Numerical Modeling of the Influence of Nanometric Ceramic Particles on the Nucleation of AlSi10MnMg Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Compositional Analysis
3.2. Heterogeneous Solidification Model for Nano-Metric Particles
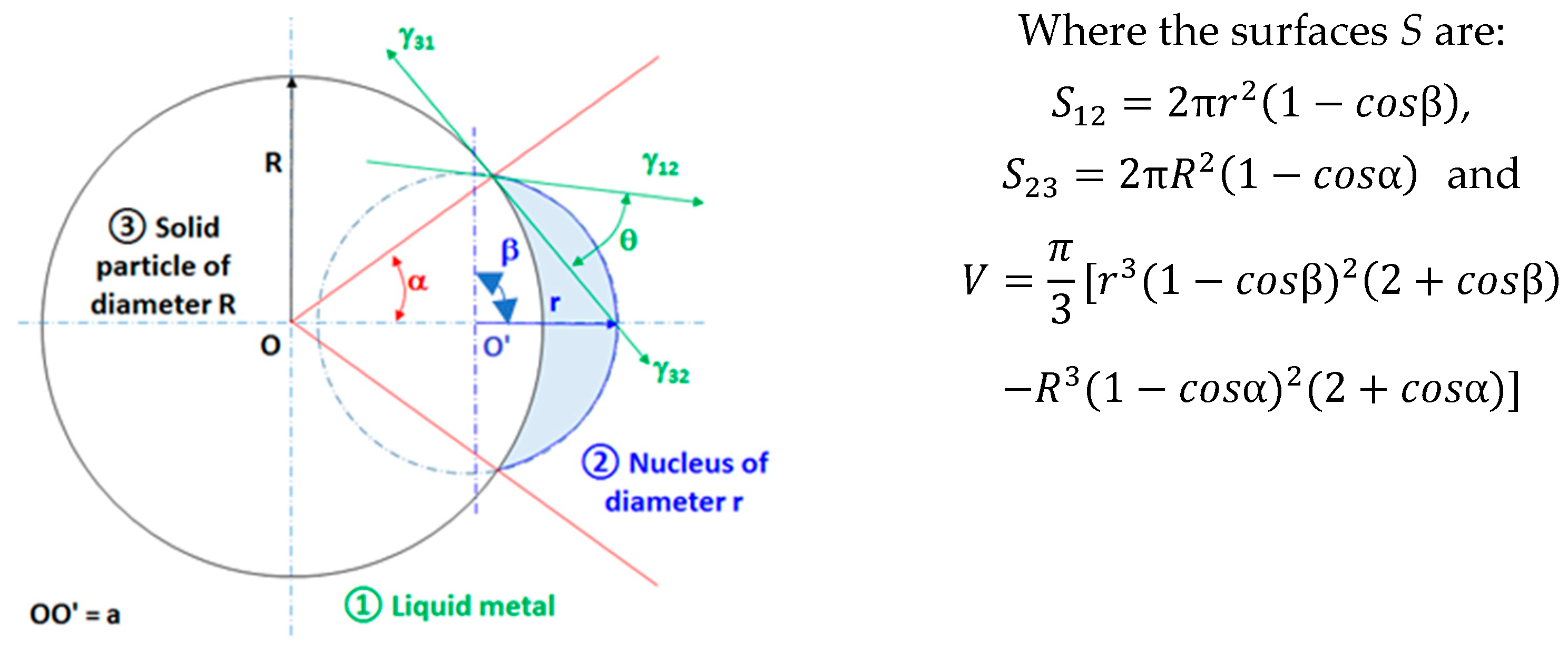
3.2.1. Spherical Particles
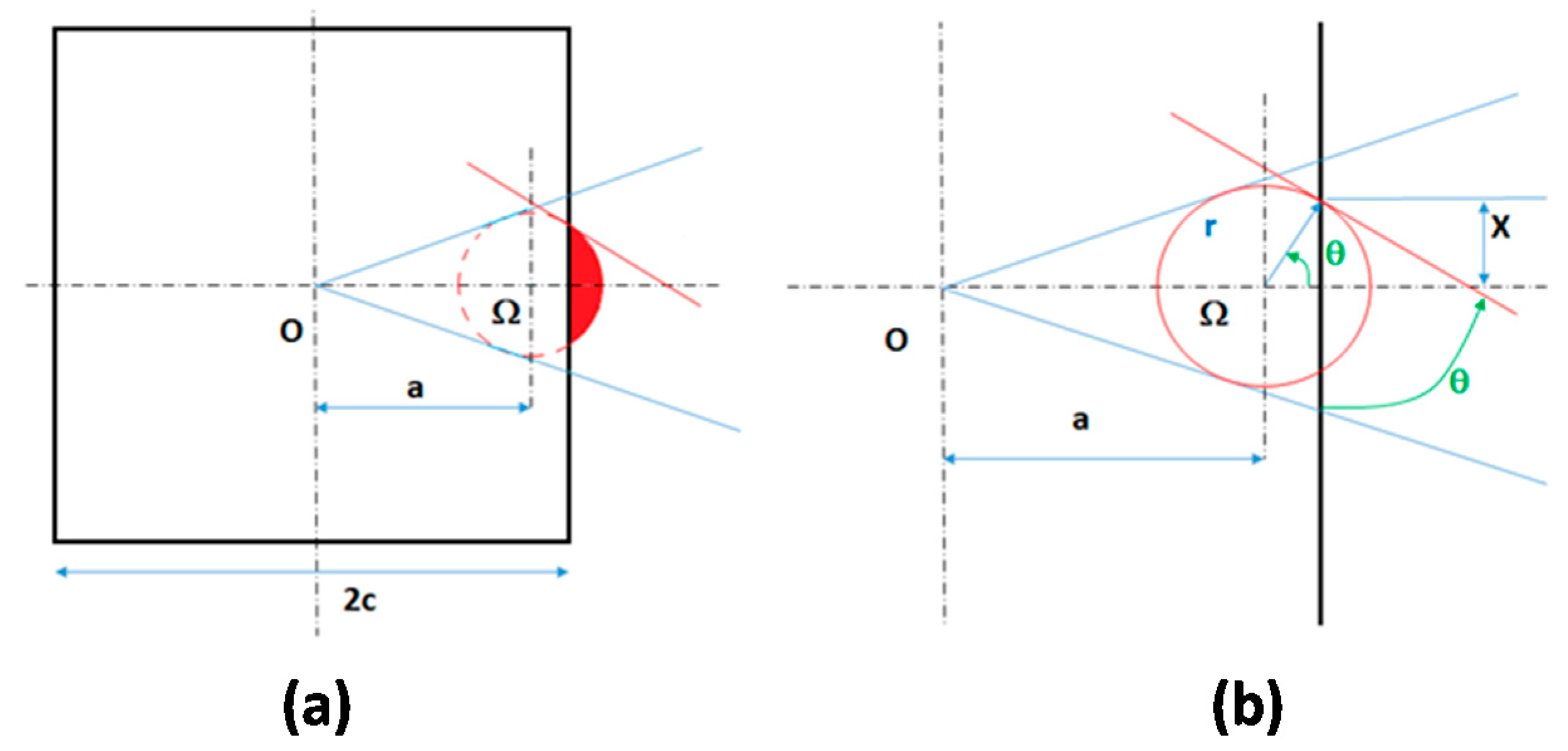
3.2.2. Cubic Particles with a Nucleus on One Face
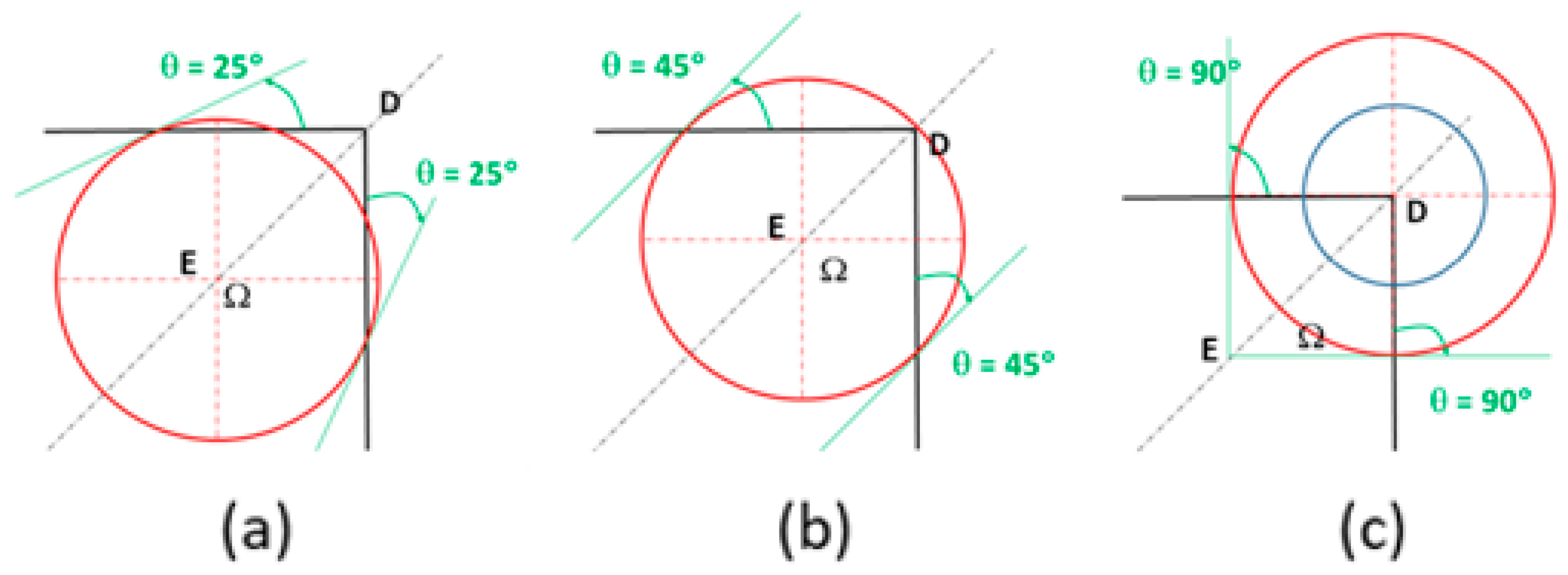
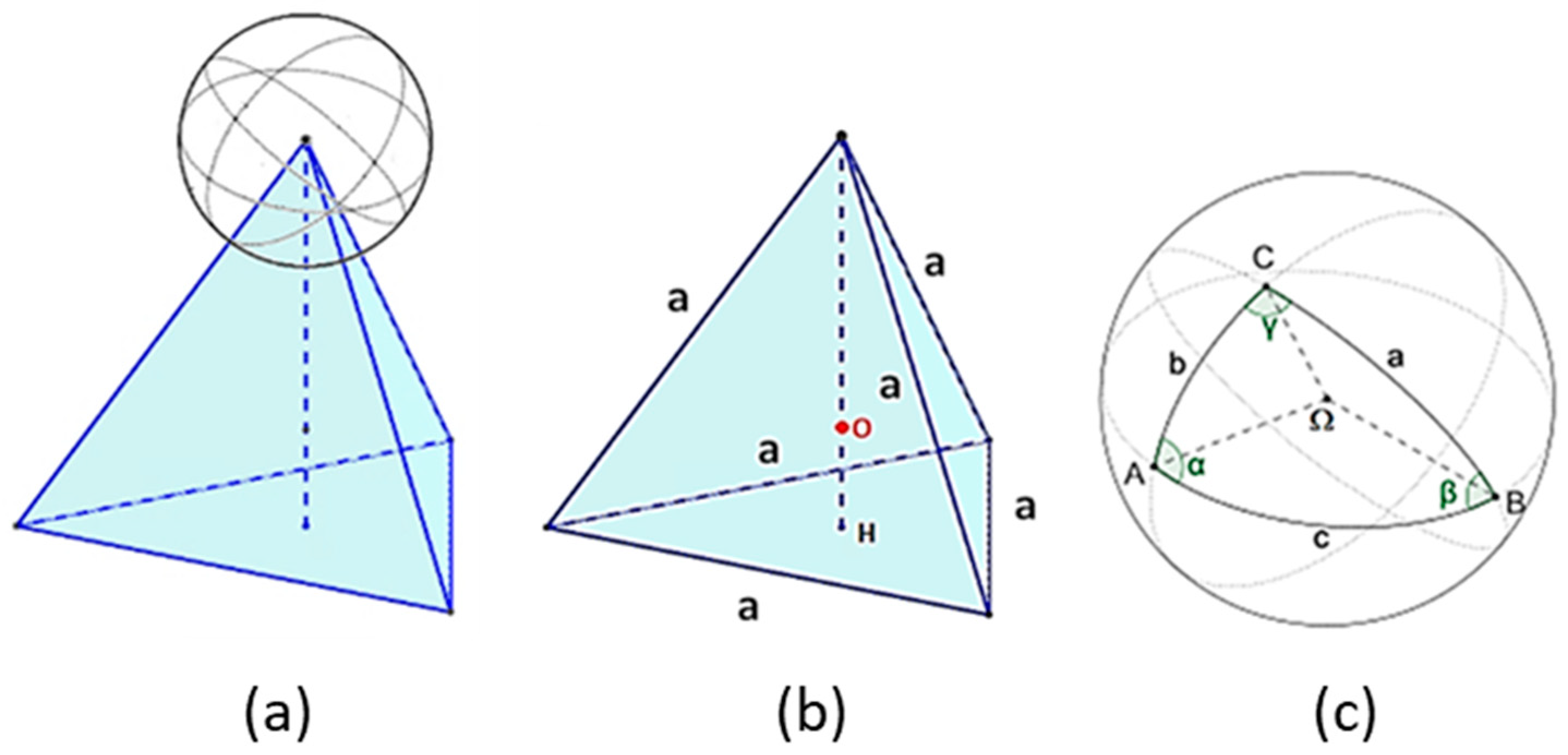
3.2.3. Cubic Particles with a Nucleus on the Vertex
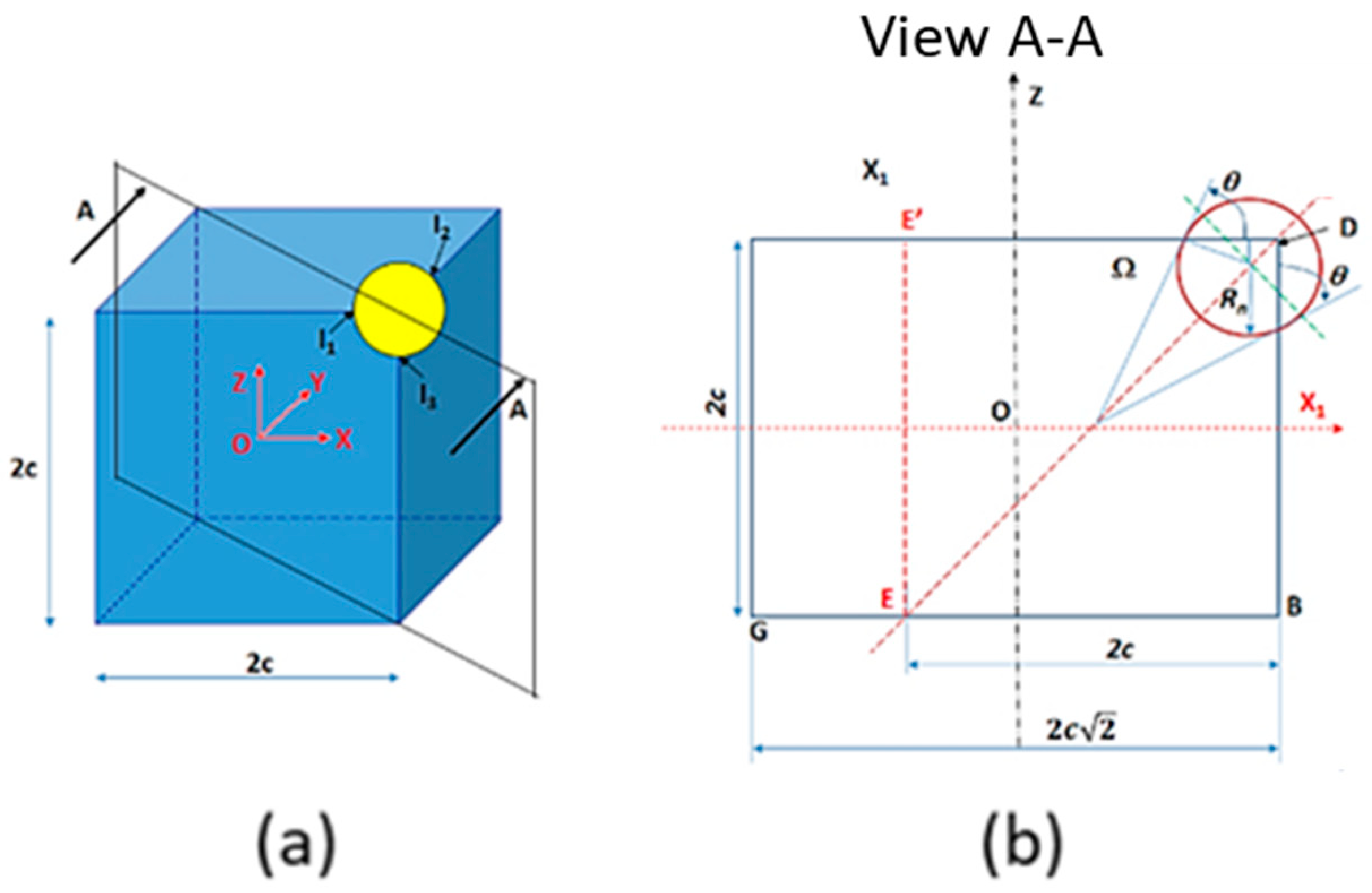
3.2.4. Pyramidal Particles
3.2.5. Numerical Modeling
| n | a | b | c |
| 1 | |||
| 2 | |||
| 3 |
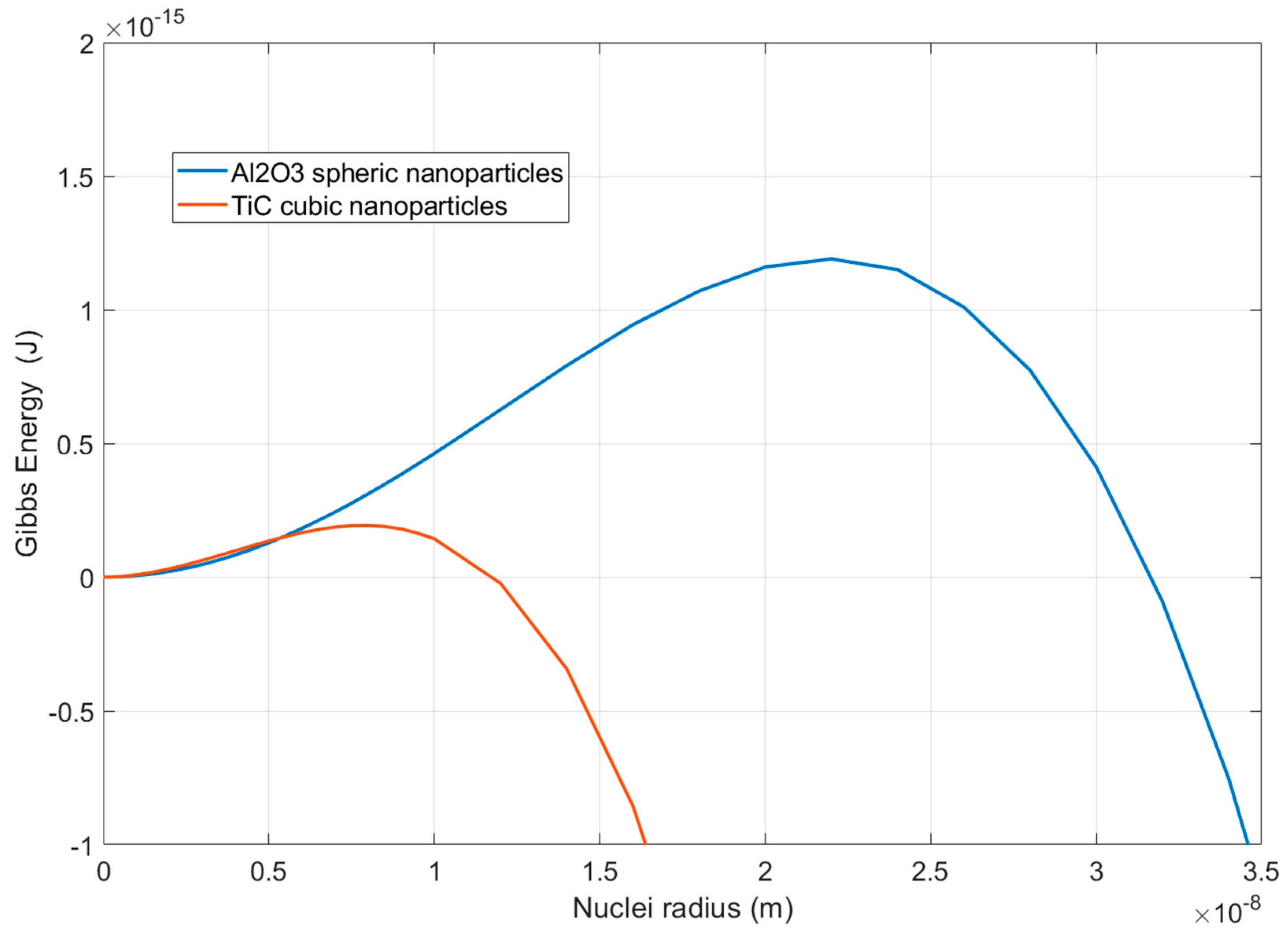
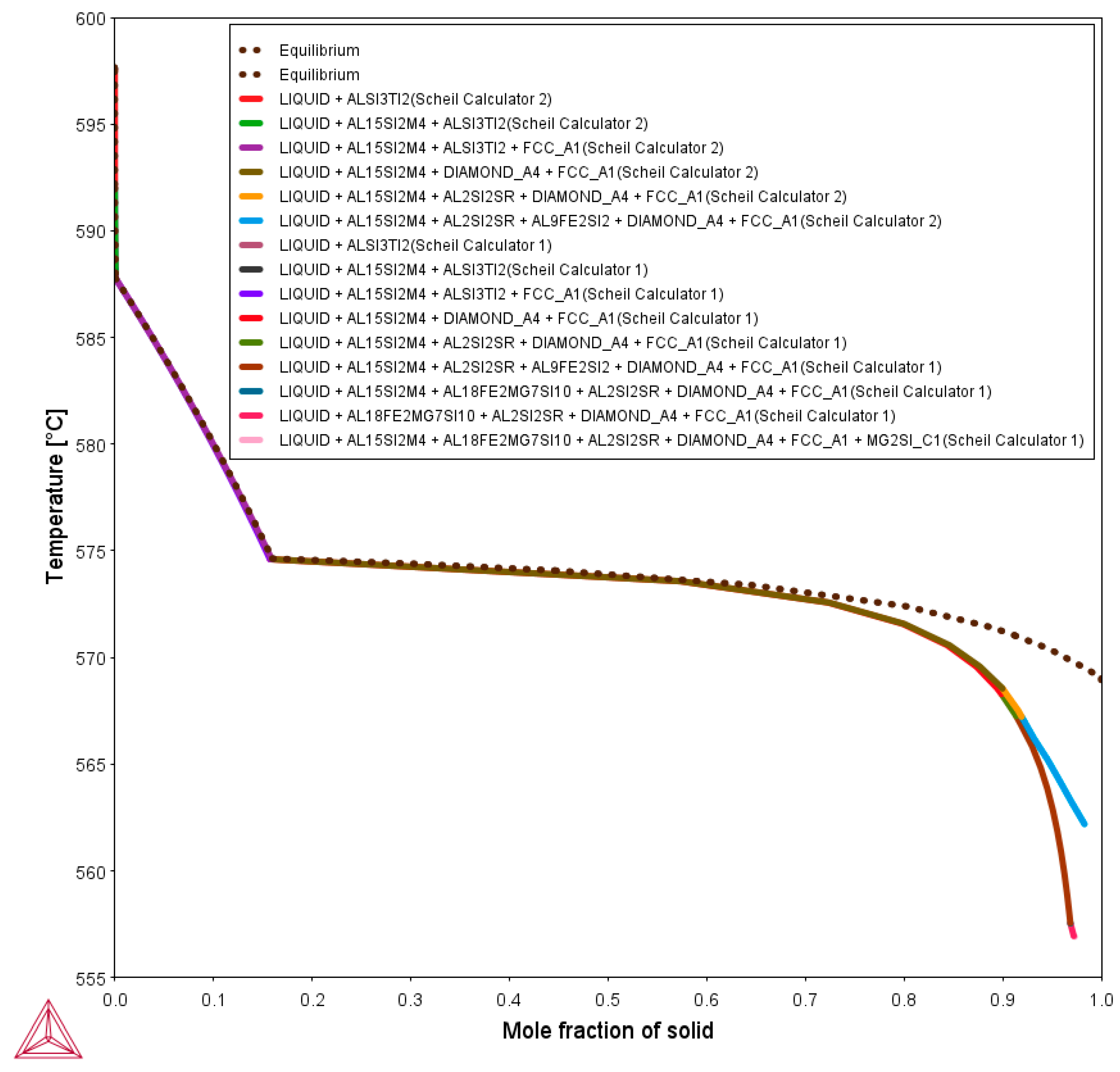
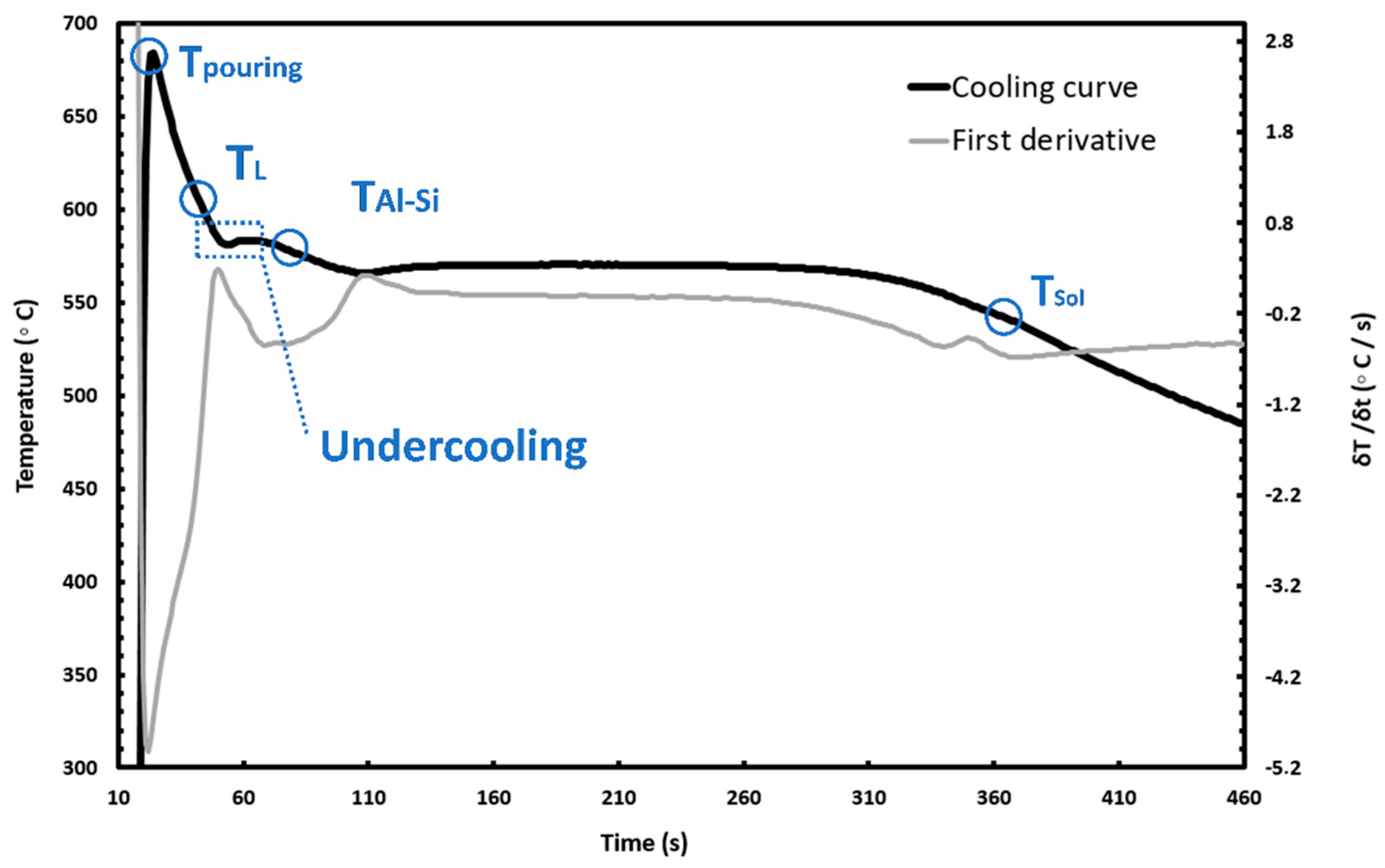
3.3. Influence of the Nanometric Particles in the Solidification Properties of the Alloys
- Liquid (L)→ L + AlSi3Ti2 at 598 °C
- L + AlSi3Ti2 + Al15Si(Mn,Fe)4 at 591 °C
- L + AlSi3Ti2 + Al15Si(Mn,Fe)4 + α-Al at 588 °C
- L + AlSi3Ti2 + Al15Si(Mn,Fe)4 + α-Al + Si at 575 °C
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koli, D.K.; Agnihotri, G.; Purohit, R. Advanced Aluminium Matrix Composites: The Critical Need of Auto-motive and Aerospace Engineering Fields. Mater. Today Proc. 2015, 2, 3032–3041. [Google Scholar] [CrossRef]
- Lazarova, R.; Bojanova, N.; Dimitrova, R.; Panov, I.; Manolov, V. Influence of Nanoparticles Introducing in the Melt of Aluminum Alloys on Castings Microstructure and Properties. Int. J. Met. 2016, 10, 466–476. [Google Scholar] [CrossRef] [Green Version]
- Zhukov, I.A.; Kozulin, A.A.; Khrustalyov, A.P.; Kahidze, N.I.; Khmeleva, M.G.; Moskvichev, E.N.; Lychagin, D.V.; Vorozhtsov, A.B. Pure Aluminum Structure and Mechanical Properties Modified by Al2O3 Nanoparticles and Ultrasonic Treatment. Metals 2019, 9, 1199. [Google Scholar] [CrossRef] [Green Version]
- Nishida, Y. Introduction to Metal Matrix Composites: Fabrication and Recycling; Springer: New York, NY, USA, 2013. [Google Scholar]
- Sajjadi, S.; Ezatpour, H.; Parizi, M.T. Comparison of microstructure and mechanical properties of A356 aluminum alloy/Al2O3 composites fabricated by stir and compo-casting processes. Mater. Des. 2012, 34, 106–111. [Google Scholar] [CrossRef]
- Hamedan, A.D.; Shahmiri, M. Production of A356–1wt% SiC nanocomposite by the modified stir casting method. Mater. Sci. Eng. A 2012, 556, 921–926. [Google Scholar] [CrossRef]
- Mahata, A.; Zaeem, M.A.; Baskes, M.I. Understanding homogeneous nucleation in solidification of aluminum by molecular dynamics simulations. Model. Simul. Mater. Sci. Eng. 2018, 26, 025007. [Google Scholar] [CrossRef]
- Nicolas, M.; Deschamps, A. Characterisation and modelling of precipitate evolution in an Al–Zn–Mg alloy during non-isothermal heat treatments. Acta Mater. 2003, 51, 6077–6094. [Google Scholar] [CrossRef] [Green Version]
- Qian, M. Heterogeneous nucleation on potent spherical substrates during solidification. Acta Mater. 2006, 55, 943–953. [Google Scholar] [CrossRef]
- Stefanescu, D.M. Science and Engineering of Casting Solidification; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Myhr, O.R.; Grong, Ø. Modelling of non-isothermal transformations in alloys containing a particle distribution. Acta Mater. 2000, 48, 1605–1615. [Google Scholar] [CrossRef]
- Perez, M.; Dumont, M.; Acevedo-Reyes, D. Implementation of classical nucleation and growth theories for precipitation. Acta Mater. 2008, 56, 2119–2132. [Google Scholar] [CrossRef]
- Kampmann, R.; Wagner, R. Chapter Kinetics of precipitation in metastable binary alloys—Theory and application to Cu-1.9 at% Ti and Ni-14 at% Al. In Decomposition of Alloys: The Early Stages; Haasen, P., Gerold, V., Wagner, R., Ashby, Y.M.F., Eds.; Pergamon: Sonnenbergm, Germany, 1984; pp. 91–103. [Google Scholar] [CrossRef]
- Oxtoby, D.W. Nucleation of Crystals from the Melt. Adv. Chem. Phys. 1988, 70, 263–296. [Google Scholar] [CrossRef]
- Vehkamäki, H.; Määttänen, A.; Lauri, A.; Napari, I.; Kulmala, M. Technical Note: The heterogeneous Zeldovich factor. Atmos. Chem. Phys. 2007, 7, 309–313. [Google Scholar] [CrossRef] [Green Version]
- Kolmasiak, C.; Łągiewka, M. Solidification of the Al alloy composite reinforced with graphite particles. Metalurgija 2021, 60, 399–402. [Google Scholar]
- Andersson, J.-O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Mosisa, E.; Bazhin, V.Y.; Savchenkov, Y.S. Review on nano particle reinforced aluminum metal matrix compo-sites. J. Appl. Sci. Res. 2016, 11, 188–196. [Google Scholar] [CrossRef]
- Sunyaev, R.A. (Ed.) On the Theory of New Phase Formation. Cavitation. In Selected Works of Yakov Borisovich Zeldovich, Volume I: Chemical Physics and Hydrodynanics; Princeton University Press: Princeton, NJ, USA, 2014; pp. 120–137. [Google Scholar] [CrossRef]
- Bracco, G.; Holst, B. (Eds.) Surface Science Techniques: 51; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Karliga, B.; Tokeser, U. Girard Type Theorems for de Sitter Triangles with non-null Edges. arXiv 2017, arXiv:1412.5507. [Google Scholar]
- Backenrud, L.; Krol, E.; Tamminem, J. Solidification Characteristics of Aluminium Alloys; American Foundrymans Society/Skanaluminium: Oslo, Norway, 1990; Volume 2. [Google Scholar]
| Ref. | Al | Fe | Si | Mn | Cr | Ni | Zn | Mg | Ti | Sr | V |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Base | Bal. | 0.16 | 10.92 | 0.55 | 0.01 | 0.01 | 0.01 | 0.24 | 0.05 | 0.01 | 0.01 |
| Base + TiC | Bal. | 0.19 | 11.31 | 0.54 | 0.01 | 0.01 | 0.01 | 0.25 | 0.10 | 0.01 | 0.01 |
| Base + Al2O3 | Bal. | 0.17 | 11.12 | 0.54 | 0.01 | 0.01 | 0.01 | 0.24 | 0.05 | 0.01 | 0.01 |
| Particle Shape | Volume | Contact Surface |
|---|---|---|
| Cubic, with a nucleus on one face | ||
| Cubic, with a nucleus on the vertex θ ≥ 45° | ||
| Pyramidal |
| Particle Shape | Critical Gibbs Energy | Form Factor |
|---|---|---|
| Spherical | and x = R/r*; m = cosθ | |
| Cubic, with a nucleus on one face | ||
|
Cubic, with a nucleus on the vertex θ ≥ 45° | ||
| Pyramidal |
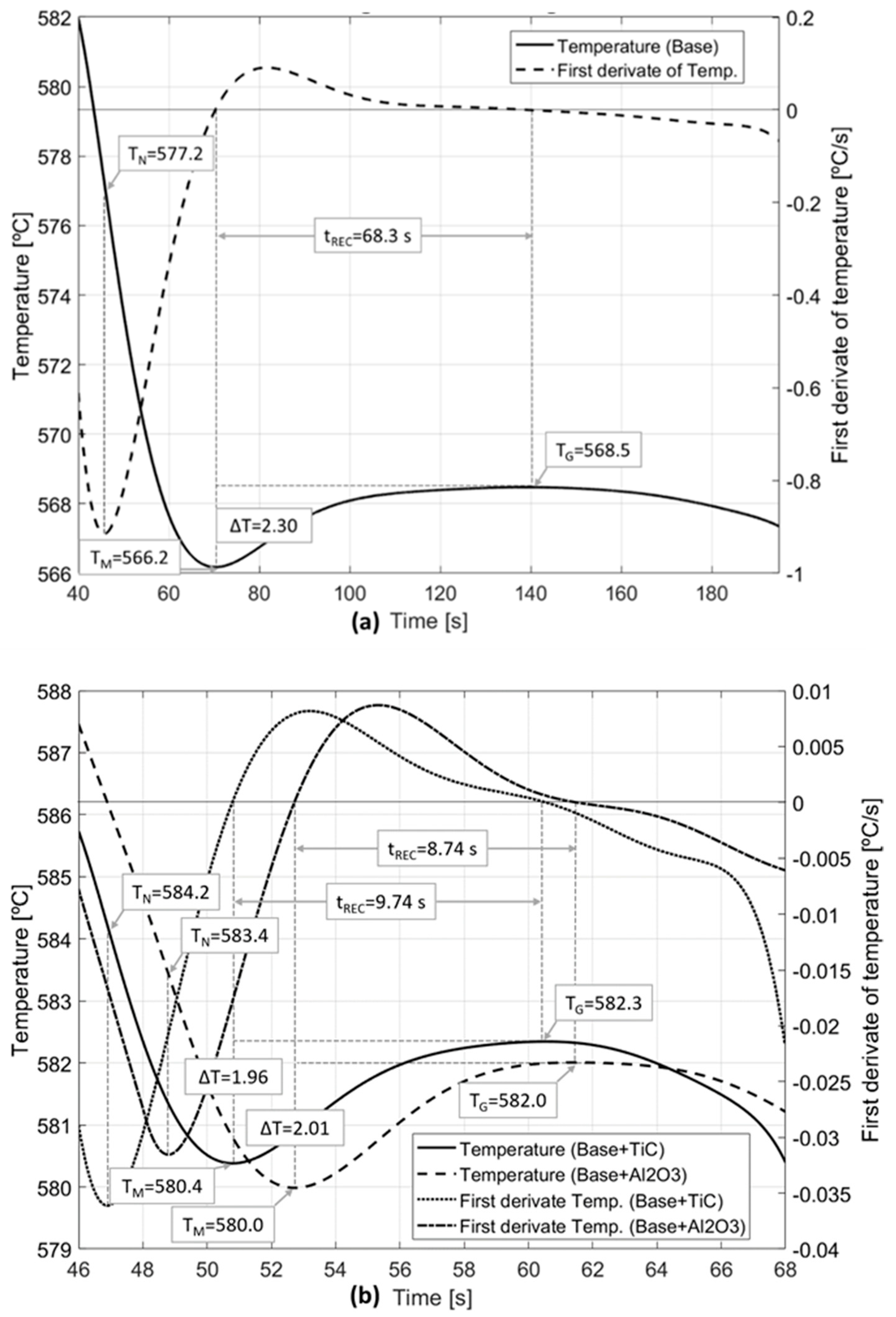
| Calculation | Liquidus (°C) | TAl-Si (°C) | TN (°C) | TG (°C) | TM (°C) | ∆T (°C) | tREC (s) |
|---|---|---|---|---|---|---|---|
| Thermo-Calc | 597 | 575 | - | - | - | - | - |
| Base | 598 | 576 | 577.2 | 568.5 | 566.2 | 2.3 | 68.35 |
| Base + Al2O3 | 590 | 577 | 583.4 | 582.0 | 580.0 | 2.0 | 8.74 |
| Base + TiC | 591 | 578 | 584.2 | 582.3 | 580.4 | 1.9 | 9.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez, A.; Sanchez, J.M.; Girot, F.; Renderos, M.; Egizabal, P. Numerical Modeling of the Influence of Nanometric Ceramic Particles on the Nucleation of AlSi10MnMg Alloy. Metals 2022, 12, 855. https://doi.org/10.3390/met12050855
Jimenez A, Sanchez JM, Girot F, Renderos M, Egizabal P. Numerical Modeling of the Influence of Nanometric Ceramic Particles on the Nucleation of AlSi10MnMg Alloy. Metals. 2022; 12(5):855. https://doi.org/10.3390/met12050855
Chicago/Turabian StyleJimenez, Ane, Jon Mikel Sanchez, Franck Girot, Mario Renderos, and Pedro Egizabal. 2022. "Numerical Modeling of the Influence of Nanometric Ceramic Particles on the Nucleation of AlSi10MnMg Alloy" Metals 12, no. 5: 855. https://doi.org/10.3390/met12050855
APA StyleJimenez, A., Sanchez, J. M., Girot, F., Renderos, M., & Egizabal, P. (2022). Numerical Modeling of the Influence of Nanometric Ceramic Particles on the Nucleation of AlSi10MnMg Alloy. Metals, 12(5), 855. https://doi.org/10.3390/met12050855







