Antisolvent Precipitation for Metal Recovery from Citric Acid Solution in Recycling of NMC Cathode Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Leaching of NMC111
2.3. Antisolvent Precipitation from Single Metal Systems
2.4. Antisolvent Precipitation from NMC111 Leach Liquor
2.5. Analysis
3. Results and Discussion
3.1. Antisolvent Precipitation from Synthetic Solutions (Single Metal Systems)
3.2. Antisolvent Precipitation from NMC111 Leach Solution (Mixed Metal Systems)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melin, H.E.; Rajaeifar, M.A.; Ku, A.Y.; Kendall, A.; Harper, G.; Heidrich, O. Global implications of the EU battery regulation. Science 2021, 373, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Boyden, A.; Soo, V.K.; Doolan, M. The Environmental Impacts of Recycling Portable Lithium-Ion Batteries. Procedia CIRP 2016, 48, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Chagnes, A.; Swiatowska, J. Lithium Process Chemistry: Resources, Extraction, Batteries, and Recycling; Elsevier Science: San Diego, CA, USA, 2015; ISBN 978-0-12-801417-2. [Google Scholar]
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of Lithium-Ion Batteries—Current State of the Art, Circular Economy, and Next Generation Recycling. Adv. Energy Mater. 2022; in press. [Google Scholar] [CrossRef]
- Moldoveanu, G.A.; Demopoulos, G.P. Organic solvent-assisted crystallization of inorganic salts from acidic media. J. Chem. Technol. Biotechnol. 2015, 90, 686–692. [Google Scholar] [CrossRef]
- Weingaertner, D.A.; Lynn, S.; Hanson, D.N. Extractive crystallization of salts from concentrated aqueous solution. Ind. Eng. Chem. Res. 1991, 30, 490–501. [Google Scholar] [CrossRef]
- Ma, Y.; Svärd, M.; Xiao, X.; Gardner, J.M.; Olsson, R.; Forsberg, K. Precipitation and crystallization used in the production of metal salts for Li-ion battery materials: A review. Metals 2020, 10, 1609. [Google Scholar] [CrossRef]
- Korkmaz, K.; Alemrajabi, M.; Rasmuson, Å.C.; Forsberg, K.M. Separation of valuable elements from NiMH battery leach liquor via antisolvent precipitation. Sep. Purif. Technol. 2020, 234, 115812. [Google Scholar] [CrossRef]
- Cruz, P.; Alvarez, C.; Rocha, F.; Ferreira, A. Tailoring the crystal size distribution of an active pharmaceutical ingredient by continuous antisolvent crystallization in a planar oscillatory flow crystallizer. Chem. Eng. Res. Des. 2021, 175, 115–123. [Google Scholar] [CrossRef]
- Di Profio, G.; Stabile, C.; Caridi, A.; Curcio, E.; Drioli, E. Antisolvent membrane crystallization of pharmaceutical compounds. J. Pharm. Sci. 2009, 98, 4902–4913. [Google Scholar] [CrossRef] [PubMed]
- Diab, S.; Gerogiorgis, D.I. Process modelling, simulation and technoeconomic evaluation of crystallisation antisolvents for the continuous pharmaceutical manufacturing of rufinamide. Comput. Chem. Eng. 2018, 111, 102–114. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; He, J.; Zhou, P.; Huang, L.; Zhou, J. A high-throughput system combining microfluidic hydrogel droplets with deep learning for screening the antisolvent-crystallization conditions of active pharmaceutical ingredients. Lab A Chip 2020, 20, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.X.; Fujiwara, M.; Woo, X.Y.; Rusli, E.; Tung, H.-H.; Starbuck, C.; Davidson, O.; Ge, Z.; Braatz, R.D. Direct Design of Pharmaceutical Antisolvent Crystallization through Concentration Control. Cryst. Growth Des. 2006, 6, 892–898. [Google Scholar] [CrossRef]
- Xuan, W.; de Souza Braga, A.; Chagnes, A. Development of a Novel Solvent Extraction Process to Recover Cobalt, Nickel, Manganese, and Lithium from Cathodic Materials of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 582–593. [Google Scholar] [CrossRef]
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries: Technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Xuan, W.; Otsuki, A.; Chagnes, A. Investigation of the leaching mechanism of NMC 811 (LiNi0.8 Mn0.1 Co0.1 O2) by hydrochloric acid for recycling lithium ion battery cathodes. RSC Adv. 2019, 9, 38612–38618. [Google Scholar] [CrossRef] [Green Version]
- Xuan, W.; de Souza Braga, A.; Korbel, C.; Chagnes, A. New insights in the leaching kinetics of cathodic materials in acidic chloride media for lithium-ion battery recycling. Hydrometallurgy 2021, 204, 105705. [Google Scholar] [CrossRef]
- Aktas, S.; Fray, D.J.; Burheim, O.; Fenstad, J.; Açma, E. Recovery of metallic values from spent Li ion secondary batteries. Miner. Process. Extr. Metall. 2006, 115, 95–100. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching. Chem. Eng. J. 2015, 281, 418–427. [Google Scholar] [CrossRef]
- Pant, D.; Dolker, T. Green and facile method for the recovery of spent Lithium Nickel Manganese Cobalt Oxide (NMC) based Lithium ion batteries. Waste Manag. 2017, 60, 689–695. [Google Scholar] [CrossRef]
- Xiao, X.; Hoogendoorn, B.W.; Ma, Y.; Ashoka Sahadevan, S.; Gardner, J.M.; Forsberg, K.; Olsson, R.T. Ultrasound-assisted extraction of metals from Lithium-ion batteries using natural organic acids. Green Chem. 2021, 23, 8519–8532. [Google Scholar] [CrossRef]
- Punt, T.; Akdogan, G.; Bradshaw, S.; van Wyk, P. Development of a novel solvent extraction process using citric acid for lithium-ion battery recycling. Miner. Eng. 2021, 173, 107204. [Google Scholar] [CrossRef]
- Okonkwo, E.G.; Wheatley, G.; He, Y. The role of organic compounds in the recovery of valuable metals from primary and secondary sources: A mini-review. Resour. Conserv. Recycl. 2021, 174, 105813. [Google Scholar] [CrossRef]
- Horeh, N.B.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger. J. Power Sources 2016, 320, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Or, T.; Gourley, S.W.D.; Kaliyappan, K.; Yu, A.; Chen, Z. Recycling of mixed cathode lithium-ion batteries for electric vehicles: Current status and future outlook. Carbon Energy 2020, 2, 6–43. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Liu, C.; Cao, H.; Zheng, X.; Lin, X.; Wang, H.; Zhang, Y.; Sun, Z. Comprehensive evaluation on effective leaching of critical metals from spent lithium-ion batteries. Waste Manag. 2018, 75, 477–485. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of lithium and cobalt from spent lithium ion batteries (LIBs) using organic acids as leaching reagents: A review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Li, L.; Zhai, L.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Recovery of valuable metals from spent lithium-ion batteries by ultrasonic-assisted leaching process. J. Power Sources 2014, 262, 380–385. [Google Scholar] [CrossRef]
- Deng, Y.-F.; Zhou, Z.-H.; Wan, H.-L.; Ng, S.W. Δ-Aqua-S-citrato(2–) manganese(II). Acta Crystallographica. Sect. E Struct. 2003, 59, m310–m312. [Google Scholar] [CrossRef]
- Oustadakis, P.; Agatzini-Leonardou, S.; Tsakiridis, P.E. Nickel and cobalt precipitation from sulphate leach liquor using MgO pulp as neutralizing agent. Miner. Eng. 2006, 19, 1204–1211. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Amiri, H.; Jazi, B. Dielectric Constants of Water, Methanol, Ethanol, Butanol and Acetone: Measurement and Computational Study. J. Solut. Chem. 2010, 39, 701–708. [Google Scholar] [CrossRef]
- Doki, N.; Kubota, N.; Yokota, M.; Kimura, S.; Sasaki, S. Production of Sodium Chloride Crystals of Uni-Modal Size Distribution by Batch Dilution Crystallization. J. Chem. Eng. Jpn. 2002, 35, 1099–1104. [Google Scholar] [CrossRef]
- Nefedova, K.V.; Zhuravlev, V.D.; Khaliullin, S.M.; Tyutyunnik, A.P.; Buldakova, L.Y. Study of the Composition of a Precipitate Formed from Solutions for the Synthesis of Cathodic Materials Containing Manganese and Citric Acid. Theor. Found. Chem. Eng. 2021, 55, 117–122. [Google Scholar] [CrossRef]
- Lackner, M. Combustion Synthesis: Novel Routes to Novel Materials; Bentham Science Publication: Vienna, Austria, 2010; pp. 55–71. ISBN 978-1-60805-155-7. [Google Scholar]
- Bohlender, C.; Kahnes, M.; Müller, R.; Töpfer, J. Phase formation, magnetic properties, and phase stability in reducing atmosphere of M-type strontium hexaferrite nanoparticles synthesized via a modified citrate process. J. Mater. Sci. 2019, 54, 1136–1146. [Google Scholar] [CrossRef]
- Demirel, H.S.; Svärd, M.; Uysal, D.; Doğan, Ö.M.; Uysal, B.Z.; Forsberg, K. Antisolvent crystallization of battery grade nickel sulphate hydrate in the processing of lateritic ores. Sep. Purif. Technol. 2022, 286, 120473. [Google Scholar] [CrossRef]
- Matzapetakis, M.; Raptopoulou, C.P.; Tsohos, A.; Papaefthymiou, V.; Moon, N.; Salifoglou, A. Synthesis, Spectroscopic and Structural Characterization of the First Mononuclear, Water Soluble Iron−Citrate Complex, (NH4)5Fe(C6H4O7)2·2H2O. J. Am. Chem. Soc. 1998, 120, 13266–13267. [Google Scholar] [CrossRef]
- Dou, S.; Wang, W. Synthesis and electrochemical properties of layered LiNi0.5 − x Mn0.5 − x Co2x O2 for lithium-ion battery from nickel manganese cobalt oxide precursor. J. Solid State Electrochem. 2011, 15, 399–404. [Google Scholar] [CrossRef]
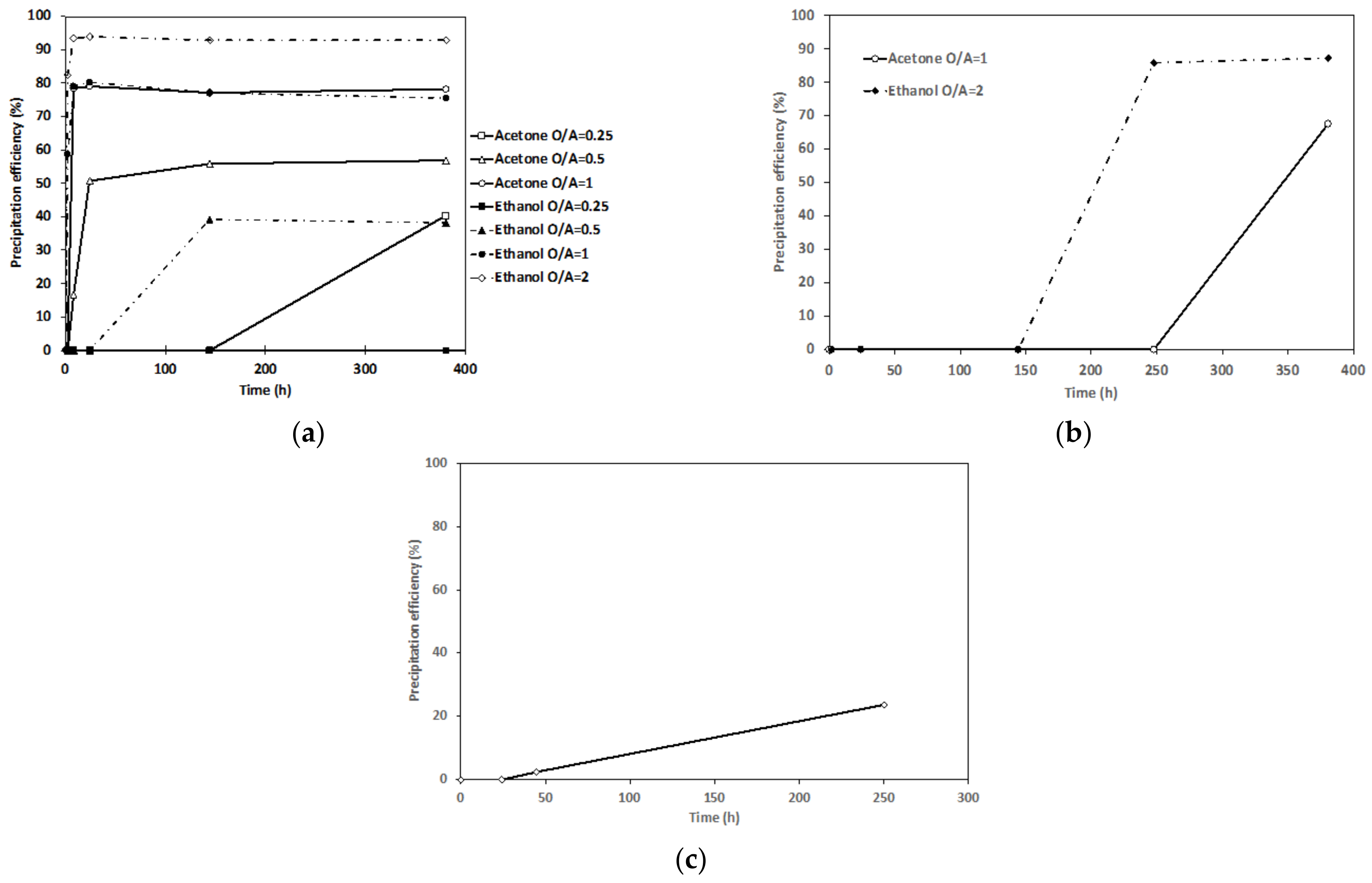

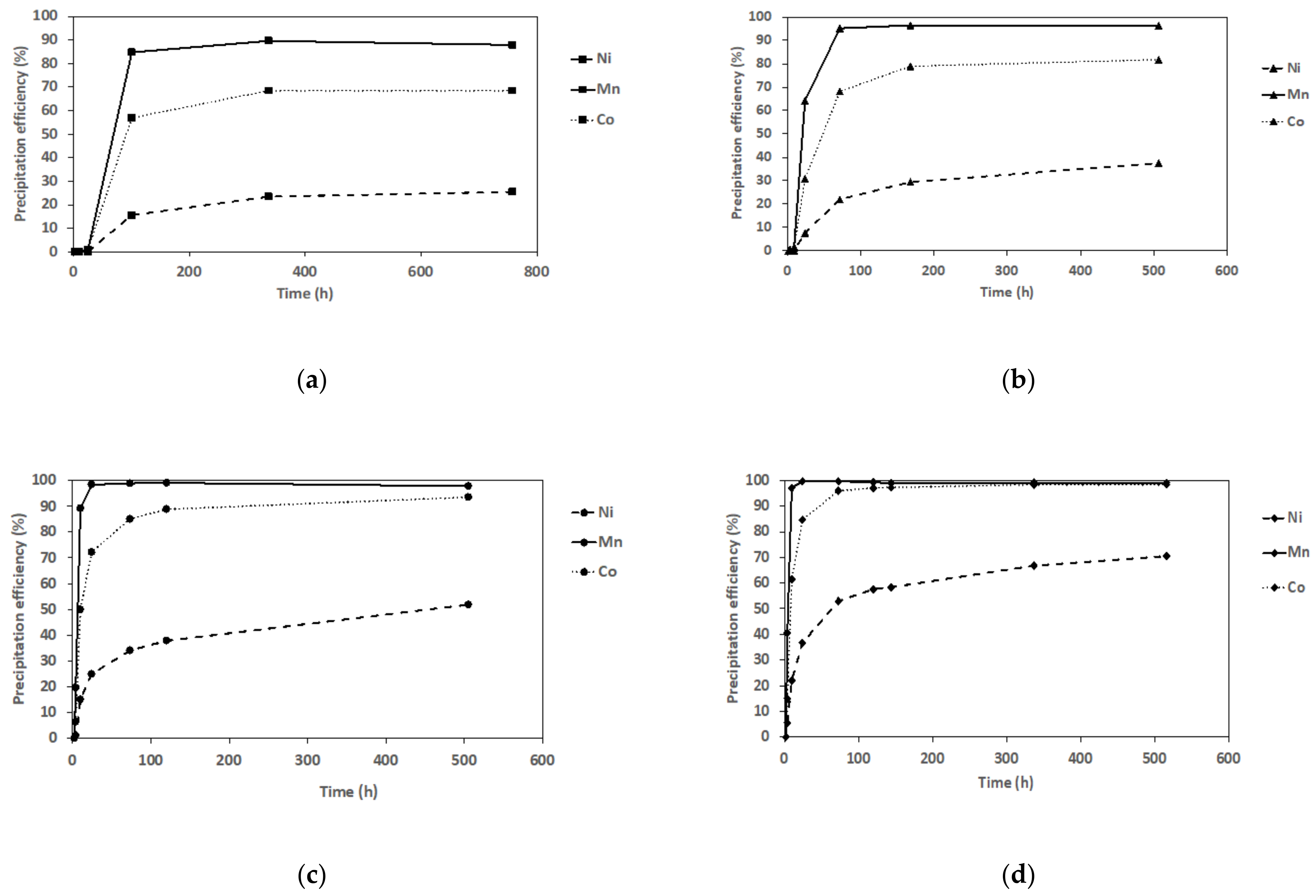
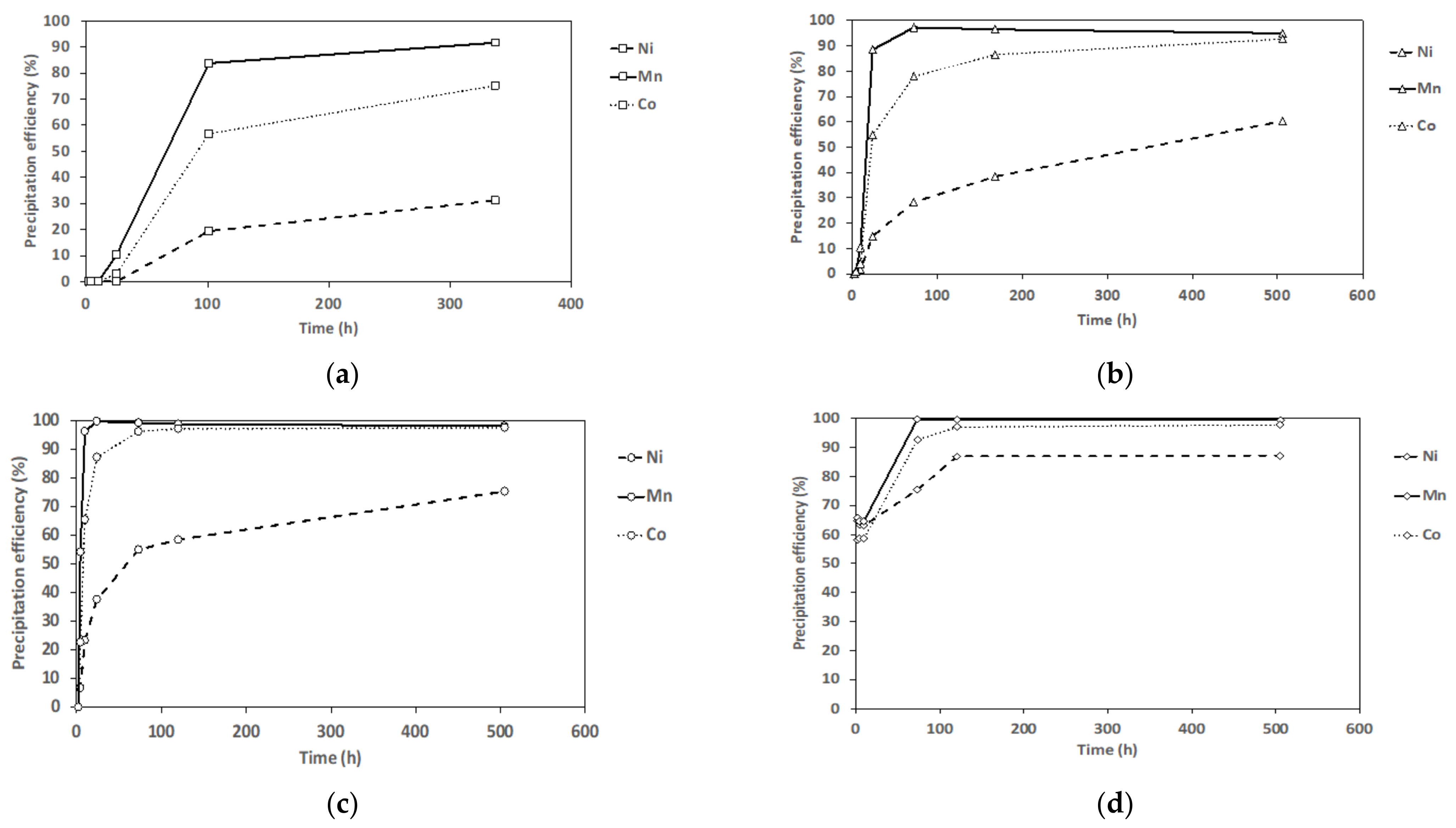



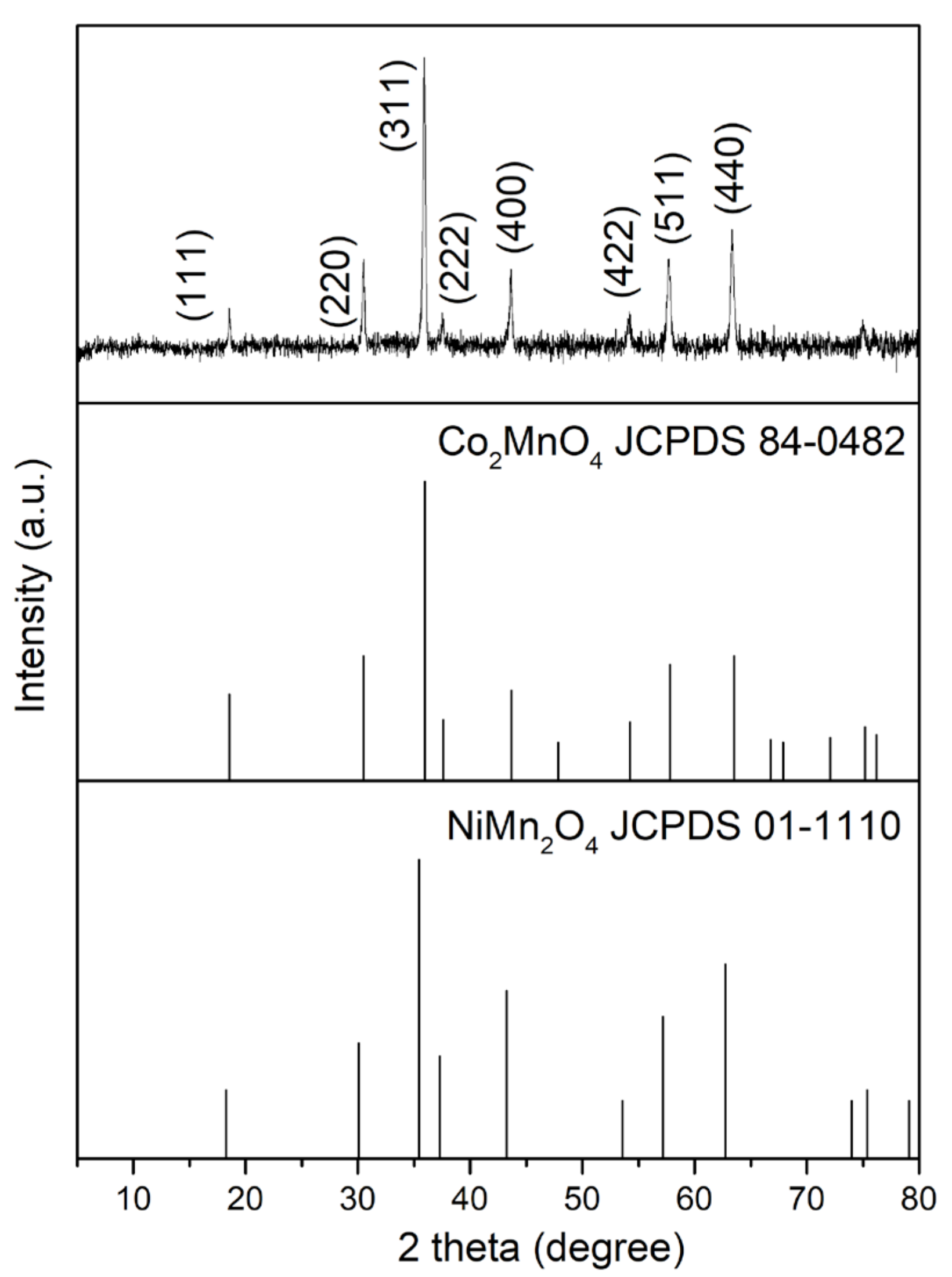
| Exp. | S/L (g/L) | pH | Leachate Composition Molality (mmol Kg−1) | Leaching Efficiency (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Li | Ni | Mn | Co | Li | Ni | Mn | Co | |||
| 1 * | 40 | 2.91 | 404 | 99 | 24 | 62 | 87 | 63 | 15 | 39 |
| 2 | 20 | 2.53 | 189 | 57 | 73 | 63 | 82 | 74 | 95 | 81 |
| Metal Ion | Salt | Temperature (°C) | Agitation Time (h) | Final Metal Concentration (mg L−1) |
|---|---|---|---|---|
| Lithium(I) | LiOH | 25 | 8 | 1455 |
| Nickel(II) | Ni(OH)2 | 60 | 10 | 3648 |
| Manganese(II) | MnCO3 | 25 | 1 | 3786 |
| Cobalt(II) | CoCO3 | 60 | 10 | 3873 |
| Exp. | Metal Ion | Initial Total Metal Concentration (mmol Kg−1) | Antisolvent | Antisolvent Addition Rate (mL min−1) | Final Antisolvent-to-Aqueous (O/A) Volumetric Ratio | Time of Experiment (h) |
|---|---|---|---|---|---|---|
| L1 | Li | 189.5 | Acetone | One-pot | 0.25 | 336 |
| L2 | Li | 189.5 | Acetone | One-pot | 0.5 | 336 |
| L3 | Li | 189.5 | Acetone | One-pot | 1 | 336 |
| L4 | Li | 189.5 | Ethanol | One-pot | 0.25 | 336 |
| L5 | Li | 189.5 | Ethanol | One-pot | 0.5 | 336 |
| L6 | Li | 189.5 | Ethanol | One-pot | 1 | 336 |
| L7 | Li | 189.5 | Ethanol | One-pot | 2 | 336 |
| N1 | Ni | 56.3 | Acetone | One-pot | 0.25 | 380 |
| N2 | Ni | 56.3 | Acetone | One-pot | 0.5 | 380 |
| N3 | Ni | 56.3 | Acetone | One-pot | 1 | 380 |
| N4 | Ni | 56.3 | Ethanol | One-pot | 0.25 | 380 |
| N5 | Ni | 56.3 | Ethanol | One-pot | 0.5 | 380 |
| N6 | Ni | 56.3 | Ethanol | One-pot | 1 | 380 |
| N7 | Ni | 56.3 | Ethanol | One-pot | 2 | 380 |
| N8 | Ni | 182.9 | Acetone | 0.5 | 2 | 250 |
| M1 | Mn | 62.5 | Acetone | One-pot | 0.25 | 380 |
| M2 | Mn | 62.5 | Acetone | One-pot | 0.5 | 380 |
| M3 | Mn | 62.5 | Acetone | One-pot | 1 | 380 |
| M4 | Mn | 62.5 | Ethanol | One-pot | 0.25 | 380 |
| M5 | Mn | 62.5 | Ethanol | One-pot | 0.5 | 380 |
| M6 | Mn | 62.5 | Ethanol | One-pot | 1 | 380 |
| M7 | Mn | 62.5 | Ethanol | One-pot | 2 | 380 |
| C1 | Co | 59.6 | Acetone | One-pot | 0.25 | 380 |
| C2 | Co | 59.6 | Acetone | One-pot | 0.5 | 380 |
| C3 | Co | 59.6 | Acetone | One-pot | 1 | 380 |
| C4 | Co | 59.6 | Ethanol | One-pot | 0.25 | 380 |
| C5 | Co | 59.6 | Ethanol | One-pot | 0.5 | 380 |
| C6 | Co | 59.6 | Ethanol | One-pot | 1 | 380 |
| C7 | Co | 59.6 | Ethanol | One-pot | 2 | 380 |
| C8 | Co | 59.6 | Acetone | 0.5 | 2 | 321 |
| Exp. | Antisolvent | Antisolvent Addition Rate (mL/min) | Final Antisolvent-to-Aqueous Volumetric Ratio (O/A) | Experiment Duration (h) |
|---|---|---|---|---|
| LNMC1 | Acetone | One-pot | 0.25 | 758 |
| LNMC2 | Acetone | One-pot | 0.5 | 506 |
| LNMC3 | Acetone | One-pot | 1 | 505 |
| LNMC4 | Acetone | One-pot | 2 | 516 |
| LNMC5 | Ethanol | One-pot | 0.25 | 758 |
| LNMC6 | Ethanol | One-pot | 0.5 | 506 |
| LNMC7 | Ethanol | One-pot | 1 | 505 |
| LNMC8 | Ethanol | One-pot | 2 | 516 |
| LNMC9 | Acetone | 0.5 | 1 | 24 |
| LNMC10 | Acetone | 0.5 | 2 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, W.; Chagnes, A.; Xiao, X.; Olsson, R.T.; Forsberg, K. Antisolvent Precipitation for Metal Recovery from Citric Acid Solution in Recycling of NMC Cathode Materials. Metals 2022, 12, 607. https://doi.org/10.3390/met12040607
Xuan W, Chagnes A, Xiao X, Olsson RT, Forsberg K. Antisolvent Precipitation for Metal Recovery from Citric Acid Solution in Recycling of NMC Cathode Materials. Metals. 2022; 12(4):607. https://doi.org/10.3390/met12040607
Chicago/Turabian StyleXuan, Wen, Alexandre Chagnes, Xiong Xiao, Richard T. Olsson, and Kerstin Forsberg. 2022. "Antisolvent Precipitation for Metal Recovery from Citric Acid Solution in Recycling of NMC Cathode Materials" Metals 12, no. 4: 607. https://doi.org/10.3390/met12040607
APA StyleXuan, W., Chagnes, A., Xiao, X., Olsson, R. T., & Forsberg, K. (2022). Antisolvent Precipitation for Metal Recovery from Citric Acid Solution in Recycling of NMC Cathode Materials. Metals, 12(4), 607. https://doi.org/10.3390/met12040607









