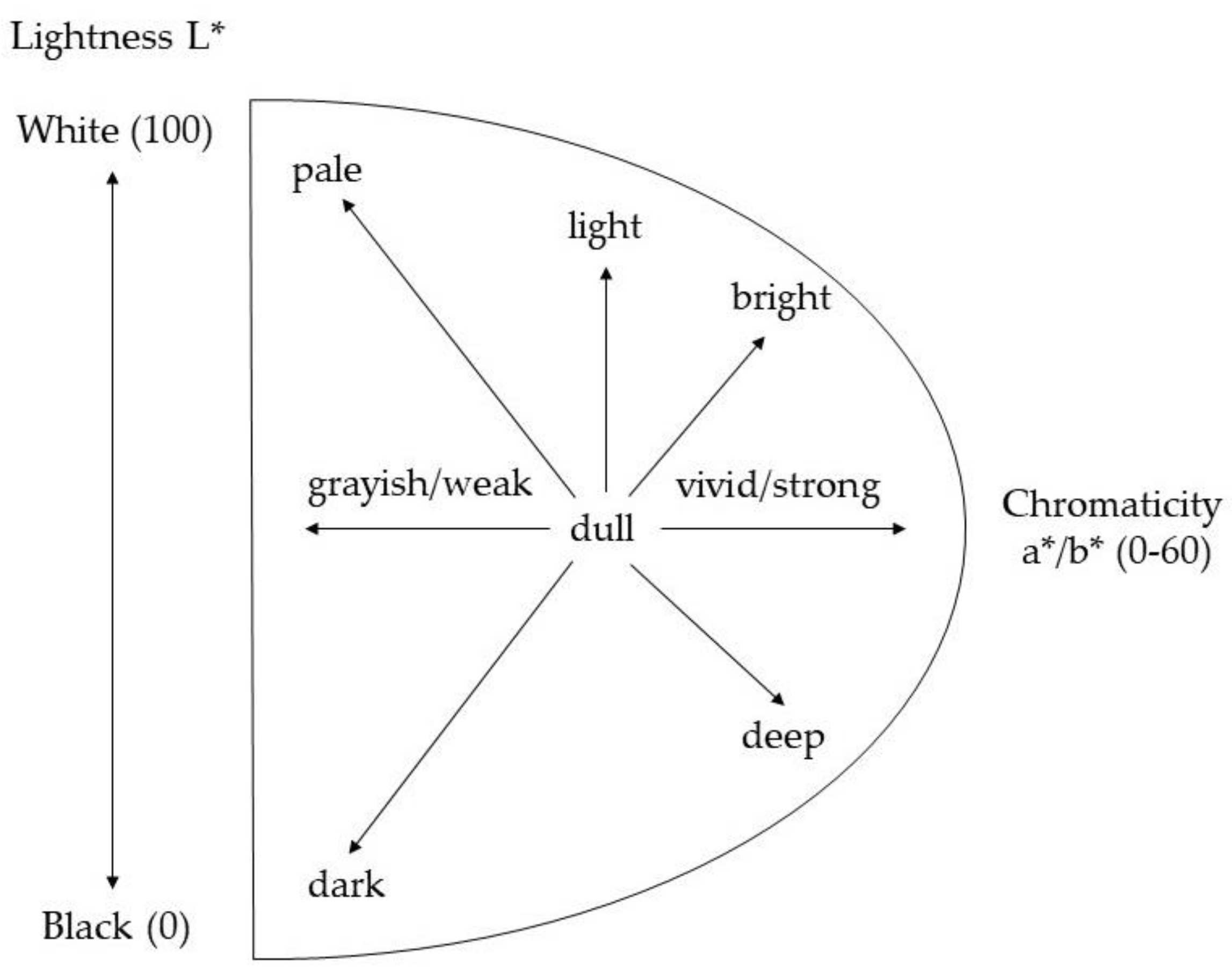
2.3. Evaluation of Color Differences and Similarities: ΔE and Hue
2.4. Micro-Raman Analysis
2.5. Optical Microscope
2.6. X-ray Diffraction
3. Results
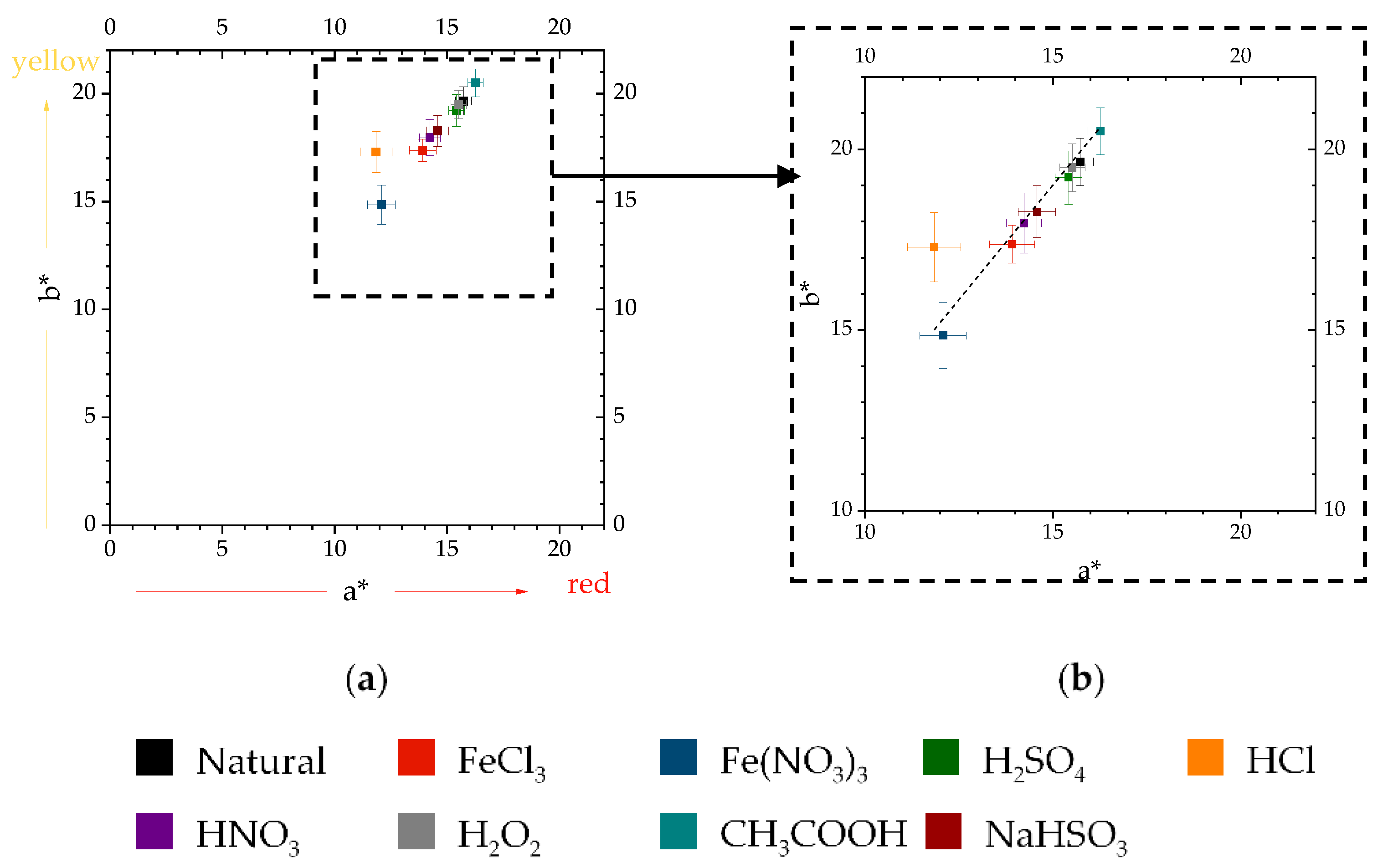
3.1. Color Analysis
3.1.1. Color Evolution of the Natural Patina
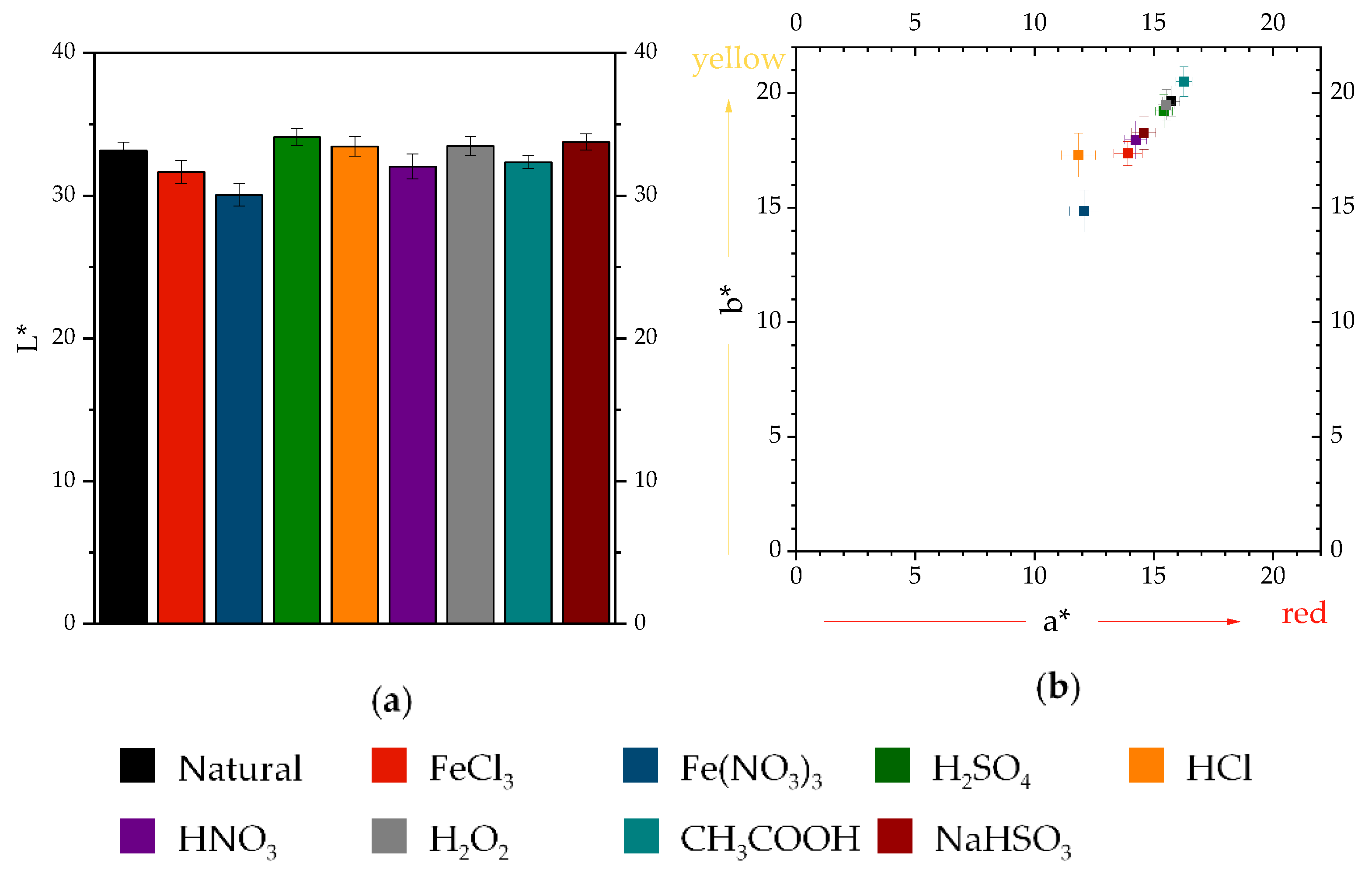
3.1.2. Color Evolution of Accelerated Patinas
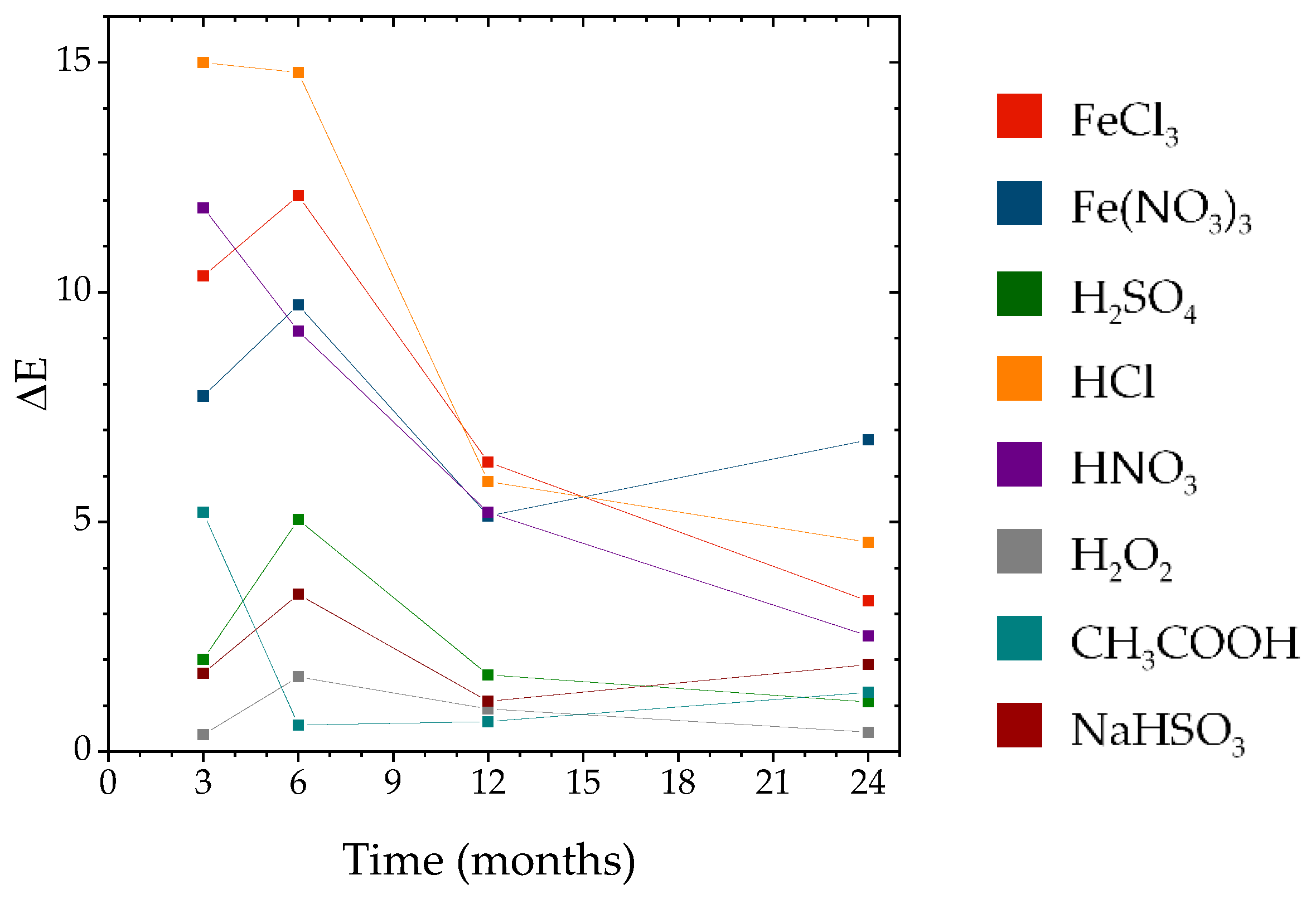
3.1.3. Color Differences and Similarities
3.2. Raman Spectroscopy
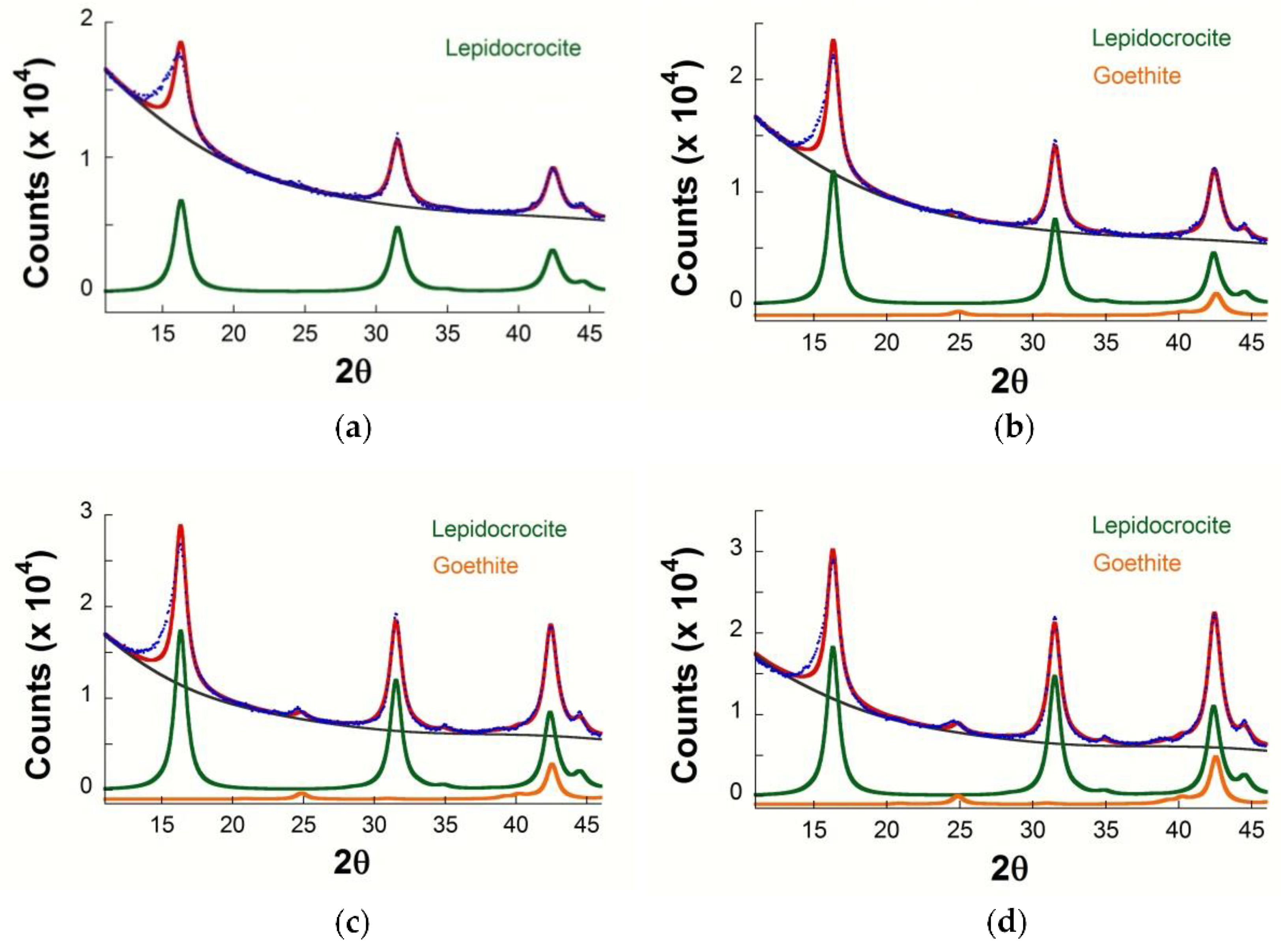
3.3. X-ray Diffraction
4. Discussion
4.1. Aesthetic Properties Evolution of the Natural Patina
4.2. Aesthetic Properties Evolution of Accelerated Patinas
4.3. Evaluation of Color Differences and Similarities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murata, T.T. Weathering steel. In Uhlig’s Corrosion Handbook, 2nd ed.; Revie, R.W., Ed.; John Wiley & Sons: Toronto, ON, Canada, 2000; pp. 621–632. [Google Scholar]
- Morcillo, M.; Díaz, I.; Chico, B.; Cano, H.; de la Fuente, D. Weathering steels: From empirical development to scientific design. A review. Corros. Sci. 2014, 83, 6–31. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liu, J.; Wu, L.; Han, R.; Sun, Y. Study of the corrosion behavior of weathering steels in atmospheric environments. Corros. Sci. 2013, 67, 1–10. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; Díaz, I.; de la Fuente, D.; Alcántara, J.; Simancas, J. La Corrosión Atmosférica del Acero al Carbono en Ambientes Costeros, 1st ed.; Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2018. [Google Scholar]
- Cook, D.C. Spectroscopic identification of protective and non-protective corrosion coatings on steel structures in marine environments. Corros. Sci. 2005, 47, 2550–2570. [Google Scholar] [CrossRef]
- Matsushima, I.; Ishizu, Y.; Ueno, T.; Kanazashi, M.; Horikawa, K. Effect of Structural and Environmental Factors in the Practical Use of Low-Alloy Weathering Steel. Corros. Eng. 1974, 23, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Morcillo, M.; Díaz, I.; Cano, H.; Chico, B.; de la Fuente, D. Atmospheric corrosion of weathering steels. Overview for engineers. Part I: Basic concepts. Constr. Build. Mater. 2019, 213, 723–737. [Google Scholar] [CrossRef]
- Morcillo, M.; Díaz, I.; Cano, H.; Chico, B.; de la Fuente, D. Atmospheric corrosion of weathering steels. Overview for engineers. Part II: Testing, inspection, maintenance. Constr. Build. Mater. 2019, 222, 750–765. [Google Scholar] [CrossRef]
- Bernardi, E.; Vassura, I.; Raffo, S.; Nobili, L.; Passarini, F.; de la Fuente, D.; Morcillo, M. Influence of inorganic anions from atmospheric depositions on weathering steel corrosion and metal release. Constr. Build. Mater. 2020, 236, 117515. [Google Scholar] [CrossRef]
- Scott, J. Conservation of weathering steel sculpture. In Proceedings of the Saving the Twentieth Century: The Conservation of Modern Materials, Ottawa, ON, Canada, 15–20 September 1991. [Google Scholar]
- Gallagher, W.P. The Performance of Weathering Steel in Sculpture. Mater. Perform. 2001, 40, 58–61. [Google Scholar]
- Golfomitsou, S. The role of conservation in new contemporary art installations in new contexts: The case of Richard Serra’s East–West/West–East in Qatar. Stud. Conserv. 2016, 61, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Crespo, A. Estudio de Herrumbres Aceleradas en Escultura Contemporánea de Acero Patinable. Ph.D. Thesis, Carlos III University, Madrid, Spain, 2020. [Google Scholar]
- Pullen, D.; Heuman, J. Modern and Contemporary Outdoor Sculpture Conservation: Challenges and Advances. GCI Newsl. 2007, 22, 1–7. [Google Scholar]
- Chemello, C.; Cano, E.; Crespo, A.; Mardikian, P.; Patterson, C. Conservation and Investigation of Cometh the Sun: A Monumental Weathering Steel Sculpture. In Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Neuchatel, Switzerland, 2–6 September 2019. [Google Scholar]
- Aramendia, J.; Gomez-Nubla, L.; Castro, K.; Madariaga, J.M. Structural and chemical analyzer system for the analysis of deposited airborne particles and degradation compounds present on the surface of outdoor weathering steel objects. Microchem. J. 2015, 123, 267–275. [Google Scholar] [CrossRef]
- Aramendia, J.; Gomez-Nubla, L.; Castro, K.; Martínez-Arkarazo, I.; Vega, D.; De Heredia, A.S.L.; De Opakua, A.G.I.; Madariaga, J.M. Portable Raman study on the conservation state of four CorTen steel-based sculptures by Eduardo Chillida impacted by urban atmospheres. J. Raman Spectrosc. 2012, 43, 1111–1117. [Google Scholar] [CrossRef]
- Aramendia, J.; Gomez-Nubla, L.; Bellot-Gurlet, L.; Castro, K.; Paris, C.; Colomban, P.; Madariaga, J.M. Protective ability index measurement through Raman quantification imaging to diagnose the conservation state of weathering steel structures. J. Raman Spectrosc. 2014, 45, 1076–1084. [Google Scholar] [CrossRef]
- Angelini, E.; Grassini, S.; Parvis, M.; Zucchi, F. An in situ investigation of the corrosion behavior of a weathering steel work of art. Surf. Interface Anal. 2012, 44, 942–946. [Google Scholar] [CrossRef]
- Angelini, E.; Assante, D.; Grassini, S.; Parvis, M. EIS measurements for the assessment of the conservation state of metallic works of art. Int. J. Circuits Syst. Signal Process. 2014, 8, 240–245. [Google Scholar]
- Crespo, A.; Ramírez-Barat, B.; Díaz, I.; Cano, E. Electrochemical evaluation of the patina of the weathering steel sculpture Once Modulo. Ge-Conservacion 2020, 17, 153–159. [Google Scholar] [CrossRef]
- Daukšys, M.; Bytautaitė, E.; Mockienė, J.; Nizevičienė, D. NaCl and Na2SO4 solution effect on weathering steel visual appearance when the ambient temperature changes cyclically. Cogent Eng. 2019, 6, 1659124. [Google Scholar] [CrossRef]
- Travassos, S.J.; Tomachuk, C.R.; de Melo, H.G. EIS investigation and patina characterization of weathering steel exposed to each of the four seasons in the São Paulo metropolitan area. Electrochim. Acta 2019, 325, 134885. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; Díaz, I.; Cano, H.; de la Fuente, D. Atmospheric corrosion data of weathering steels. A review. Corros. Sci. 2013, 77, 6–24. [Google Scholar] [CrossRef] [Green Version]
- Díaz, I.; Cano, H.; Crespo, D.; Chico, B.; de la Fuente, D.; Morcillo, M. Atmospheric corrosion of ASTM A-242 and ASTM A-588 weathering steels in different types of atmosphere. Corros. Eng. Sci. Technol. 2018, 53, 449–459. [Google Scholar] [CrossRef]
- McLaren, K. The Development of the CIE 1976 (L*a*b*) Uniform Colour Space and Colour-difference Formula. J. Soc. Dye Colour. 1976, 92, 338–341. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venâncio Silva, S.; De Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Criado, M.; Martínez-Ramirez, S.; Bastidas, J.M. A Raman spectroscopy study of steel corrosion products in activated fly ash mortar containing chlorides. Constr. Build. Mater. 2015, 96, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Villars, P.; Cenzual, K. Pearson’s Crystal Data: Crystal Structure Database for Inorganic Compounds; ASM International: Materials Park, OH, USA, 2010. [Google Scholar]
- Balzar, D.; Ledbetter, H. Voigt-function modeling in Fourier analysis of size- and strain-broadened X-ray diffraction peaks. J. Appl. Crystallogr. 1993, 26, 97–103. [Google Scholar] [CrossRef]
- Morcillo, M.; de La Fuente, D.; Díaz, I.; Cano, H. Atmospheric corrosion of mild Steel. Rev. Metal. 2011, 47, 426–444. [Google Scholar] [CrossRef] [Green Version]
- de la Fuente, D.; Alcántara, J.; Chico, B.; Díaz, I.; Jiménez, J.A.; Morcillo, M. Characterisation of rust surfaces formed on mild steel exposed to marine atmospheres using XRD and SEM/Micro-Raman techniques. Corros. Sci. 2016, 110, 253–264. [Google Scholar] [CrossRef]
- Alcántara, J.; Chico, B.; Simancas, J.; Díaz, I.; de la Fuente, D.; Morcillo, M. An attempt to classify the morphologies presented by different rust phases formed during the exposure of carbon steel to marine atmospheres. Mater. Charact. 2016, 118, 65–78. [Google Scholar] [CrossRef]
- Crespo, A.; Díaz, I.; Neff, D.; Llorente, I.; Martínez-Ramírez, S.; Cano, E. Effect of Sulfuric Acid Patination Treatment on Atmospheric Corrosion of Weathering Steel. Metals 2020, 10, 591. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; John Wiley & Sons: Weinheim, Germany, 2003. [Google Scholar]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory: PREPARATION and Characterization; John Wiley & Sons: Weinheim, Germany, 2000. [Google Scholar]
- Scheinost, A.C.; Chavernas, A.; Barrón, V.; Torrent, J. Use and limitations of second-derivative diffuse reflectance spectroscopy in the visible to near-infrared range to identify and quantify Fe oxide minerals in soils. Clays Clay Miner. 1998, 46, 528–536. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Schwertmann, U. Color identification of iron oxides and hydroxysulfates: Use and limitations. Soil Sci. Soc. Am. J. 1999, 63, 1463–1471. [Google Scholar] [CrossRef]
- Elias, E.; Chartier, C.; Prévot, G.; Garay, H.; Vignaud, C. The colour of ochres explained by their composition. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2006, 127, 70–80. [Google Scholar] [CrossRef]
- Fasiska, E.J. Structural aspects of the oxides and oxyhydrates of iron. Corros. Sci. 1967, 7, 833–839. [Google Scholar] [CrossRef]
- Alcántara, J.; de la Fuente, D.; Chico, B.; Simancas, J.; Díaz, I.; Morcillo, M. Marine atmospheric corrosion of carbon steel: A review. Materials 2014, 10, 406. [Google Scholar] [CrossRef] [Green Version]
- Cano, H.; Neff, D.; Morcillo, M.; Dillmann, P.; Diaz, I.; de la Fuente, D. Characterization of corrosion products formed on Ni 2.4 wt%-Cu 0.5 wt%-Cr 0.5 wt% weathering steel exposed in marine atmospheres. Corros. Sci. 2014, 87, 438–451. [Google Scholar] [CrossRef] [Green Version]
- Ghelardi, E.; Degano, I.; Colombini, M.P.; Mazurek, J.; Schilling, M.; Khanjian, H.; Learner, T. A multi-analytical study on the photochemical degradation of synthetic organic pigments. Dye. Pigment. 2015, 123, 396–403. [Google Scholar] [CrossRef]
- Mahy, M.; Van Eycken, L.; Oosterlinck, A. Evaluation of Uniform Color Spaces Developed after the Adoption of CIELAB and CIELUV. Color Res. Appl. 1994, 19, 105–121. [Google Scholar]
- Sharma, G.; Wu, W.; Dalal, E.N. The CIEDE2000 color-difference formula: Implementation notes, supplementary test data, and mathematical observations. Color Res. Appl. 2005, 30, 21–30. [Google Scholar] [CrossRef]



| Element | Fe | C | Si | Mn | P | S | Cu | Cr | Ni | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| % | 97.453 | 0.091 | 0.60 | 0.42 | 0.109 | 0.0123 | 0.302 | 0.807 | 0.181 | 0.025 |
| Treatment | Molarity (mol/L) |
|---|---|
| FeCl3 | 1.47 |
| Fe(NO3)3 | 0.99 |
| H2SO4 | 1.87 |
| HCl | 1.01 |
| HNO3 | 1.09 |
| H2O2 | 9.70 |
| CH3COOH | 1.74 |
| NaHSO3 | 1.42 |
| Time | L* | L* Error | a* | a* Error | b* | b* Error |
|---|---|---|---|---|---|---|
| 3 months | 33.4 | 0.3 | 15.4 | 0.2 | 23.7 | 0.4 |
| 6 months | 28.7 | 0.5 | 17.1 | 0.3 | 23.3 | 0.6 |
| 12 months | 33.1 | 0.4 | 14.8 | 0.2 | 19.6 | 0.5 |
| 24 months | 33.2 | 0.6 | 15.7 | 0.3 | 19.7 | 0.7 |
| Months | Slope | Standard Error | Pearson’s r |
|---|---|---|---|
| Zero time | 1.32 | 0.13 | 0.9692 |
| 3 | 1.32 | 0.08 | 0.9859 |
| 6 | 1.28 | 0.06 | 0.9922 |
| 12 | 1.26 | 0.03 | 0.9981 |
| 24 | 1.27 | 0.02 | 0.9990 |
| Treatment | Zero Time | 3 Months | 6 Months | 12 Months | 24 Months |
|---|---|---|---|---|---|
| Natural | - | L | L | L | L |
| FeCl3 | L, A | L, A | L, A | L, A | L, A |
| Fe(NO3)3 | L, G | L, G | L, G | L, G | L, G |
| H2SO4 | L | L | L | L | L |
| HCl | L, A | L, A | L, A | L, A | L |
| HNO3 | L, G | L, G | L, G | L | L |
| H2O2 | L | L | L | L | L |
| CH3COOH | L | L | L | L | L |
| NaHSO3 | L | L | L | L | L |
| Classification | 3 Months | 6 Months | 12 Months | 24 Months |
|---|---|---|---|---|
| Indistinguishable | ||||
| (ΔE ≤ 1) | H2O2 | H2O2 | H2O2, CH3COOH | H2O2 |
| Similar | ||||
| (1 < ΔE ≤ 3) | H2SO4, NaHSO3 | CH3COOH | H2SO4, NaHSO3 | H2SO4, HNO3, CH3COOH, NaHSO3 |
| Distinguishable | ||||
| (3 < ΔE ≤ 5) | NaHSO3 | FeCl3, HCl | ||
| Different | ||||
| (5 < ΔE) | FeCl3, Fe(NO3)3, HCl, HNO3, CH3COOH | FeCl3, Fe(NO3)3, H2SO4, HCl, HNO3 | FeCl3, Fe(NO3)3, HCl, HNO3 | Fe(NO3)3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespo, A.; Pérez, G.; Jiménez, J.A.; Llorente, I.; Martínez-Ramírez, S.; Cano, E.; Díaz, I. Evolution and Evaluation of Aesthetic Properties in Weathering Steel Accelerated Patinas: The Role of Lepidocrocite. Metals 2022, 12, 977. https://doi.org/10.3390/met12060977
Crespo A, Pérez G, Jiménez JA, Llorente I, Martínez-Ramírez S, Cano E, Díaz I. Evolution and Evaluation of Aesthetic Properties in Weathering Steel Accelerated Patinas: The Role of Lepidocrocite. Metals. 2022; 12(6):977. https://doi.org/10.3390/met12060977
Chicago/Turabian StyleCrespo, Ana, Gloria Pérez, José A. Jiménez, Irene Llorente, Sagrario Martínez-Ramírez, Emilio Cano, and Iván Díaz. 2022. "Evolution and Evaluation of Aesthetic Properties in Weathering Steel Accelerated Patinas: The Role of Lepidocrocite" Metals 12, no. 6: 977. https://doi.org/10.3390/met12060977
APA StyleCrespo, A., Pérez, G., Jiménez, J. A., Llorente, I., Martínez-Ramírez, S., Cano, E., & Díaz, I. (2022). Evolution and Evaluation of Aesthetic Properties in Weathering Steel Accelerated Patinas: The Role of Lepidocrocite. Metals, 12(6), 977. https://doi.org/10.3390/met12060977







