Jarosites: Formation, Structure, Reactivity and Environmental
Abstract
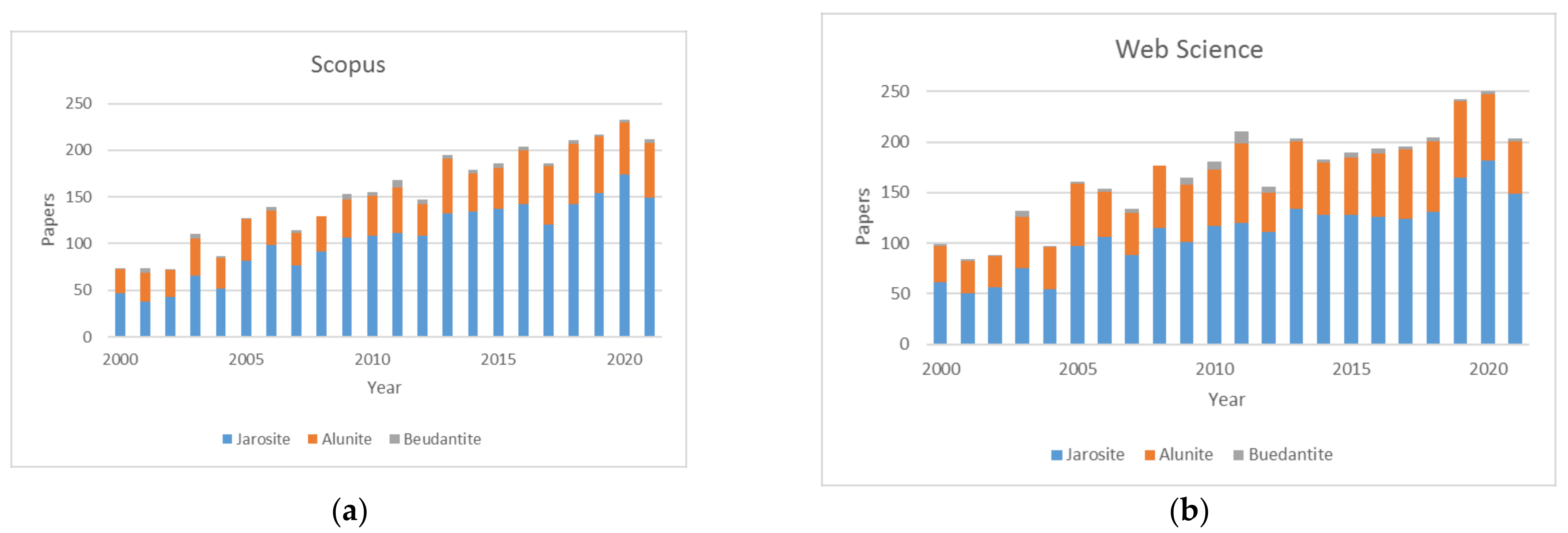
1. Introduction
2. Formation of Jarosites
2.1. Natural Jarosites
2.2. Synthetic Jarosites
2.2.1. Chemical Synthesis of Jarosite
2.2.2. Biological Synthesis of Jarosite
2.2.3. Synthesis of Jarosite in Hydrometallurgical Circuits
2.2.4. Synthesis of Alunite
3. Structure and Thermodynamics of Jarosite–Beudantite–Alunite Solids
3.1. Structure of Jarosite-Type Compounds
3.2. Thermodynamics of Jarosites
4. Reactivity of Jarosites
4.1. Reactivity in Alkaline Media
4.2. Reactivity in Acid Media
4.3. Transformation by Thermal Treatments
5. Immobilization of Metals in Alunite-Type Structures
6. Jarosite Passivation of Chalcopyrite during Leaching or Bioleaching
7. Jarosites on Mars
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutrizac, J.E.; Kaiman, S. Synthesis and properties of jarosite-type compounds. Can. Mineral. 1976, 14, 151–158. [Google Scholar]
- Jambor, J.L. Nomenclature of the alunite supergroup. Can. Mineral. 1999, 37, 1323–1341. [Google Scholar]
- Botinelly, T. A review of the minerals of the alunite–jarosite, beudantite, and plumbogummite groups. J. Res. US Geol. Surv. 1976, 4, 213–216. [Google Scholar]
- Scott, K.M. Nomenclature of the alunite supergroup: Discussion. Can. Mineral. 2000, 38, 1295–1297. [Google Scholar] [CrossRef]
- Smith, D.K.; Roberts, A.C.; Bayliss, P.; Liebau, F. A systematic approach to general and structure-type formulas for minerals and other inorganic phases. Am. Mineral. 1998, 83, 126–132. [Google Scholar] [CrossRef]
- Viñals, J.; Sunyer, A.; Torres, E.; Beltran, V.; Llorca, N. Arsenic inertization from copper pyrometallurgy through phases of the alunite supergroup. World Metall.-Erzmetall 2010, 63, 310. [Google Scholar]
- Sunyer, A.; Viñals., J. Arsenate substitution in natroalunite: A Potential Medium for Arsenic Immobilization. Part 1: Synthesis and Compositions. Hydrometallurgy 2011, 109, 54–64. [Google Scholar] [CrossRef]
- Eftekhari, N.; Kargar, M.; Zamin, F.R.; Rastakhiz, N.; Manofi, Z. A review on various aspects of jarosite and its utilization potentials. Ann. Chim. Sci. Mater. 2020, 44, 43–52. [Google Scholar] [CrossRef]
- Das, G.K.; Acharya, S.; Anand, S.; Das, R.P. Jarosites: A Review. Miner. Proccess. Extr. Metall. Rev. 1996, 16, 185–210. [Google Scholar] [CrossRef]
- Hoeber, L.; Steinlechner, S. A comprehensive review of processing strategies for iron precipitation, residues from zinc hydrometallurgy. Clean. Eng. Technol. 2021, 4, 100214. [Google Scholar] [CrossRef]
- Smith, A.M.L.; Hudson-Edwards, K.A.; Dubbin, W.E.; Wright, K. Dissolution of jarosite [KFe3(SO4)2(OH)6] at pH 2 and 8: Insights from Batch Experiments and Computational Modelling. Geochim. Cosmochim. Acta 2006, 70, 608–621. [Google Scholar] [CrossRef]
- Hofstra, A.H.; Snee, L.W.; Rye, R.O.; Folger, H.W.; Phinisey, J.D.; Loranger, R.J.; Dahl, A.R.; Naeser, C.W.; Stein, H.J.; Lewchuk, M.T. Age constraints on Jerritt Canyon and other Carlin-type gold deposits in the Western United States: Relationship to mid-Tertiary extension and magmatism. Econ. Geol. 1999, 94, 769–802. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Jambor, J. Jarosites and their application in Hydrometallurgy. Rev. Mineral. Geochem. 2000, 40, 405–452. [Google Scholar] [CrossRef]
- Velasco, F.; Herrero, J.M.; Suárez, S.; Yusta, I.; Alvaro, A.; Tornos, F. Supergene features and evolution of gossans capping massive sulphide deposits in the Iberian Pyrite Belt. Ore Geol. Rev. 2013, 53, 181–203. [Google Scholar] [CrossRef]
- Viñals, J.; Roca, A.; Cruells, M.; Núñez, C. Characterization and cyanidation of Rio Tinto gossan ores. Can. Metall. Q. 1995, 34, 115–122. [Google Scholar] [CrossRef]
- Roca, A.; Viñals, J.; Arranz, M.; Calero, J. Characterization and Alkaline Decomposition/Cyanidation of Beudantite–Jarosite Materials from Rio Tinto Gossan Ores. Can. Metall. Q. 1999, 38, 93–103. [Google Scholar] [CrossRef]
- Savage, K.S.; Bird, D.K.; O’Day, P.A. Arsenic speciation in synthetic jarosite. Chem. Geol. 2005, 215, 473–498. [Google Scholar] [CrossRef]
- West, L.; McGown, D.J.; Onstott, T.C.; Morris, R.V.; Suchecki, P.; Pratt, L.M. High Lake gossan deposit: An Arctic Analogue for Ancient Martian Surficial Processes? Planet. Space Sci. 2009, 57, 1302–1311. [Google Scholar] [CrossRef]
- Dill, H.G.; Melcher, F.; Kaufhold, S.; Techmer, A.; Weber, B.; Bäumler, W. Post-Miocene and Bronze-age supergene Cu-Pb arsenate-humate-oxalate-carbonate mineralization at Mega Livadi, Serifos, Greece. Can. Mineral. 2010, 48, 163–181. [Google Scholar] [CrossRef]
- Reynolds, G.A. The Jarosite Group of Compounds—Stability, Decomposition and Conversion. Master’s Thesis, University of Newcastle, Newcastle, NSW, Australia, 2007. Available online: https://nova.newcastle.edu.au/vital/access/services/Download/uon:743/ATTACHMENT02 (accessed on 24 March 2022).
- Holley, E.A.; Monecke, T.; Bissig, T.; Reynolds, T.J. Evolution of high-level magmatic-hydrothermal systems: New Insights from Ore Paragenesis of the Veladero High-Sulfidation Epithermal Au–Ag Deposit, El Indio-Pascua Belt, Argentina. Econ. Geol. 2017, 112, 1747–1771. [Google Scholar] [CrossRef]
- Cutruneo, C.M.N.L.; Oliveira, M.L.S.; Ward, C.R.; Hower, J.C.; de Brum, I.A.S.; Sampaio, C.H.; Kautzmann, R.M.; Taffarel, S.R.; Teixeira, E.C.; Silva, L.F.O. A mineralogical and geochemical study of three Brazilian coal cleaning rejects: Demonstration of electron beam applications. Int. J. Coal Geol. 2014, 130, 33–52. [Google Scholar] [CrossRef]
- Montero, I.C.; Brimhall, G.H.; Alpers, C.N.; Swayze, G.A. Characterization of waste rock associated with acid drainage at the Penn Mine, California, by ground-based visible to short-wave infrared reflectance spectroscopy assisted by digital mapping. Chem. Geol. 2005, 215, 453–472. [Google Scholar] [CrossRef]
- Acero, P.; Ayora, C.; Torrentó, C.; Nieto, J.M. The behavior of trace elements during schwertmannite precipitation and subsequent transformation into goethite and jarosite. Geochim. Cosmochim. Acta 2006, 70, 4130–4139. [Google Scholar] [CrossRef]
- Smith, A.M.; Dubbin, W.E.; Wright, K.; Hudson-Edwards, K.A. Dissolution of lead- and lead-arsenic-jarosites at pH 2 and 8 and 20 °C: Insights from batch experiments. Chem. Geol. 2006, 229, 344–361. [Google Scholar] [CrossRef]
- Azzali, E.; Marescotti, P.; Frau, F.; Dinelli, E.; Carbone, C.; Capitani, G.; Luchetti, G. Mineralogical and chemical variations of ochreous precipitates from acid sulphate waters/asw) at the Rosia Montana gold mine (Romania). Environ. Earth Sci. 2014, 72, 3567–3584. [Google Scholar] [CrossRef]
- Malakooti, S.J.; Shahhosseini, M.; Ardejani, F.D.; Tonkaboni, S.Z.S.; Noaparast, M. Hydrochemical characterisation of water quality in the Sarcheshmeh copper complex, SE Iran. Environ. Earth Sci. 2015, 74, 3171–3190. [Google Scholar] [CrossRef]
- Liu, J.; He, L.; Dong, F.; Hudson-Edwards, K.A. The role of nano-sized manganese coatings on bone char in removing arsenic (V) from solution: Implications for Permeable Reactive Barrier Technologies. Chemosphere 2016, 153, 146–154. [Google Scholar] [CrossRef]
- Zhou, L.; Dong, F.; Liu, J.; Hudson-Edwards, K.A. Coupling effect of Fe3+(aq) and biological, nano-sized FeS-coated limestone on the removal of redox-sensitive contaminants (As,Sb and Cr): Implications for In Situ Passive Treatment of Acid Mine Drainage. Appl. Geochem. 2017, 80, 102–111. [Google Scholar] [CrossRef]
- Mitscherlich. J. Prakt. Chem. Leipz. 1861, 83, 474. Available online: https://www.mindat.org/min-10725.html (accessed on 24 March 2022).
- Fairchild, J.G. Artificial Jarosites-the separation of potassium from Cesium. Am. Mineral. J. Earth Planet. Mater. 1933, 18, 543–547. Available online: http://www.minsocam.org/ammin/AM18/AM18_543.pdf (accessed on 24 March 2022).
- Dutrizac, J.E. Factors affecting alkali jarosite precipitation. Metall. Trans. B 1983, 14, 531–539. [Google Scholar] [CrossRef]
- Hernández-Lazcano, E.; Cerecedo-Sáenz, E.; Hernández-Ávila, J.; Toro, N.; Karthik, T.V.K.; Mendoza-Anaya, D.; Fernández-García, M.E.; Rodríguez-Lugo, V.; Salinas-Rodríguez, E. Synthesis of Hydronium-Potassium Jarosites: The Effect of pH and Aging Time on Their Structural, Morphological, and Electrical Properties. Minerals 2021, 11, 80. [Google Scholar] [CrossRef]
- Cruells, M.; Roca, A.; Patiño, F.; Salinas, E.; Rivera, I. Cyanidation kinetics of argentian jarosite in alkaline media. Hydrometallurgy 2000, 55, 153–163. [Google Scholar] [CrossRef]
- Dutrizac, J.E. The effect of seeding on the rate of precipitation of ammonium jarosite and sodium jarosite. Hydrometallurgy 1996, 42, 293–312. [Google Scholar] [CrossRef]
- Dutrizac, J.E. Comparative rates of precipitation of ammonium jarosite and sodium jarosite in ferric sulfate-sulfuric acid media. Can. Metall. Q. 2010, 49, 121–130. [Google Scholar] [CrossRef]
- Patiño, F.; Salinas, E.; Cruells, M.; Roca, A. Alkaline decomposition-cyanidation kinetics of argentian natrojarosite. Hydrometallurgy 1998, 49, 323–336. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Chen, T.T. The behaviour of scandium, yttrium and uranium during jarosite precipitation. Hydrometallurgy 2009, 98, 128–135. [Google Scholar] [CrossRef]
- Dutrizac, J.E. Factors affecting the precipitation of potassium jarosite in sulfate and chloride media. Metall. Trans. B 2008, 39, 771–783. [Google Scholar] [CrossRef]
- Roca, A.; Patiño, F.; Viñals, J.; Núñez, C. Alkaline decomposition-cyanidation kinetics of argentojarosite. Hydrometallurgy 1993, 33, 341–357. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Dinardo, O.; Kaiman, S. Factors affecting lead jarosite formation. Hydrometallurgy 1980, 5, 305–324. [Google Scholar] [CrossRef]
- Alcobé, X.; Bassas, J.; Tarruella, I.; Roca, A.; Viñals, J. Structural characterization of synthetic beudantite-type phases by Rietveld refinement. Mater. Sci. Forum 2001, 378, 671–676. [Google Scholar] [CrossRef]
- Islas, H.; Patiño, F.; Flores, M.U.; Reyes, I.A.; Reyes, M.C.M.; Hernández, J. Synthesis and characterization of beudantite. Eur. Metall. Conf. EMC 2013, 1, 303–312. Available online: https://www.researchgate.net/profile/Ivan-Reyes-8/publication/273319348_Synthesis_and_Characterization_of_Beudantite/links/54fe09070cf2741b69ef9a71/Synthesis-and-Characterization-of-Beudantite.pdf (accessed on 24 March 2022).
- Eftekhari, N.; Kargar, M. Assessment of optimal iron concentration in the precipitation of jarosite and the activity of acidithiobacillus ferroxidans. J. Biotechnol. 2018, 9, 525–529. [Google Scholar]
- Wang, H.; Bigham, J.M.; Jones, F.S.; Tuovinen, O.H. Synthesis and properties of ammoniojarosites prepared with iron-oxidizing acidophilic microorganisms at 22–65 °C. Geochim. Cosmochim. Acta 2007, 71, 155–164. [Google Scholar] [CrossRef]
- Bigham, J.M.; Jones, F.S.; Özkaya, B.; Sahinkaya, E.; Puhakka, J.A.; Tuovinen, O.H. Characterization of jarosites produced by chemical synthesis over a temperature gradient from 2 to 40 °C. Int. J. Miner. Proccess. 2010, 94, 121–128. [Google Scholar] [CrossRef]
- Bigham, J.M.; Algur, Ö.F.; Jones, F.S.; Tuovinen, O.H. Solid-phase controls on lead partitioning in laboratory bioleaching solutions. Hydrometallurgy 2013, 136, 27–30. [Google Scholar] [CrossRef]
- Jones, F.S.; Bigham, J.M.; Gramp, J.P.; Tuovinen, O.H. Synthesis and properties of ternary (K,NH4,H3O)-jarosites precipitated from Acidithiobacillus ferrooxidans cultures in simulated bioleaching solutions. Mater. Sci. Eng. C 2014, 44, 391–399. [Google Scholar] [CrossRef]
- Crabbe, H.; Fernandez, N.; Jones, F. Crystallization of jarosite in the presence of amino acids. J. Cryst. Growth 2015, 416, 28–33. [Google Scholar] [CrossRef]
- Sinclair, S.J. The Extractive Metallurgy of Zinc, 1st ed.; Spectrum Series; The Australasian Institute of Mining and Metallurgy: Victoria, Australia, 2005; Volume 13. [Google Scholar]
- Han, H.; Sun, W.; Hu, Y.; Jia, B.; Tang, H. Anglesite and silver recovery from jarosite residues through roasting and sulfidization-flotation in zinc hydrometallurgy. J. Hazard. Mater. 2014, 278, 49–54. [Google Scholar] [CrossRef]
- Kerolli-Mustafa, M.; Mandić, V.; Ćurković, L.; Šipušić, J. Investigation of thermal decomposition of jarosite tailing waste—A prerequisite for comprehensive jarosite reuse and waste minimization. J. Therm. Anal. Calorim. 2016, 123, 421–430. [Google Scholar] [CrossRef]
- Antrekowitsch, J. Recycling of Poly-Metallic Residues from Metal Industry—Current Status and Future Developments. In REWAS 2016; Blanplain, B., Meskers, C., Olivetti, E., Apelian, D., Mishra, B., Howarter, J., Kvithyld, A., Neelameggham, N.R., Spangenberger, J., Eds.; TMS Series; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Steinlechner, S. Present status in the recycling of industrial residues from lead, zinc and copper industry and their possible future contribution to the supply of selected minor elements, like silver. In Proceedings of the METAL 2017—26th International Conference on Metallurgy and Materials, Brno, Czech Republic, 24–26 May 2017; pp. 1469–1474. Available online: http://www.scopus.com/inward/record.url?scp=85043358656&partnerID=8YFLogxK. (accessed on 24 March 2022).
- Wu, J.; Chai, L.; Lin, Z.; Wei, Y.; Shi, M.; Peng, J.; Peng, N.; Yan, X. Fe (II)-induced transformation of Jarosite residues generated from zinc hydrometallurgy: Influence on Metals Behaviors During Acid Washing. Hydrometallurgy 2020, 200, 105523. [Google Scholar] [CrossRef]
- Paszkowicz, W.; Dynowska, E.; Świerkocki, J. X-ray study of synthetic alunite. Acta Phys. Pol. A 1994, 86, 621–627. Available online: https://www.infona.pl/resource/bwmeta1.element.bwnjournal-article-appv86z420kz. [CrossRef]
- Luo, Z.Q.; Zhou, X.T.; Jia, Q.M.; Chen, X.F.; Tao, Z.C.; Liu, S.Q. Preparation of arsenical-natroalunite solid solutions with high crystallinity by hydrothermal method. Mater. Res. Innov. 2015, 19, 26–29. [Google Scholar] [CrossRef]
- Wang, L.; Xue, N.; Zhang, Y.; Hu, P. Controlled Hydrothermal Precipitation of Alunite and Natroalunite in High-Aluminum Vanadium-Bearing Aqueous System. Minerals 2021, 11, 892. [Google Scholar] [CrossRef]
- Zhu, Y.; Xuan, H.; Liang, Y.; Yan, Q.; Zhu, Z.; Jiang, Z.; Zhang, L.; Liu, L.; Tang, S. Dissolution and Solubility of the Synthetic Natroalunite and the Arsenic-Incorporated Natroalunite at pH of 2.00–5.60 and 25–45 °C. J. Chem. 2019, 2019, 9568360. [Google Scholar] [CrossRef]
- Cloutis, E.A.; Hawthorne, F.C.; Mertzman, S.A.; Krenn, K.; Craig, M.A.; Marcino, D.; Methot, M.; Strong, J.; Mustard, J.F.; Blaney, D.L.; et al. Detection and discrimination of sulfate minerals using reflectance spectroscopy. Icarus 2006, 184, 121–157. [Google Scholar] [CrossRef]
- Aguilar-Carrillo, J.; Villalobos, M.; Pi-Puig, T.; Escobar-Quiroz, I.N.; Romero, F.M. Synergistic arsenic (V) and lead (II) retention on synthetic jarosite. Simultaneous structural incorporation behaviour and mechanism. Enviromental. Sci. Process Impact 2018, 20, 354–369. [Google Scholar] [CrossRef]
- Szymanski, J.T. The Crystal structure of beudantite, Pb(Fe,Al)3[(As,S)O4](OH)6. Can. Mineral. 1988, 26, 923–932. [Google Scholar]
- Frost, R.L.; Wills, R.A.; Weier, M.L.; Martens, W.; Mills, S. A Raman spectroscopic study of selected natural jarosites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 63, 18. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, Y.; Zhang, J.; Wang, Y.; Hickmott, D.D.; Daemen, L.L.; Hartl, M.A.; Wang, L. Anisotropic elasticity of jarosite: A high-P synchrotron XRD study. Am. Mineral. 2010, 95, 19–23. [Google Scholar] [CrossRef]
- Sunyer, A.; Viñals, J. Arsenate substitution in natroalunite: A Potential Mèdium for Arsenic Immobilization. Part 2: Cell Parameters and Stability Tests. Hydrometallurgy 2011, 109, 106–115. [Google Scholar] [CrossRef]
- Frost, R.L.; Wain, D. A Thermogravimetric and Infrared Emission Spectros-copic Study of Alunite. J. Therm. Anal. Calorim. 2008, 91, 267–274. [Google Scholar] [CrossRef]
- Drouet, C.; Navrotsky, A. Synthesis, characterization and thermochemistry of K-Na_H3O jarosites. Geochim. Cosmochim. Acta 2003, 67, 2063–2076. [Google Scholar] [CrossRef]
- Perez-Labra, M.; Romero-Serrano, A.; Salinas-Rodriguez, E.; Avila-Dávila, E.O.; Reyes-Pérez, M. Synthesis, thermodynamics and kinetics of rubidium jarosite decomposition in calcium hydroxide solutions. Metall. Mater. Trans. B 2013, 43, 773–780. [Google Scholar] [CrossRef]
- Forray, F.L.; Smith, A.M.L.; Drouet, C.; Navrotsky, A.; Wright, K.; Hudson-Edwards, K.A.; Dubbin, W.E. Synthesis, characterization and thermo-chemistry of a Pb-jarosite. Geochim. Cosmochim. Acta 2010, 74, 215–224. [Google Scholar] [CrossRef]
- Forray, F.L.; Smith, A.M.L.; Navrotsky, A.; Wright, K.; Hudson-Edwards, K.A.; Dubbin, W.E. Synthesis, Characterization and Thermochemistry of Synthetic Pb-As, Pb-Cu and Pb-Zn Jarosites. Geochim. Cosmochim. Acta 2014, 127, 107–119. [Google Scholar] [CrossRef]
- Patiño, F.; Viñals, J.; Roca, A.; Núñez, C. Alkaline decomposition-cyanidation kinetics of argentian plumbojarosite. Hydrometallurgy 1994, 34, 279–291. [Google Scholar] [CrossRef]
- Roca, A.; Cruells, M.; Patiño, F.; Rivera, I.; Plata, M. Kinetic model for the cyanidation of silver ammonium jarosite in NaOH medium. Hydrometallurgy 2006, 81, 15–23. [Google Scholar] [CrossRef]
- Patiño, F.; Cruells, M.; Roca, A.; Salinas, E.; Pérez, M. Kinetics of alkaline decomposition and cyanidation of argentian ammonium jarosite in lime medium. Hydrometallurgy 2003, 70, 153–161. [Google Scholar] [CrossRef]
- Salinas, E.; Roca, A.; Cruells, M.; Patiño, F.; Córdoba, D.A. Characterization and alkaline decomposition-cyanidation kinetics of industrial ammonium jarosite in NaOH media. Hydrometallurgy 2001, 60, 237–246. [Google Scholar] [CrossRef]
- Ordóñez, S.; Flores, M.U.; Patiño, F.; Reyes, I.A.; Islas, H.; Reyes, M.; Méndez, E.; Palacios, E.G. Kinetic Analysis of the Decomposition Reaction of the Mercury Jarosite in NaOH Medium. Int. J. Chem. Kinet. 2017, 49, 798–809. [Google Scholar] [CrossRef]
- Salinas, E.; Cerecedo, E.; Ramirez, M.; Patiño, F.; Pérez, M. Kinetics of Alkaline Decomposition and Cyaniding of Argentian Rubidium Jarosite in NaOH Medium. Metall. Mater. Trans. B-Process Metall. Mater. Proccess. Sci. 2012, 43, 1027–1033. [Google Scholar] [CrossRef]
- Cerecedo, E.; Salinas, E.; Longoria, L.C.; Carrillo, F.R.; Hernández, J. Kinetics Study of Alkaline Decomposition of Rubidium Jarosite in Ca(OH)2. In Proceedings of the 2011 TMS Annual Meeting & Exhibition, Symposium: Hydrometallurgy Fundamentals and Applications, San Diego, CA, USA, 27 February–3 March 2011. [Google Scholar] [CrossRef]
- Reyes, I.A.; Mireles, I.; Patiño, F.; Pandiyan, T.; Flores, M.U.; Palacios, E.G.; Gutiérrez, E.J.; Reyes, M. A study on the dissolution rates of K-Cr(VI)-jarosites: Kinetic Analysis and Implications. Geochem. Trans. 2016, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Patiño, F.; Reyes, I.A.; Flores, M.U.; Pandiyan, T.; Roca, A.; Reyes, M.; Hernández, J. Kinetic modeling and experimental design of the sodium arsenojarosite decomposition in alkaline media: Implications. Hydrometallurgy 2013, 137, 115–125. [Google Scholar] [CrossRef]
- Patiño, F.; Flores, M.U.; Reyes, I.A.; Reyes, M.; Hernández, J.; Rivera, I.; Juárez, J.C. Alkaline decomposition of synthetic jarosite with arsenic. Geochem. Trans. 2013, 14, 2. Available online: https://geochemicaltransactions.biomedcentral.com/articles/10.1186/1467-4866-14-2. (accessed on 24 March 2022). [CrossRef] [PubMed]
- Patiño, F.; Flores, M.U.; Reyes, I.A.; Ordóñez, S.; Méndez, J.E.; Flores, V.H.; Islas, H.; Reyes, M. Kinetic modeling of the decomposition of beudantite in NaOH medium. React. Kinet. Mech. Catal. 2016, 119, 367–379. [Google Scholar] [CrossRef]
- Viñals, J.; Roca, A.; Cruells, M.; Núñez, C. Recovery of Gold and Silver from Plumbojarosite-Containing Hematite Tailings by Alkaline Pretreatment and Cyanidation. In EMC’91: Non-Ferrous Metallurgy, Present and Future; Springer: Dordrecht, The Netherlands, 1991; pp. 11–18. [Google Scholar]
- González-Ibarra, A.A.; Nava-Alonso, F.; Fuentes-Aceituno, J.C.; Uribe-Salas, A. Hydrothermal decomposition of industrial jarosite in alkaline media: The Rate Determining Step of the Process Kinetics. J. Min. Metall. Sect. B Metall. 2016, 52, 135–142. [Google Scholar] [CrossRef][Green Version]
- Celep, O.; Serbest, V. Characterization of an iron oxy/hydroxide (gossan type) bearing refractory gold and silver ore by diagnostic leaching. Trans. Nonferrous Met. Soc. China 2015, 25, 1286–1297. [Google Scholar] [CrossRef]
- Viñals, J.; Núñez, C. Dissolution kinetics of argentian plumbojarosite from old tailings of sulfatizing roasting pyrites by HCl-CaCl2 leaching. Metall. Trans. B 1988, 19, 365–373. [Google Scholar] [CrossRef]
- Kendall, M.R.; Madden, A.S.; Madden, M.E.; Hu, Q. Effects of arsenic incorporation on jarosite dissolution rates and reaction products. Geochim. Cosmochim. Acta 2013, 112, 192–207. [Google Scholar] [CrossRef]
- Islas, H.; Flores, M.U.; Reyes, I.A.; Juárez, J.C.; Reyes, M.; Teja, A.M.; Palacios, E.G.; Pandiyan, T.; Aguilar-Carrillo, J. Determination of the dissolution rate of hazardous jarosites in different conditions using the shrinking core kinetic model. J. Hazard. Mater. 2020, 386, 121664. [Google Scholar] [CrossRef] [PubMed]
- Calla-Choque, D.; Lapidus, G.T. Jarosite dissolution kinetics in the presence of acidic thiourea and oxalate media. Hydrometallurgy 2021, 200, 105565. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Anaerobic bioleaching of jarosites by Shewanella putrefaciens, influence of chelators and biofilm formation. Hydrometallurgy 2017, 168, 56–63. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Anaerobic Bioreduction of Jarosites and Biofilm Formation by a Natural Microbial Consortium. Minerals 2019, 9, 81. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Sunyer, A. Hematite formation from jarositetype compounds by hidrotermal conversion. Can. Metall. Q. 2012, 51, 11–23. [Google Scholar] [CrossRef]
- Frost, R.L.; Wills, R.A.; Weier, M.L.; Musumeci, A.W.; Martens, W. Thermal decomposition of natural and synthetic plumbojarosite: Importance in “Archeochemistry”. Thermochim. Acta 2005, 432, 30–35. [Google Scholar] [CrossRef][Green Version]
- Frost, R.L.; Wills, R.A.; Kloprogge, J.T.; Martens, W. Thermal decomposition of hydronium jarosite (H3O)Fe3(SO4)2(OH)6. J. Therm. Anal. Calorim. 2006, 83, 213–218. [Google Scholar] [CrossRef]
- Vu, H.N.; Dvořák, P.; Síta, T. Study of conversion of waste jarosite precipitates to hematite. Inz. Miner. 2014, 15, 275–280. Available online: http://www.potopk.com.pl/Full_text/2014_full/2014_2_46.pdf.
- Ma, X.; Tan, H.; Dong, F.; Li, B.; Liu, J.; Chen, Y.; Wang, L. Preparation of Pyrrhotite from Ammonium Jarosite and Estimation of Activation Energy in Reducing Atmosphere. Int. J. Chem. React. Eng. 2019, 17, 20180149. [Google Scholar] [CrossRef]
- Ma, X.; Tan, H.; Dong, F.; Li, B.; Wang, J.; He, X.; Liu, C. Preparation of ammonium jarosite and estimated activation energy of thermal decomposition in reducing atmosphere. J. Therm. Anal. Calorim. 2019, 135, 2565–2572. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Hou, X.; Gao, W.; Gui, H.; Liu, Q. The effect of pellet technology on direct reduction of jarosite residues from zinc hydrometallurgy. Physicochem. Probl. Miner. Proccess. 2019, 55, 802–811. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Zhang, G.; Kang, J.; Wang, C. Comprehensive recovery and recycle of jarosite residues from zinc hydrometallurgy. Chem. Eng. J. Adv. 2020, 3, 100023. [Google Scholar] [CrossRef]
- Kolitsch, U.; Pring, A. Crystal chemistry of the crandallite, beudantite and alunite groups: A Review and Evaluation of the Suitability as Storage Materials for Toxic Metals. J. Mineral. Petrol. Sci. 2001, 96, 67–78. [Google Scholar] [CrossRef]
- Roussel, C.; Neel, C.; Bril, H. Minerals controlling arsenic and lead solubility in an abandoned gold mine tailings. Sci. Total Environ. 2000, 263, 209–219. [Google Scholar] [CrossRef]
- Smeaton, C.M.; Walshe, G.E.; Smith, A.M.L.; Hudson-Edwards, K.A.; Dubbin, W.E.; Wright, K.; Beale, A.M.; Fryer, B.J.; Weisener, C.G. Simultaneous Release of Fe and as during the Reductive Dissolution of Pb−As Jarosite by Shewanella putrefaciens CN32. Environ. Sci. Technol. 2012, 46, 12823–12831. [Google Scholar] [CrossRef]
- Alarcón, R.; Gaviria, J.; Dold, B. Liberation of Adsorbed and Co-Precipitated Arsenic from Jarosite, Schwertmannite, Ferrihydrite, and Goethite in Seawater. Minerals 2014, 4, 603–620. [Google Scholar] [CrossRef]
- Zhu, Y.; Wei, W.; Tang, S.; Zhu, Z.; Yan, Q.; Zhang, L.; Deng, H. A comparative study on the dissolution and stability of beudantite and hidalgoite at pH 2–12 and 25–45 °C for the possible long-term simultaneous immobilization of arsenic and lead. Chemosphere 2021, 263, 128386. [Google Scholar] [CrossRef]
- Hudson-Edwards, K.A. Uptake and release of arsenic and antimony in alunite–jarosite and beudantite group minerals. Am. Mineral. 2019, 104, 5. [Google Scholar] [CrossRef]
- Johnston, S.G.; Burton, E.D.; Keene, A.F.; Planer-Friedrich, B.; Voegelin, A.; Blackford, M.G.; Lumpkin, G.R. Arsenic mobilization and iron transformations during sulfidization of As(V)-bearing jarosite. Chem. Geol. 2012, 334, 9–24. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Passivation of chalcopyrite durin gits chemical leaching with ferric ion at 68 °C. Miner. Eng. 2009, 22, 229–235. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Comparative kinetic study of the silver-catalyzed chalcopyrite leaching at 35 °C and 68 °C. Int. J. Miner. Process. 2009, 92, 137–143. [Google Scholar] [CrossRef]
- Kartal, M.; Xia, F.; Ralph, D.; Rickard, W.D.A.; Renard, F.; Li, W. Enhancing chalcopyrite leaching by tetrachloroethylene-assisted removal of sulfur passivation and the mechanisms of jarosite formation. Hydrometallurgy 2020, 191, 105192. [Google Scholar] [CrossRef]
- Quezada, V.; Roca, A.; Benavente, O.; Cruells, M.; Melo, E. The effects of sulphuric acid and sodium chloride agglomeration and curing on chalcopyrite leaching. Metals 2021, 11, 873. [Google Scholar] [CrossRef]
- Quezada, V.; Roca, A.; Benavente, O.; Cruells, M.; Melo, E.; Hernández, M. Pretreatment to leaching for a primary copper sulphide ore in chloride media. Metals 2021, 11, 1260. [Google Scholar] [CrossRef]
- Sasaki, K.; Nakamuta, Y.; Hirajima, T.; Tuovinen, O.H. Raman characterization of secondary minerals formed during chalcopyrite leaching with Acidithiobacillus ferrooxidans. Hydrometallurgy 2009, 95, 153–158. [Google Scholar] [CrossRef]
- Vakylabad, A.B.; Nazari, S.; Darezereshki, E. Bioleaching of copper from chalcopyrite ore at higher NaCl concentrations. Miner. Eng. 2022, 175, 107281. [Google Scholar] [CrossRef]
- Morris, R.V.; Golden, D.C.; Bell III, J.F.; Shelfer, T.D.; Scheinost, A.C.; Hinman, N.W.; Furniss, G.; Mertzman, S.A.; Bishop, J.L.; Ming, D.W.; et al. Mineralogy, composition, and alteration of Mars Pathfinder Rocks and soils’ Evidence from multispectral, elemental, and magnetic data on terrestrial analogue, SNC meteorite, and Pathfinder samples. J. Geophys. Res. 2000, 105, 1757–1817. [Google Scholar] [CrossRef]
- Klingelhöfer, G.; Morris, R.V.; Bernhardt, B.; Schröder, C.; Rodionov, D.S.; de Souza, P.A.; Yen, A.; Gellert, R.; Evlanov, E.N.; Zubkov, B.; et al. Jarosite and Hematite at Meridiani Planum from Opportunity’s Mössbauer Spectrometer. Science 2004, 306, 1740–1745. [Google Scholar] [CrossRef]
- Madden, M.E.E.; Madden, A.S.; Rimstidt, J.D.; Zahrai, S.; Kendall, M.R.; Miller, M.A. Jarosite dissolution rates and nanoscale mineralogy. Geochim. Cosmochim. Acta 2013, 91, 306–321. [Google Scholar] [CrossRef]
- Rampe, E.B.; Ming, D.W.; Blake, D.F.; Bristow, T.F.; Chipera, S.J.; Grotzinger, J.P.; Morris, R.V.; Morrison, S.M.; Vaniman, D.T.; Yen, A.S.; et al. Mineralogy of an ancient lacustrine mudstone succession from the Murray formation, Gale crater, Mars. Earth Planet. Sci. Lett. 2017, 471, 172–185. [Google Scholar] [CrossRef]
- Potter-McIntyre, S.L.; McCollom, T.M. Jarosite and Alunite in Ancient Terrestrial Sedimentary Rocks: Reinterpreting Martian Depositional and Diagenetic Environmental Conditions. Life 2018, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, L.; Fernández-Martínez, M.A.; Moreno-Paz, M.; Carrizo, D.; García-Villadangos, M.; Manchado, J.M.; Stoker, C.R.; Glass, B.; Parro, V. Simulating Mars Drilling Mission for Searching for Life: Ground-Truthing Lipids and Other Complex Microbial Biomarkers in the Iron-Sulfur Rich Río Tinto Analog. Astrobiology 2020, 20, 1029–1047. [Google Scholar] [CrossRef] [PubMed]
- Baccolo, G.; Delmonte, B.; Niles, P.B.; Cibin, G.; Di Stefano, E.; Hampai, D.; Keller, L.; Maggi, V.; Marcelli, A.; Michalski, J.; et al. Jarosite formation in deep Antarctic ice provides a window into acidic, water-limited weathering on Mars. Nat. Commun. 2021, 12, 436. [Google Scholar] [CrossRef] [PubMed]
- Grasby, S.E.; Percival, J.B.; Bilot, I.; Ardakani, O.H.; Smith, I.R.; Galloway, J.; Bringué, M.; McLoughlin-Coleman, T. Extensive jarosite deposits formed through auto-combustion and weathering of pyritiferous mudstone, Smoking Hills (Ingniryuat), Northwest Territories, Canadian Arctic—A potential Mars analogue. Chem. Geol. 2022, 587, 2022. [Google Scholar] [CrossRef]

| Name | Formula | Name | Formula |
|---|---|---|---|
| Alunite group | Beudantite group | ||
| Alunite | KAl3(SO4)2(OH)6 | Beudantite | PbFe3[(S,As)O4)2(OH,H2O)6 |
| Ammonium alunite | (NH4)Al3(SO4)2(OH)6 | Hidalgoite | PbAl3[(S,As)O4)2(OH,H2O)6 |
| Jarosite | KFe3(SO4)2(OH)6 | Crandallite group | |
| Plumbojarosite | PbFe6(SO4)4(OH)12 | Crandallite | CaAl3[PO3(O1/2(OH)1/2)]2(OH)6 |
| Argentojarosite | AgFe3(SO4)2(OH)6 | Plumbogummite | PbAl3(PO4)2(OH,H2O)6 |
| Location | Mineral | Species Detected | Source |
|---|---|---|---|
| Tintic Standard mine, Utah (USA) | Oxidized sulfide deposits | Argentojarosite | Dutrizac and Jambor [13] |
| Cook’s Peek, NewMexico (USA) | Id. | Plumbojarosite | Id. |
| Shinkolowbwe (Zaire) | Id. | Jarosite, natrojarosite | Id. |
| Yellowstone Natural Park (USA) | Hypogene mineral | Jarosite | Id. |
| Sulfur Bank hot-spring Hg dep. CA (USA) | Id. | Ammonium jarosite | Id. |
| Iberian Pyrite Belt (Spain) | Iron caps, gossan | Jarosite | Velasco et al. [14] |
| Rio Tinto (Spain) | Gossan | As-potassium jarosite Beudantite | Viñals et al. [15] Roca et al. [16] |
| Mother Lode Gold California (USA) | Outcrops, mine Tailings | As-potassium jarosite | Savage et al. [17] |
| High Lake, Nunavut (Canada) | Gossan, outcrops | Jarosite | West et al. [18] |
| Mega, Livaldi, Serifos Greece | As-rich tennan- tite, As-pyrite | Beudantite | Dill et al. [19] |
| Veladero, Andean Cordillera, Argentina | Fine grained quartz | Alunite, jarosite | Holley et al. [21] |
| Santa Caterina State (Brazil) | Tailings coal industry | Jarosite | Cutruneo et al. [22] |
| Penn Mine, Sierra Nevada CA (USA) | Secondary minerals | Jarosite | Montero et al. [23] |
| Monte Romero mine IBP (Spain) | Mineral precipitated from acid rock drainage | Hydronium jarosite | Acero et al. [24] |
| Sulfide deposits galena | Id. | As-plumbojarosite | Smith et al. [25] |
| Rosia Montana Gold (Romania) | Ochreous precipitates | Jarosite | Azzali et al. [26] |
| Sarsheshmeh copper Mine (Iran) | Tailings mine | Jarosite, alunite | Malakooti et al. [27] |
| Jarosite (Alunite) | Fe3+ (Al3+) (mol/L) | Metal Sulfate (mol/L) | Sulfuric Acid (mol/L) | Temperature(°C) | Reference |
|---|---|---|---|---|---|
| Na | 0.20 | 0.30 | 0.01 | 97 | [32,36] |
| K | 0.20 | 0.30 | 0.01 | 97 | [34] |
| NH4+ | 0.30 | 0.20 | (pH 1.6) | 98 | [35] |
| H3O+ | 0.27 | --- | --- | 140 | [1] |
| Ag | 0.30 | 0.048 | 0.01 | 97 | [40] |
| Pb | 0.054 | [Pb(NO3)2] | 0.01 | 95 | [1,25] |
| K | 0.16 (without) Fe(II) nor Bacteria | K+, 0.27; SO42−, 0.20; PO43−, 0.025; Mg, 2.3·10−3; Ca2+, 6·10−4; NO3− 1.2·10−4 | (pH 1.4) | 2 to 40 | [15] |
| K-NH4-H3O Ternary solid solutions | 0.16 (as Fe2+) Acidithio-bacillus ferroxidans | PO43–, 3.67·10−3; and as sulfates: Mg2+, 1.62·10−3; NH4+, 3.03·10−3 | 0.01 | 22 | [46] |
| Natroalunite | 0.063 | Na+: 0.021 | (pH 2.8–2.9) | 160 | [7] |
| Alunite | 0.60 | K+: 0.6 | pH 0.4) | 220 | [56] |
| Sample | Elements—Atomic Ratio | Parameter a | Parameter c |
|---|---|---|---|
| 1 | 0.99K; 0.01H3O+; 0.63Fe; 2.19Al | 7.064 ± 0.004 | 17.049 ± 0.009 |
| 2 | 0.79K; 0.21H3O+; 1.99Fe; 0.44Al | 7.248 ± 0.003 | 17.087 ± 0.006 |
| 3 | 0.68Na; 0.32H3O+; 2.66Fe; 0.07Al | 7.327 ± 0.001 | 16.654 ± 0.002 |
| 4 | 0.66Na; 0.34H3O+; 0Fe; 2.65Al | 6.987 ± 0.001 | 16.635 ± 0.002 |
| 5 | 0.48K; 0.29Na; 0.23H3O+; 2.63Al | 6.985 ± 0.0036 | 16.905 ± 0.088 |
| 6 | 0.24K; 0.59Na; 0.17H3O+; 2.74Al | 6.981 ± 0.003 | 16.776 ± 0.008 |
| Jarosite | Ratio (mol) Cation/H3O+ | Medium | Reaction Order [OH−] | Activation Energy (kJ/mol) | Temperature (°C) | References |
|---|---|---|---|---|---|---|
| Ag | 0.78–0.22 | NaOH | 0.5 | 42.0 | 23–60 | [40] |
| Ag | 0.78–0.22 | Ca(OH)2 | 0.5 | 42.0 | 30–60 | [40] |
| Pb | 0.32–0.35 | NaOH | 1 | 53.0 | 25–55 | [71] |
| Pb | 0.32–0.35 | Ca(OH)2 | 0 | 47.0 | 22–55 | [71] |
| Na | 0.675–0.320 | NaOH | 0.4 | 96.0 | 25–45 | [37] |
| Na | 0.675–0.320 | Ca(OH)2 | 0.5 | 40.0 | 25–60 | [37] |
| K | 0.91–0.083 | NaOH | 0.6 | 43.0 | 25–60 | [34] |
| K | 0.91–0.083 | Ca(OH)2 | 0.5 | 80.0 | 20–60 | [34] |
| NH4+ | 0.71–0.25 | NaOH | 0.6 | 60.0 | 30–50 | [72] |
| NH4+ | 0.71–0.25 | Ca(OH)2 | 0.4 | 70.0 | 25–50 | [73] |
| Id. Ind 1 | 0.59–0.31 with 0.07Na, 0.02K, 0.007Pb | NaOH | 1.1 | 77.0 | 30–50 | [74] |
| Hg | 0.39–0.22 | NaOH | 1 | 56.9 | 25–60 | [75] |
| Rb | 0.82–0.17 | NaOH | 0.94 | 91.3 | 25–55 | [76] |
| Rb | 0.94–0.06 | Ca(OH)2 | 0.42 | 98.7 | 25–50 | [77] |
| K with Cr 2 | 0.95–0.56K 0.05–0.44H3O+ 1.99–0Cr | NaOH | 1.1 | 75.7 | 20–75 | [78] |
| Na-As | 0.87–0.13Na 0.05As | NaOH | 0.75 | 57.1 | 20–60 | [79] |
| Na-As | 0.87–0.13Na 0.05As | Ca(OH)2 | 1.56 | 48.2 | 20–70 | [79] |
| K-As | Low As | NaOH | 1.86 | 60.3 | 21–60 | [80] |
| K-As | Low As | Ca(OH)2 | 1.15 | 74.4 | 21–70 | [80] |
| K-As Gossan RT | 0.56–0 0.37Pb | Ca(OH)2 | --- | 86.5 | 75–95 | [16] |
| Beudantite Gossan RT | 0.39Pb/0.22 and 0.16As | NaOH | 0.99 | 96.9 | 20–70 | [81] |
| Jarosite | Medium | Molar Ratio | Kexp·d0 (s−1·μm) | References |
|---|---|---|---|---|
| K | NaOH | 0.86K; 0.13H3O+ | 0.090 | [34] |
| NH4+ | NaOH | 0.71NH4+; 0.28H3O+ | 0.028 | [72] |
| Ag | NaOH | 0.79Ag; 0.21H3O+ | 0.025 | [40] |
| NH4+ Ind. | NaOH | 0.59NH4+; 0.07 Na; 0.31H3O+; 0.02K 0.05As | 0.018 | [74] |
| As-Potassium Gossan RT | NaOH | 0.44Pb; 0.10Na; 0.42K; 0.40As | Negligible (at 50 °C) | [16] |
| Beudantite Gossan RT | NaOH | 0.97Pb; 0.03K; 0.97As | Negligible (50 °C) | [16] |
| Potassium | Ca(OH)2 | 0.86K; 0.13H3O | 0.080 | [34] |
| Sodium | Ca(OH)2 | 0.65Na; 0.34H3O | 0.020 | [37] |
| Ammonium | Ca(OH)2 | 0.71NH4; 0.28H3O | 0.018 | [73] |
| Silver | Ca(OH)2 | 0.79Ag; 0.21H3O | 0.010 | [40] |
| Lead | Ca(OH)2 | 0.31Pb; 0.37H3O | <0.001 | [70] |
| As-Potassium Gossan Rio Tinto | Ca(OH)2 | 0.44Pb; 0.10Na; 0.42K; 0.40As | Negligible (50 °C) | [16] |
| Ammonium Industrial | Ca(OH)2 | 0.59NH4; 0.31H3O; 0.07Na; 0.02K; 0.05As | Negligible (50 °C) | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruells, M.; Roca, A. Jarosites: Formation, Structure, Reactivity and Environmental. Metals 2022, 12, 802. https://doi.org/10.3390/met12050802
Cruells M, Roca A. Jarosites: Formation, Structure, Reactivity and Environmental. Metals. 2022; 12(5):802. https://doi.org/10.3390/met12050802
Chicago/Turabian StyleCruells, Montserrat, and Antoni Roca. 2022. "Jarosites: Formation, Structure, Reactivity and Environmental" Metals 12, no. 5: 802. https://doi.org/10.3390/met12050802
APA StyleCruells, M., & Roca, A. (2022). Jarosites: Formation, Structure, Reactivity and Environmental. Metals, 12(5), 802. https://doi.org/10.3390/met12050802






