Study on Stress Corrosion Cracking Behavior of Incoloy825/X65 Bimetallic Composite Pipe Welded Joint in Wet Hydrogen Sulfide Environment
Abstract
:1. Introduction
2. Experimental
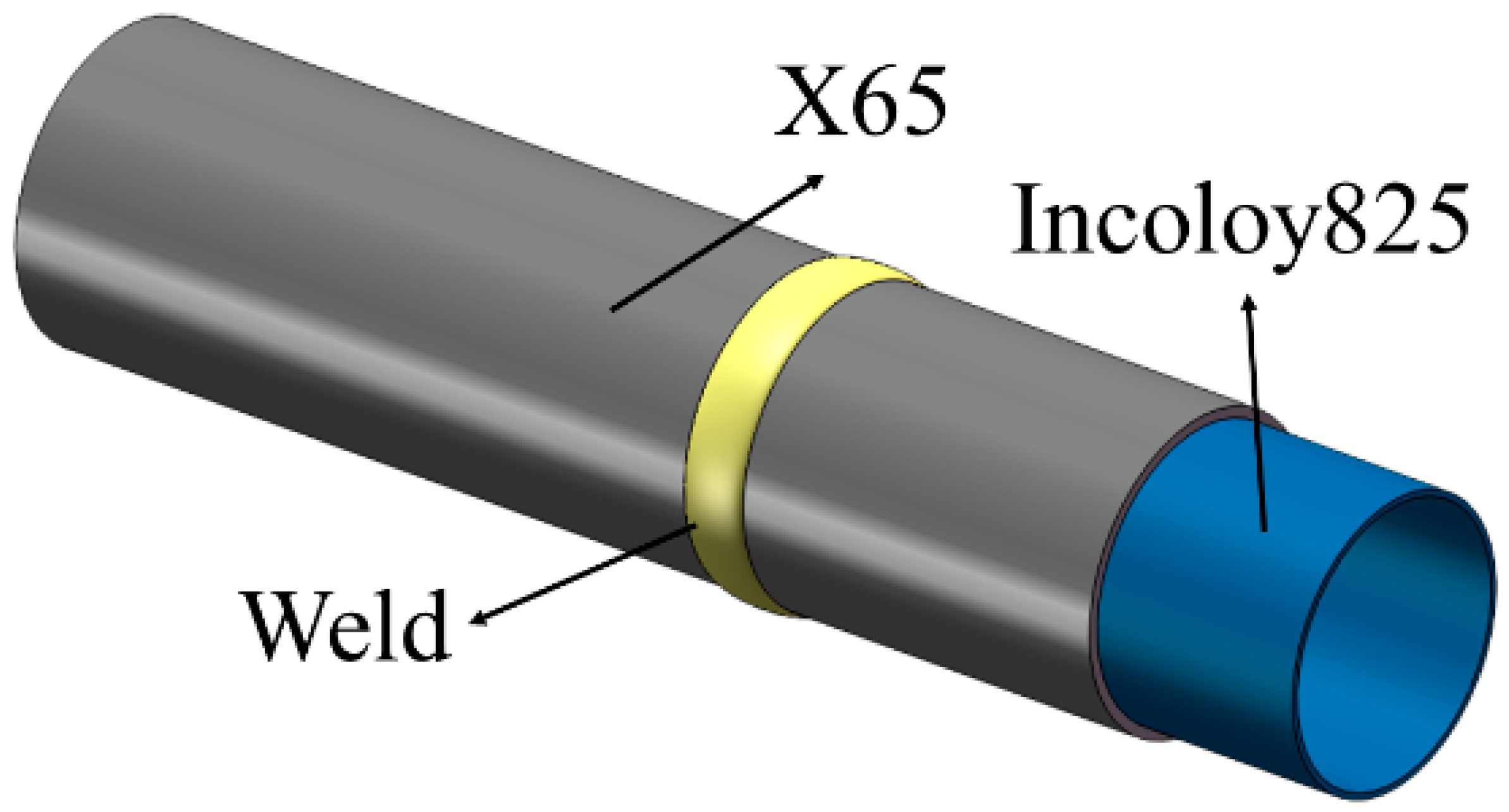
2.1. Experimental Material
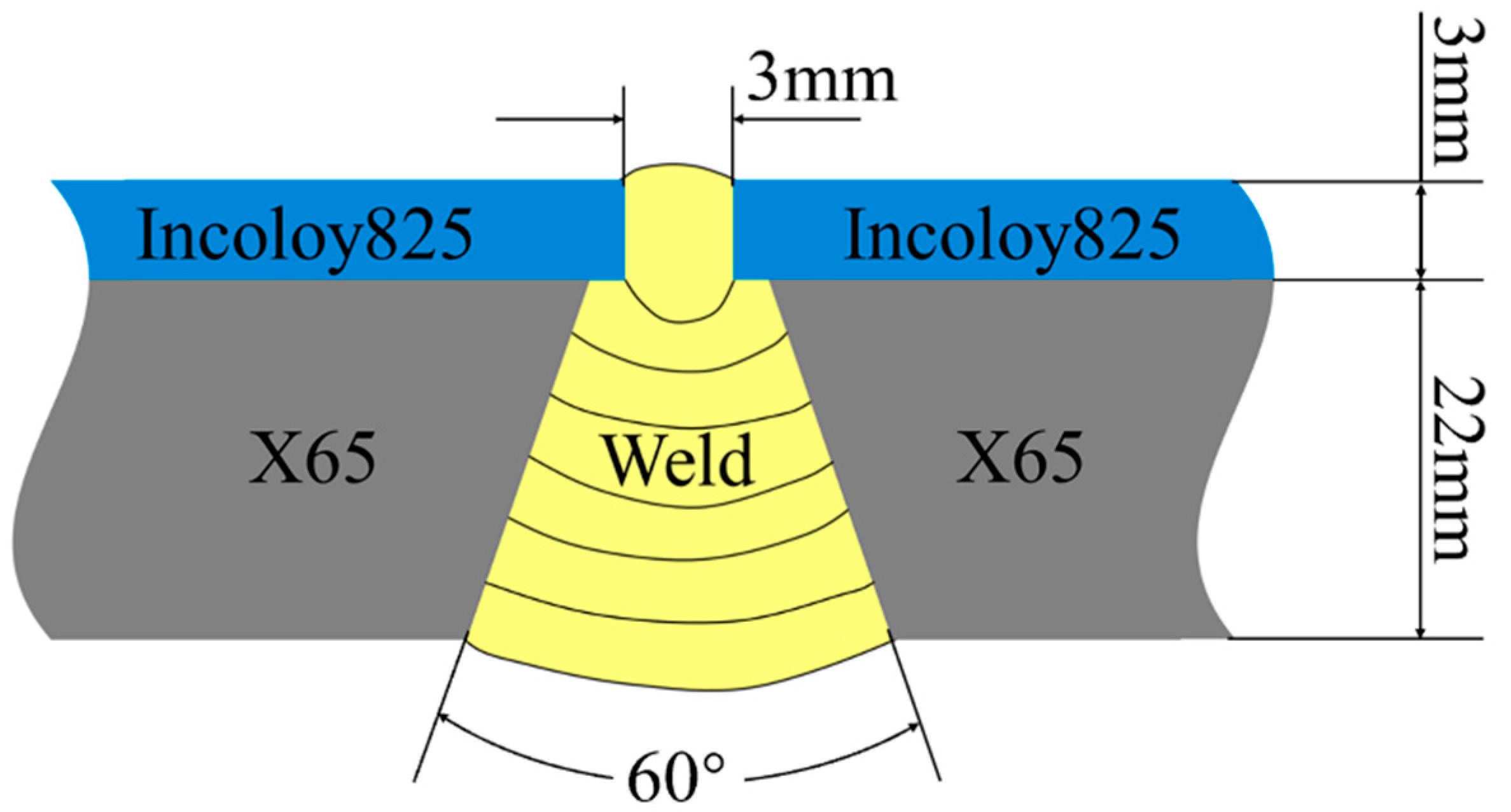
2.2. Welding Parameters
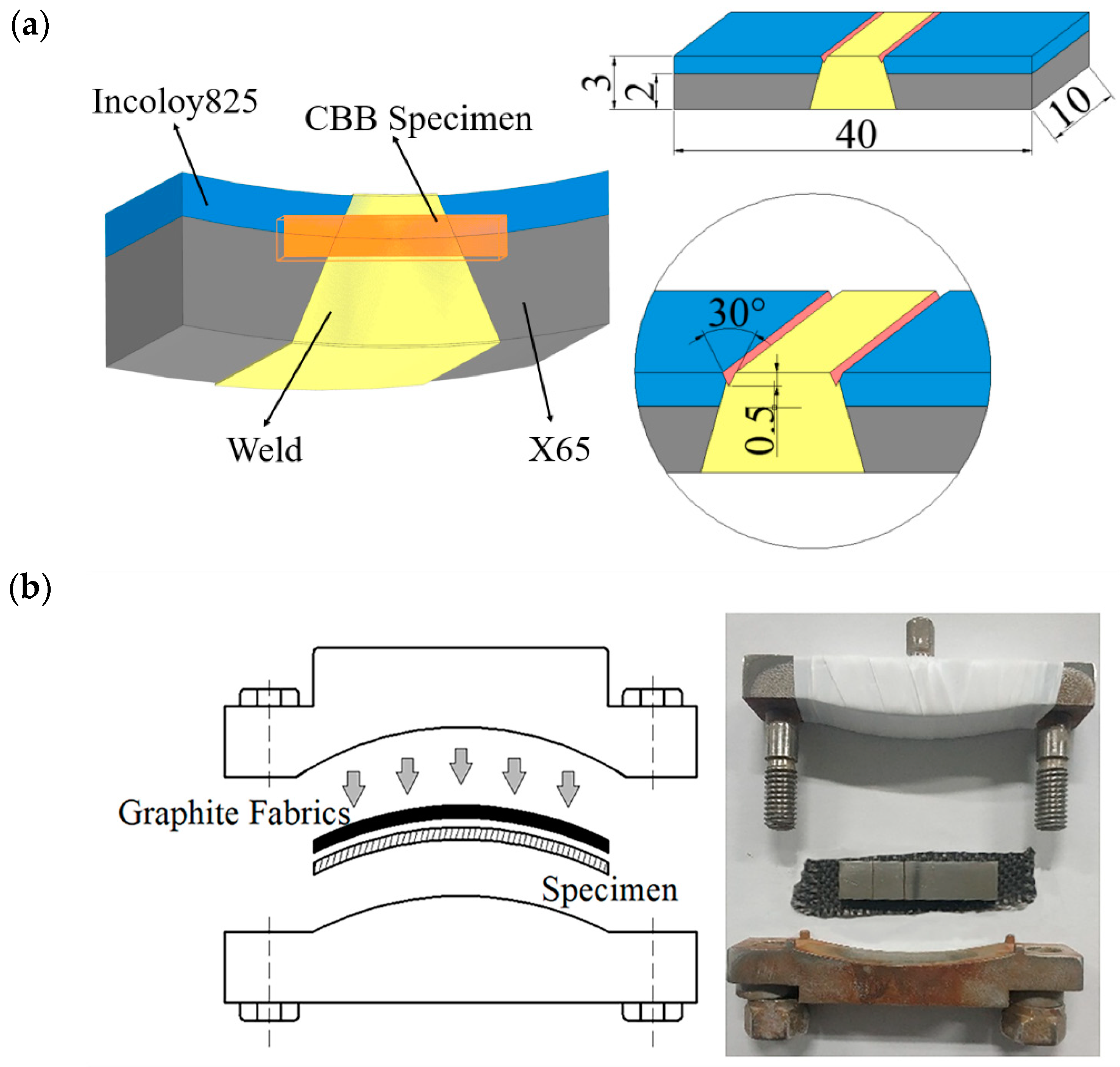
2.3. CBB Test
2.4. Characterization Test
3. Results and Discussion
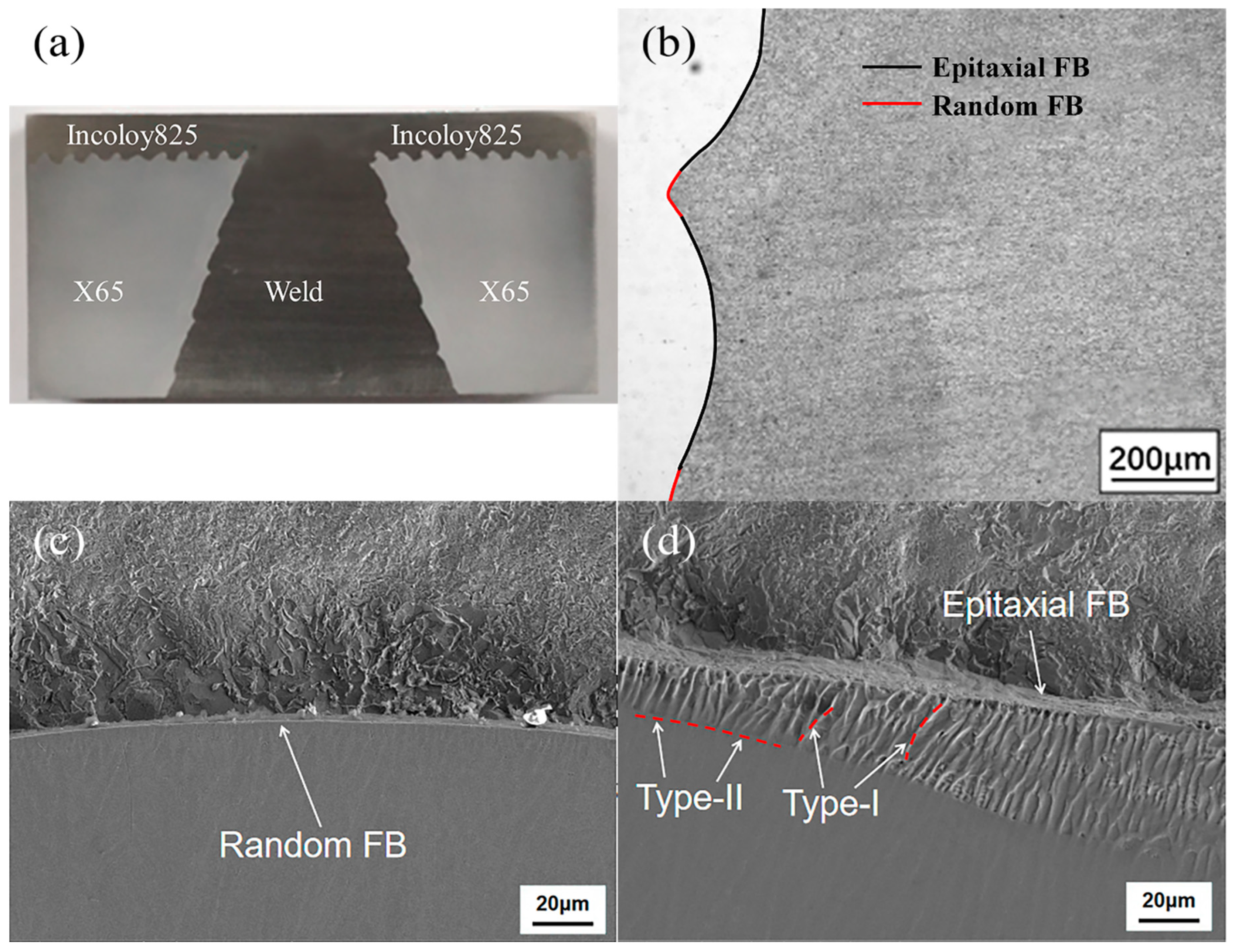
3.1. Microstructure Analysis
3.1.1. Microstructure Analysis of the Welded Joint
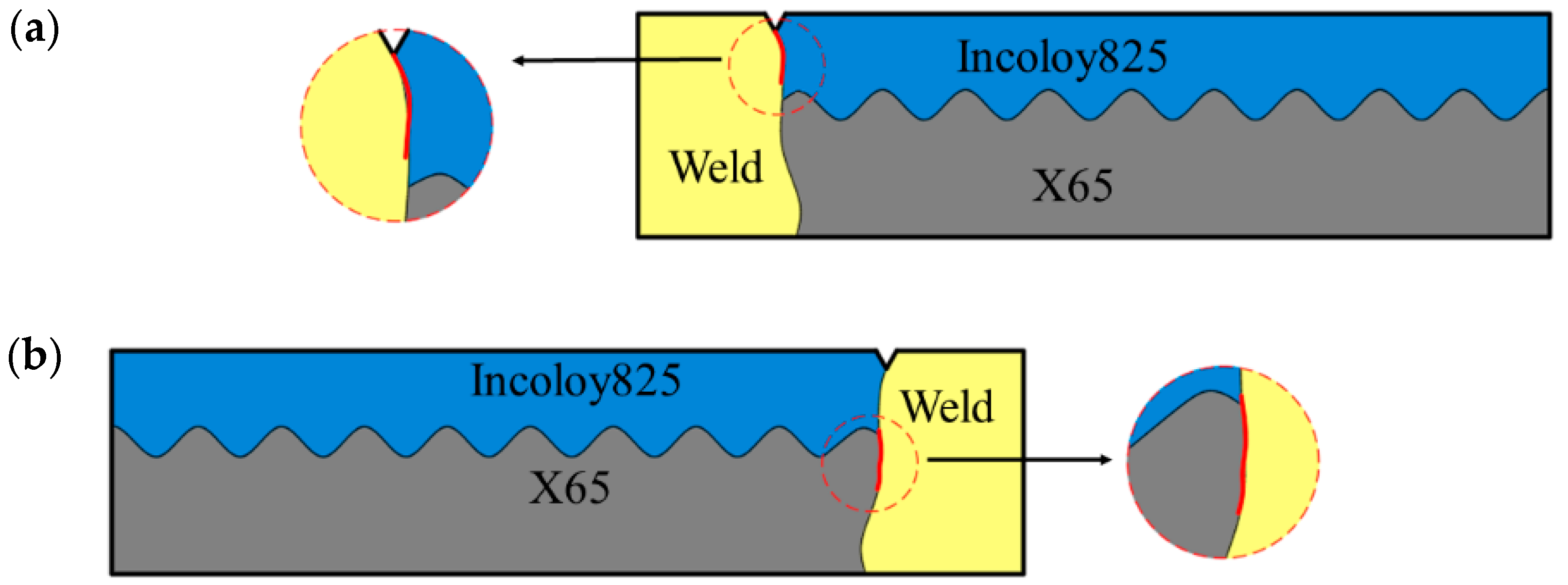
3.1.2. SCC Behavior Analysis
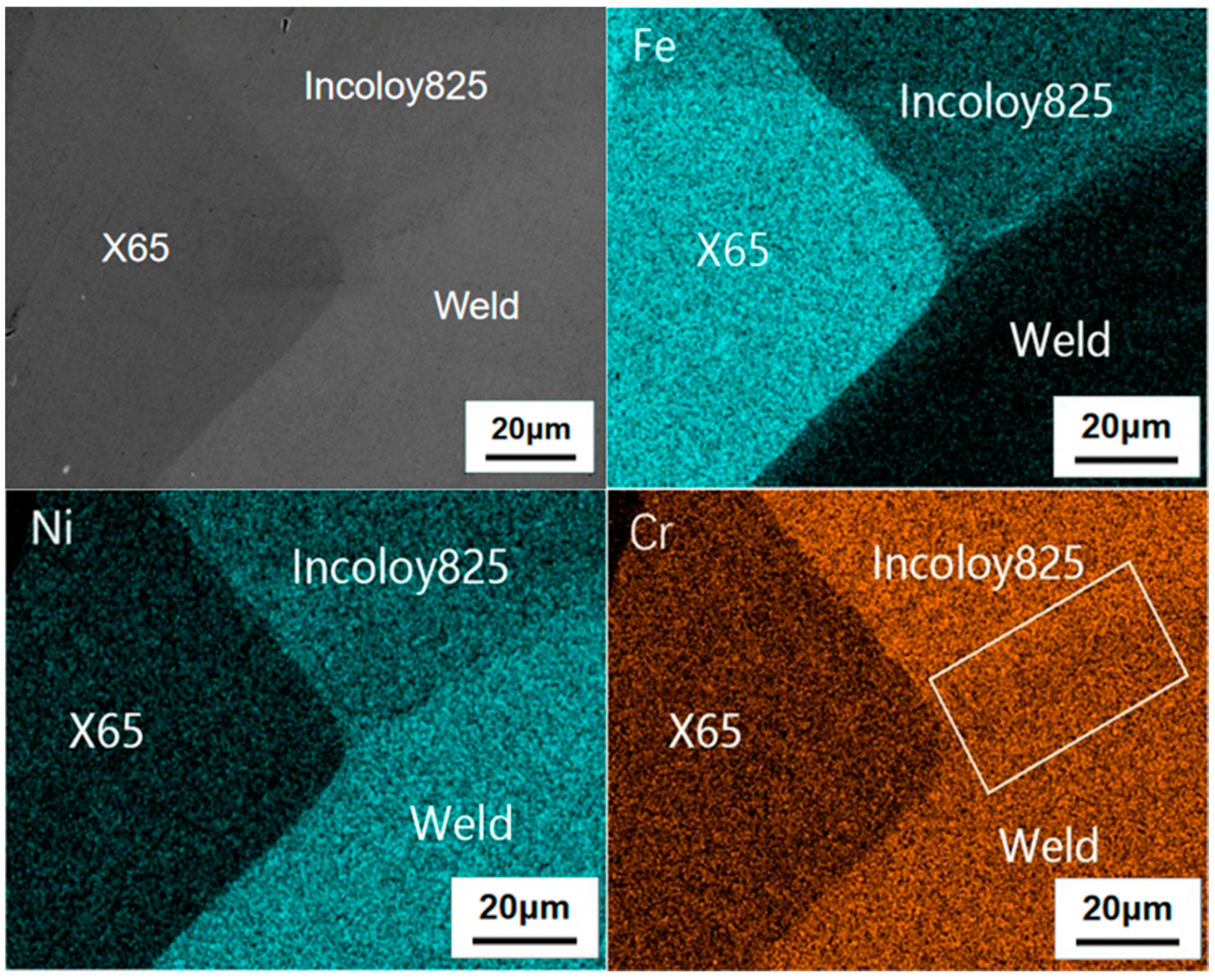
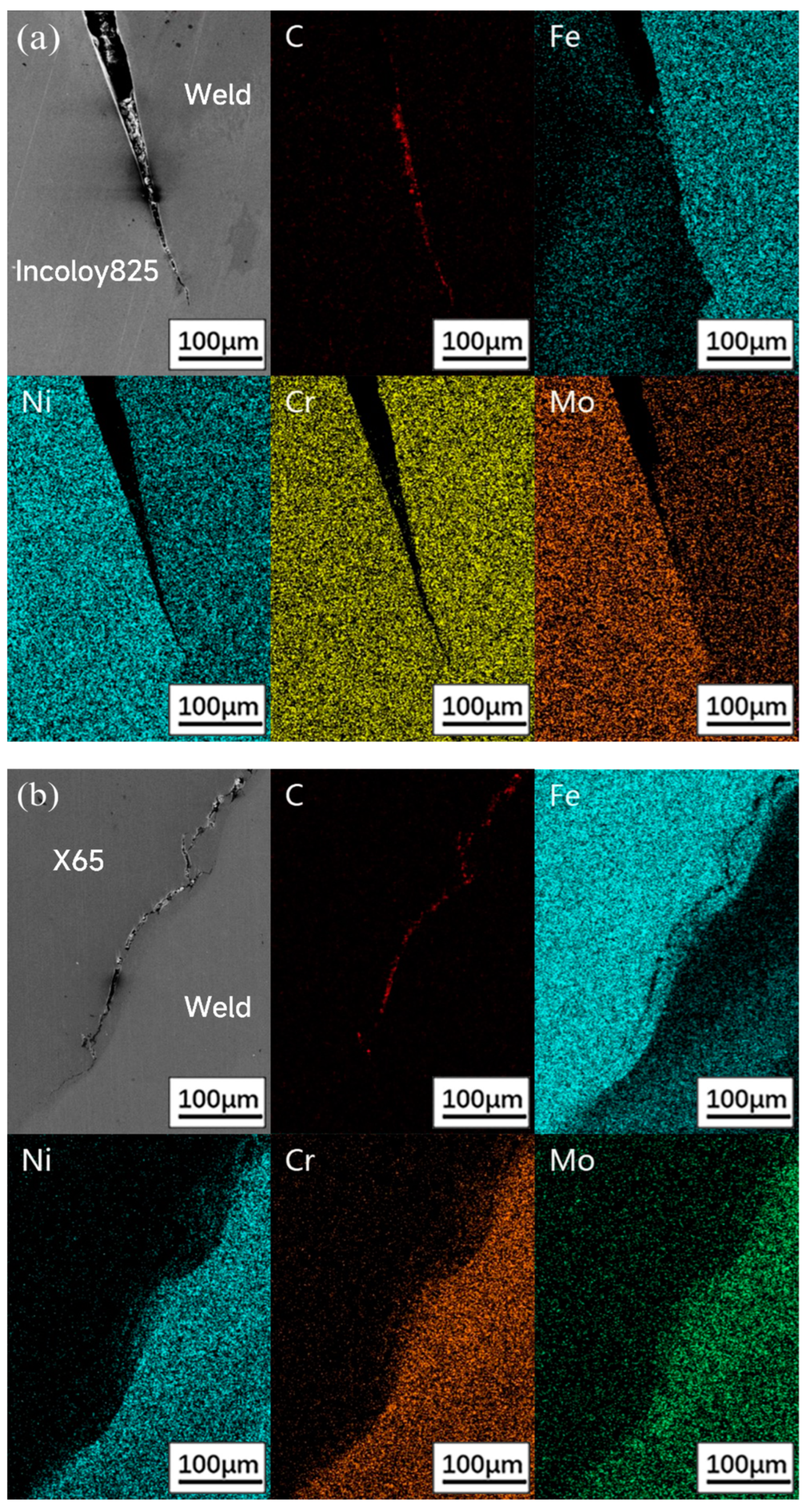
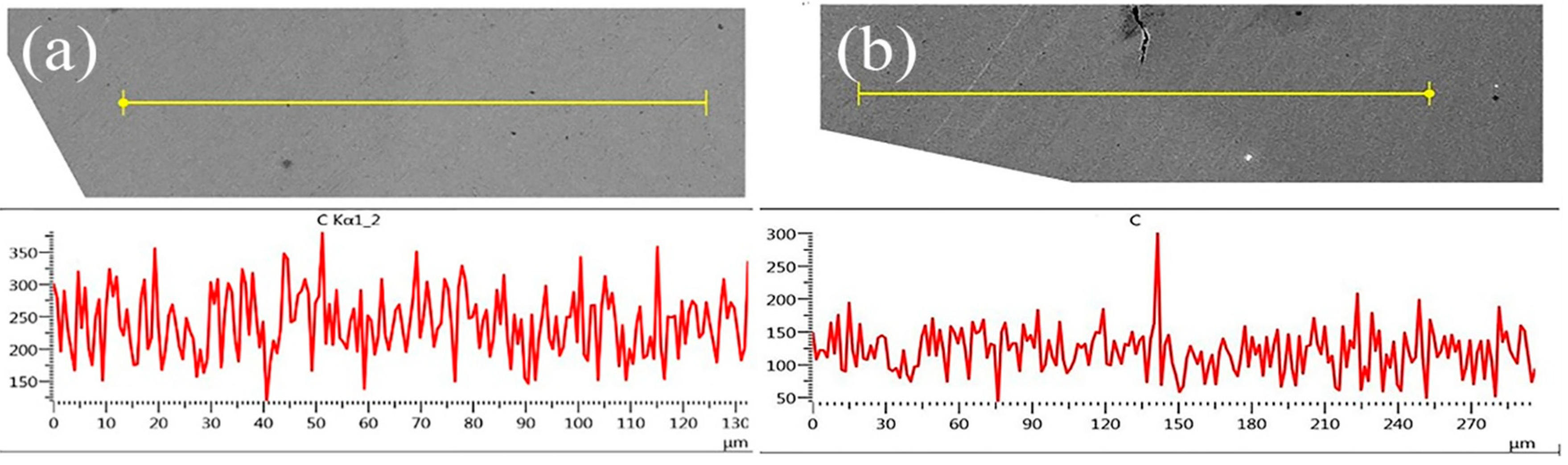
3.2. Element Diffusion Analysis
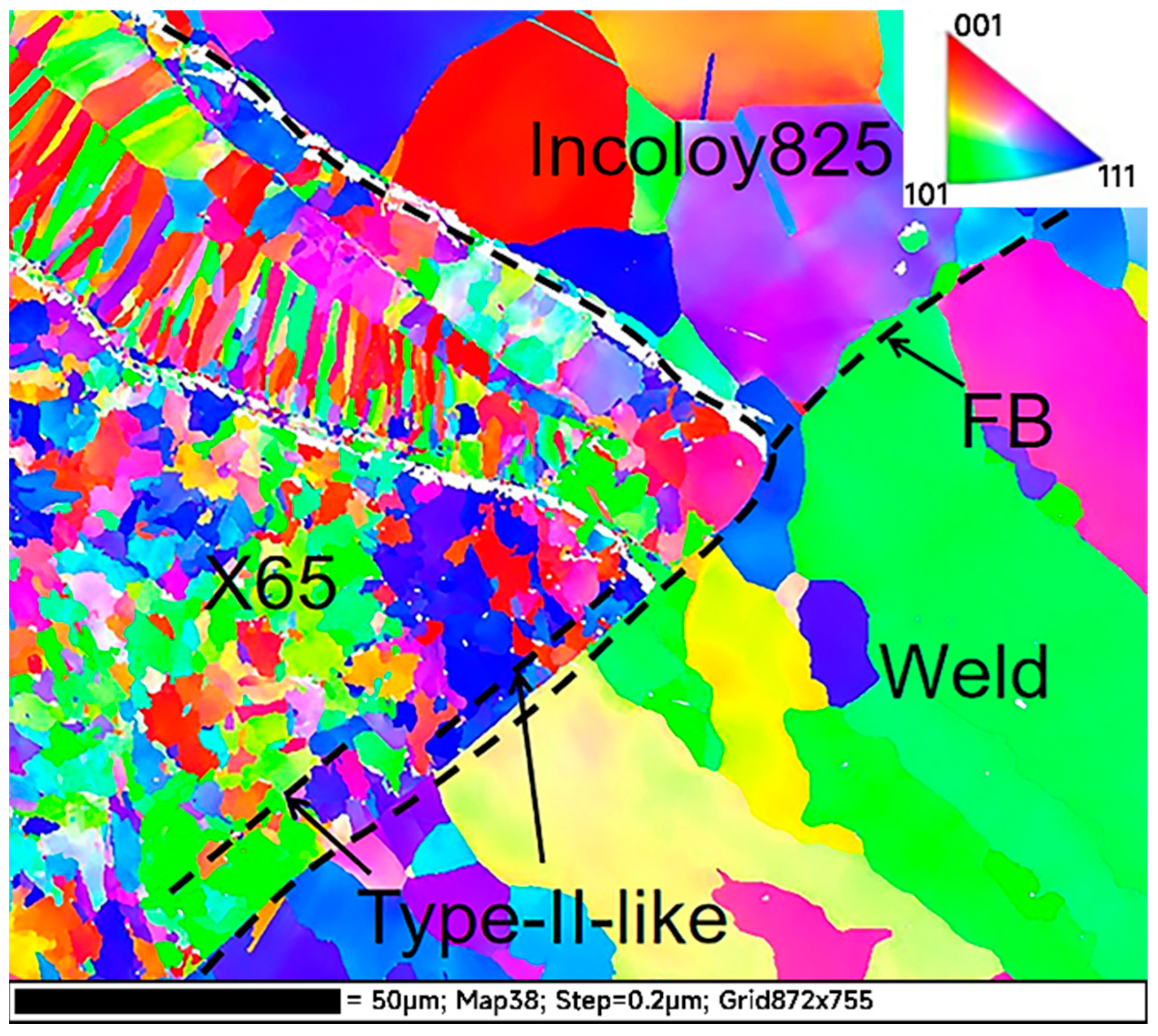
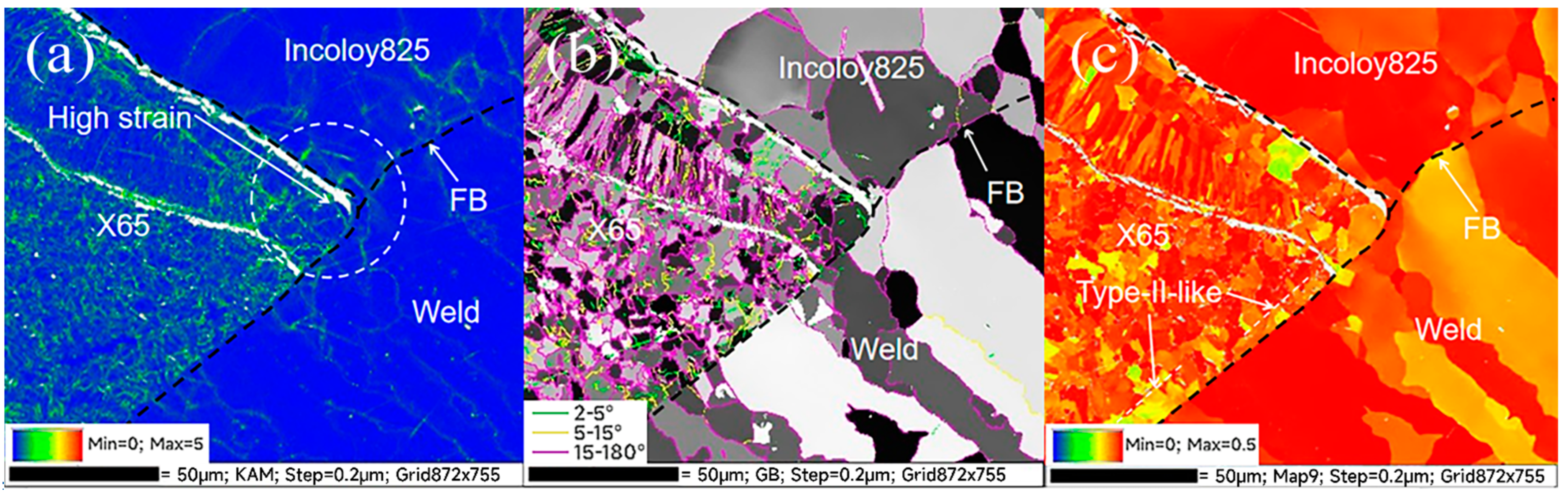
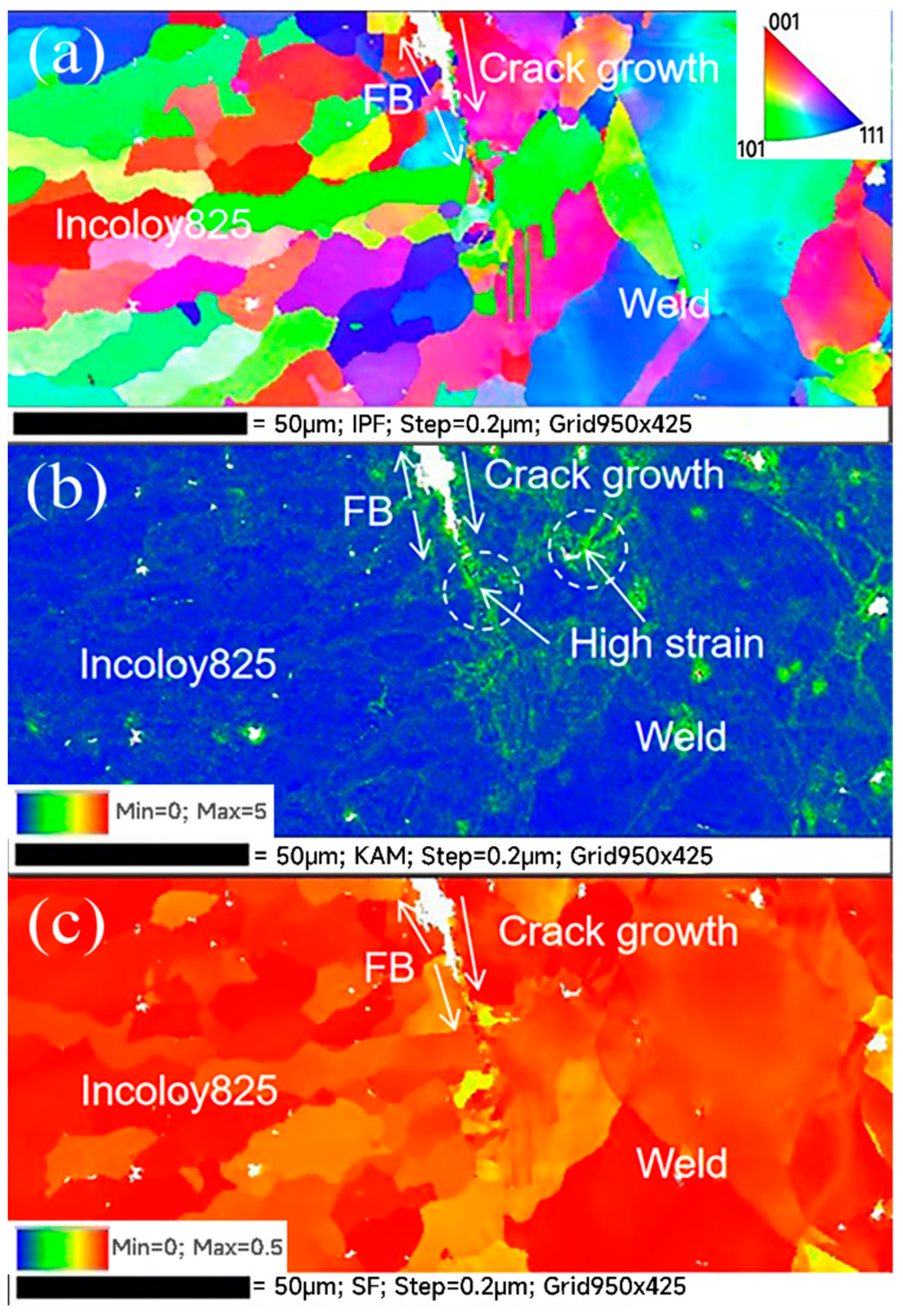
3.3. EBSD Results Analysis
4. Conclusions
- (1)
- Crack 1 initiated from the notch. The main reasons for cracking are the stress and strain concentration. The mutation of the electrode potential was led by the uneven distribution of Fe, Ni and Cr at the interface and the direct reaction between metal and H2S. The mechanism is the anodic dissolved stress corrosion cracking.
- (2)
- Crack 2 initiated from the intersection of the three zones, where there was residual strain concentration, lack of Cr and the effect of hydrogen generated by crack 1 during the corrosion process on the intersection of the three zones. The synergistic effect of strain concentration, grain boundary embrittlement and hydrogen embrittlement promoted the initiation of cracks.
- (3)
- The welded joint has high angle grain boundaries, Type-I, Type-II and the Type-II-like, which had high SCC sensitivity and provided intergranular channels for crack propagation. After initiation, the two kinds of cracks propagated along the FB and the Type-II-like boundary, and the crack tips were pitted and passivated, thus stopping the propagation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fragiel, A.; Serna, S.; Malo-Tamayo, J.; Silva, P.; Campillo, B.; Martínez-Martínez, E.; Cota, L.; Staia, M.; Puchi-Cabrera, E.S.; Perez, R. Effect of microstructure and temperature on the stress corrosion cracking of two microalloyed pipeline steels in H2S environment for gas transport. Eng. Fail. Anal. 2019, 105, 1055–1068. [Google Scholar] [CrossRef]
- Carneiro, R.A.; Ratnapuli, R.C.; Lins, V. The influence of chemical composition and microstructure of API linepipe steels on hydrogen induced cracking and sulfide stress corrosion cracking. Mater. Sci. Eng. A 2003, 357, 104–110. [Google Scholar] [CrossRef]
- Wen, X.; Bai, P.; Luo, B.; Zheng, S.; Chen, C. Review of recent progress in the study of corrosion products of steels in a hydrogen sulphide environment. Corros. Sci. 2018, 139, 124–140. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, X.; Li, G.; Chen, S.; Quan, Z.; Zhao, S.; Niu, L. The influence of hydrogen sulfide on corrosion of iron under different conditions. Corros. Sci. 2000, 42, 1669–1683. [Google Scholar] [CrossRef]
- Song, C.; Ling, J. Effects of H2S Partial Pressure on Stress Corrosion Cracking of Nickel-based Alloy G3. Corros. Prot. Petrochem. Ind. 2020, 37, 21–23. [Google Scholar]
- Zhao, X.H.; Han, Y.; Bai, Z.Q.; Wei, B. The experiment research of corrosion behaviour about Ni-based alloys in simulant solution containing H2S/CO2. Electrochim. Acta 2011, 56, 7725–7731. [Google Scholar] [CrossRef]
- Alekseeva, E.; Galata, L.; Lapechenkov, A.; Kovalev, M. Evaluation of Corrosion Resistance of Nickel-based Alloy EP718 for use in Hydrogen Sulphide Containing Environment. E3S Web Conf. 2021, 225, 03001. [Google Scholar] [CrossRef]
- Lu, C.-H. PWHT welding procedure for nickel alloy cladding pipe. Weld. Technol. 2021, 49, 51–54. [Google Scholar]
- Wang, B.; Lei, B.-B.; Wang, W.; Xu, M.; Wang, L. Investigations on the crack formation and propagation in the dissimilar pipe welds involving L360QS and N08825. Eng. Fail. Anal. 2015, 58, 56–63. [Google Scholar] [CrossRef]
- Wang, H.T.; Wang, G.Z.; Xuan, F.Z.; Tu, S.T. An experimental investigation of local fracture resistance and crack growth paths in a dissimilar metal welded joint. Mater. Des. 2013, 44, 179–189. [Google Scholar] [CrossRef]
- Jang, C.; Lee, J.; Kim, J.S.; Jin, T.E. Mechanical property variation within Inconel 82/182 dissimilar metal weld between low alloy steel and 316 stainless steel. Int. J. Press. Vessel. Pip. 2008, 85, 635–646. [Google Scholar] [CrossRef]
- Blouin, A.; Chapuliot, S.; Marie, S.; Niclaeys, C.; Bergheau, J.-M. Brittle fracture analysis of Dissimilar Metal Welds. Eng. Fract. Mech. 2014, 131, 58–73. [Google Scholar] [CrossRef]
- Celin, R.; Tehovnik, F. Degradation of a Ni-Cr-Fe alloy in a pressurised-water nuclear power plant. Mater. Technol. 2011, 45, 151–157. [Google Scholar]
- Yoshida, K.; Kojim, M.; Iida, M.; Takahashi, I. Fracture toughness of weld metals in steel piping for nuclear power plants. Int. J. Press. Vessel. Pip. 1990, 43, 273–284. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.; Ding, X.; Shoji, T. Microstructures and microhardness at fusion boundary of 316 stainless steel/Inconel 182 dissimilar welding. Mater. Charact. 2015, 107, 255–261. [Google Scholar] [CrossRef]
- Nelson, T.W.; Lippold, J.C.; Mills, M.J. Nature and evolution of the fusion boundary in ferritic–austenitic dissimilar weld metals, part 2: On-cooling transformations. Weld. J. 2000, 79, 267S–277S. [Google Scholar]
- Hou, J.; Peng, Q.; Takeda, Y.; Kuniya, J.; Shoji, T. Microstructure and stress corrosion cracking of the fusion boundary region in an alloy 182-A533B low alloy steel dissimilar weld joint. Corros. Sci. 2010, 52, 3949–3954. [Google Scholar] [CrossRef]
- Nishikawa, S.; Horii, Y.; Ikeuchi, K. Effect of Chromium Content on Stress Corrosion Cracking Susceptibility of Shielded Metal Arc Weld Metals for 600 Type Alloy in High Temperature Pressurized Pure Water. Weld. Int. 2013, 27, 450–459. [Google Scholar] [CrossRef]
- Mayuzumi, M.; Ishiyama, N.; Mizutani, Y.; Tani, J.-I.; Kako, K. Incubation Time of Stress Corrosion Cracking for Type 316L and Type 316 Stainless Steels by CBB Method in High Temperature Water. Corros. Eng. 2008, 57, 230–234. [Google Scholar] [CrossRef]
- Nelson, T.W.; Lippold, J.C.; Mills, M.J. Nature and evolution of the fusion boundary in ferritic-austenitic dissimilar weld metals. Part 1—Nucleation and growth. Weld. J. 1999, 78, 329s–337s. [Google Scholar]
- Lu, Z.; Shoji, T.; Meng, F.; Xue, H.; Qiu, Y.; Takeda, Y.; Negishi, K. Characterization of microstructure and local deformation in 316NG weld heat-affected zone and stress corrosion cracking in high temperature water. Corros. Sci. 2011, 53, 1916–1932. [Google Scholar] [CrossRef]
- Ghosh, M.; Santosh, R.; Das, S.K.; Das, G.; Mahato, B.; Korody, J.; Kumar, S.; Singh, P.K. Effect of Structural Heterogeneity on In Situ Deformation of Dissimilar Weld Between Ferritic and Austenitic Steel. Metall. Mater. Trans. A 2015, 46, 3555–3568. [Google Scholar] [CrossRef]
- Nelson, T.W.; Lippold, J.C.; Mills, M.J. Investigation of boundaries and structures in dissimilar metal welds. Sci. Technol. Weld. Join. 1998, 3, 249–255. [Google Scholar] [CrossRef]
- Hou, J.; Peng, Q.; Shoji, T.; Wang, J.; Ke, W.; Han, E. Study of Microstructure and Stress Corrosion Cracking Behavior in Welding Transition Zone of Ni-Based Alloys. Acta Metall. Sin. 2010, 46, 1258–1266. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, W.; Kish, J.R. Stress corrosion cracking susceptibility of thermally-aged Type 310S stainless steels in supercritical water. Corros. Sci. 2018, 135, 1–11. [Google Scholar] [CrossRef]
- Dong, L.; Peng, Q.; Xue, H.; Han, E.-H.; Ke, W.; Wang, L. Correlation of microstructure and stress corrosion cracking initiation behaviour of the fusion boundary region in a SA508 Cl. 3-Alloy 52M dissimilar weld joint in primary pressurized water reactor environment. Corros. Sci. 2017, 132, 9–20. [Google Scholar] [CrossRef]

| Material | C | Si | Mn | P | S | Ni | Cr | Mo | Cu | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| X65 | 0.08 | 0.26 | 0.98 | 0.06 | 0.10 | 0.29 | 0.24 | 0.04 | 0.24 | 0.00 | 97.71 |
| Incoloy825 | 0.01 | 0.49 | 0.73 | 0.05 | 0.01 | 37.2 | 22.23 | 3.91 | 3.11 | 0.70 | 32.29 |
| Weld | 0.03 | 0.46 | 0.32 | 0.06 | 0.10 | 59.62 | 20.33 | 9.32 | 0.33 | 0.35 | 9.08 |
| Pass (Welding Process) | Filler Metal | Diameter (mm) | Power Polarity | Current (A) | Voltage (V) | Weld Speed (cm/min) | Gas Flow (L/min) | |
|---|---|---|---|---|---|---|---|---|
| Front | Back | |||||||
| Root Pass (GTAW) | ERNiCrMo-3 | 2.4 | DC Straight Polarity | 90~120 | 9~12 | 5~8 | 7~13 | 15~25 |
| Filling and Covering Pass (SMAW) | ENiCrMo-3 | 3.2 | DC Reverse Polarity | 80~90 | 20~23 | 5~7 | 7~13 | 15~25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Ouyang, L.; Xu, J.; Huang, P.; Liu, E.; Yang, B. Study on Stress Corrosion Cracking Behavior of Incoloy825/X65 Bimetallic Composite Pipe Welded Joint in Wet Hydrogen Sulfide Environment. Metals 2022, 12, 632. https://doi.org/10.3390/met12040632
Wang B, Ouyang L, Xu J, Huang P, Liu E, Yang B. Study on Stress Corrosion Cracking Behavior of Incoloy825/X65 Bimetallic Composite Pipe Welded Joint in Wet Hydrogen Sulfide Environment. Metals. 2022; 12(4):632. https://doi.org/10.3390/met12040632
Chicago/Turabian StyleWang, Bingying, Li Ouyang, Jianxing Xu, Peng Huang, Enyang Liu, and Bin Yang. 2022. "Study on Stress Corrosion Cracking Behavior of Incoloy825/X65 Bimetallic Composite Pipe Welded Joint in Wet Hydrogen Sulfide Environment" Metals 12, no. 4: 632. https://doi.org/10.3390/met12040632
APA StyleWang, B., Ouyang, L., Xu, J., Huang, P., Liu, E., & Yang, B. (2022). Study on Stress Corrosion Cracking Behavior of Incoloy825/X65 Bimetallic Composite Pipe Welded Joint in Wet Hydrogen Sulfide Environment. Metals, 12(4), 632. https://doi.org/10.3390/met12040632






