Eutectic Reaction and Microstructure Stability in CoCrFeNiNbx High-Entropy Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
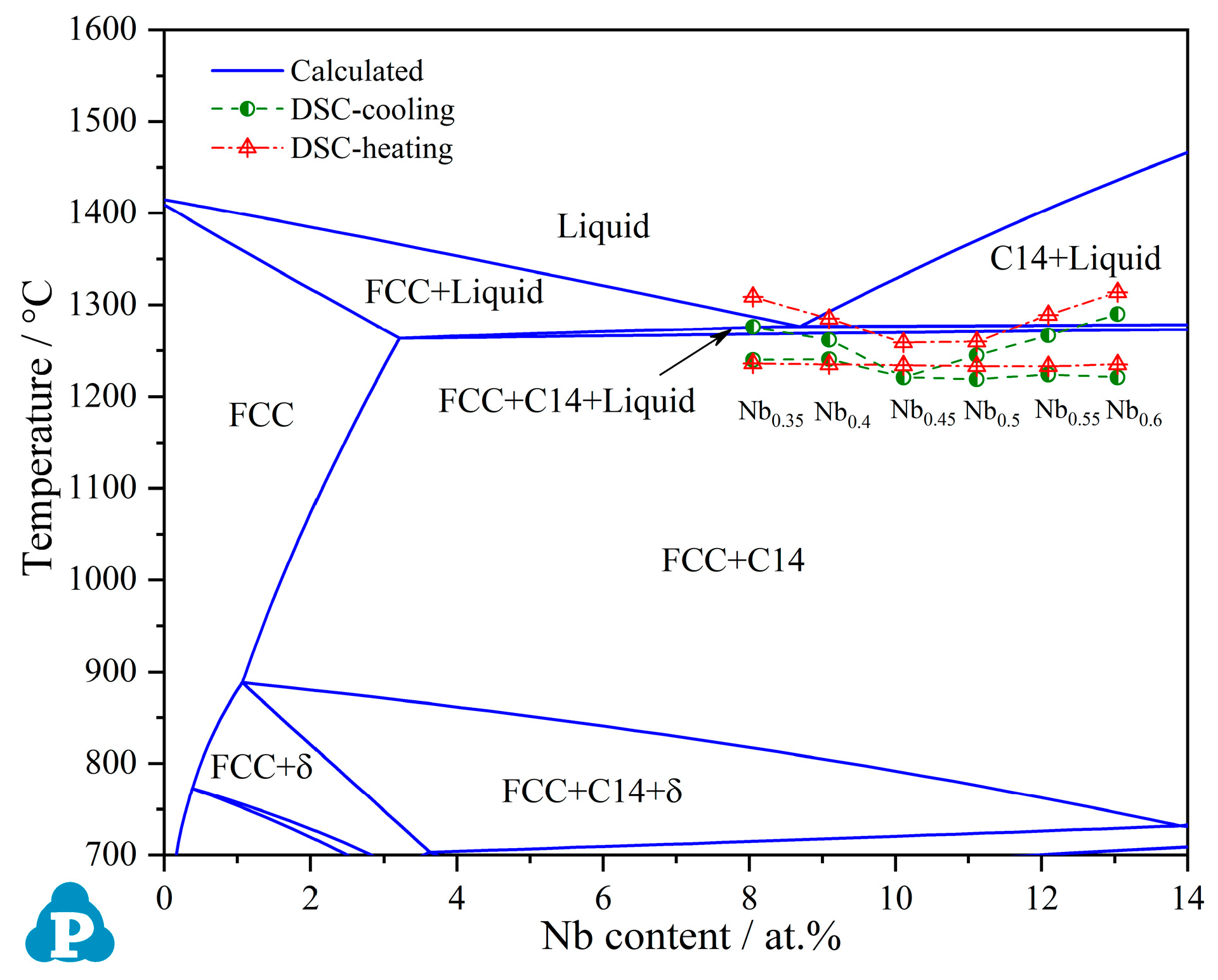
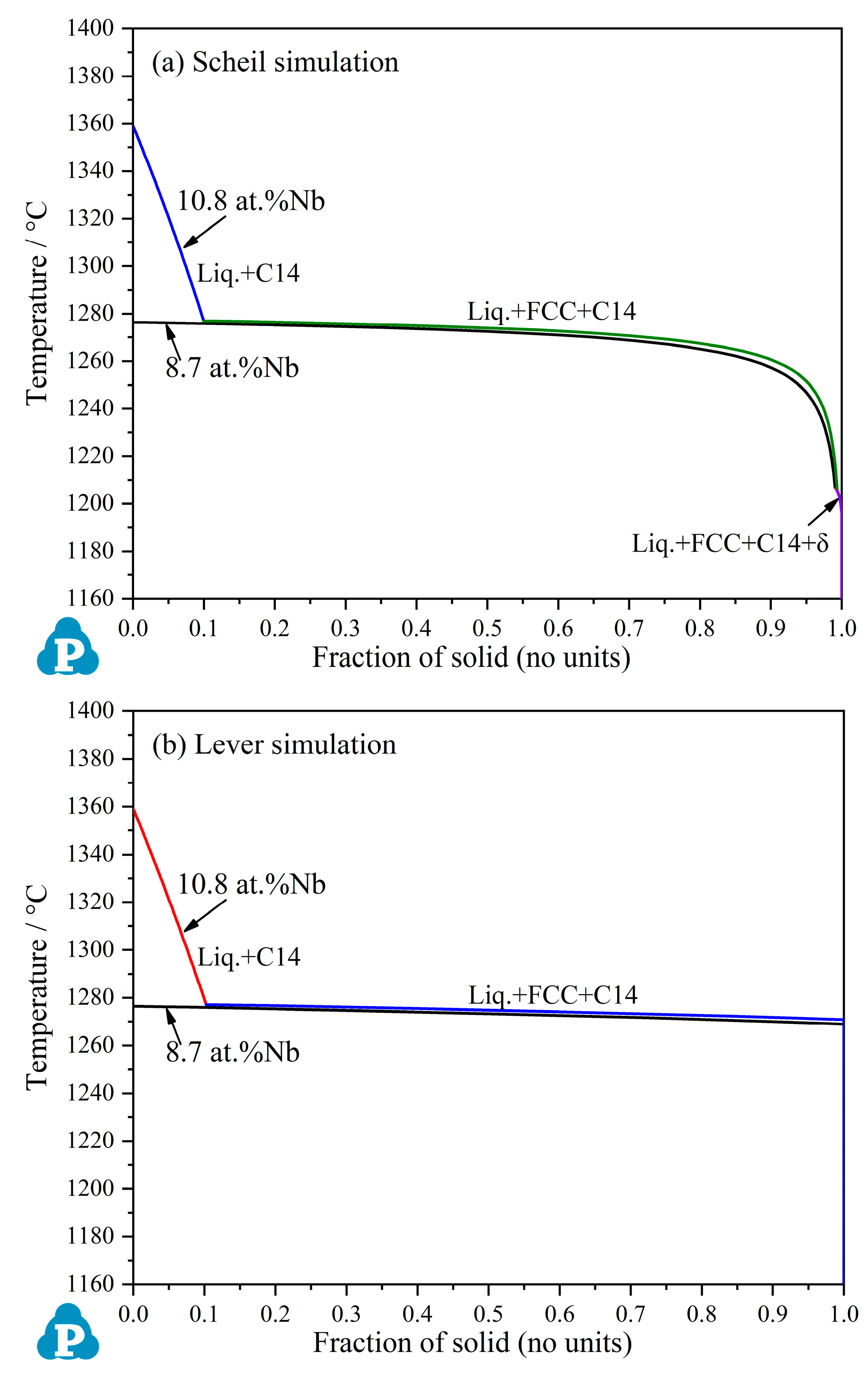
3.1. Thermodynamic Calculation Based on PanNi Database
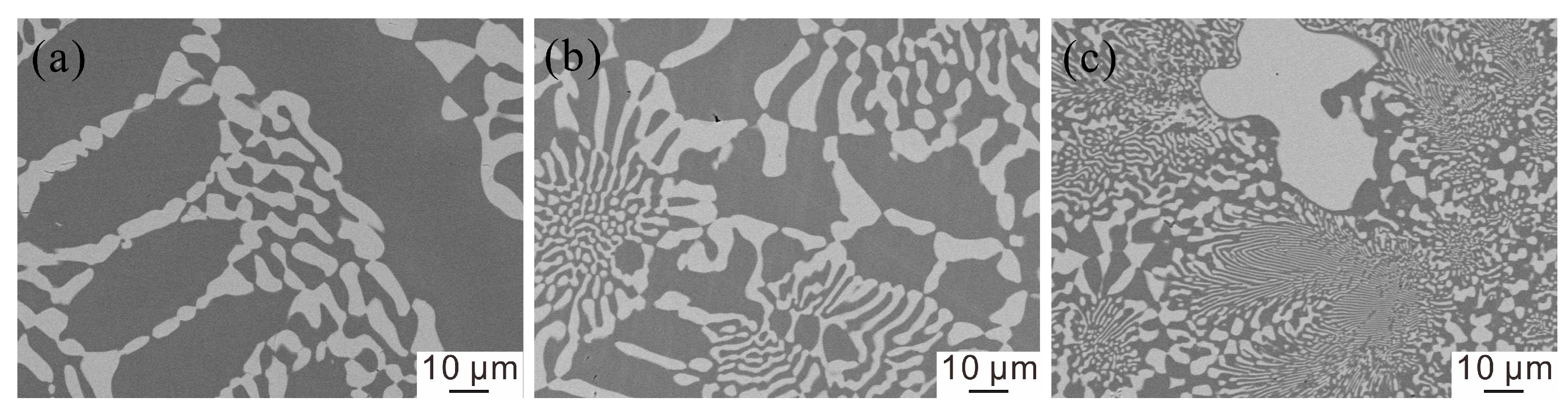
3.2. Microstructure and Phase Constituent of the As-Cast Alloys
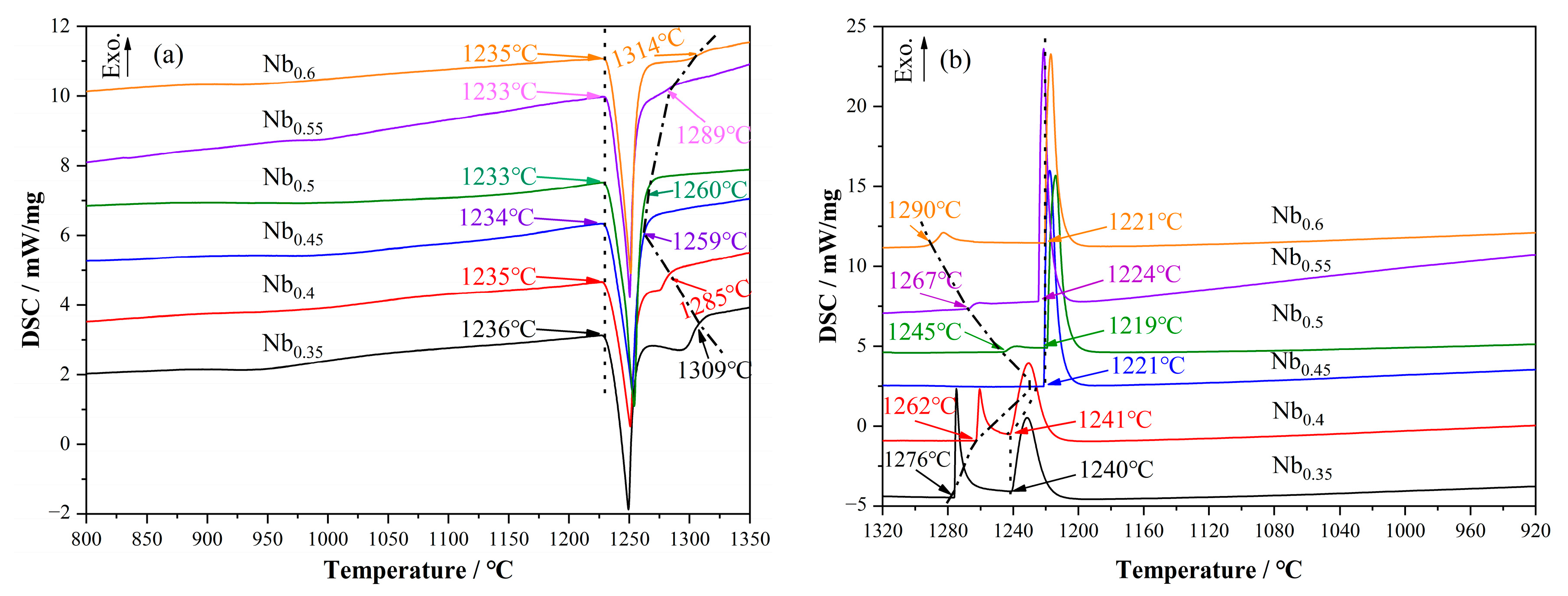
3.3. Heating and Cooling DSC Test
3.4. Microstructure after the DSC Test
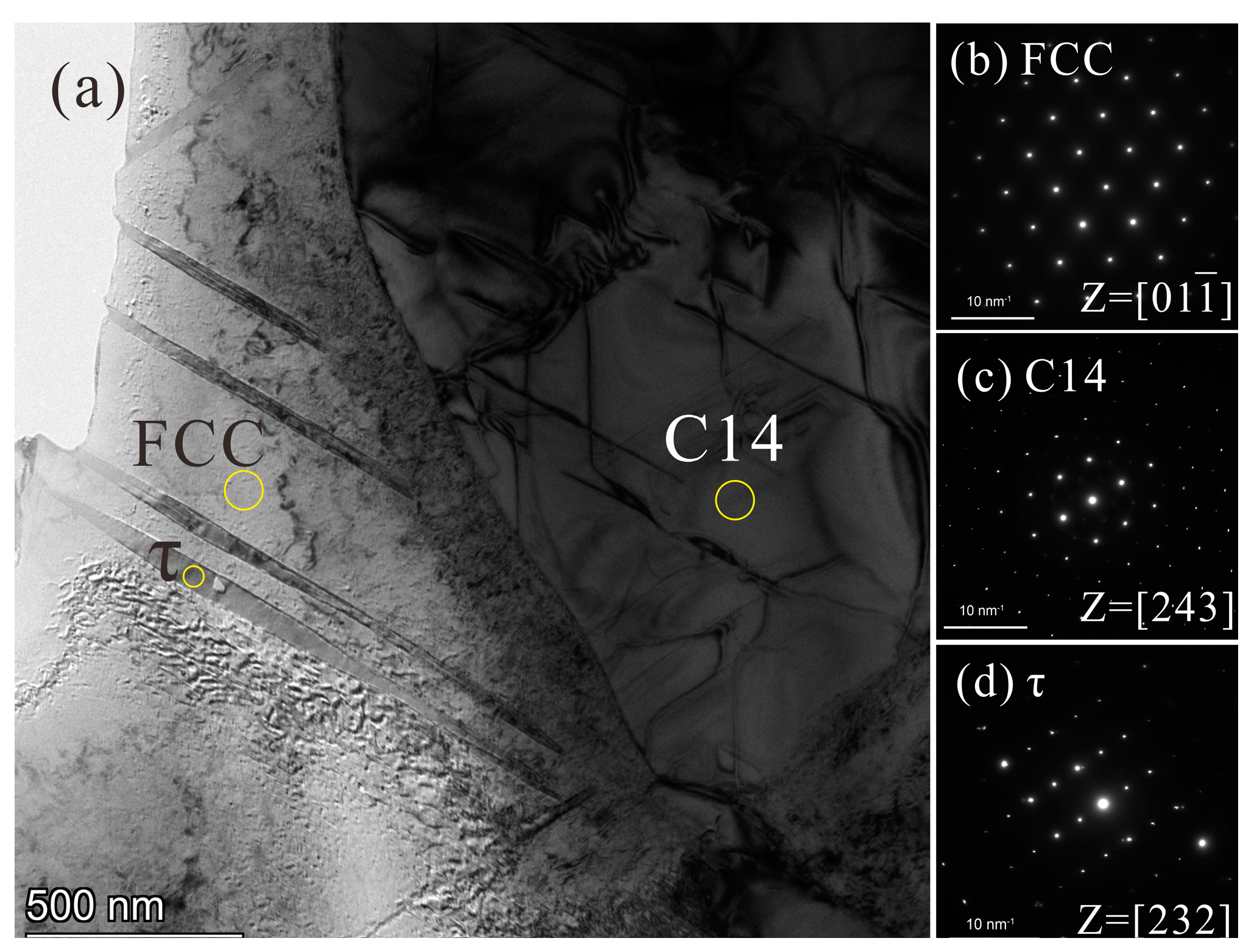
3.5. Microstructure and Phase Constituents of the 1000 or 800 °C Annealed Alloys
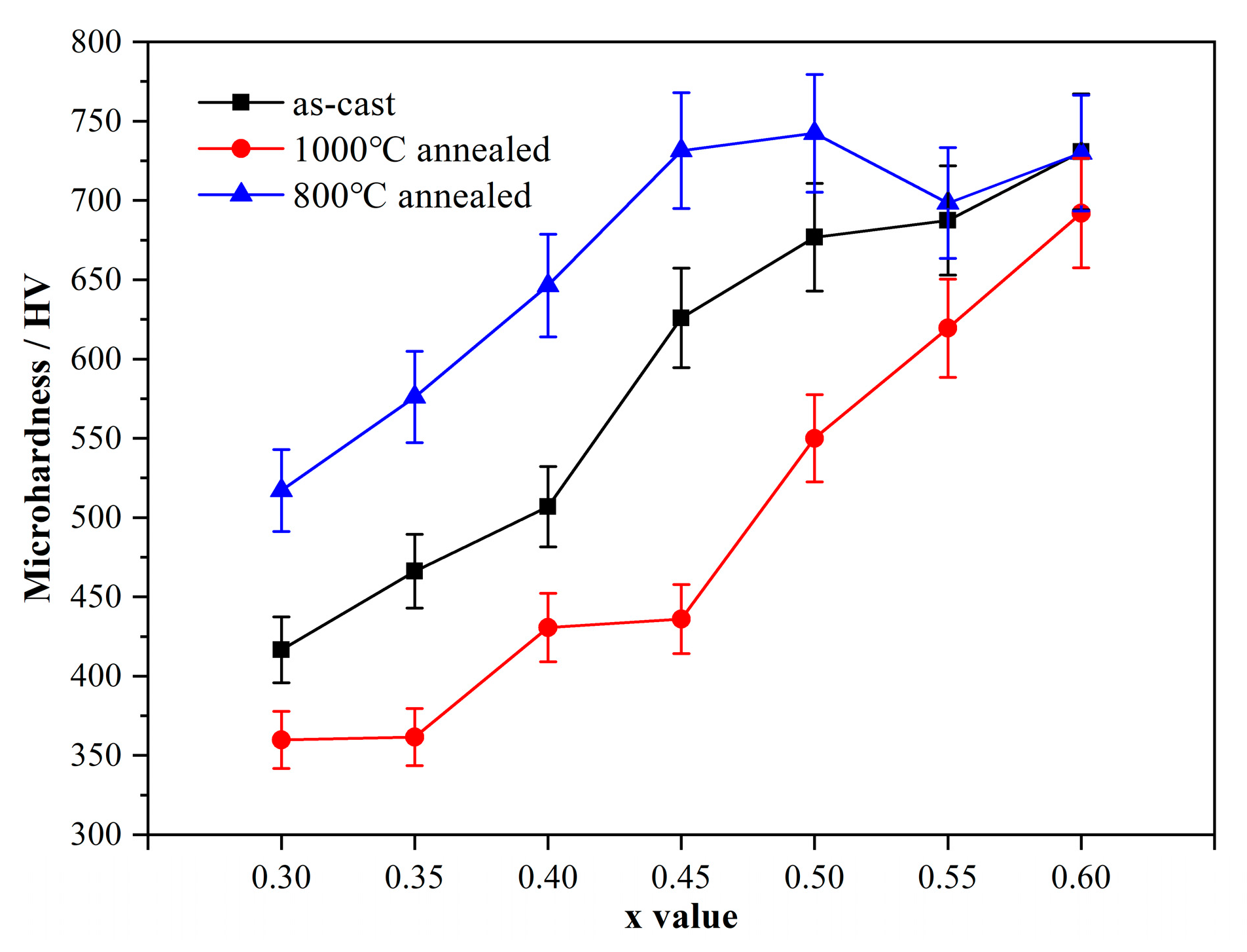
3.6. Microhardness
4. Discussion
5. Conclusions
- (1)
- All the as-cast or 1000 °C annealed CoCrFeNiNbx (x = 0.3–0.6) alloys are composed of FCC and C14 Laves phases. Their compositions change minimally with x values, cooling rates or annealing treatments. Moreover, the Nb content in the C14 phase stays at around 24.5 at.%.
- (2)
- The eutectic reaction of Liquid → FCC + C14 occurs in a narrow temperature range and starts at 1234 °C near CoCrFeNiNb0.45 in the CoCrFeNi–Nb pseudo-binary system.
- (3)
- The microstructure of the CoCrFeNiNbx alloys is greatly affected by the cooling rate and annealing treatment. The C14 phase is easily spheroidized during annealing. Special attention should be paid to the effects of the cooling rate and annealing treatment when preparing Co–Cr–Fe–Ni–Nb alloys.
- (4)
- The solubility of Nb in the FCC phase decreases with decreasing temperature. It has been confirmed that, after annealing at 800 °C for 120 h, the needle-like nano Mg3Cd-type τ phase, with a composition of 2.9Cr–4.5Fe–24.1Co–41.1Ni–27.4Nb (at.%), precipitated from the pro-eutectic FCC phase and led to an increase in alloy microhardness for ~100 HV. This may be a good method to strengthen the FCC phase.
- (5)
- The microhardness of the CoCrFeNiNbx alloys increases with the x value. The increased rate in hypo-eutectic alloys (x < 0.45) is a little higher than that in hyper-eutectic alloys (x > 0.45).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Lin, S.J.; Chin, T.S.; Gan, J.Y.; Chen, S.K.; Shun, T.T.; Tsau, C.H.; Chou, S.Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Metall. Mater. Trans. A 2004, 35, 2533–2536. [Google Scholar] [CrossRef]
- Maity, T.; Prashanth, K.G.; Balcı, O.; Kim, J.T.; Schöberl, T.; Wang, Z.; Eckert, J. Influence of severe straining and strain rate on the evolution of dislocation structures during micro-/nanoindentation in high entropy lamellar eutectics. Int. J. Plast. 2018, 109, 121–136. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Fan, J.; Yu, P.; Li, G. Novel Co-free CrFeNiNb0.1Tix high-entropy alloys with ultra high hardness and strength. Mater. Sci. Eng. A 2019, 764, 138212. [Google Scholar] [CrossRef]
- Průša, F.; Cabibbo, M.; Šenková, A.; Kučera, V.; Veselka, Z.; Školáková, A.; Vojtěch, D.; Cibulková, J.; Čapek, J. High-strength ultrafine-grained CoCrFeNiNb high-entropy alloy prepared by mechanical alloying: Properties and strengthening mechanism. J. Alloys Compd. 2020, 835, 155308. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Wang, W.; Zhou, K.; He, F.; Wang, Z. Superior Slurry Erosion Behavior of a Casting NiCoCrFeNb0.45 Eutectic High Entropy Alloy. Acta Metall. Sinica Engl. Lett. 2020, 33, 1111–1116. [Google Scholar] [CrossRef]
- Ma, M.; Han, A.; Zhang, Z.; Lian, Y.; Zhao, C.; Zhang, J. The role of Si on microstructure and high-temperature oxidation of CoCr2FeNb0.5Ni high-entropy alloy coating. Corros. Sci. 2021, 185, 109417. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Wang, J.; Wu, Q.; Chen, D.; Han, B.; Li, J.; Wang, J.; Kai, J.J. Abnormal γ″-ε phase transformation in the CoCrFeNiNb0.25 high entropy alloy. Scr. Mater. 2018, 146, 281–285. [Google Scholar] [CrossRef]
- Chanda, B.; Das, J. An assessment on the stability of the eutectic phases in high entropy alloys. J. Alloys Compd. 2019, 798, 167–173. [Google Scholar] [CrossRef]
- Wang, J.; Wu, S.; Fu, S.; Liu, S.; Yan, M.; Lai, Q.; Lan, S.; Hahn, H.; Feng, T. Ultrahigh hardness with exceptional thermal stability of a nanocrystalline CoCrFeNiMn high-entropy alloy prepared by inert gas condensation. Scr. Mater. 2020, 187, 335–339. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef] [Green Version]
- Chanda, B.; Verma, A.; Das, J. Nano-/Ultrafine Eutectic in CoCrFeNi(Nb/Ta) High-Entropy Alloys. Trans. Indian Inst. Met. 2018, 71, 2717–2723. [Google Scholar] [CrossRef]
- Liu, W.H.; He, J.Y.; Huang, H.L.; Wang, H.; Lu, Z.P.; Liu, C.T. Effects of Nb additions on the microstructure and mechanical property of CoCrFeNi high-entropy alloys. Intermetallics 2015, 60, 1–8. [Google Scholar] [CrossRef]
- Varvenne, C.; Luque, A.; Curtin, W.A. Theory of strengthening in fcc high entropy alloys. Acta Mater. 2016, 118, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Sun, Y.; Liu, Y.; Tu, H. Effect of Long-Time Annealing at 1000 °C on Phase Constituent and Microhardness of the 20Co-Cr-Fe-Ni Alloys. Materials 2019, 12, 1700. [Google Scholar] [CrossRef] [Green Version]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Senkov, O.N.; Jensen, J.K.; Pilchak, A.L.; Miracle, D.B.; Fraser, H.L. Compositional variation effects on the microstructure and properties of a refractory high-entropy superalloy AlMo0.5NbTa0.5TiZr. Mater. Des. 2018, 139, 498–511. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, H.; Huang, T.; Lu, Y.; Wang, T.; Li, T. Microstructures and mechanical properties of Co2MoxNi2VWx eutectic high entropy alloys. Mater. Des. 2016, 109, 539–546. [Google Scholar] [CrossRef]
- Yang, H.; Tan, Y.; Qiao, J.; Hawk, J.A.; Zhang, Y.; Gao, M.; Liaw, P.K. Optimize the Mechanical Properties of Al0.6CoCrFeNi High-Entropy Alloys by Thermo-Mechanical Processing. Metals 2022, 12, 178. [Google Scholar] [CrossRef]
- Yu, Y.; He, F.; Qiao, Z.H.; Wang, Z.J.; Liu, W.M.; Yang, J. Effects of temperature and microstructure on the triblogical properties of CoCrFeNiNbx eutectic high entropy alloys. J. Alloys Compd. 2019, 775, 1376–1385. [Google Scholar] [CrossRef]
- Chanda, B.; Potnis, G.; Jana, P.P.; Das, J. A review on nano-/ultrafine advanced eutectic alloys. J. Alloys Compd. 2020, 827, 154226. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.; Wang, T.; Wen, B.; Wang, Z.; Jie, J.; Cao, Z.; et al. A Promising New Class of High-Temperature Alloys: Eutectic High-Entropy Alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, Y.; Dong, Y.; Wang, T.; Cao, Z.; Li, T. Effects of Nb addition on structural evolution and properties of the CoFeNi2V0.5 high-entropy alloy. Appl. Phys. A 2015, 119, 291–297. [Google Scholar] [CrossRef]
- Shi, P.; Li, R.; Li, Y.; Wen, Y.; Zhong, Y.; Ren, W.; Shen, Z.; Zheng, T.; Peng, J.; Liang, X. Hierarchical crack buffering triples ductility in eutectic herringbone high-entropy alloys. Science 2021, 373, 912–918. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Z.; Hu, X.; Zheng, T.; Yang, Z.; He, F.; Li, J.; Wang, J. Uncovering the eutectics design by machine learning in the Al–Co–Cr–Fe–Ni high entropy system. Acta Mater. 2020, 182, 278–286. [Google Scholar] [CrossRef]
- Xiong, T.; Yang, W.; Zheng, S.; Liu, Z.; Lu, Y.; Zhang, R.; Zhou, Y.; Shao, X.; Zhang, B.; Wang, J.; et al. Faceted Kurdjumov-Sachs interface-induced slip continuity in the eutectic high-entropy alloy, AlCoCrFeNi2.1. J. Mater. Sci. Technol. 2021, 65, 216–227. [Google Scholar] [CrossRef]
- Jin, X.; Zhou, Y.; Zhang, L.; Du, X.Y.; Li, B.S. A novel Fe20Co20Ni41Al19 eutectic high entropy alloy with excellent tensile properties. Mater. Lett. 2018, 216, 144–146. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, H.; Guo, S.; Wang, T.; Cao, Z.; Li, T. A new strategy to design eutectic high-entropy alloys using mixing enthalpy. Intermetallics 2017, 91, 124–128. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Jiang, H.; Wang, Z.; Cao, Z.; Guo, S.; Wang, T.; Li, T.; Liaw, P.K. Promising properties and future trend of eutectic high entropy alloys. Scr. Mater. 2020, 187, 202–209. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, X.; Lu, Y.; Wang, T.; Li, T. A multi-component AlCrFe2Ni2 alloy with excellent mechanical properties. Mater. Lett. 2016, 169, 62–64. [Google Scholar] [CrossRef]
- Wei, C.B.; Du, X.H.; Lu, Y.P.; Jiang, H.; Li, T.J.; Wang, T.M. Novel as-cast AlCrFe2Ni2Ti0.5 high-entropy alloy with excellent mechanical properties. Int. J. Miner. Metall. Mater. 2020, 27, 1312–1317. [Google Scholar] [CrossRef]
- Jiang, H.; Han, K.; Gao, X.; Lu, Y.; Cao, Z.; Gao, M.C.; Hawk, J.A.; Li, T. A new strategy to design eutectic high-entropy alloys using simple mixture method. Mater. Des. 2018, 142, 101–105. [Google Scholar] [CrossRef]
- Jiang, H.; Qiao, D.; Lu, Y.; Ren, Z.; Cao, Z.; Wang, T.; Li, T. Direct solidification of bulk ultrafine-microstructure eutectic high-entropy alloys with outstanding thermal stability. Scr. Mater. 2019, 165, 145–149. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, L.; Qiao, Q.; Lu, Y.; Wang, T.; Cao, Z.; Li, T. Effect of Niobium on Microstructure and Properties of the CoCrFeNb x Ni High Entropy Alloys. J. Mater. Sci. Technol. 2017, 33, 712–717. [Google Scholar] [CrossRef]
- An, X.; Chu, C.; Zhao, H.; Shen, B.; Zhou, L.; Chu, P.K. CoNiFeNb0.45 eutectic multi-principal element alloy with excellent mechanical properties and corrosion resistance. Mater. Sci. Eng. A 2020, 777, 139026. [Google Scholar] [CrossRef]
- Chanda, B.; Das, J. Composition Dependence on the Evolution of Nanoeutectic in CoCrFeNiNbx (0.45 ≤ x ≤ 0.65) High Entropy Alloys. Adv. Eng. Mater. 2018, 20, 1700908. [Google Scholar] [CrossRef]
- Chung, D.; Ding, Z.Y.; Yang, Y. Hierarchical Eutectic Structure Enabling Superior Fracture Toughness and Superb Strength in CoCrFeNiNb0.5 Eutectic High Entropy Alloy at Room Temperature. Adv. Eng. Mater. 2019, 21, 1801060. [Google Scholar] [CrossRef]
- He, Q.F.; Ding, Z.Y.; Ye, Y.F.; Yang, Y. Design of High-Entropy Alloy: A Perspective from Nonideal Mixing. JOM. 2017, 69, 2092–2098. [Google Scholar] [CrossRef]
- Xiang, K.; Chen, L.Y.; Chai, L.; Guo, N.; Wang, H. Microstructural characteristics and properties of CoCrFeNiNbx high-entropy alloy coatings on pure titanium substrate by pulsed laser cladding. Appl. Surf. Sci. 2020, 517, 146214. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Cheng, P.; Wang, Q.; Li, J.; Dang, Y.; Wang, J.; Liu, C.T. Designing eutectic high entropy alloys of CoCrFeNiNbx. J. Alloys Compd. 2016, 656, 284–289. [Google Scholar] [CrossRef]
- Wu, C.J.; Zhou, C.; Zeng, J.F.; Liu, Y.; Tu, H.; Su, X.P. Effects of annealing at 800 and 1000 °C on phase precipitates and hardness of Al7Cr20FexNi73−x alloys. Trans. Nonferrous Metal. Soc. China 2021, 31, 734–743. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, C.J.; Peng, H.P.; Liu, Y.; Wang, J.H.; Su, X.P. Phase Constituent and Microhardness of As-Cast and Long-Time Annealed AlxCo2-xCrFeNi Multicomponent Alloys. J. Phase Equilib. Diffus. 2019, 40, 706–714. [Google Scholar] [CrossRef]
- Nagase, T.; Takemura, M.; Matsumuro, M.; Maruyama, T. Solidification microstructure of AlCoCrFeNi2.1 eutectic high entropy alloy ingots. Mater. Trans. 2018, 59, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Nassar, A.; Mullis, A.; Cochrane, R.; Aslam, Z.; Micklethwaite, S.; Cao, L. Rapid solidification of AlCoCrFeNi2.1 High-entropy Alloy. J. Alloys Compd. 2022, 900, 163350. [Google Scholar] [CrossRef]
- Wu, C.J.; Xiong, W.; Zhou, C.; Liu, Y.; Su, X. Effect of annealing temperature on microstructure and hardness of Al-Co-Cr-Fe-Ni high-entropy alloy. J. Changzhou Univ. (Nat. Sci. Ed.) 2021, 33, 1–8. [Google Scholar]
- Fan, A.C.; Li, J.H.; Tsai, M.H. On the phase constituents of three CoCrFeNiX (X = V, Nb, Ta) high-entropy alloys after prolonged annealing. J. Alloys Compd. 2020, 823, 153524. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Niu, S.; Wu, Q.; Li, J.; Wang, J.; Liu, C.T.; Dang, Y. Strengthening the CoCrFeNiNb0.25 high entropy alloy by FCC precipitate. J. Alloys Compd. 2016, 667, 53–57. [Google Scholar] [CrossRef]
- Chanda, B.; Das, J. Evolution of microstructure homogeneity and mechanical properties in nano-/ultrafine eutectic CoCrFeNiNbx (0.45 ≤ x ≤ 0.65) high entropy alloy ingots and cast rods. J. Alloys Compd. 2022, 910, 163610. [Google Scholar] [CrossRef]
- PanNi-Thermodynamic Database for Nickel-Based Superalloys for Pandat. 2021. Available online: https://computherm.com/pannickel (accessed on 20 March 2022).
- Shaipov, R.K.; Kerimov, E.Y.; Slyusarenko, E.M. Isothermal sections of the Co-Nb-Ni phase diagram at 1200 and 1375 K. J. Alloys Compd. 2018, 742, 466–479. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, C.; Li, J.; Li, C.; Du, Z. Key Experiments and Thermodynamic Description of the Co-Nb-Ni System. Metall. Mater. Trans. A 2020, 51, 5892–5911. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, C.; Jiang, L.; Jin, Z.; Zhao, J.C. Experimental determination of the phase diagrams of the Co-Ni-X (X = W, Mo, Nb, Ta) ternary systems using diffusion multiples. Intermetallics 2018, 93, 20–29. [Google Scholar] [CrossRef]
- Maity, T.; Prashanth, K.G.; Balçi, O.; Wang, Z.; Jia, Y.D.; Eckert, J. Plastic deformation mechanisms in severely strained eutectic high entropy composites explained via strain rate sensitivity and activation volume. Compos. Part B Eng. 2018, 150, 7–13. [Google Scholar] [CrossRef]



| Alloys | Region/Phase | Chemical Composition (at.%) | ||||
|---|---|---|---|---|---|---|
| Cr | Fe | Co | Ni | Nb | ||
| Nb0.3 | alloy | 22.5 | 22.3 | 22.8 | 24.5 | 7.9 |
| FCC | 23.9 | 26.6 | 23.6 | 23.5 | 2.4 | |
| C14 Laves | 14.2 | 16.3 | 23.6 | 23.0 | 22.9 | |
| Eutectic | 19.7 | 21.1 | 23.3 | 23.2 | 12.7 | |
| Nb0.35 | alloy | 22.8 | 22.2 | 23.1 | 23.1 | 8.8 |
| FCC | 24.8 | 25.3 | 23.3 | 24.3 | 2.3 | |
| C14 Laves | 14.9 | 15.4 | 23.1 | 24.3 | 22.3 | |
| Eutectic | 20.7 | 22.5 | 23.2 | 22.3 | 11.3 | |
| Nb0.4 | alloy | 22.1 | 21.7 | 22.2 | 24.1 | 9.9 |
| FCC | 24.5 | 25.2 | 23.2 | 24.3 | 2.8 | |
| C14 Laves | 14.6 | 16.2 | 23.9 | 22.4 | 22.9 | |
| Eutectic | 20.4 | 21.4 | 22.2 | 24.3 | 11.7 | |
| Nb0.45 | alloy/Eutectic | 21.6 | 22.3 | 22.9 | 22.7 | 10.5 |
| Region C | 21.1 | 21.1 | 22.8 | 24.2 | 10.8 | |
| Nb0.5 | alloy | 20.8 | 22.5 | 21.7 | 23.2 | 11.8 |
| Eutectic | 21.4 | 22.5 | 21.1 | 24.3 | 10.7 | |
| Nb0.55 | alloy | 20.3 | 21.2 | 21.5 | 24.2 | 12.8 |
| FCC | 22.8 | 22.6 | 20.9 | 29.2 | 4.5 | |
| C14 Laves | 15.6 | 19.9 | 23.9 | 16.9 | 23.7 | |
| Eutectic | 22.9 | 21.3 | 22.2 | 23.9 | 9.7 | |
| Nb0.6 | alloy | 21.1 | 21.2 | 21.3 | 23.2 | 13.2 |
| FCC | 24.5 | 22.9 | 21.1 | 26.8 | 4.7 | |
| C14 Laves | 16.4 | 19.4 | 22.9 | 16.9 | 24.4 | |
| Eutectic | 22.3 | 21.3 | 21.1 | 24.5 | 10.8 | |
| Alloys | States | Phases | Chemical Composition (at.%) | ||||
|---|---|---|---|---|---|---|---|
| Cr | Fe | Co | Ni | Nb | |||
| Nb0.4 | after DSC | FCC | 24.3 | 25.3 | 23.5 | 24.1 | 2.8 |
| C14 Laves | 14.6 | 16.2 | 23.4 | 21.1 | 24.7 | ||
| Nb0.45 | after DSC | FCC | 24.9 | 25.6 | 23.2 | 23.9 | 2.4 |
| C14 Laves | 15.7 | 16.6 | 22.6 | 20.2 | 24.9 | ||
| Nb0.5 | after DSC | FCC | 24.7 | 23.8 | 22.7 | 26.2 | 2.6 |
| C14 Laves | 14.9 | 16.9 | 22.8 | 20.5 | 24.9 | ||
| Nb0.35 | 1000 °C annealed | FCC | 25.1 | 25.9 | 24.2 | 22.8 | 2.0 |
| C14 Laves | 15.2 | 15.9 | 26.3 | 18.1 | 24.5 | ||
| Nb0.45 | 1000 °C annealed | FCC | 25.9 | 25.9 | 21.5 | 24.6 | 2.1 |
| C14 Laves | 16.6 | 16.2 | 23.2 | 21.4 | 22.6 | ||
| Nb0.5 | 1000 °C annealed | FCC | 24.7 | 24.9 | 21.4 | 26.5 | 2.5 |
| C14 Laves | 14.7 | 17.5 | 22.1 | 20.5 | 25.2 | ||
| Nb0.55 | 1000 °C annealed | FCC | 26.3 | 23.1 | 20.8 | 27.4 | 2.4 |
| C14 Laves | 17.7 | 16.3 | 22.2 | 18.9 | 24.9 | ||
| Nb0.35 | 800 °C annealed | FCC a | 26.9 | 25.4 | 22.1 | 23.9 | 1.7 |
| C14 Laves a | 13.9 | 17.4 | 26.3 | 18.1 | 24.3 | ||
| Mg3Cd-type τ a | 2.9 | 4.5 | 24.1 | 41.1 | 27.4 | ||
| Nb0.55 | 800 °C annealed | FCC | 24.7 | 25.6 | 21.9 | 25.2 | 2.6 |
| C14 Laves | 13.8 | 17.2 | 26.2 | 18.5 | 24.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Wu, C.; Liu, Y.; Peng, H.; Su, X. Eutectic Reaction and Microstructure Stability in CoCrFeNiNbx High-Entropy Alloys. Metals 2022, 12, 756. https://doi.org/10.3390/met12050756
Cao X, Wu C, Liu Y, Peng H, Su X. Eutectic Reaction and Microstructure Stability in CoCrFeNiNbx High-Entropy Alloys. Metals. 2022; 12(5):756. https://doi.org/10.3390/met12050756
Chicago/Turabian StyleCao, Xu, Changjun Wu, Ya Liu, Haoping Peng, and Xuping Su. 2022. "Eutectic Reaction and Microstructure Stability in CoCrFeNiNbx High-Entropy Alloys" Metals 12, no. 5: 756. https://doi.org/10.3390/met12050756
APA StyleCao, X., Wu, C., Liu, Y., Peng, H., & Su, X. (2022). Eutectic Reaction and Microstructure Stability in CoCrFeNiNbx High-Entropy Alloys. Metals, 12(5), 756. https://doi.org/10.3390/met12050756






