Crystallization Kinetics of Hypo, Hyper and Eutectic Ni–Nb Glassy Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Structural Characterization
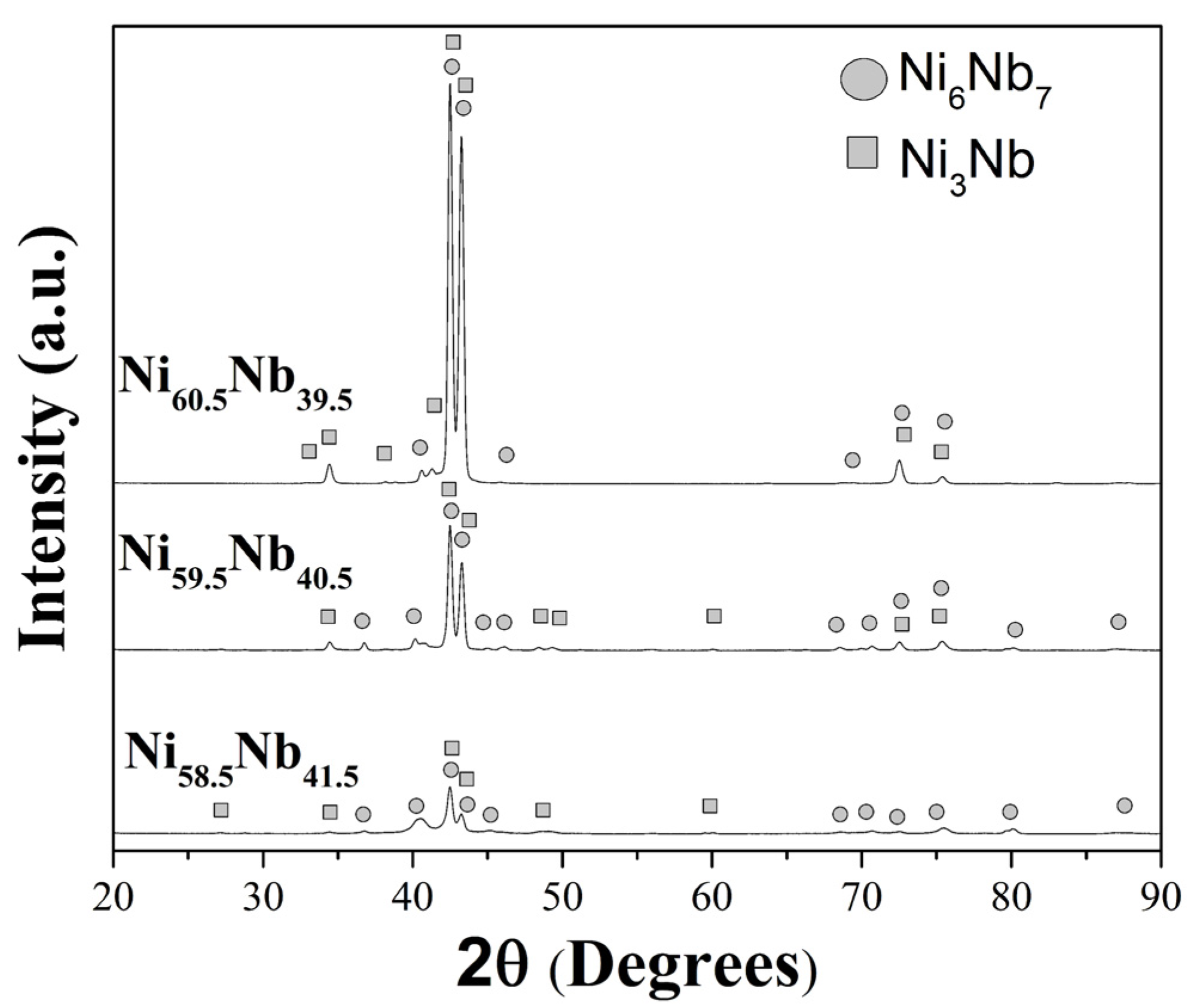
3.1.1. Ingots Ni58.5Nb41.5, Ni59.5Nb40.5, and Ni60.5Nb39.5 Alloys
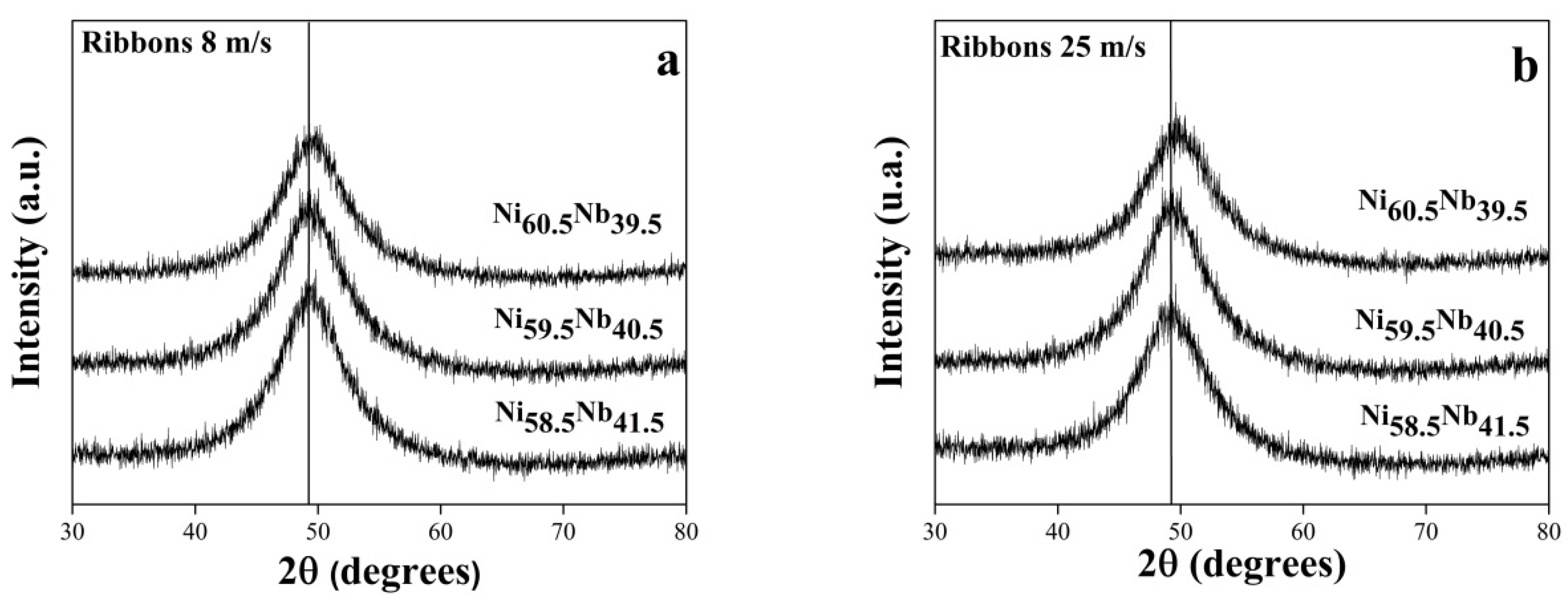
3.1.2. Ribbons Cast at 8 m/s and 25 m/s
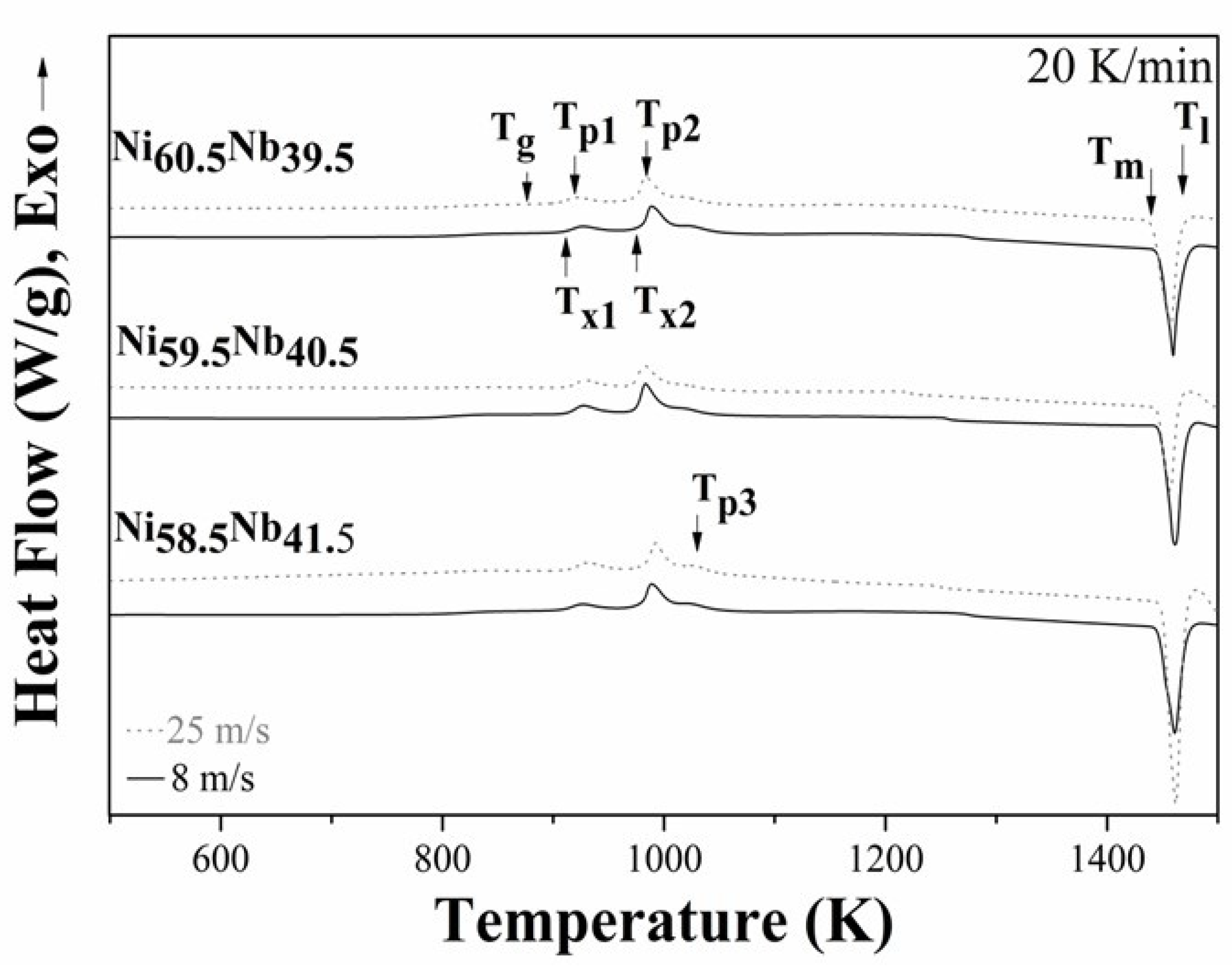
3.2. Thermal Characterization
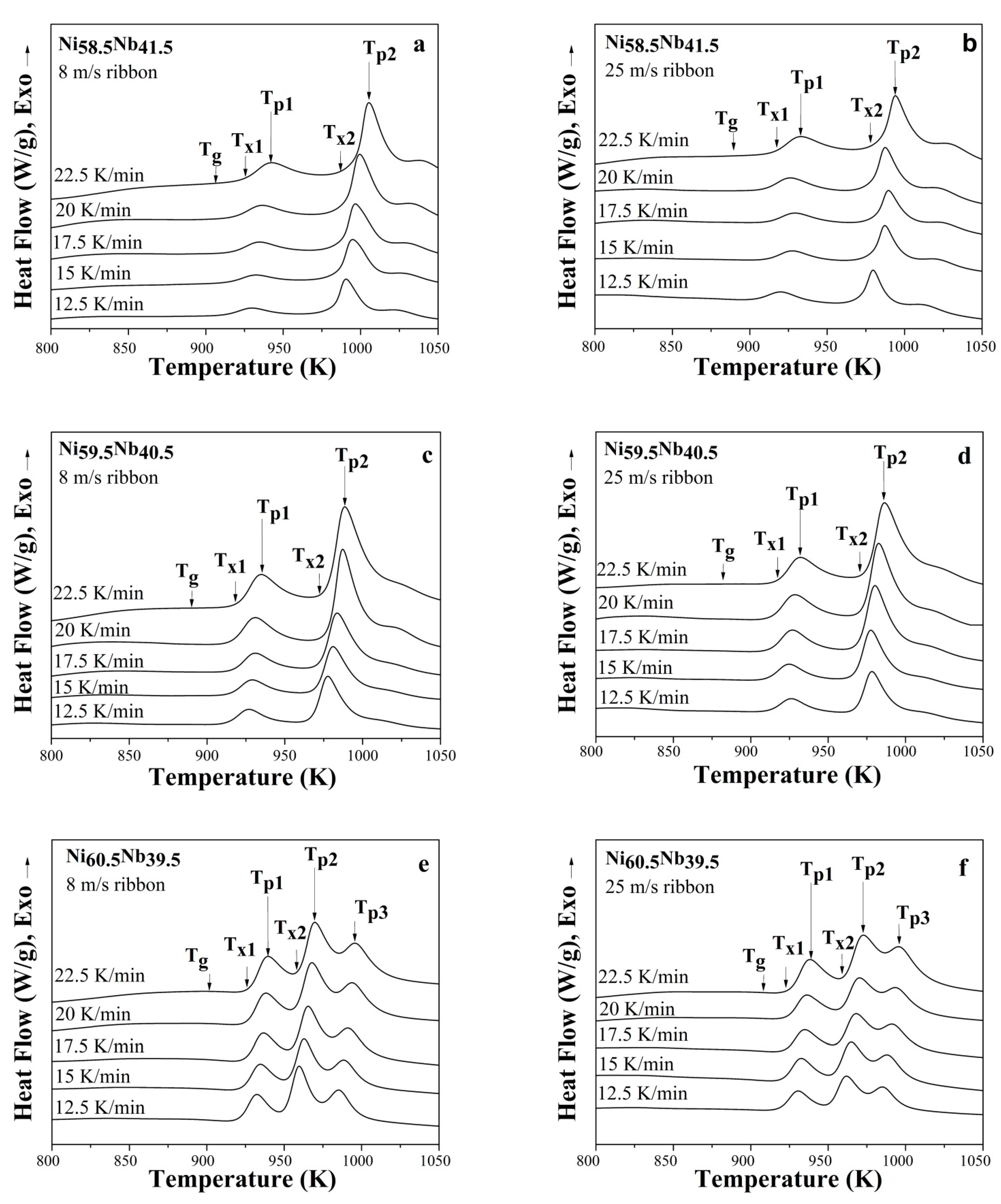
3.2.1. Hypereutectic Ni58.5Nb41.5 Alloy
3.2.2. Eutectic Ni59.5Nb40.5 Alloy
3.2.3. Hypoeutectic Ni59.5Nb40.5 Alloy
3.3. Iso-Conversional Kinetics
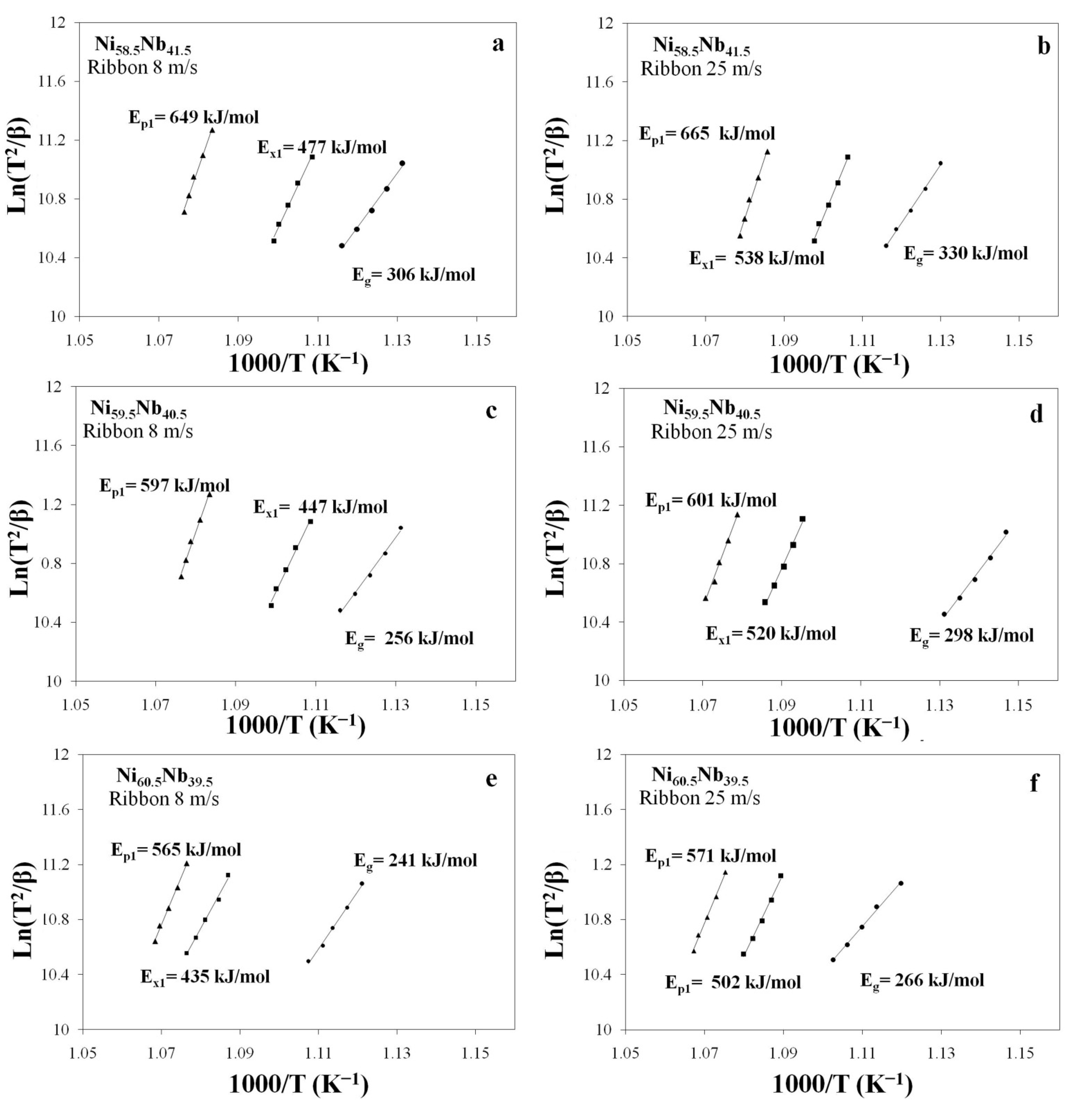
3.3.1. Kissinger Method for Ribbons Cast at 8 and 25 m/s
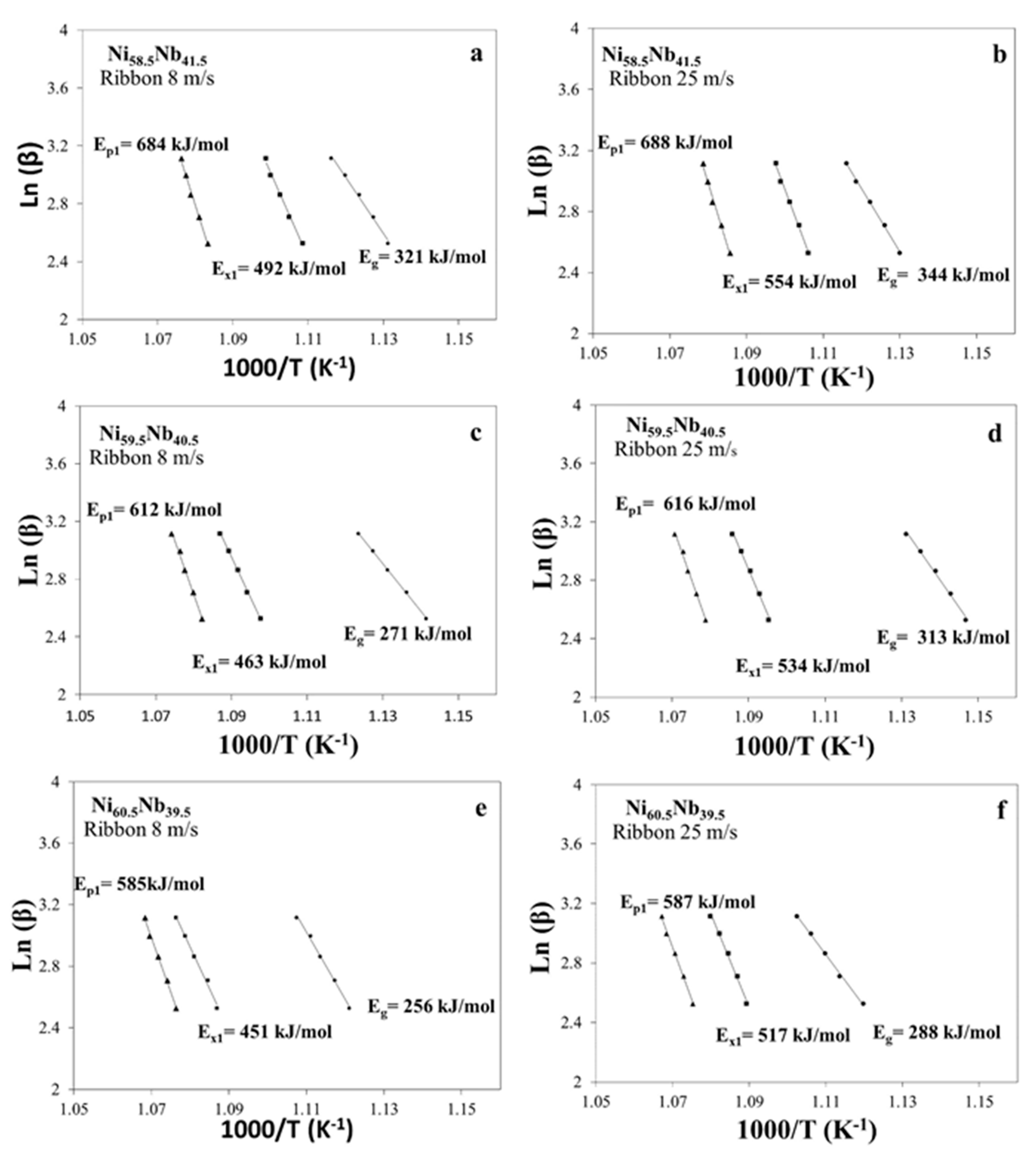
3.3.2. Kissinger Method for Ribbons Cast at 8 and 25 m/s
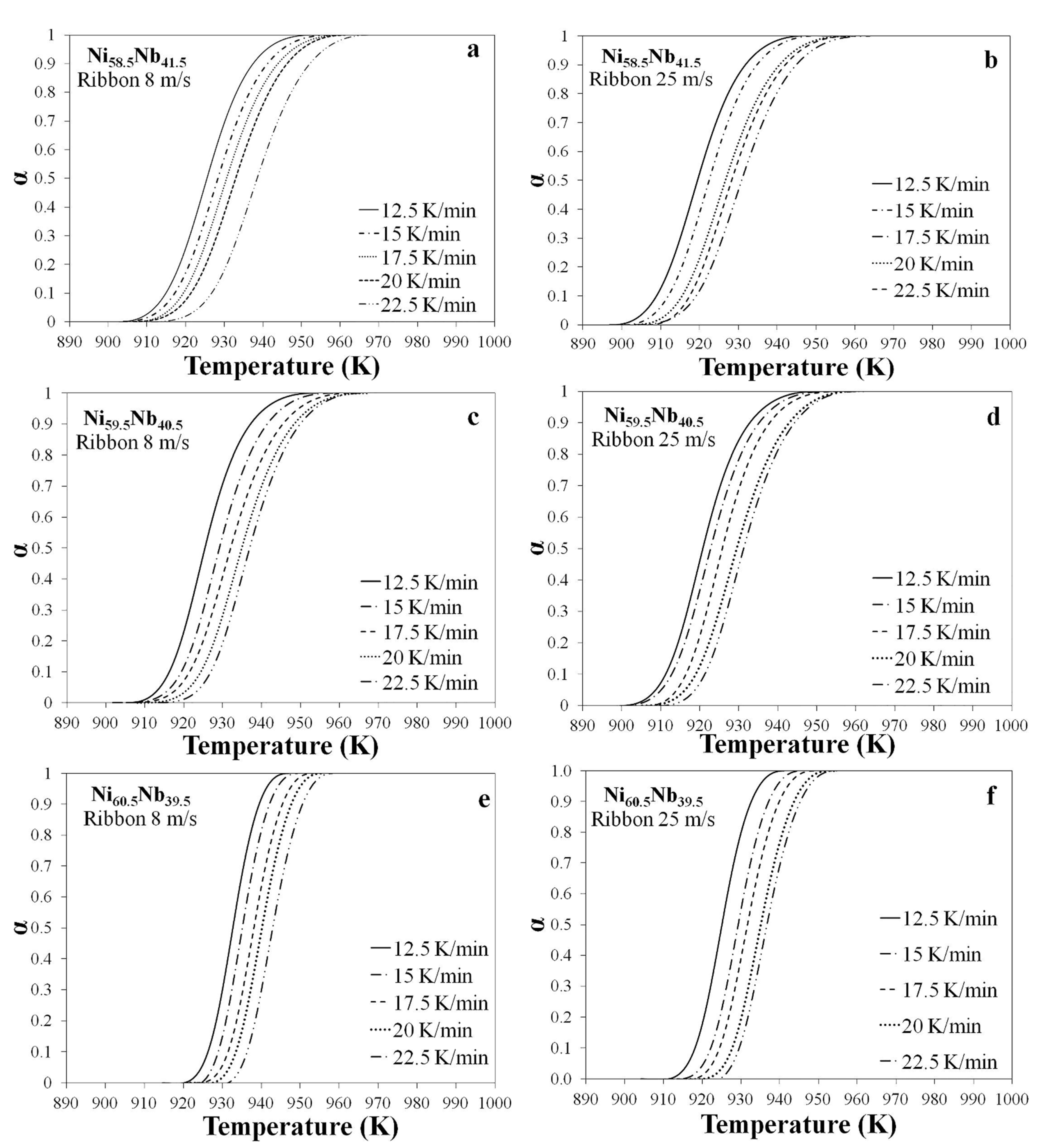
3.4. Crystallization Fraction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oreshkin, A.; Mantesevich, V.; Savinov, S.; Oreshkin, S.; Panov, V.; Maslova, N.; Louzguine-Louzgin, D. Direct visualization of Ni-Nb metallic glasses surface: From initial nucleation to full crystallization. J. Appl. Phys. Lett. 2012, 101, 181601. [Google Scholar] [CrossRef]
- Krimer, Y.; Aronhime, N.; Ohodnicki, P.; McHenry, M.E. Prediction of good glass forming ability in amorphous soft magnetic alloys by thermocalc simulation with experimental validation. J. Alloys Compd. 2020, 814, 152294. [Google Scholar] [CrossRef]
- Zhang, C.; Ji, X.; Wang, J.; Lu, L.; Yang, Z.; Lyu, P.; Guan, Q.; Cai, J. Amorphization and nano-crystallization of Ni-Nb coating on GH3039 alloys by high current pulsed electron beam. Nanomaterials 2021, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- Nagase, T.; Ueda, M.; Umakoshi, Y. Preparation of Ni-Nb-based metallic glass wires by arc-melt-type melt-extraction method. J. Alloys Compd. 2009, 485, 304–312. [Google Scholar] [CrossRef]
- Arai, K.; Zhang, W.; Jia, F.; Inoue, A. Synthesis and thermal stability of new Ni-based bulk glassy alloy with excellent mechanical properties. Mater. Trans. 2006, 17, 2358–2362. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Bae, D.; Kim, W.; Kim, D. Ni-based refractory bulk amorphous alloys with high thermal stability. Mater. Trans. 2003, 4, 2084–2087. [Google Scholar] [CrossRef] [Green Version]
- Suryanarayana, C.; Seki, I.; Inoue, A. A critical analysis of the Glass-forming ability of alloys. J. Non-Cryst. Solids. 2009, 355, 355–360. [Google Scholar] [CrossRef]
- Li, J.H.; Dai, Y.; Cui, Y.Y.; Liu, B.X. Atomistic theory for predicting the binary metallic glass formation. Mater. Sci. Eng. R. 2011, 72, 1–28. [Google Scholar] [CrossRef]
- Amini, N.; Pries, J.; Cheng, Y.; Persch, C.; Wutting, M.; Stolpe, M.; Wei, S. Thermodynamics and kinetics of glassy and liquid phase-change materials. Mater. Sci. Semicond. Process. 2021, 135, 106094. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Kalb, J.; Spaaepen, F. Caloric measurements of phase transformatios in thin films of amorphous Te alloys used for optical data storage. J. Appl. Phys. 2003, 93, 2389. [Google Scholar] [CrossRef]
- Lozada-Flores, O.; Figueroa, I.A.; Lara, G.A.; Gonzalez, G.; Borja-Soto, C.; Verduzco, J.A. Crystallization kinetics of Cu55Hf45 glassy alloy. J. Non-Cryst. Solids. 2017, 460, 1–5. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Li, D.; Liu, F.; Liang, S.H. Crystallization kinetics of Cu33Zr67 amorphous alloy during cooling annealing. Mater. Lett. 2016, 181, 292–295. [Google Scholar] [CrossRef]
- Mukherjee, S.; Zhou, Z.; Johnson, W.L.; Rhim, W.K. Thermophysical properties of Ni-Nb and Ni-Nb-Sn Bulk metallic glass-forming melts by containerless electrostatic levitation processing. J. Non-Cryst. Solids. 2004, 337, 21–28. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, H.; Pan, D.; Sun, W.; Hu, Z. Fabrication of Binary Ni-Nb bulk Metallic Glass with High strength and compressive plasticity. Adv. Eng. Mater. 2006, 8, 953–957. [Google Scholar] [CrossRef]
- Reddy, K.V.; Pal, S. Contribution of Nb towards enhancement of glass forming ability and plasticity of Ni-Nb binary metallic glass. J. Non-Cryst. Solids. 2017, 471, 243–250. [Google Scholar] [CrossRef]
- Kreuch, G.; Hafner, J. Concentration dependence of the local order in amorphous Ni-Nb alloys. J. Non-Cryst. Solids. 1995, 189, 227–241. [Google Scholar] [CrossRef]
- Leonhardt, M.; Löser, W.; Lindenkreuz, H.G. Solidification kinetics and phase formation of undercooled eutectic Ni-Nb melts. Acta Mater. 1999, 47, 2961–2968. [Google Scholar] [CrossRef]
- Xia, L.; Li, W.H.; Fang, S.S.; Wei, B.C.; Dong, Y.D. Binary Ni-Nb bulk metallic glasses. J. Appl. Phys. 2006, 99, 026103. [Google Scholar] [CrossRef] [Green Version]
- Nowosielski, R.; Januszka, A.; Pilarczyk, W. Glass forming ability of binary Ni60+xNb40−x (x = 0; 1; 2) alloys. J. Achiev. Mater. Manuf. Eng. 2010, 42, 73–80. [Google Scholar]
- Lesz, S.; Dercz, G. Study on crystallization phenomenon and thermal stability of binary Ni-Nb amorphous alloy. J. Therm. Anal. Cal. 2016, 126, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Oreshkin, A.I.; Mantsevich, V.N.; Savinov, S.V.; Oreshkin, S.I.; Panov, V.I.; Yavari, A.R.; Miracle, D.B.; Louzguine-Luzgin, D.V. In situ visualization of Ni-Nb bulk metallic glasses phase transition. Acta Mater. 2018, 61, 5216–5222. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.J.; Park, E.S.; Jung, Y.S.; Kim, M.K.; Kim, D.H. The effect of Zr addition in glass forming ability of Ni-Nb alloy system. J. Alloys Compd. 2007, 434–435, 156–159. [Google Scholar] [CrossRef]
- Santos, F.S.; Kiminami, C.S.; Bolfarini, C.; de Oliveira, M.F.; Botta, W.J. Evaluation of glass forming ability in the Ni-Nb-Zr alloy system by the topological instability (λ) criterion. J. Alloys Compd. 2010, 495, 313–315. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Z.; Perepezco, J.H.; Voyles, P.M. Vitrification, crystallization, and atomic structure of deformed and quenched Ni60Nb40 metallic glass. J. Non-Cryst. Solids. 2018, 491, 133–140. [Google Scholar] [CrossRef]
- Enayati, M.H. Crystallization behavior of Ni-Nb amorphous alloys. Sci. Iranica. 2002, 9, 157–161. [Google Scholar]
- Afonso, C.R.M.; Martinez-Orozco, K.; Amigó, V.; Della, C.A.; Spinelli, J.; Kiminami, C.S. Characterization, corrotion resistance and hardness of rapidly solidified Ni-Nb alloys. J. Alloys Compd. 2020, 829, 154529. [Google Scholar] [CrossRef]
- Yuan, C.C.; Yang, Y.F.; Xi, X.K. Atomic and electronic structure of Ni-Nb metallic glasses. J. Appl. Phys. 2013, 114, 213511. [Google Scholar] [CrossRef]
- Xu, T.D.; Wang, X.D.; Zhang, H.; Cao, Q.P.; Zhang, D.X.; Jiang, J.Z. Structural evolution and atomic dynamics in Ni-Nb metallic glasses: A molecular dynamics study. J. Chem. Phys. 2017, 147, 144503. [Google Scholar] [CrossRef]
- Vazquez, J.; Villares, P.; Jiménez-Garay, R. A theoretical method for deducing the evolution with time of the fraction crystallized and obtaining the kinetic parameters by DSC, using non-isothermal techniques. J. Alloys Compd. 1997, 257, 259–265. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Criado, J.M.; Pérez-Maqueda, L.A. Kissinger Kinetic Analysis of Data Obtained Under Different Heating Schedules. J. Therm. Anal. Cal. 2008, 94, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Khond, A.; Adil, S.; Guruvidyatrh, K.; Murty, B.; Majumdar, B.; Bhatt, J.; Srvastav, A. Kinetics and phase formation during crystallization of Hf64Cu18Ni18 amorphous alloy. Phase Transit. 2021, 94, 110–121. [Google Scholar] [CrossRef]
- ICDD. International Center for Difraction Data ICDD. Available online: http://www.icdd.com/ (accessed on 21 February 2022).
- Massalski, T. Binary Alloy Phase Diagrams, 2nd ed.; American Society for Metals: Russell Township: Geauga County, OH, USA, 1986; pp. 1681–1682. [Google Scholar]
- Turnbull, D. Metastable Structures in Metallurgy. Metall. Mater. Trans. A. 1980, 12A, 695–708. [Google Scholar]
- Lu, Z.P.; Liu, C.T. A new glass-forming ability criterion for bulk metallic glasses. Acta Mater. 2002, 50, 3501–3512. [Google Scholar] [CrossRef]
- Opfermann, J.; Kaisersberger, E. An advantageus variant of the Ozawa-Flynn-Wall analysis. Thermochim. Acta. 1992, 203, 167–175. [Google Scholar] [CrossRef]
- Han, X.; Ding, F.; Qin, Y.; Wu, D.; Xing, H.; Shi, Y.; Song, K.; Cao, C. Compositional dependence of crystallization kinetics in Zr-Ni-Al metallic glasses. Vacuum 2018, 151, 30–38. [Google Scholar] [CrossRef]
- Collins, L.E.; Grant, N.J.; Vander, J.B. Crystallization of amorphous Ni60Nb40. J. Mater. Sci. 1983, 18, 804–814. [Google Scholar] [CrossRef]
- Pratten, N.A.; Scott, M.G. Satability of some metalloid-free metallic glasses. Scr. Metall 1977, 12, 2. [Google Scholar]
- Starink, M.J. The determination of activation energy from linear heating rateexperiments: A comparision of the accuracy of isoconversión methods. Thermochim. Acta. 2003, 404, 163–176. [Google Scholar] [CrossRef] [Green Version]
| Alloy | Heating Rate (K/min) | Tg (K) | Tx1 (K) | Tp1 (K) | Tx2 (K) | Tp2 (K) | Tp3 (K) | ΔTx (K) | Tm (K) | Tl (K) | Trg | γ | Thickness (μm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni58.5Nb41.5 | 12.5 | 884 | 902 | 923 | 982 | 990 | --- | 18 | 1447 | 1472 | 0.60 | 0.39 | 105 |
| 15 | 887 | 905 | 925 | 985 | 995 | --- | 18 | 0.60 | 0.39 | ||||
| 17.5 | 890 | 907 | 927 | 988 | 999 | --- | 17 | 0.60 | 0.39 | ||||
| 20 | 893 | 909 | 928 | 991 | 1002 | --- | 16 | 0.61 | 0.39 | ||||
| 22.5 | 896 | 910 | 929 | 993 | 1005 | --- | 14 | 0.61 | 0.39 | ||||
| Ni59.5Nb40.5 | 12.5 | 876 | 911 | 924 | 969 | 977 | --- | 35 | 1445 | 1472 | 0.60 | 0.39 | 130 |
| 15 | 880 | 914 | 926 | 972 | 981 | --- | 34 | 0.60 | 0.39 | ||||
| 17.5 | 884 | 916 | 928 | 974 | 985 | --- | 32 | 0.60 | 0.39 | ||||
| 20 | 887 | 918 | 929 | 976 | 988 | --- | 31 | 0.60 | 0.39 | ||||
| 22.5 | 890 | 920 | 931 | 978 | 991 | --- | 30 | 0.60 | 0.39 | ||||
| Ni60.5Nb39.5 | 12.5 | 887 | 920 | 929 | 951 | 960 | 977 | 28 | 1449 | 1474 | 0.60 | 0.39 | 140 |
| 15 | 891 | 922 | 931 | 954 | 963 | 980 | 27 | 0.60 | 0.39 | ||||
| 17.5 | 895 | 925 | 933 | 956 | 965 | 983 | 27 | 0.61 | 0.39 | ||||
| 20 | 899 | 927 | 935 | 958 | 968 | 986 | 27 | 0.61 | 0.39 | ||||
| 22.5 | 902 | 929 | 936 | 960 | 970 | 988 | 26 | 0.61 | 0.39 |
| Alloy | Heating Rate (K/min) | Tg (K) | Tx1 (K) | Tp1 (K) | Tx2 (K) | Tp2 (K) | Tp3 (K) | ΔTx (K) | Tm (K) | Tl (K) | Trg | γ | Thickness (μm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni58.5Nb41.5 | 12.5 15 17.5 20 22.5 | 885 888 891 894 896 | 904 906 908 910 911 | 921 923 925 926 927 | 969 977 979 975 982 | 980 987 990 987 994 | --- --- --- --- --- | 19 18 17 16 15 | 1450 | 1471 | 0.60 0.60 0.60 0.61 0.61 | 0.38 0.38 0.38 0.38 0.38 | 45 |
| Ni59.5Nb40.5 | 12.5 15 17.5 20 22.5 | 872 875 878 881 884 | 913 915 917 919 921 | 927 929 931 932 934 | 968 972 967 972 978 | 979 982 977 982 989 | --- --- --- --- --- | 41 40 39 38 37 | 1447 | 1466 | 0.60 0.60 0.60 0.60 0.60 | 0.39 0.39 0.39 0.39 0.39 | 50 |
| Ni60.5Nb39.5 | 12.5 15 17.5 20 22.5 | 893 898 901 904 907 | 918 920 922 924 926 | 930 932 934 936 937 | 962 964 967 970 972 | 986 988 992 994 997 | 995 997 1003 1007 1012 | 25 22 21 20 19 | 1450 | 1469 | 0.61 0.61 0.61 0.61 0.61 | 0.39 0.39 0.39 0.39 0.39 | 60 |
| Alloy | Ribbon (m/s) | Eg | Ex1 | Ep1 |
|---|---|---|---|---|
| (kJ/mol) | ||||
| Ni58.5Nb41.5 | 8 | 306 | 477 | 649 |
| 25 | 330 | 538 | 665 | |
| Ni59.5Nb40.5 | 8 | 256 | 447 | 597 |
| 25 | 298 | 520 | 601 | |
| Ni60.5Nb39.5 | 8 | 241 | 435 | 565 |
| 25 | 266 | 502 | 571 | |
| Alloy | Ribbon (m/s) | Eg | Ex1 | Ep1 |
|---|---|---|---|---|
| (kJ/mol) | ||||
| Ni58.5Nb41.5 | 8 | 321 | 492 | 684 |
| 25 | 344 | 554 | 688 | |
| Ni59.5Nb40.5 | 8 | 271 | 463 | 612 |
| 25 | 313 | 534 | 616 | |
| Ni60.5Nb39.5 | 8 | 256 | 451 | 585 |
| 25 | 288 | 517 | 587 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza, L.E.; Hernández, J.M.; González, J.G.; Orgaz, E.; Lozada, O.; Figueroa, I.A. Crystallization Kinetics of Hypo, Hyper and Eutectic Ni–Nb Glassy Alloys. Metals 2022, 12, 808. https://doi.org/10.3390/met12050808
Mendoza LE, Hernández JM, González JG, Orgaz E, Lozada O, Figueroa IA. Crystallization Kinetics of Hypo, Hyper and Eutectic Ni–Nb Glassy Alloys. Metals. 2022; 12(5):808. https://doi.org/10.3390/met12050808
Chicago/Turabian StyleMendoza, Laura Esmeralda, José Manuel Hernández, José Gonzalo González, Emilio Orgaz, Octavio Lozada, and Ignacio Alejandro Figueroa. 2022. "Crystallization Kinetics of Hypo, Hyper and Eutectic Ni–Nb Glassy Alloys" Metals 12, no. 5: 808. https://doi.org/10.3390/met12050808
APA StyleMendoza, L. E., Hernández, J. M., González, J. G., Orgaz, E., Lozada, O., & Figueroa, I. A. (2022). Crystallization Kinetics of Hypo, Hyper and Eutectic Ni–Nb Glassy Alloys. Metals, 12(5), 808. https://doi.org/10.3390/met12050808







