Corrosion Behavior of Ancient White Cast Iron Artifacts from Marine Excavations at Atmospheric Condition
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Phenomenon of Embrittlement and Pulverization
3.2. Materials and Corrosion Micromorphology of the Iron Pot
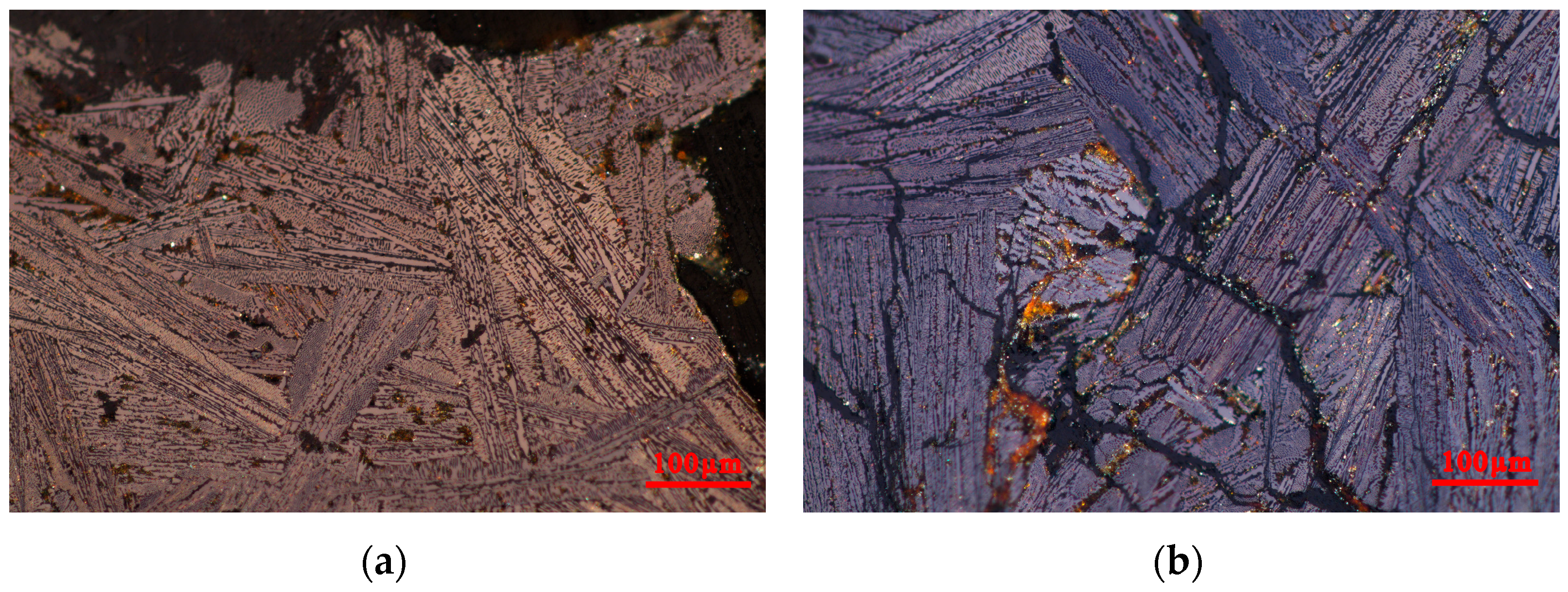
3.2.1. Materials of the Iron Pot
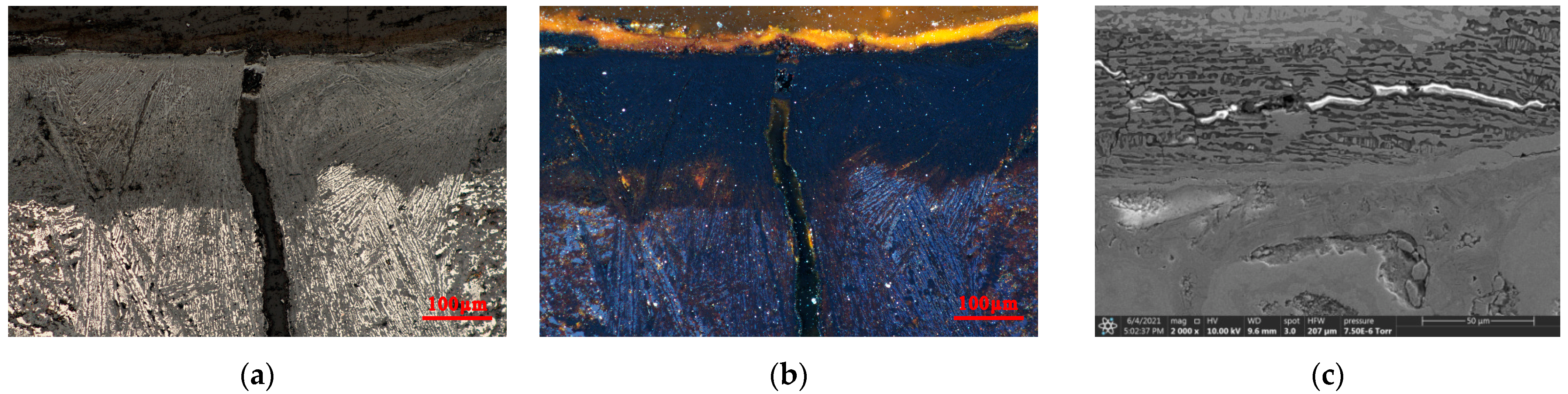
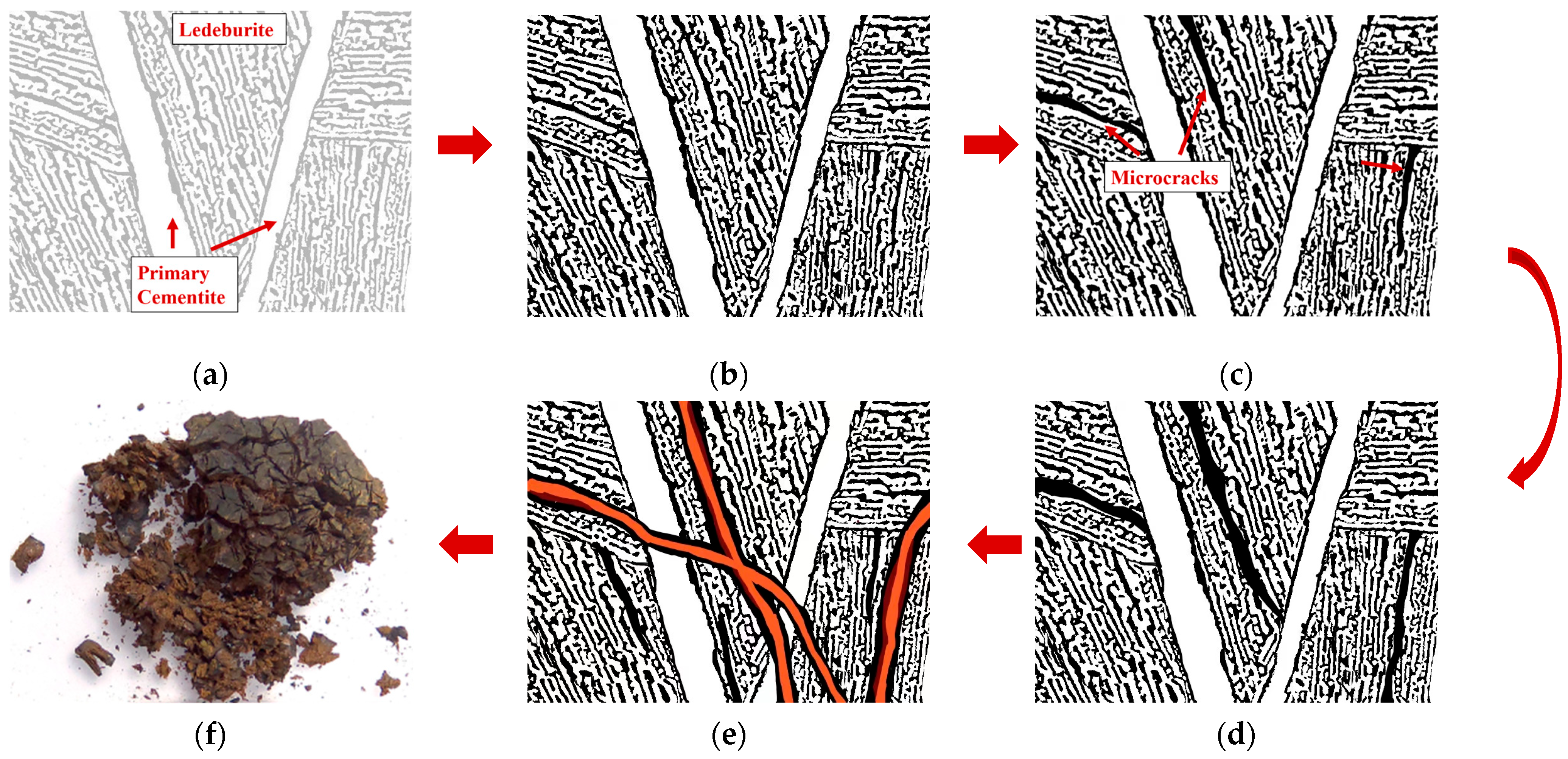
3.2.2. Micromorphology of Inner Cracks of the Iron Pot
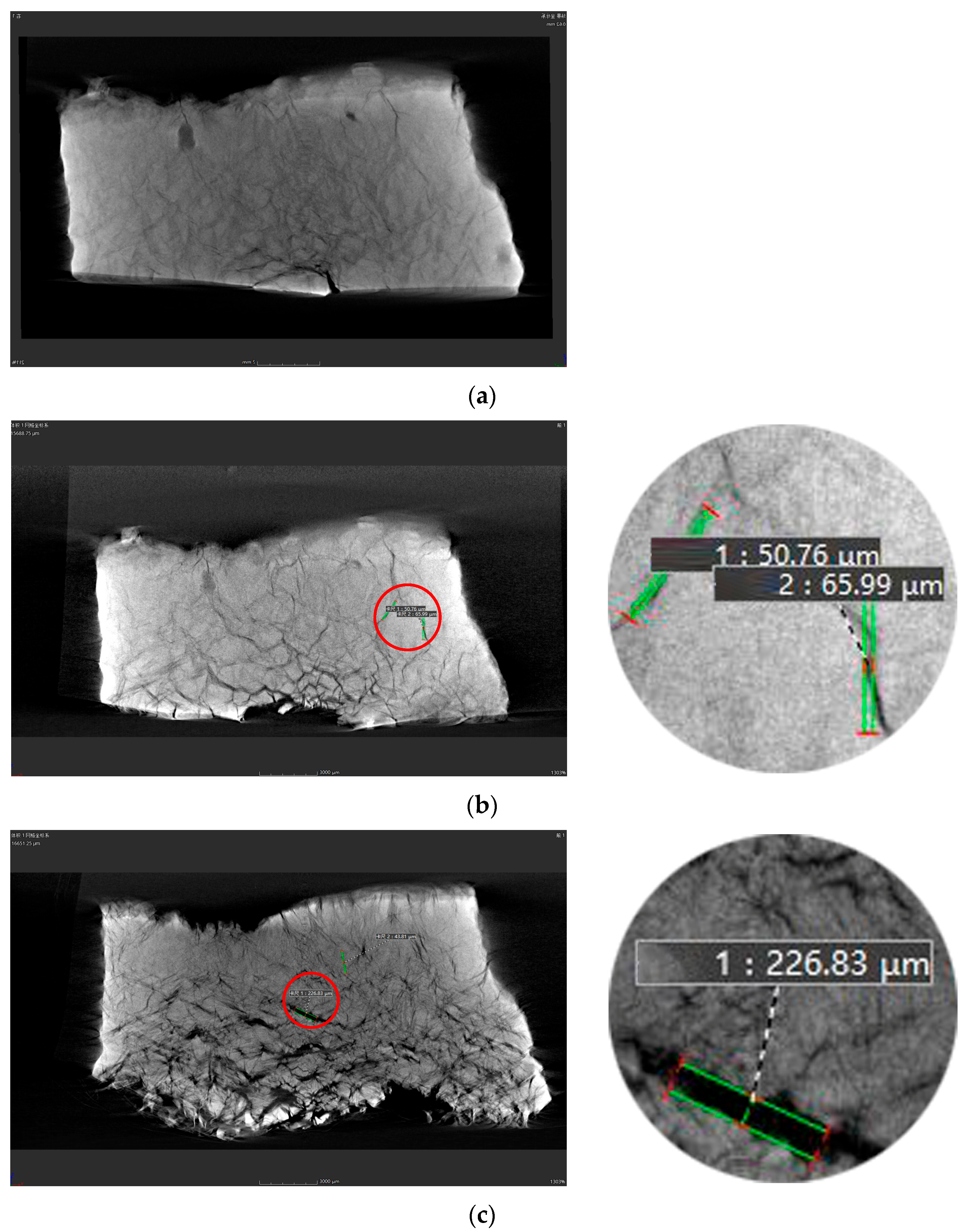
3.3. Distribution and Development of Fractures
3.4. Composition and Structure of Rust in Fractures
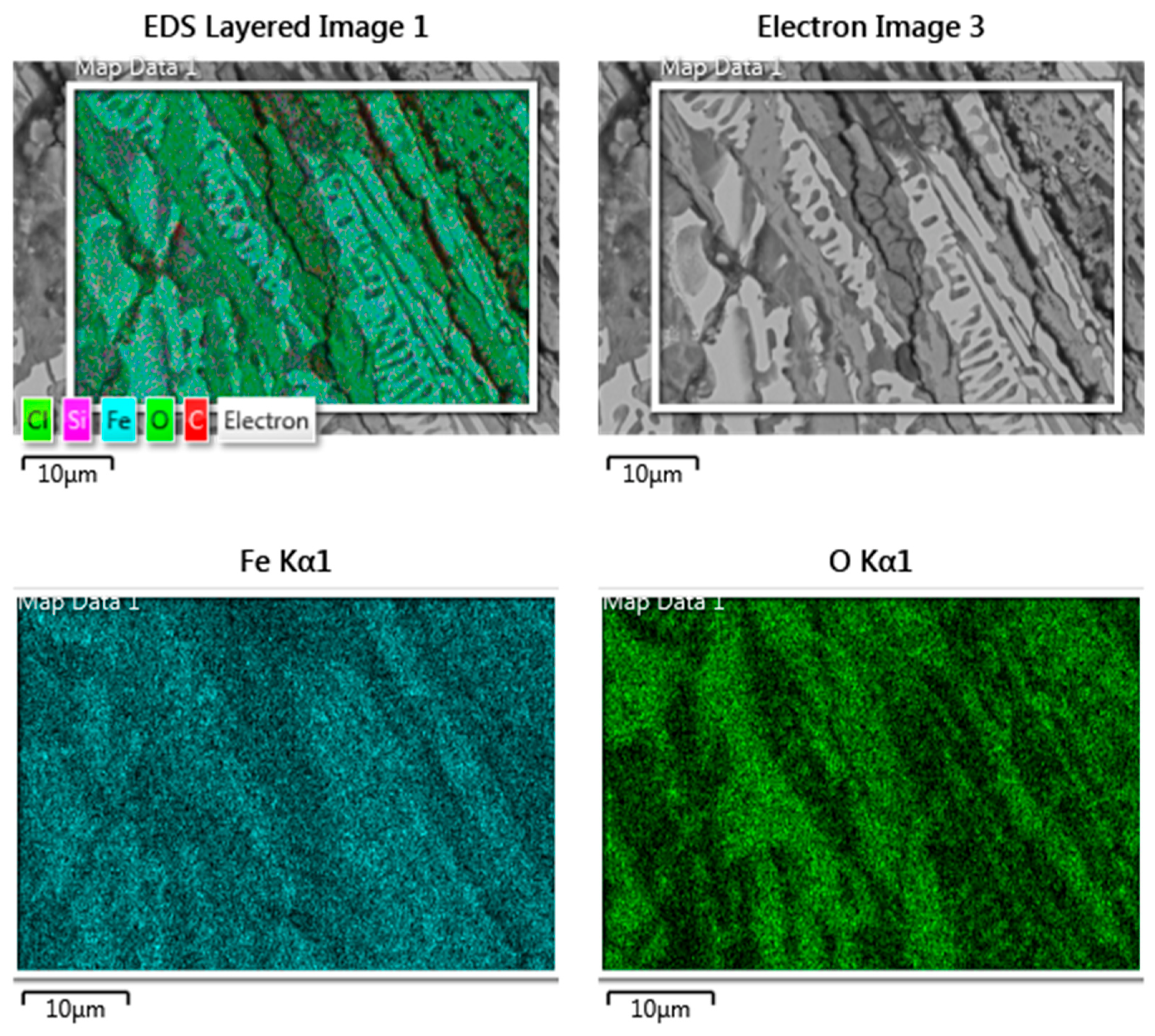
3.4.1. Component Analysis of Fractures
3.4.2. Rust Conversion in Fracture Development
3.5. Mechanism of Corrosion Embrittlement and Pulverization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dillmann, P.; Watkinson, D.; Angelini, E.; Adriaens, A. Corrosion and Conservation of Cultural Heritage Metallic Artefacts; Woodhead Publishing Limited: Cambridge, UK, 2013. [Google Scholar]
- Remazeilles, C.; Neff, D.; Bourdoiseau, J.A.; Sabot, R.; Jeannin, M.; Refait, P. Role of previously formed corrosion product layers on sulfide-assisted corrosion of iron archaeological artefacts in soil. Corros. Sci. 2017, 129, 169–178. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Zhang, L.; Zhang, L.; Wang, Q.; Wang, T. Structure of the rust layer of weathering steel in A high chloride environment: A detailed characterization via HRTEM, STEM-EDS, and FIB-SEM. Corros. Sci. 2020, 177, 108997. [Google Scholar] [CrossRef]
- Refait, P.; Memet, J.B.; Bon, C.; Sabot, R.; Genin, J.M.R. Formation of the Fe(II)-Fe(III) hydroxysulphate green rust during marine corrosion of steel. Corros. Sci. 2003, 45, 833–845. [Google Scholar] [CrossRef]
- Garcia, K.E.; Morales, A.L.; Barrero, C.A.; Greneche, J.M. New contributions to the understanding of rust layer formation in steels exposed to a total immersion test. Corros. Sci. 2006, 48, 2813–2830. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Zheng, Y.Y. Electrochemical techniques for determining corrosion rate of rusted steel in seawater. Corros. Sci. 2011, 53, 208–216. [Google Scholar] [CrossRef]
- Hoerle, S.; Mazaudier, F.; Dillmann, P.; Santarini, G. Advances in understanding atmospheric corrosion of iron. II. Mechanistic modelling of wet-dry cycles. Corros. Sci. 2004, 46, 1431–1465. [Google Scholar] [CrossRef]
- Remazeilles, C.; Neff, D.; Kergourlay, F.; Foy, E.; Conforto, E.; Guilminot, E.; Reguer, S.; Refait, P.; Dillmann, P. Mechanisms of long-term anaerobic corrosion of iron archaeological artefacts in seawater. Corros. Sci. 2009, 51, 2932–2941. [Google Scholar] [CrossRef]
- Li, N.; Chen, Y.; Shen, D. Conservation Research on Excavation Site of “Nanhai I” Shipwreck 2014–2016; Science Press: Beijing, China, 2017. [Google Scholar]
- Zhou, Y.; Wang, K.; Jin, Y.; Sun, J.; Cui, Y.; Hu, D. Chemical and microstructural comparison of the export porcelain from five different kilns excavated from Nanhai I shipwreck. Ceram. Int. 2019, 45, 12880–12887. [Google Scholar] [CrossRef]
- Wan, X.; Mao, Z.; Zhang, Z.; Li, X. Analysis of iron pans and iron nails unearthed from Nanhai I shipwreck. China Cult. Herit. Sci. Res. 2016, 2, 46–51. [Google Scholar]
- Wu, J.J.; Wang, P.; Zhang, D.; Chen, S.Q.; Sun, Y.; Wu, J.Y. Catalysis of oxygen reduction reaction by an iron-reducing bacterium isolated from marine corrosion product layers. J. Electroanal. Chem. 2016, 774, 83–87. [Google Scholar] [CrossRef]
- Dong, B.; Liu, W.; Zhang, T.; Chen, L.; Fan, Y.; Zhao, Y.; Yang, W.; Banthukul, W. Corrosion failure analysis of low alloy steel and carbon steel rebar in tropical marine atmospheric environment: Outdoor exposure and indoor test. Eng. Fail. Anal. 2021, 129, 105720. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, Z.; Chen, Y.; Nong, Y.; Yi, C. Molecular insight into iron corrosion induced by chloride and sulphate. Comput. Mater. Sci. 2022, 209, 111429. [Google Scholar] [CrossRef]
- Selwyn, L.S.; Sirois, P.J.; Argyropoulos, V. The corrosion of excavated archaeological iron with details on weeping and akaganeite. Stud. Conserv. 1999, 44, 217–232. [Google Scholar]
- Moore, J.D., III. Long-Term Corrosion Processes of Iron and Steel Shipwrecks in the Marine Environment: A Review of Current Knowledge. J. Marit. Archaeol. 2015, 10, 191–204. [Google Scholar] [CrossRef]
- Bao, C.; Jia, S.; Li, J.; Fu, Y.; Li, G. Conservation study of iron cannons excavated from tropical marine. Corros. Sci. Prot. Technol. 2016, 28, 189–192. [Google Scholar]
- Kergourlay, F.; Reguer, S.; Neff, D.; Foy, E.; Picca, F.E.; Saheb, M.; Hustache, S.; Mirambet, F.; Dillmann, P. Stabilization treatment of cultural heritage artefacts: In situ monitoring of marine iron objects dechlorinated in alkali solution. Corros. Sci. 2018, 132, 21–34. [Google Scholar] [CrossRef]
- Ouyang, W.; Cao, X.; Wang, N. A mathematical model for electrochemical chloride removal from marine cast iron artifacts. Acta Metall. Sin-Engl. 2009, 22, 91–99. [Google Scholar] [CrossRef]
- Kergourlay, F.; Remazeilles, C.; Neff, D.; Foy, E.; Conforto, E.; Guilminot, E.; Reguer, S.; Dillmann, P.; Nicot, F.; Mielcarek, F.; et al. Mechanisms of the dechlorination of iron archaeological artefacts extracted from seawater. Corros. Sci. 2011, 53, 2474–2483. [Google Scholar] [CrossRef]
- Qian, B.; Hou, B.; Zheng, M. The inhibition effect of tannic acid on mild steel corrosion in seawater wet/dry cyclic conditions. Corros. Sci. 2013, 72, 1–9. [Google Scholar] [CrossRef]
- Qi, Y.; Li, Z.; Feng, S. Study on corrosion inhibition-sealing process on iron substrate. Mater. Prot. 2019, 52, 96–99. [Google Scholar]
- Wang, H.; Zhang, H.; Liang, H.; Huo, H.; Han, B. Conservation and restoration of unearthed iron with heavy corrosion and mineralization—A case study of the conservation and restoration of iron cauldron unearthed from Weijiazhuang in Ji’nan. Jianghan Arch. 2017, 5, 108–116. [Google Scholar]
- Ashkenazi, D.; Nusbaum, I.; Shacham-Diamand, Y.; Cvikel, D.; Kahanov, Y.; Inberg, A. A method of conserving ancient iron artefacts retrieved from shipwrecks using a combination of silane self-assembled monolayers and wax coating. Corros. Sci. 2017, 123, 88–102. [Google Scholar] [CrossRef]
- Neff, D.; Reguer, S.; Bellot-Gurlet, L.; Dillmann, P.; Bertholon, R. Structural characterization of corrosion products on archaeological iron: An integrated analytical approach to establish corrosion forms. J. Raman Spectrosc. 2004, 35, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Neff, D.; Bellot-Gurlet, L.; Dillmann, P.; Reguer, S.; Legrand, L. Raman imaging of ancient rust scales on archaeological iron artefacts for long-term atmospheric corrosion mechanisms study. J. Raman Spectrosc. 2006, 37, 1228–1237. [Google Scholar] [CrossRef]
- Chen, L. Comparative Study on the Microstructure and the Volume Expansion Ratio of Corrosion Product under Different Corrosion Environments. Master’s Thesis, Shenzhen University, Shenzhen, China, 2016. [Google Scholar]



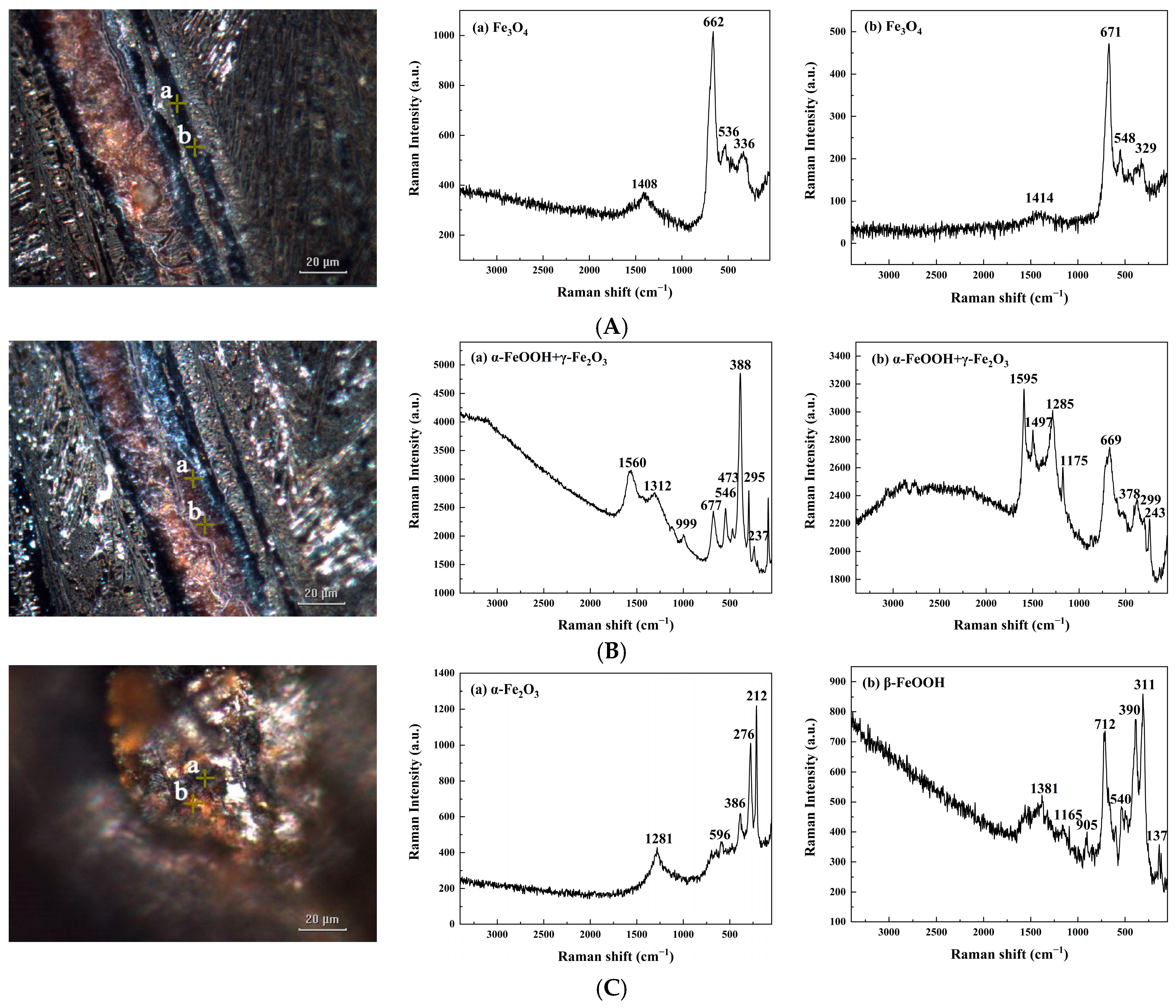
| Parameters | Fe3O4 | α-FeOOH + γ-Fe2O3 | β-FeOOH + α-Fe2O3 |
|---|---|---|---|
| Density/(g·cm−3) | 5.18 (Fe3O4) | 4.26 (α-FeOOH) 4.87 (γ-Fe2O3) | 3.56 (β-FeOOH) 5.24 (α-Fe2O3) |
| Volumetric expansion rate | 210% (Fe3O4) | 295% (α-FeOOH) 232% (γ-Fe2O3) | 353% (β-FeOOH) 215% (α-Fe2O3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, P.; Jia, M.; Li, M.; Sun, J.; Cui, Y.; Hu, D.; Hu, G. Corrosion Behavior of Ancient White Cast Iron Artifacts from Marine Excavations at Atmospheric Condition. Metals 2022, 12, 921. https://doi.org/10.3390/met12060921
Hu P, Jia M, Li M, Sun J, Cui Y, Hu D, Hu G. Corrosion Behavior of Ancient White Cast Iron Artifacts from Marine Excavations at Atmospheric Condition. Metals. 2022; 12(6):921. https://doi.org/10.3390/met12060921
Chicago/Turabian StyleHu, Pei, Minghao Jia, Mohan Li, Jian Sun, Yong Cui, Dongbo Hu, and Gang Hu. 2022. "Corrosion Behavior of Ancient White Cast Iron Artifacts from Marine Excavations at Atmospheric Condition" Metals 12, no. 6: 921. https://doi.org/10.3390/met12060921
APA StyleHu, P., Jia, M., Li, M., Sun, J., Cui, Y., Hu, D., & Hu, G. (2022). Corrosion Behavior of Ancient White Cast Iron Artifacts from Marine Excavations at Atmospheric Condition. Metals, 12(6), 921. https://doi.org/10.3390/met12060921






