Corrosion Inhibition and Rust Conversion of Catechin on Archaeological Iron of Nanhai I
Abstract
:1. Introduction
2. Materials and Methods
2.1. Archaeological Iron
2.2. Samples Preparation
2.3. Characterization
3. Results and Discussion
3.1. Metallographic Structure
3.2. Original Rust Composition
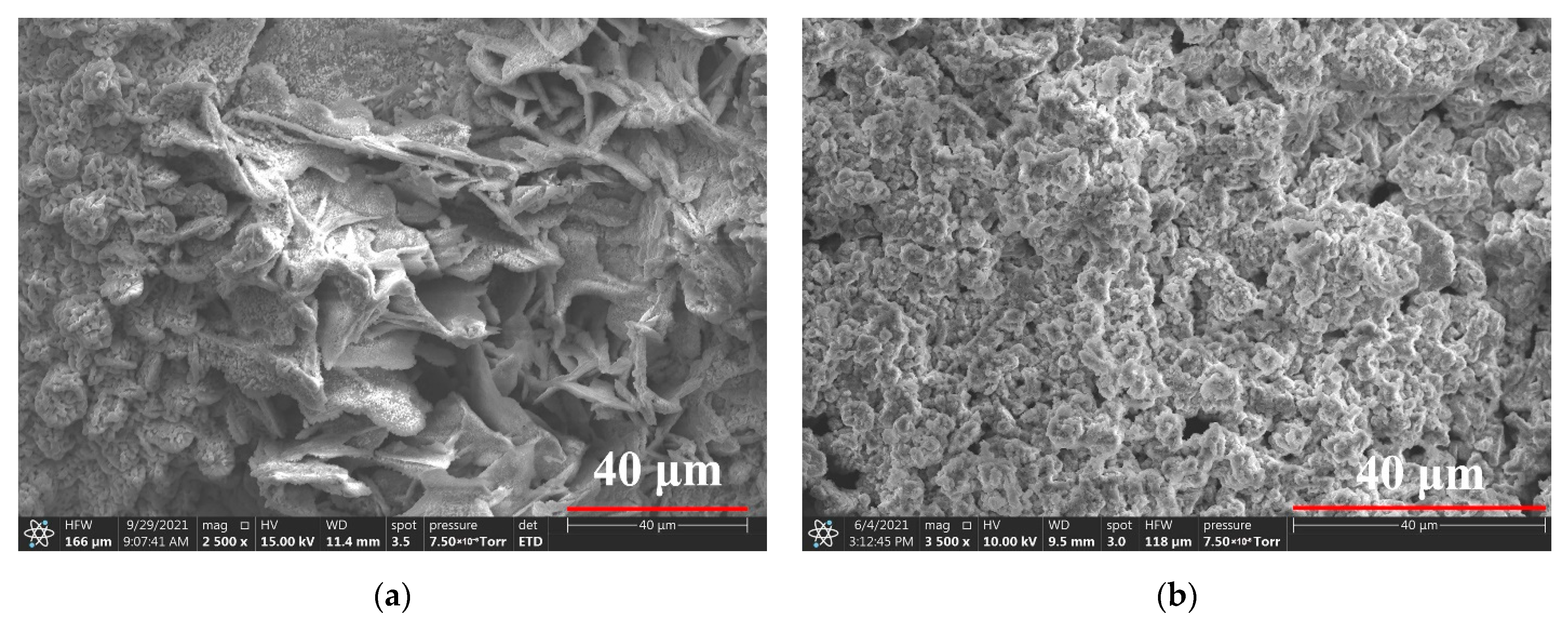
3.3. Rust Conversion
3.4. Surface Modification
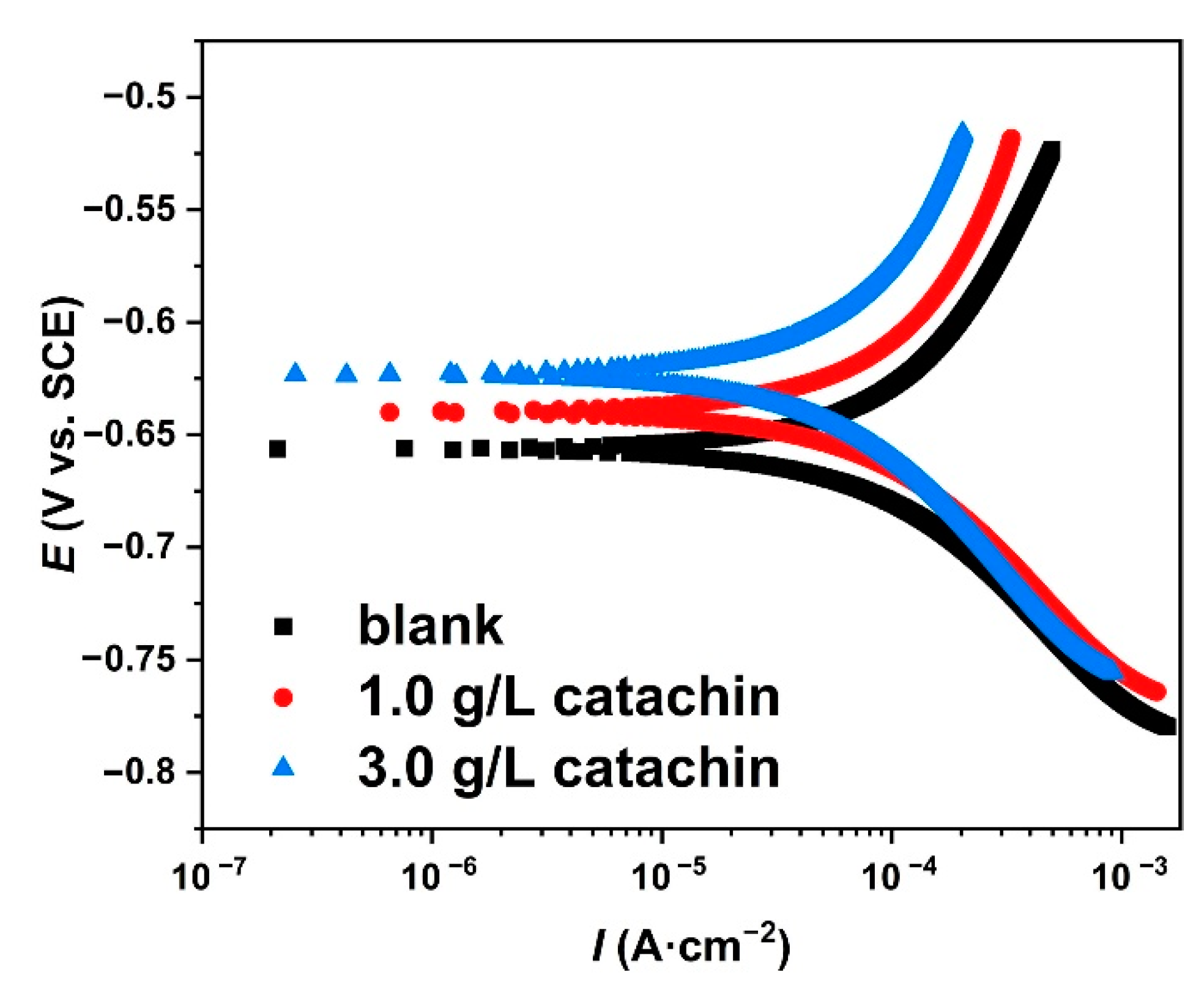
3.5. Potentiodynamic Polarization
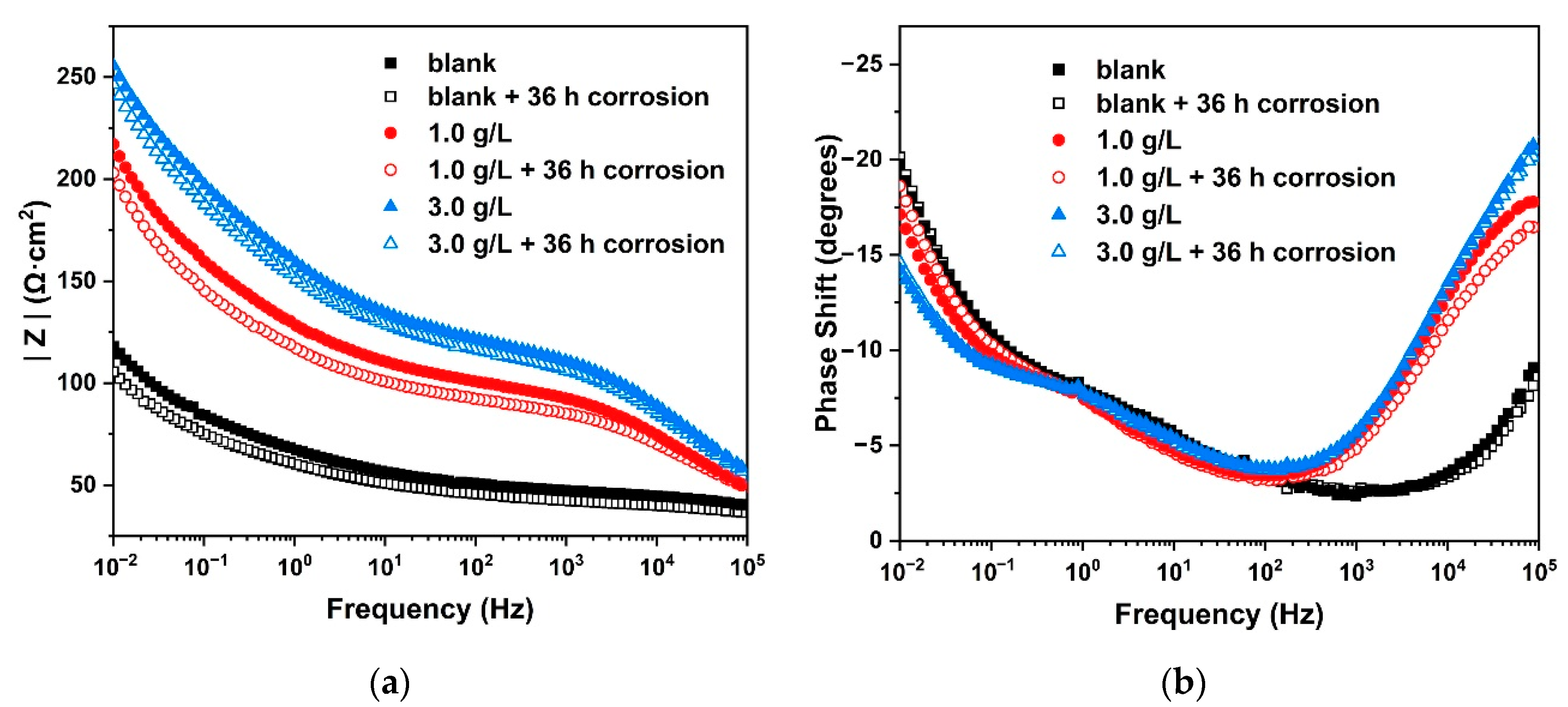
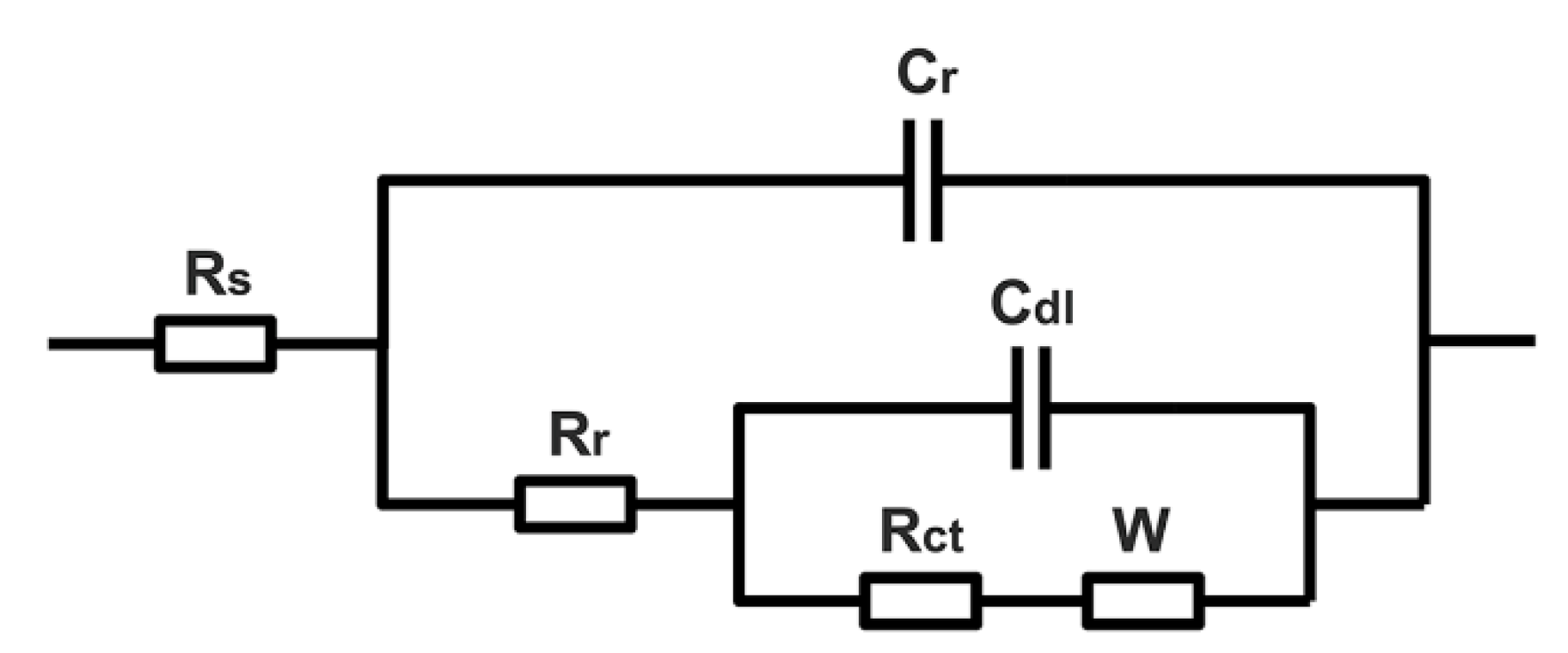
3.6. Electrochemical Impedance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batis, G.; Zacharopoulou, A.; Zacharopoulou, E.; Siova, H.; Argyropoulos, V. Dechlorination of large marine iron artifact using a novel technique involving impressed current. Anti-Corros. Methods Mater. 2015, 62, 259–269. [Google Scholar] [CrossRef]
- Parra, R.; Covelo, A.; Ramirez, R.J.; Tejeda, A.; Ortega, A.; Hernandez, M. Characterization of superficial modification of ferrous rusted substrates subjected to dechlorination-electrochemical process. J. Adhes. Sci. Technol. 2018, 32, 1341–1358. [Google Scholar] [CrossRef]
- Blahova, L.; Horak, J.; Prikryl, R.; Pekarek, J.; Tkacz, J.; Mencik, P.; Krcma, F. A novel technology for the corrosion protection of iron archaeological artefacts using parylene base removable bilayer. J. Cult. Herit. 2020, 42, 28–35. [Google Scholar] [CrossRef]
- Armetta, F.; Saladino, M.L.; Scherillo, A.; Caponetti, E. Microstructure and phase composition of bronze Montefortino helmets discovered Mediterranean seabed to explain an unusual corrosion. Sci. Rep. 2021, 11, 23022. [Google Scholar] [CrossRef]
- Bernabale, M.; Nigro, L.; Vaccaro, C.; Nicoli, M.; Montanari, D.; Bigini, P.; De Vito, C. Micro-Raman spectroscopy and complementary techniques for the study of iron weapons from Motya and Lilybaeum (Sicily, Italy): Corrosion patterns in lagoon-like and calcarenitic hypogea environments. J. Raman Spectrosc. 2022, 53, 272–287. [Google Scholar] [CrossRef]
- Hao, X.L.; Zhu, T.Q.; Xu, J.J.; Wang, Y.R.; Zhang, X.W. Microscopic study on the concretion of ceramics in the “Nanhai I” shipwreck of China, Southern Song Dynasty (1,127-1,279 AD). Microsc. Res. Techniq. 2018, 81, 486–493. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, D. The excavation of the “Nanhai No. 1” shipwreck of the Song Dynasty in 2014. Archaeology 2016, 12, 56–83. [Google Scholar]
- Remazeilles, C.; Leveque, F.; Conforto, E.; Refait, P. Long-term alteration processes of iron fasteners extracted from archaeological shipwrecks aged in biologically active waterlogged media. Corros. Sci. 2021, 181, 109231. [Google Scholar] [CrossRef]
- Simon, H.J.; Cibin, G.; Reinhard, C.; Liu, Y.; Schofield, E.; Freestone, I.C. Influence of microstructure on the corrosion of archaeological iron observed using 3D synchrotron micro-tomography. Corros. Sci. 2019, 159, 108132. [Google Scholar] [CrossRef]
- Zhao, X.D.; Cheng, Y.F.; Fan, W.; Vladimir, C.; Volha, V.; Alla, T. Inhibitive Performance of a Rust Converter on Corrosion of Mild Steel. J. Mater. Eng. Perform. 2014, 23, 4102–4108. [Google Scholar] [CrossRef]
- Flores Merino, S.; Jose Caprari, J.; Vasquez Torres, L. Inhibitive action of tara tannin in rust converter formulation. Anti-Corros. Methods Mater. 2017, 64, 136–147. [Google Scholar] [CrossRef]
- Remazeilles, C.; Neff, D.; Bourdoiseau, J.A.; Sabot, R.; Jeannin, M.; Refait, P. Role of previously formed corrosion product layers on sulfide-assisted corrosion of iron archaeological artefacts in soil. Corros. Sci. 2017, 129, 169–178. [Google Scholar] [CrossRef]
- Gao, X.L.; Han, Y.; Fu, G.Q.; Zhu, M.Y.; Zhang, X.Z. Evolution of the Rust Layers Formed on Carbon and Weathering Steels in Environment Containing Chloride Ions. Acta Metall. Sin-Engl. 2016, 29, 1025–1036. [Google Scholar] [CrossRef] [Green Version]
- Kamimura, T.; Hara, S.; Miyuki, H.; Yamashita, M.; Uchida, H. Composition and protective ability of rust layer formed on weathering steel exposed to various environments. Corros. Sci. 2006, 48, 2799–2812. [Google Scholar] [CrossRef]
- Saleh, S.A. Corrosion Mechanism of Iron Objects in Marine Environment an Analytical Investigation Study by Raman Spectrometry. Eur. J. Sci. Theol. 2017, 13, 185–206. [Google Scholar]
- Lair, V.; Antony, H.; Legrand, L.; Chausse, A. Electrochemical reduction of ferric corrosion products and evaluation of galvanic coupling with iron. Corros. Sci. 2006, 48, 2050–2063. [Google Scholar] [CrossRef]
- Alsabagh, A.M.; Migahed, M.A.; Abdelraouf, M.; Khamis, E.A. Utilization of Green Tea as Environmentally Friendly Corrosion Inhibitor for Carbon Steel in acidic media. Inter. J. Electrochem. Sci. 2015, 10, 1855–1872. [Google Scholar]
- Aourabi, S.; Driouch, M.; Sfaira, M.; Mahjoubi, F.; Hammouti, B.; Verma, C.; Ebenso, E.E.; Guo, L. Phenolic fraction of Ammi visnaga extract as environmentally friendly antioxidant and corrosion inhibitor for mild steel in acidic medium. J. Mol. Liq. 2021, 323, 114950. [Google Scholar] [CrossRef]
- Pradipta, I.; Kong, D.; Tan, J.B.L. Natural organic antioxidants from green tea inhibit corrosion of steel reinforcing bars embedded in mortar. Constr. Build. Mater. 2019, 227, 117058. [Google Scholar] [CrossRef]
- Abdallah, M. Rhodanine azosulpha drugs as corrosion inhibitors for corrosion of 304 stainless steel in hydrochloric acid solution. Corros. Sci. 2002, 44, 717–728. [Google Scholar] [CrossRef]
- Hussin, M.H.; Kassim, M.J. The corrosion inhibition and adsorption behavior of Uncaria gambir extract on mild steel in 1 M HCl. Mater. Chem. Phys. 2011, 125, 461–468. [Google Scholar] [CrossRef]
- Tang, Z. A review of corrosion inhibitors for rust preventative fluids. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100759. [Google Scholar] [CrossRef]
- Tan, K.W.; Kassim, M.J.; Oo, C.W. Possible improvement of catechin as corrosion inhibitor in acidic medium. Corros. Sci. 2012, 65, 152–162. [Google Scholar] [CrossRef]
- Hussin, M.H.; Kassim, M.J. Electrochemical, Thermodynamic and Adsorption Studies of (+)-Catechin Hydrate as Natural Mild Steel Corrosion Inhibitor in 1 M HCl. Inter. J. Electrochem. Sci. 2011, 6, 1396–1414. [Google Scholar]
- Wang, D.Y.; Nie, B.L.; Li, H.J.; Wang, F.; Zhang, W.Z.; Wu, Y.C. Experimental and theoretical investigation of inhibition behavior of bisflavanol for Q235 steel in hydrochloric acid solution. J. Mol. Liq. 2021, 342, 117490. [Google Scholar] [CrossRef]
- Ramos-Tejada, M.M.; Duran, J.D.G.; Ontiveros-Ortega, A.; Espinosa-Jimenez, M.; Perea-Carpio, R.; Chibowski, E. Investigation of alumina/(+)-catechin system properties. Part I: A study of the system by FTIR-UV-Vis spectroscopy. Colloids Surf. B 2002, 24, 297–308. [Google Scholar] [CrossRef]
- Alam, M.; Ali, S.; Ashraf, G.M.; Bilgrami, A.L.; Yadav, D.K.; Hassan, M.I. Epigallocatechin 3-gallate: From green tea to cancer therapeutics. Food Chem. 2022, 379, 132135. [Google Scholar] [CrossRef] [PubMed]
- Gallia, M.C.; Bachmeier, E.; Ferrari, A.; Queralt, I.; Mazzeo, M.A.; Bongiovanni, G.A. Pehuen (Araucaria araucana) seed residues are a valuable source of natural antioxidants with nutraceutical, chemoprotective and metal corrosion-inhibiting properties. Bioorg. Chem. 2020, 104, 104175. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, J.R.; Li, H.B.; Wu, D.T.; Geng, F.; Corke, H.; Wei, X.L.; Gan, R.Y. Green Extraction of Antioxidant Polyphenols from Green Tea (Camellia sinensis). Antioxidants 2020, 9, 785. [Google Scholar] [CrossRef]
- Abdelaziz, S.; Benamira, M.; Messaadia, L.; Boughoues, Y.; Lahmar, H.; Boudjerda, A. Green corrosion inhibition of mild steel in HCl medium using leaves extract of Arbutus unedo L. plant: An experimental and computational approach. Colloids Surf. A Physicochem. Eng. Asp. 2021; 619, 126496. [Google Scholar]
- Lekbach, Y.; Dong, Y.; Li, Z.; Xu, D.; El Abed, S.; Yi, Y.; Li, L.; Koraichi, S.I.; Su, T.; Wang, F. Catechin hydrate as an eco-friendly biocorrosion inhibitor for 304L stainless steel with dual-action antibacterial properties against Pseudomonas aeruginosa biofilm. Corros. Sci. 2019, 157, 98–108. [Google Scholar] [CrossRef]
- Mazur, A.; Gasik, M.M.; Mazur, V.I. Thermal analysis of eutectic reactions of white cast irons. Scand. J. Metall. 2005, 34, 245–249. [Google Scholar] [CrossRef]
- Rocca, E.; Faiz, H.; Dillmann, P.; Neff, D.; Mirambet, F. Electrochemical behavior of thick rust layers on steel artefact: Mechanism of corrosion inhibition. Electrochim. Acta 2019, 316, 219–227. [Google Scholar] [CrossRef]
- Calero, J.; Alcantara, J.; Chico, B.; Diaz, I.; Simancas, J.; de la Fuente, D.; Morcillo, M. Wet/dry accelerated laboratory test to simulate the formation of multilayered rust on carbon steel in marine atmospheres. Corros. Eng. Sci. Technol. 2017, 52, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, T.; Katayama, H.; Noda, K.; Kodama, T. Electrochemical behavior of rust formed on carbon steel in a wet/dry environment containing chloride ions. Corrosion 2000, 56, 935–941. [Google Scholar] [CrossRef]
- Selvi, I.K.; Nagarajan, S. Separation of catechins from green tea (Camellia sinensis L.) by microwave assisted acetylation, evaluation of antioxidant potential of individual components and spectroscopic analysis. LWT 2018, 91, 391–397. [Google Scholar] [CrossRef]
- Zhang, R.N.; Li, L.; Liu, J.X. Synthesis and characterization of ferric tannate as a novel porous adsorptive-catalyst for nitrogen removal from wastewater. RSC Adv. 2015, 5, 40785–40791. [Google Scholar] [CrossRef]
- Veneranda, M.; Aramendia, J.; Bellot-Gurlet, L.; Colomban, P.; Castro, K.; Madariaga, J.M. FTIR spectroscopic semi-quantification of iron phases: A new method to evaluate the protection ability index (PAI) of archaeological artefacts corrosion systems. Corros. Sci. 2018, 133, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.W.; Wang, Z.Y.; Wei, Y.H. Influence of Seawater on the Carbon Steel Initial Corrosion Behavior. Int. J. Electrochem. Sci. 2019, 14, 1147–1162. [Google Scholar] [CrossRef]
- Labbe, J.P.; Ledion, J.; Hui, F. Infrared spectrometry for solid phase analysis: Corrosion rusts. Corros. Sci. 2008, 50, 1228–1234. [Google Scholar] [CrossRef]
- Huang, L.; Yang, K.P.; Zhao, Q.; Li, H.J.; Wang, J.Y.; Wu, Y.C. Corrosion resistance and antibacterial activity of procyanidin B2 as a novel environment-friendly inhibitor for Q235 steel in 1 M HCl solution. Bioelectrochemistry 2022, 143, 107969. [Google Scholar] [CrossRef]
- Han, D.; Jiang, R.J.; Cheng, Y.F. Mechanism of electrochemical corrosion of carbon steel under deoxygenated water drop and sand deposit. Electrochim. Acta 2013, 114, 403–408. [Google Scholar] [CrossRef]
- Orazem, M.E.; Frateur, I.; Tribollet, B.; Vivier, V.; Marcelin, S.; Pebere, N.; Bunge, A.L.; White, E.A.; Riemer, D.P.; Musiani, M. Dielectric Properties of Materials Showing Constant-Phase-Element (CPE) Impedance Response. J. Electrochem. Soc. 2013, 160, C215–C225. [Google Scholar] [CrossRef] [Green Version]
- Dhaiveegan, P.; Elangovan, N.; Nishimura, T.; Rajendran, N. Electrochemical Characterization of Carbon and Weathering Steels Corrosion Products to Determine the Protective Ability Using Carbon Paste Electrode (CPE). Electroanalysis 2014, 26, 2419–2428. [Google Scholar] [CrossRef]
- Sharifi-Asl, S.; Taylor, M.L.; Lu, Z.; Engelhardt, G.R.; Kursten, R.; Macdonald, D.D. Modeling of the electrochemical impedance spectroscopic behavior of passive iron using a genetic algorithm approach. Electrochim. Acta 2013, 102, 161–173. [Google Scholar] [CrossRef]
- Palomar-Pardave, M.; Romero-Romo, M.; Herrera-Hernandez, H.; Abreu-Quijano, M.A.; Likhanova, N.V.; Uruchurtu, J.; Juarez-Garcia, J.M. Influence of the alkyl chain length of 2 amino 5 alkyl 1,3,4 thiadiazole compounds on the corrosion inhibition of steel immersed in sulfuric acid solutions. Corros. Sci. 2012, 54, 231–243. [Google Scholar] [CrossRef]




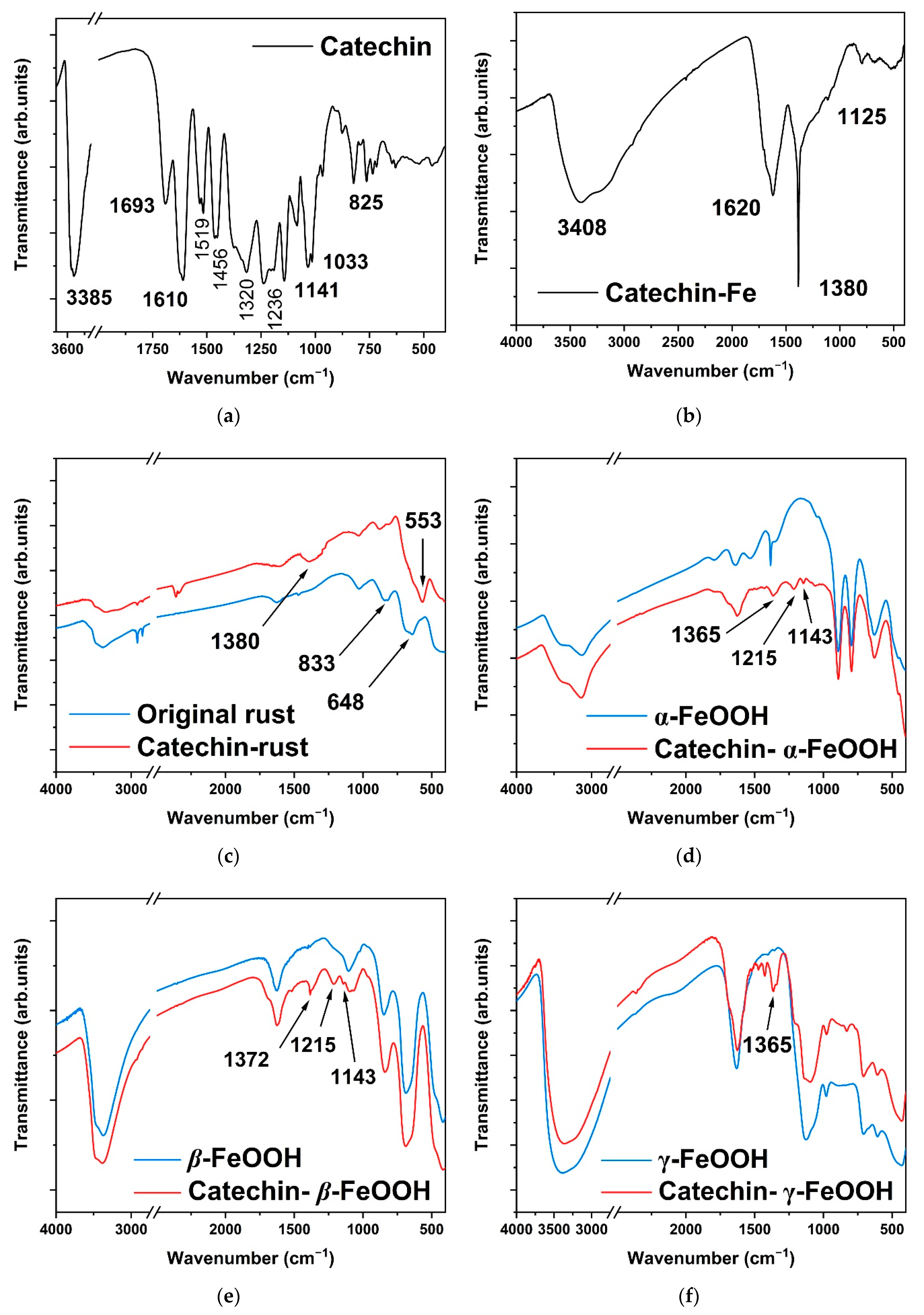

| Chemical Bonding | IR Peaks (cm−1) |
|---|---|
| stretching vibration of the −OH bond | 3385 |
| C=C bond of aromatic ring | 1456, 1519 and 1693 |
| stretching vibration of the C−O−Fe bond (in the pure catechin−Fe composite) | 1380 |
| stretching vibration of the C−O−Fe bond (in the catechin−FeOOH composite) | 1365–1372 |
| bending vibration of the C−O bond (connected to the −OH on phenolic substances) | 1320 |
| bending vibration of the C−O bond | 1236 |
| stretching vibration of the phenolic C−O−C bond | 1141 and 1033 |
| bending vibration of the =C−H bond (on the out-of-plane benzene ring) | 823 |
| vibration of the Fe−O bond in β-FeOOH | 1627 |
| bending vibration of the −OH bond in γ-FeOOH | 1026 |
| bending vibration of the −OH bond in β-FeOOH | 833 |
| vibration of iron oxides | 553 |
| Samples | Ecorr (mV vs. SCE) | Icorr (μA·cm−2) | βa (mV·dec−1) | βc (mV·dec−1) | vcorr (mmPY) | χ2 |
|---|---|---|---|---|---|---|
| blank | −656.25 | 156.89 | 257.87 | 148.95 | 1.8435 | 0.2043 |
| 1.0 g/L | −639.81 | 135.14 | 272.51 | 135.29 | 1.5758 | 0.4098 |
| 3.0 g/L | −623.58 | 98.64 | 289.47 | 146.71 | 1.1229 | 0.4110 |
| Samples | Rs (Ω·cm2) | Rr (Ω·cm2) | Rct (Ω·cm2) | Rw (Ω·cm2) | CPEr (sn·Ω−1·cm−2 × 106) | CPEdl (sn·Ω−1·cm−2 × 106) | nr | ndl | χ2 | ηct | ηr |
|---|---|---|---|---|---|---|---|---|---|---|---|
| blank | 17.54 | 28.11 | 78.52 | 81.92 | 1.677 | 17.637 | 0.59 | 0.34 | 0.013 | / | / |
| 1.0 g/L | 23.49 | 76.87 | 85.59 | 417.52 | 22.063 | 8.867 | 0.53 | 0.48 | 0.021 | 8.26% | 63.43% |
| 3.0 g/L | 16.70 | 105.12 | 100.35 | 518.41 | 20.212 | 6.005 | 0.50 | 0.51 | 0.019 | 21.75% | 73.26% |
| blank + corr. | 18.52 | 21.76 | 61.14 | 78.96 | 1.451 | 19.271 | 0.60 | 0.31 | 0.014 | / | / |
| 1.0 g/L+ corr. | 21.90 | 70.44 | 70.12 | 391.60 | 28.854 | 9.514 | 0.51 | 0.49 | 0.015 | 12.81% | 69.11% |
| 3.0 g/L+ corr. | 18.84 | 97.39 | 94.08 | 410.45 | 20.023 | 6.665 | 0.51 | 0.49 | 0.018 | 35.01% | 77.66% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Hu, P.; Gong, Z.; Sun, J.; Cui, Y.; Hu, D.; Hu, G. Corrosion Inhibition and Rust Conversion of Catechin on Archaeological Iron of Nanhai I. Metals 2022, 12, 714. https://doi.org/10.3390/met12050714
Jia M, Hu P, Gong Z, Sun J, Cui Y, Hu D, Hu G. Corrosion Inhibition and Rust Conversion of Catechin on Archaeological Iron of Nanhai I. Metals. 2022; 12(5):714. https://doi.org/10.3390/met12050714
Chicago/Turabian StyleJia, Minghao, Pei Hu, Zisang Gong, Jian Sun, Yong Cui, Dongbo Hu, and Gang Hu. 2022. "Corrosion Inhibition and Rust Conversion of Catechin on Archaeological Iron of Nanhai I" Metals 12, no. 5: 714. https://doi.org/10.3390/met12050714
APA StyleJia, M., Hu, P., Gong, Z., Sun, J., Cui, Y., Hu, D., & Hu, G. (2022). Corrosion Inhibition and Rust Conversion of Catechin on Archaeological Iron of Nanhai I. Metals, 12(5), 714. https://doi.org/10.3390/met12050714






