An Overview of the Production of Magnetic Core-Shell Nanoparticles and Their Biomedical Applications
Abstract
1. Introduction
2. Scope of this Review
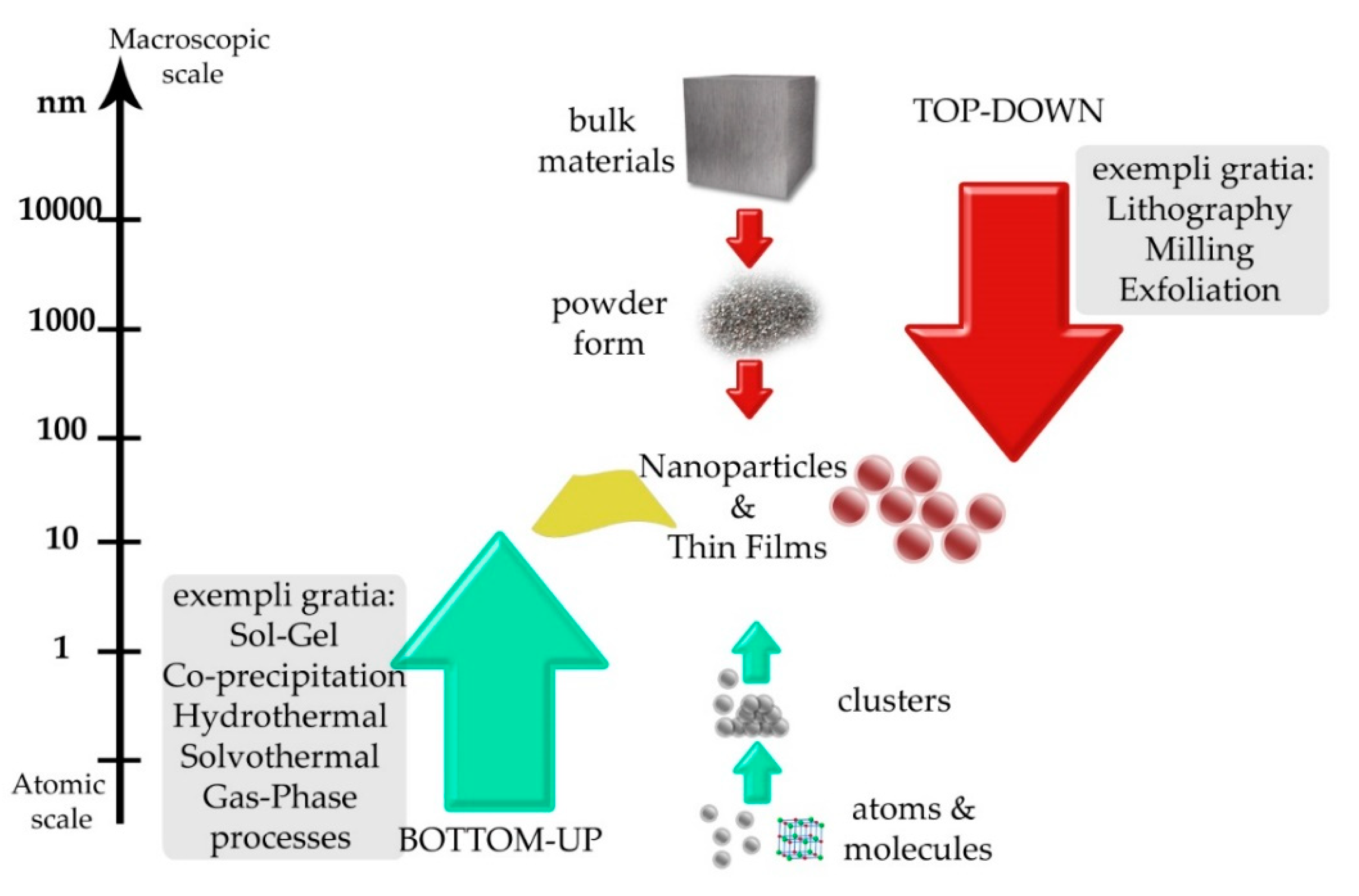
3. Basic Strategies for Producing Nanostructured Materials
4. Top-Down Processes
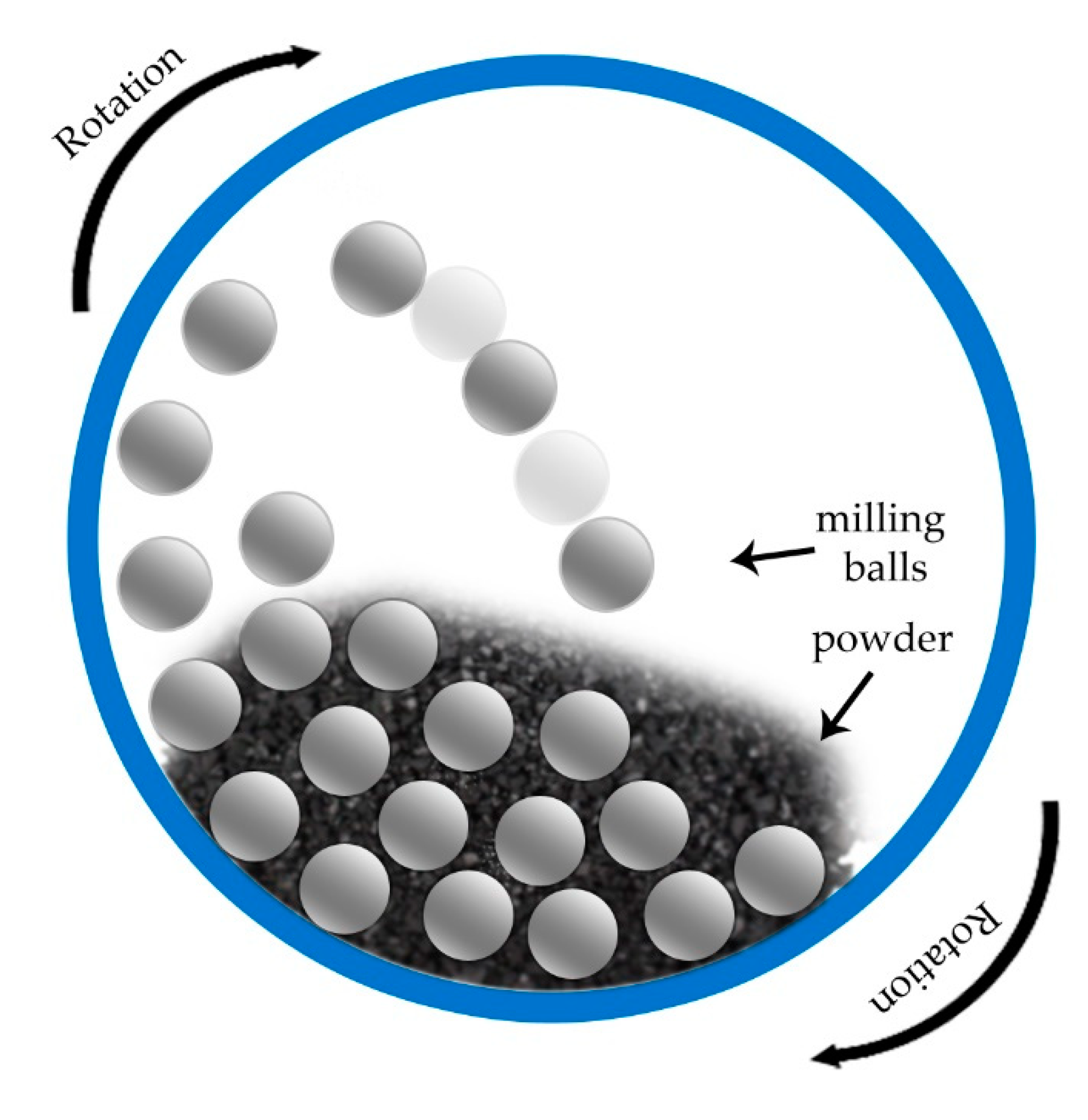
4.1. Milling Processes
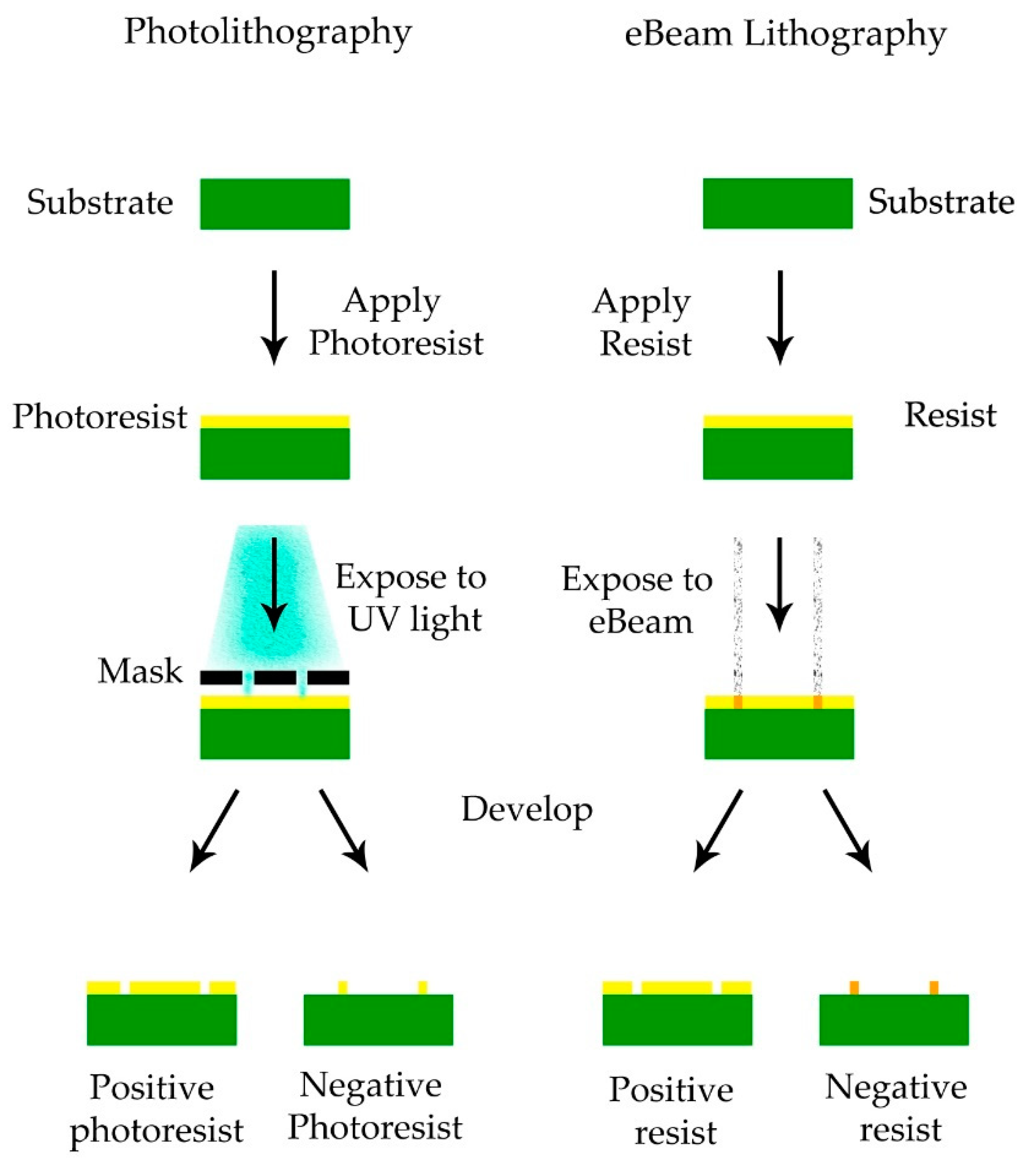
4.2. Lithographic Processes
5. Bottom-Up/Chemical Production Processes
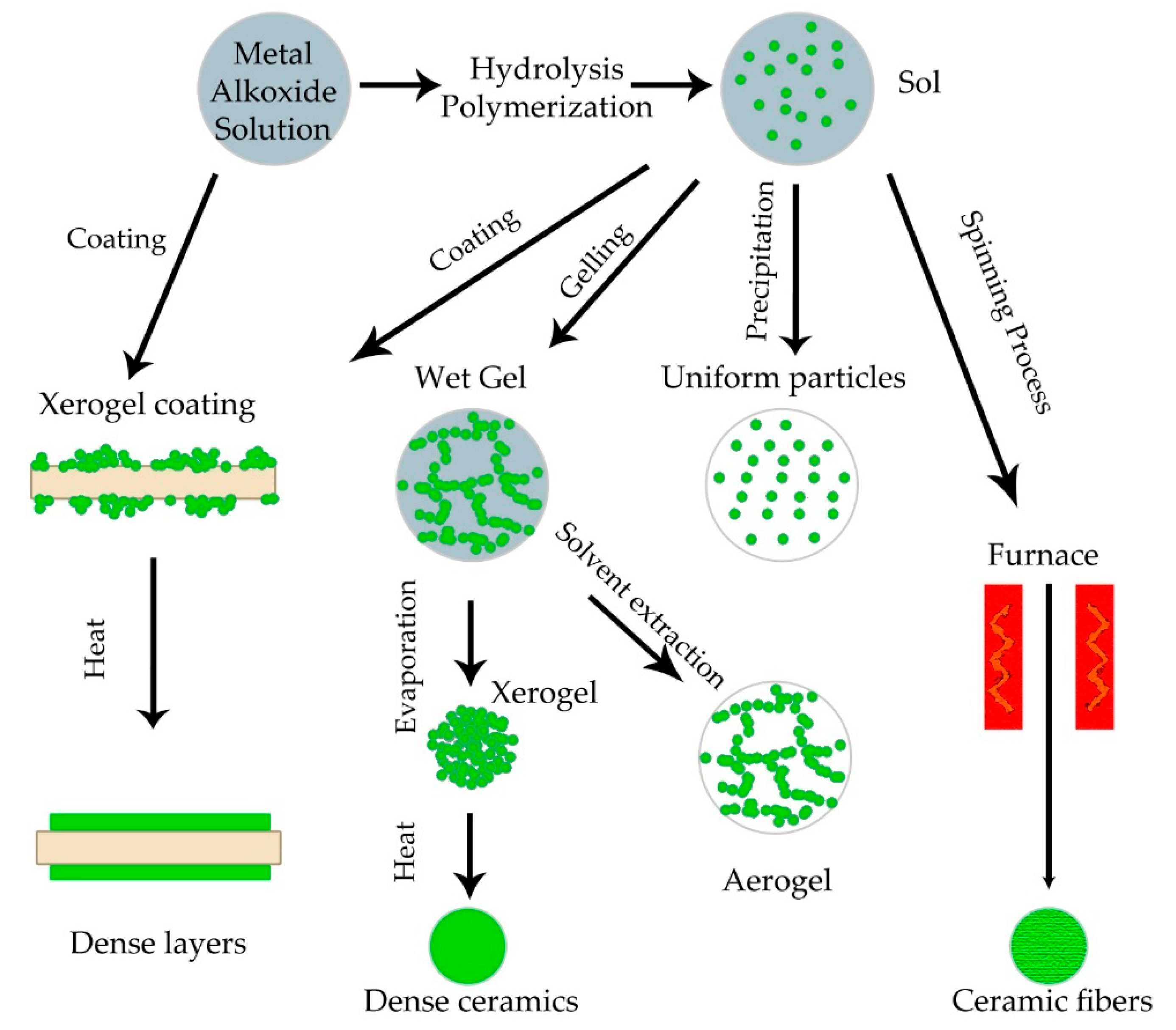
5.1. Sol-Gel
5.2. Co-Precipitation
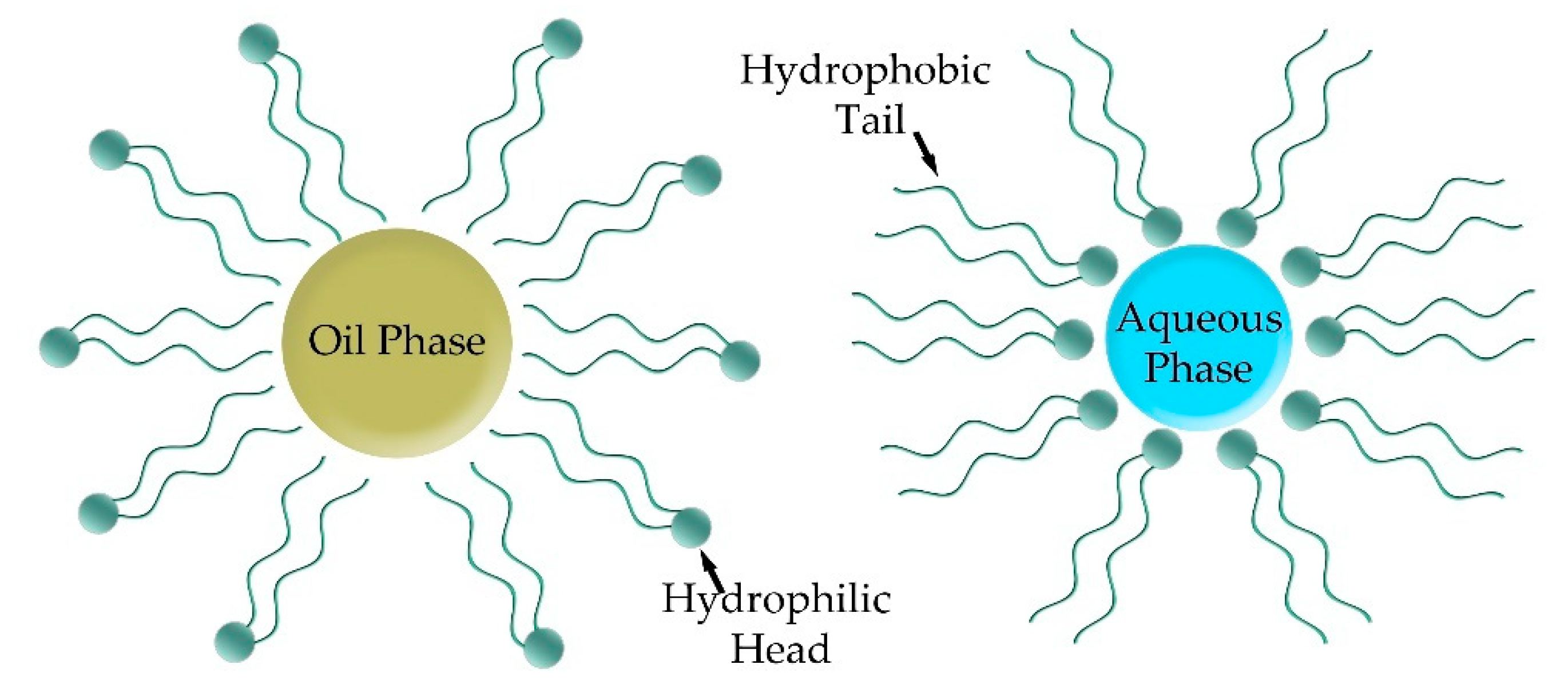
5.3. Emulsions
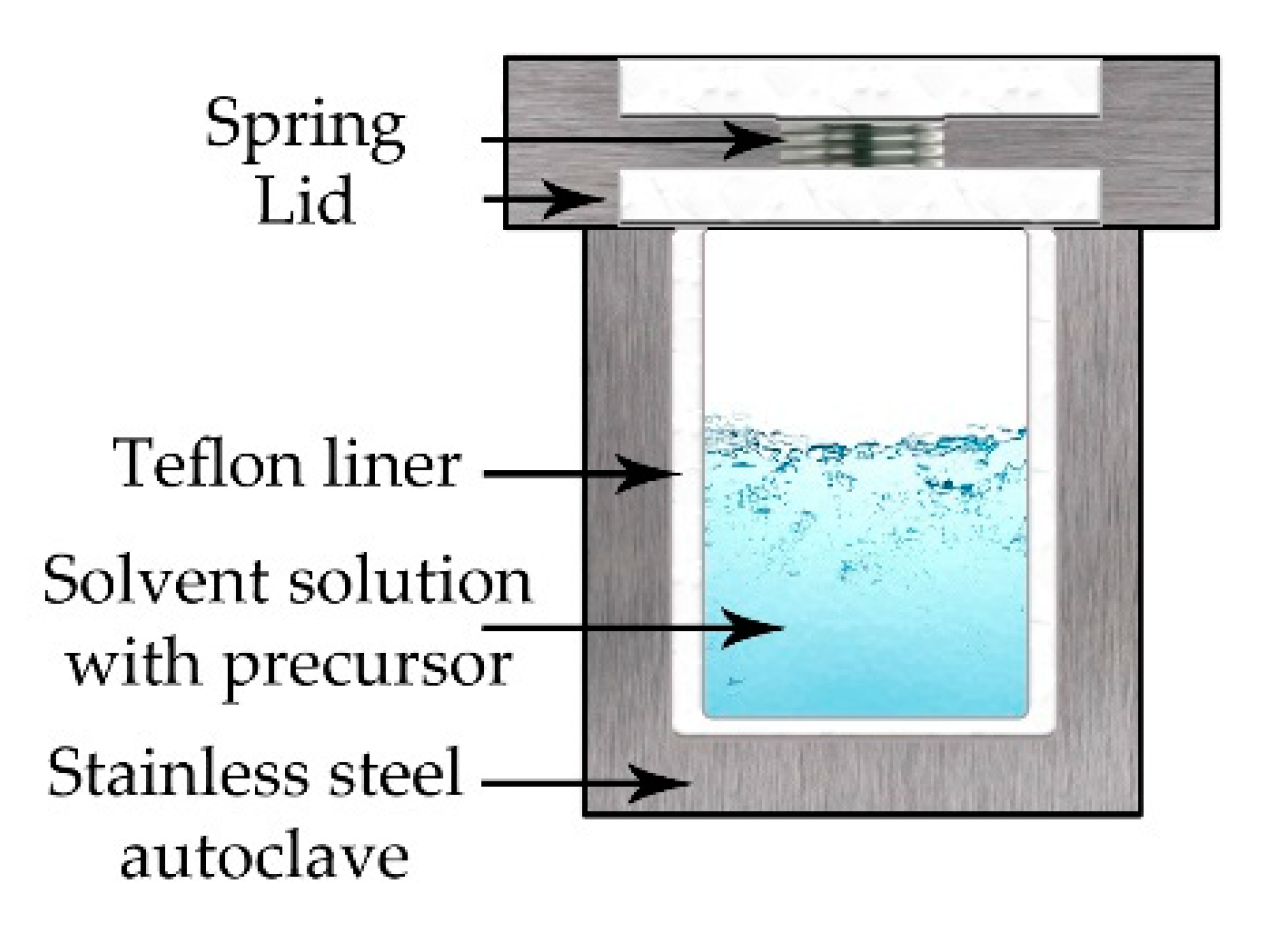
5.4. Hydrothermal—Solvothermal
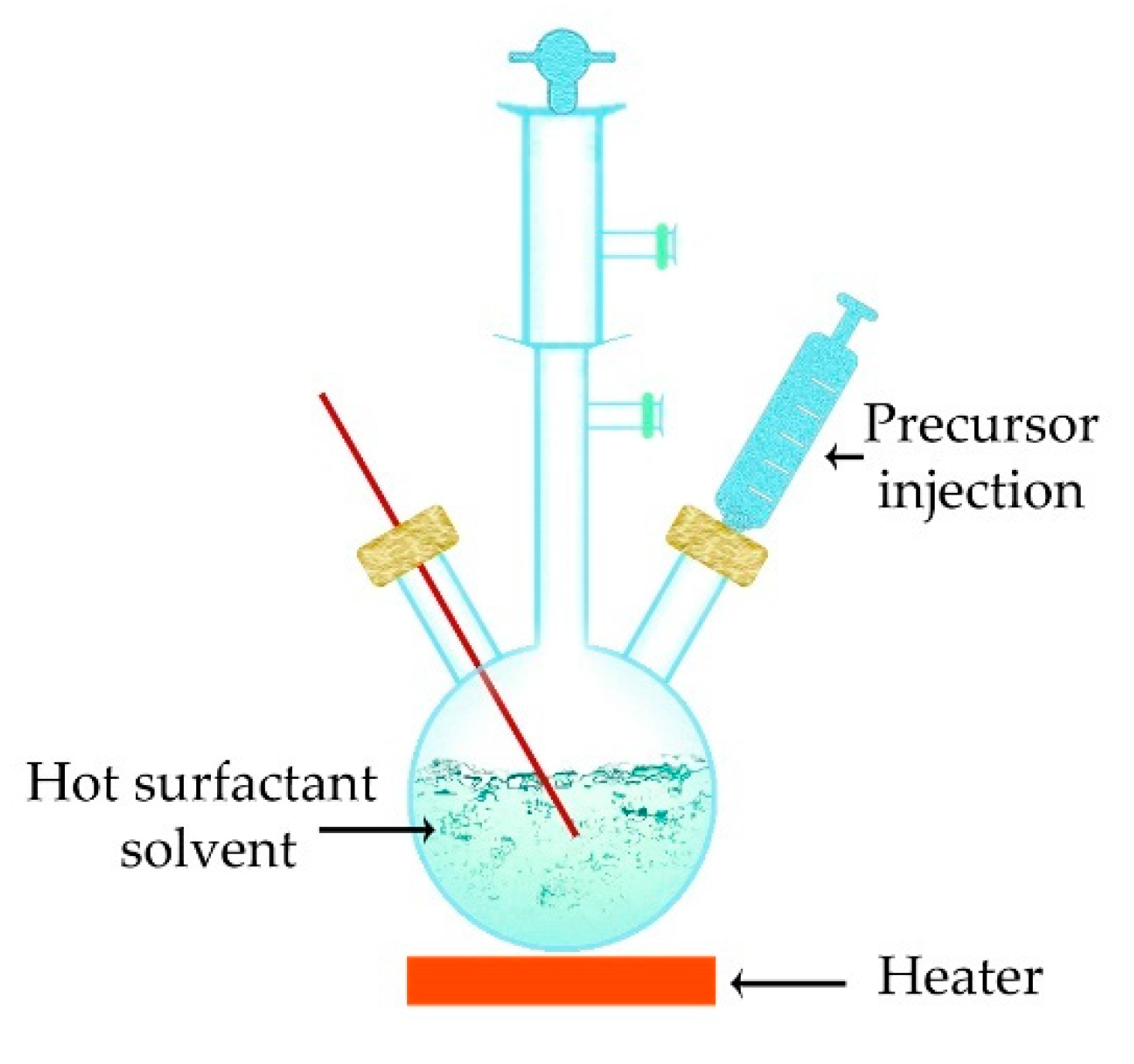
5.5. Thermal Decomposition
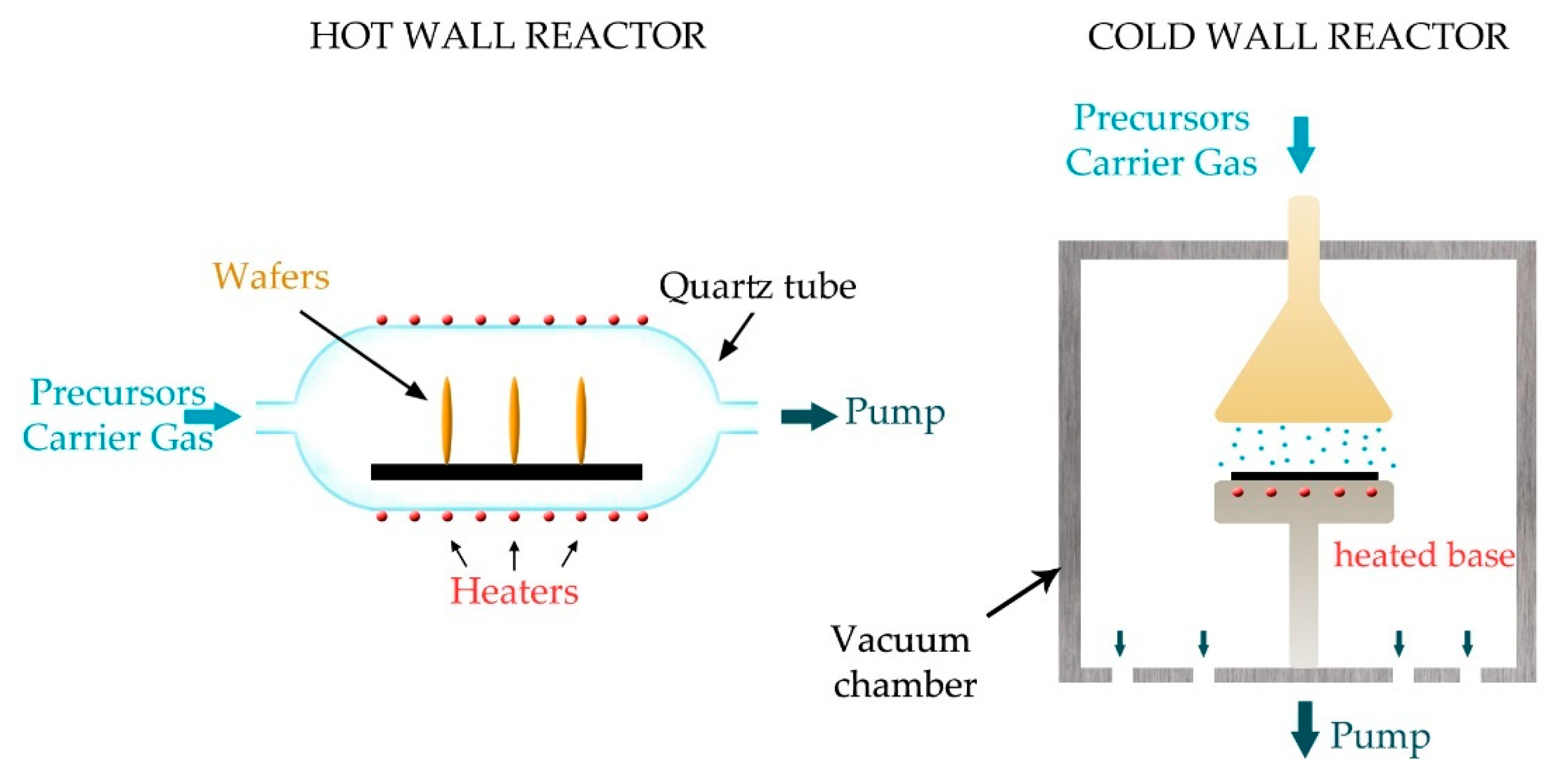
5.6. Gas-Phase—Vacuum Processes
6. Concentrated Table
7. Biomedical Applications
7.1. Hyperthermia
7.2. Drug Delivery
8. Concluding Remarks and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Freestone, I.; Meeks, N.; Sax, M.; Higgitt, C. The Lycurgus Cup—A Roman Nanotechnology. Gold Bull. 2007, 40, 270–277. [Google Scholar] [CrossRef]
- Christodoulou, E.; Nerantzaki, M.; Nanaki, S.; Barmpalexis, P.; Giannousi, K.; Dendrinou-Samara, C.; Angelakeris, M.; Gounari, E.; Anastasiou, A.D.; Bikiaris, D.N. Paclitaxel Magnetic Core–Shell Nanoparticles Based on Poly(Lactic Acid) Semitelechelic Novel Block Copolymers for Combined Hyperthermia and Chemotherapy Treatment of Cancer. Pharmaceutics 2019, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Jung, Y.S.; Cavanagh, A.S.; Kim, G.-H.; George, S.M.; Dillon, A.C.; Kim, J.K.; Lee, J. Fe3O4 Nanoparticles Confined in Mesocellular Carbon Foam for High Performance Anode Materials for Lithium-Ion Batteries. Adv. Funct. Mater. 2011, 21, 2430–2438. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Z.; Lu, M.; Wen, B.; Ouyang, Q.; Chen, Y.; Zhu, C.; Gao, P.; Li, C.; Cao, M.; et al. Graphene–Fe3O4 Nanohybrids: Synthesis and Excellent Electromagnetic Absorption Properties. J. Appl. Phys. 2013, 113, 024314. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Müllen, K. 3D Nitrogen-Doped Graphene Aerogel-Supported Fe3O4 Nanoparticles as Efficient Electrocatalysts for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef] [PubMed]
- Kirmanidou, Y.; Sidira, M.; Bakopoulou, A.; Tsouknidas, A.; Prymak, O.; Papi, R.; Choli-Papadopoulou, T.; Epple, M.; Michailidis, N.; Koidis, P.; et al. Assessment of Cytotoxicity and Antibacterial Effects of Silver Nanoparticle-Doped Titanium Alloy Surfaces. Dent. Mater. 2019, 35, e220–e233. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1223/2009 of 30 November 2009 on Cosmetic Products. Off. J. Eur. Union 2009, 342, 59–209.
- Karamanidou, T.; Bourganis, V.; Gatzogianni, G.; Tsouknidas, A. A Review of the EU’s Regulatory Framework for the Production of Nano-Enhanced Cosmetics. Metals 2021, 11, 455. [Google Scholar] [CrossRef]
- Radoń, A.; Łoński, S.; Kądziołka-Gaweł, M.; Gębara, P.; Lis, M.; Łukowiec, D.; Babilas, R. Influence of Magnetite Nanoparticles Surface Dissolution, Stabilization and Functionalization by Malonic Acid on the Catalytic Activity, Magnetic and Electrical Properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125446. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Multifunctional Magnetic Oxide Nanoparticle (MNP) Core-Shell: Review of Synthesis, Structural Studies and Application for Wastewater Treatment. Molecules 2020, 25, 4110. [Google Scholar] [CrossRef] [PubMed]
- Katz, E. Synthesis, Properties and Applications of Magnetic Nanoparticles and Nanowires—A Brief Introduction. Magnetochemistry 2019, 5, 61. [Google Scholar] [CrossRef]
- Kwizera, E.A.; Chaffin, E.; Wang, Y.; Huang, X. Synthesis and Properties of Magnetic-Optical Core–Shell Nanoparticles. RSC Adv. 2017, 7, 17137–17153. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, Functionalization, and Biomedical Applications of Multifunctional Magnetic Nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, A.A. Core/Shell Magnetic Nanoparticles for Biomedical Applications. In Magnetic Nanostructured Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 41–58. [Google Scholar]
- Mandal, S.; Chaudhuri, K. Magnetic Core-Shell Nanoparticles for Biomedical Applications. In Complex Magnetic Nanostructures; Springer International Publishing: Cham, Switzerland, 2017; pp. 425–453. [Google Scholar]
- Fu, X.; Cai, J.; Zhang, X.; Li, W.-D.; Ge, H.; Hu, Y. Top-down Fabrication of Shape-Controlled, Monodisperse Nanoparticles for Biomedical Applications. Adv. Drug Deliv. Rev. 2018, 132, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Handbook of Physical Vapor Deposition (PVD) Processing; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 9780815520375.
- Tachi, S.; Tsujimoto, K.; Okudaira, S. Low-temperature Reactive Ion Etching and Microwave Plasma Etching of Silicon. Appl. Phys. Lett. 1988, 52, 616–618. [Google Scholar] [CrossRef]
- Van Zant, P. Microchip Fabrication, Sixth Edition: A Practical Guide to Semiconductor Processing, 6th ed.; McGraw-Hill Education: New York, NY, USA, 2013. [Google Scholar]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Radoń, A.; Kądziołka-Gaweł, M.; Łukowiec, D.; Gębara, P.; Cesarz-Andraczke, K.; Kolano-Burian, A.; Włodarczyk, P.; Polak, M.; Babilas, R. Influence of Magnetite Nanoparticles Shape and Spontaneous Surface Oxidation on the Electron Transport Mechanism. Materials 2021, 14, 5241. [Google Scholar] [CrossRef]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Jampílek, J.; Kráľová, K.; Campos, E.V.R.; Fraceto, L.F. Bio-Based Nanoemulsion Formulations Applicable in Agriculture, Medicine, and Food Industry. In Nanobiotechnology in Bioformulations; Springer: Berlin/Heidelberg, Germany, 2019; pp. 33–84. [Google Scholar]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An Overview of Micro- and Nanoemulsions as Vehicles for Essential Oils: Formulation, Preparation and Stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef]
- Che Marzuki, N.H.; Wahab, R.A.; Abdul Hamid, M. An Overview of Nanoemulsion: Concepts of Development and Cosmeceutical Applications. Biotechnol. Biotechnol. Equip. 2019, 33, 779–797. [Google Scholar] [CrossRef]
- Cervera, L.; Peréz-Landazábal, J.I.; Garaio, E.; Monteserín, M.; Larumbe, S.; Martín, F.; Gómez-Polo, C. Fe-C Nanoparticles Obtained from Thermal Decomposition Employing Sugars as Reducing Agents. J. Alloys Compd. 2021, 863, 158065. [Google Scholar] [CrossRef]
- Shinde, K.P.; Ranot, M.; Choi, C.J.; Kim, H.S.; Chung, K.C. Plasma-Assisted Synthesis and Study of Structural and Magnetic Properties of Fe/C Core Shell. AIP Adv. 2017, 7, 075013. [Google Scholar] [CrossRef]
- Cao, H.; Huang, G.; Xuan, S.; Wu, Q.; Gu, F.; Li, C. Synthesis and Characterization of Carbon-Coated Iron Core/Shell Nanostructures. J. Alloys Compd. 2008, 448, 272–276. [Google Scholar] [CrossRef]
- Ma, C.; Luo, B.; Song, H.; Zhi, L. Preparation of Carbon-Encapsulated Metal Magnetic Nanoparticles by an Instant Pyrolysis Method. New Carbon Mater. 2010, 25, 199–204. [Google Scholar] [CrossRef]
- Byeon, J.H.; Kim, Y.-W. Hybrid Gas-Phase Synthesis of Nanoscale Fe–SiO2 Core–Shell Agglomerates for Efficient Transfection into Cells and Use in Magnetic Cell Patterning. RSC Adv. 2013, 3, 13685–13689. [Google Scholar] [CrossRef]
- Yuan, M.; Tao, J.; Yan, G.; Tan, M.; Qiu, G. Preparation and Characterization of Fe/SiO2 Core/Shell Nanocomposites. Trans. Nonferrous Met. Soc. China 2010, 20, 632–636. [Google Scholar] [CrossRef]
- Zhang, X.F.; Dong, X.L.; Huang, H.; Lv, B.; Zhu, X.G.; Lei, J.P.; Ma, S.; Liu, W.; Zhang, Z.D. Synthesis, Structure and Magnetic Properties of SiO2-Coated Fe Nanocapsules. Mater. Sci. Eng. A 2007, 454–455, 211–215. [Google Scholar] [CrossRef]
- Simeonidis, K.; Martinez-Boubeta, C.; Serantes, D.; Ruta, S.; Chubykalo-Fesenko, O.; Chantrell, R.; Oró-Solé, J.; LI. Balcells, A.; Kamzin, A.S.; Nazipov, R.A.; et al. Controlling Magnetization Reversal and Hyperthermia Efficiency in Core–Shell Iron–Iron Oxide Magnetic Nanoparticles by Tuning the Interphase Coupling. ACS Appl. Nano Mater. 2020, 3, 4465–4476. [Google Scholar] [CrossRef]
- Durgesh; Sharma, A.; Aggarwal, S.; Kumar, R. Effect of Incorporation of Synthesized Fe @ Ag Core-Shell Nanoparticles on Optical Parameters of Polyvinyl Alcohol. API Conf. Proc. 2019, 2093, 020017. [Google Scholar]
- Lu, L.; Zhang, W.; Wang, D.; Xu, X.; Miao, J.; Jiang, Y. Fe@Ag Core–Shell Nanoparticles with Both Sensitive Plasmonic Properties and Tunable Magnetism. Mater. Lett. 2010, 64, 1732–1734. [Google Scholar] [CrossRef]
- Malik, M.A.; Alshehri, A.A.; Patel, R. Facile One-Pot Green Synthesis of Ag–Fe Bimetallic Nanoparticles and Their Catalytic Capability for 4-Nitrophenol Reduction. J. Mater. Res. Technol. 2021, 12, 455–470. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Yan, J.-M.; Han, S.; Shioyama, H.; Xu, Q. Magnetically Recyclable Fe@Pt Core−Shell Nanoparticles and Their Use as Electrocatalysts for Ammonia Borane Oxidation: The Role of Crystallinity of the Core. J. Am. Chem. Soc. 2009, 131, 2778–2779. [Google Scholar] [CrossRef] [PubMed]
- Pisane, K.L.; Singh, S.; Seehra, M.S. Synthesis, Structural Characterization and Magnetic Properties of Fe/Pt Core-Shell Nanoparticles. J. Appl. Phys. 2015, 117, 17D708. [Google Scholar] [CrossRef]
- Burke, N.A.D.; Stöver, H.D.H.; Dawson, F.P. Magnetic Nanocomposites: Preparation and Characterization of Polymer-Coated Iron Nanoparticles. Chem. Mater. 2002, 14, 4752–4761. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, A.; Seidi, F.; Zhu, W.; Li, H.; Yin, S.; Xu, X.; Xiao, H. Core-Shell Heterostructured Nanofibers Consisting of Fe7S8 Nanoparticles Embedded into S-Doped Carbon Nanoshells for Superior Electromagnetic Wave Absorption. Chem. Eng. J. 2021, 423, 130307. [Google Scholar] [CrossRef]
- Kuang, D.; Hou, L.; Wang, S.; Yu, B.; Lianwen, D.; Lin, L.; Huang, H.; He, J.; Song, M. Enhanced Electromagnetic Wave Absorption of Ni–C Core-Shell Nanoparticles by HCP-Ni Phase. Mater. Res. Express 2018, 5, 095013. [Google Scholar] [CrossRef]
- Yang, R.-X.; Xu, L.-R.; Wu, S.-L.; Chuang, K.-H.; Wey, M.-Y. Ni/SiO2 Core–Shell Catalysts for Catalytic Hydrogen Production from Waste Plastics-Derived Syngas. Int. J. Hydrogen Energy 2017, 42, 11239–11251. [Google Scholar] [CrossRef]
- Fu, W.; Yang, H.; Chang, L.; Li, M.; Bala, H.; Yu, Q.; Zou, G. Preparation and Characteristics of Core–Shell Structure Nickel/Silica Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2005, 262, 71–75. [Google Scholar] [CrossRef]
- Tang, N.; Lü, L.; Zhong, W.; Au, C.; Du, Y. Synthesis and Magnetic Properties of Carbon-Coated Ni/SiO2 Core/Shell Nanocomposites. Sci. China Ser. G Phys. Mech. Astron. 2009, 52, 31–34. [Google Scholar] [CrossRef]
- Xia, L.; Hu, X.; Kang, X.; Zhao, H.; Sun, M.; Cihen, X. A One-Step Facile Synthesis of Ag–Ni Core–Shell Nanoparticles in Water-in-Oil Microemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 367, 96–101. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Mao, Z.; Liang, X.; Chen, B. Ag–Ni Core–Shell Nanowires with Superior Electrocatalytic Activity for Alkaline Hydrogen Evolution Reaction. J. Mater. Chem. A 2017, 5, 16646–16652. [Google Scholar] [CrossRef]
- Wang, G.; Wu, H.; Wexler, D.; Liu, H.; Savadogo, O. Ni@Pt Core–Shell Nanoparticles with Enhanced Catalytic Activity for Oxygen Reduction Reaction. J. Alloys Compd. 2010, 503, L1–L4. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, C.; Shi, Z.; Chen, D.; Chen, X.; Zhang, Q.; Pang, B.; Feng, J.; Yu, L.; Dong, L. Molten-Salt-Assisted Synthesis of Onion-like Co/CoO@FeNC Materials with Boosting Reversible Oxygen Electrocatalysis for Rechargeable Zn-Air Battery. J. Colloid Interface Sci. 2021, 596, 206–214. [Google Scholar] [CrossRef]
- Mazaleyrat, F.; Ammar, M.; LoBue, M.; Bonnet, J.-P.; Audebert, P.; Wang, G.-Y.; Champion, Y.; Hÿtch, M.; Snoeck, E. Silica Coated Nanoparticles: Synthesis, Magnetic Properties and Spin Structure. J. Alloys Compd. 2009, 483, 473–478. [Google Scholar] [CrossRef][Green Version]
- Kim, H.; Achermann, M.; Balet, L.P.; Hollingsworth, J.A.; Klimov, V.I. Synthesis and Characterization of Co/CdSe Core/Shell Nanocomposites: Bifunctional Magnetic-Optical Nanocrystals. J. Am. Chem. Soc. 2005, 127, 544–546. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, Q.; Bi, H.; Liang, C.; Yuan, K.; She, W.; Yang, Y.; Che, R. CoNi@SiO2 @TiO2 and CoNi@Air@TiO2 Microspheres with Strong Wideband Microwave Absorption. Adv. Mater. 2016, 28, 486–490. [Google Scholar] [CrossRef]
- Bao, F.; Li, J.-F.; Ren, B.; Yao, J.-L.; Gu, R.-A.; Tian, Z.-Q. Synthesis and Characterization of Au@Co and Au@Ni Core−Shell Nanoparticles and Their Applications in Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2008, 112, 345–350. [Google Scholar] [CrossRef]
- Lee, W.; Kim, M.G.; Choi, J.; Park, J.-I.; Ko, S.J.; Oh, S.J.; Cheon, J. Redox−Transmetalation Process as a Generalized Synthetic Strategy for Core−Shell Magnetic Nanoparticles. J. Am. Chem. Soc. 2005, 127, 16090–16097. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Chen, M.; Wu, Y.; Liu, G.; Qi, P.; Fu, M.; Wu, H.; Tang, Y. Rationally Designed Hierarchical ZnCo2O4/C Core-Shell Nanowire Arrays for High Performance and Stable Supercapacitors. J. Alloys Compd. 2021, 876, 160037. [Google Scholar] [CrossRef]
- Saini, L.; Gupta, V.; Patra, M.K.; Jani, R.K.; Shukla, A.; Kumar, N.; Dixit, A. Impedance Engineered Microwave Absorption Properties of Fe-Ni/C Core-Shell Enabled Rubber Composites for X-Band Stealth Applications. J. Alloys Compd. 2021, 869, 159360. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. A Greener Synthesis of Core (Fe, Cu)-Shell (Au, Pt, Pd, and Ag) Nanocrystals Using Aqueous Vitamin C. Cryst. Growth Des. 2007, 7, 2582–2587. [Google Scholar] [CrossRef]
- Gu, H.; Zheng, R.; Zhang, X.; Xu, B. Facile One-Pot Synthesis of Bifunctional Heterodimers of Nanoparticles: A Conjugate of Quantum Dot and Magnetic Nanoparticles. J. Am. Chem. Soc. 2004, 126, 5664–5665. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Li, J.; Wang, Z.L.; Liu, J.P.; Sun, S. Bimagnetic Core/Shell FePt/Fe3O4 Nanoparticles. Nano Lett. 2004, 4, 187–190. [Google Scholar] [CrossRef]
- Emadi, M.; Shams, E.; Amini, M.K. Removal of Zinc from Aqueous Solutions by Magnetite Silica Core-Shell Nanoparticles. J. Chem. 2013, 2013, 787682. [Google Scholar] [CrossRef]
- Lien, Y.-H.; Wu, T.-M. Preparation and Characterization of Thermosensitive Polymers Grafted onto Silica-Coated Iron Oxide Nanoparticles. J. Colloid Interface Sci. 2008, 326, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Tapec, R.; Theodoropoulou, N.; Dobson, J.; Hebard, A.; Tan, W. Synthesis and Characterization of Silica-Coated Iron Oxide Nanoparticles in Microemulsion: The Effect of Nonionic Surfactants. Langmuir 2001, 17, 2900–2906. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.G.; Li, Z.Q.; Wang, C.; Li, J.F.; Gu, Y.J. Synthesis and Characterization of Magnetic Nanocomposites with Fe3O4 Core. J. Phys. Conf. Ser. 2009, 152, 012041. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Youn, J.K.; Na, H.B.; Yu, T.; Kim, H.; Lee, S.-M.; Koo, Y.-M.; Kwak, J.H.; Park, H.G.; et al. Simple Synthesis of Functionalized Superparamagnetic Magnetite/Silica Core/Shell Nanoparticles and Their Application as Magnetically Separable High-Performance Biocatalysts. Small 2008, 4, 143–152. [Google Scholar] [CrossRef]
- Ta, T.K.H.; Trinh, M.-T.; Long, N.V.; Nguyen, T.T.M.; Nguyen, T.L.T.; Thuoc, T.L.; Phan, B.T.; Mott, D.; Maenosono, S.; Tran-Van, H.; et al. Synthesis and Surface Functionalization of Fe3O4-SiO2 Core-Shell Nanoparticles with 3-Glycidoxypropyltrimethoxysilane and 1,1′-Carbonyldiimidazole for Bio-Applications. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 376–383. [Google Scholar] [CrossRef]
- Adam, A.; Harlepp, S.; Ghilini, F.; Cotin, G.; Freis, B.; Goetz, J.; Bégin, S.; Tasso, M.; Mertz, D. Core-Shell Iron Oxide@stellate Mesoporous Silica for Combined near-Infrared Photothermia and Drug Delivery: Influence of PH and Surface Chemistry. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128407. [Google Scholar] [CrossRef]
- Xuan, S.; Wang, Y.-X.J.; Yu, J.C.; Leung, K.C.-F. Preparation, Characterization, and Catalytic Activity of Core/Shell Fe3O4 @Polyaniline@Au Nanocomposites. Langmuir 2009, 25, 11835–11843. [Google Scholar] [CrossRef] [PubMed]
- Nalluri, S.R.; Nagarjuna, R.; Patra, D.; Ganesan, R.; Balaji, G. Large Scale Solid-State Synthesis of Catalytically Active Fe3O4@M (M = Au, Ag and Au-Ag Alloy) Core-Shell Nanostructures. Sci. Rep. 2019, 9, 6603. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Luo, J.; Fan, Q.; Suzuki, M.; Suzuki, I.S.; Engelhard, M.H.; Lin, Y.; Kim, N.; Wang, J.Q.; Zhong, C.-J. Monodispersed Core−Shell Fe3O4 @Au Nanoparticles. J. Phys. Chem. B 2005, 109, 21593–21601. [Google Scholar] [CrossRef]
- Mikhaylova, M.; Kim, D.K.; Bobrysheva, N.; Osmolowsky, M.; Semenov, V.; Tsakalakos, T.; Muhammed, M. Superparamagnetism of Magnetite Nanoparticles: Dependence on Surface Modification. Langmuir 2004, 20, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Huong, P.T.L.; Huy, L.T.; Lan, H.; Thang, L.H.; An, T.T.; van Quy, N.; Tuan, P.A.; Alonso, J.; Phan, M.-H.; Le, A.-T. Magnetic Iron Oxide-Carbon Nanocomposites: Impacts of Carbon Coating on the As(V) Adsorption and Inductive Heating Responses. J. Alloys Compd. 2018, 739, 139–148. [Google Scholar] [CrossRef]
- Li, Z.; Hu, X.; Li, B.; Wang, X.; Shi, Z.; Lu, J.; Wang, Z. MOF-Derived Fe3O4 Hierarchical Nanocomposites Encapsulated by Carbon Shells as High-Performance Anodes for Li-Storage Systems. J. Alloys Compd. 2021, 875, 159906. [Google Scholar] [CrossRef]
- Meng, X.; Lei, W.; Yang, W.; Liu, Y.; Yu, Y. Fe3O4 Nanoparticles Coated with Ultra-Thin Carbon Layer for Polarization-Controlled Microwave Absorption Performance. J. Colloid Interface Sci. 2021, 600, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Starchikov, S.S.; Zayakhanov, V.A.; Vasiliev, A.L.; Lyubutin, I.S.; Baskakov, A.O.; Nikiforova, Y.; Funtov, K.O.; Lyubutina, M.V.; Kulikova, L.F.; Agafonov, V.N.; et al. Core@shell Nanocomposites Fe7C3/FexOy/C Obtained by High Pressure-High Temperature Treatment of Ferrocene Fe(C5H5)2. Carbon 2021, 178, 708–717. [Google Scholar] [CrossRef]
- Yu, G.; Sun, B.; Pei, Y.; Xie, S.; Yan, S.; Qiao, M.; Fan, K.; Zhang, X.; Zong, B. FexOy @C Spheres as an Excellent Catalyst for Fischer−Tropsch Synthesis. J. Am. Chem. Soc. 2010, 132, 935–937. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Z.; Xiong, J.; Xiong, Y.; Xiao, H. A Novel Biomaterial—Fe3O4:TiO2 Core-Shell Nano Particle with Magnetic Performance and High Visible Light Photocatalytic Activity. Opt. Mater. 2008, 31, 380–384. [Google Scholar] [CrossRef]
- Lu, H.; Yi, G.; Zhao, S.; Chen, D.; Guo, L.-H.; Cheng, J. Synthesis and Characterization of Multi-Functional Nanoparticles Possessing Magnetic, up-Conversion Fluorescence and Bio-Affinity Properties. J. Mater. Chem. 2004, 14, 1336. [Google Scholar] [CrossRef]
- Zhao, D.-L.; Teng, P.; Xu, Y.; Xia, Q.-S.; Tang, J.-T. Magnetic and Inductive Heating Properties of Fe3O4/Polyethylene Glycol Composite Nanoparticles with Core–Shell Structure. J. Alloys Compd. 2010, 502, 392–395. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Eshteghal, F.; Shahbazi-Alavi, H. A Facile One-Pot Ultrasound Assisted for an Efficient Synthesis of Benzo[g]Chromenes Using Fe3O4/Polyethylene Glycol (PEG) Core/Shell Nanoparticles. Ultrason. Sonochem. 2016, 33, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Choa, Y.-H.; Kim, D.H.; Kim, K.H. Magnetic Behaviors of Surface Modified Superparamagnetic Magnetite Nanoparticles. IEEE Trans. Magn. 2009, 45, 2446–2449. [Google Scholar] [CrossRef]
- Chen, F.; Gao, Q.; Hong, G.; Ni, J. Synthesis of Magnetite Core–Shell Nanoparticles by Surface-Initiated Ring-Opening Polymerization of l-Lactide. J. Magn. Magn. Mater. 2008, 320, 1921–1927. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Makino, M.; Ohura, T.; Yamamoto, K.; Enomoto, Y.; Takase, H. Surface Modification of Fe3O4 Nanoparticles with Dextran via a Coupling Reaction between Naked Fe3O4 Mechano-Cation and Naked Dextran Mechano-Anion: A New Mechanism of Covalent Bond Formation. Adv. Powder Technol. 2019, 30, 795–806. [Google Scholar] [CrossRef]
- Qin, S.; Wang, L.; Zhang, X.; Su, G. Grafting Poly(Ethylene Glycol) Monomethacrylate onto Fe3O4 Nanoparticles to Resist Nonspecific Protein Adsorption. Appl. Surf. Sci. 2010, 257, 731–735. [Google Scholar] [CrossRef]
- Omer-Mizrahi, M.; Margel, S. Synthesis and Characterization of Magnetic and Non-Magnetic Core–Shell Polyepoxide Micrometer-Sized Particles of Narrow Size Distribution. J. Colloid Interface Sci. 2009, 329, 228–234. [Google Scholar] [CrossRef]
- Yuan, W.; Yuan, J.; Zhou, L.; Wu, S.; Hong, X. Fe3O4@poly(2-Hydroxyethyl Methacrylate)-Graft-Poly(ɛ-Caprolactone) Magnetic Nanoparticles with Branched Brush Polymeric Shell. Polymer 2010, 51, 2540–2547. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Guo, C.; Wang, J.; Ma, J.; Liang, X.; Yang, L.-R.; Liu, H.-Z. Temperature-Responsive Magnetite/PEO−PPO−PEO Block Copolymer Nanoparticles for Controlled Drug Targeting Delivery. Langmuir 2007, 23, 12669–12676. [Google Scholar] [CrossRef]
- Qin, R.; Li, F.; Jiang, W.; Chen, M. Synthesis and Characterization of Diethylenetriaminepentaacetic Acid-Chitosan-Coated Cobalt Ferrite Core/Shell Nanostructures. Mater. Chem. Phys. 2010, 122, 498–501. [Google Scholar] [CrossRef]
- Tang, X.; Jia, R.; Zhai, T.; Xia, H. Hierarchical Fe3O4 @Fe2O3 Core–Shell Nanorod Arrays as High-Performance Anodes for Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 27518–27525. [Google Scholar] [CrossRef] [PubMed]
- Carnes, C.L.; Klabunde, K.J. Unique Chemical Reactivities of Nanocrystalline Metal Oxides toward Hydrogen Sulfide. Chem. Mater. 2002, 14, 1806–1811. [Google Scholar] [CrossRef]
- Shen, H.; Yao, J.; Gu, R. Fabrication and Characteristics of Spindle Fe2O3@Au Core/Shell Particles. Trans. Nonferrous Met. Soc. China 2009, 19, 652–656. [Google Scholar] [CrossRef]
- Utech, S.; Scherer, C.; Krohne, K.; Carrella, L.; Rentschler, E.; Gasi, T.; Ksenofontov, V.; Felser, C.; Maskos, M. Magnetic Polyorganosiloxane Core–Shell Nanoparticles: Synthesis, Characterization and Magnetic Fractionation. J. Magn. Magn. Mater. 2010, 322, 3519–3526. [Google Scholar] [CrossRef]
- Galperin, A.; Margel, S. Synthesis and Characterization of Radiopaque Magnetic Core-Shell Nanoparticles for X-ray Imaging Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83B, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Thünemann, A.F.; Schütt, D.; Kaufner, L.; Pison, U.; Möhwald, H. Maghemite Nanoparticles Protectively Coated with Poly(Ethylene Imine) and Poly(Ethylene Oxide)- b Lock -Poly(Glutamic Acid). Langmuir 2006, 22, 2351–2357. [Google Scholar] [CrossRef]
- Nagao, D.; Yokoyama, M.; Yamauchi, N.; Matsumoto, H.; Kobayashi, Y.; Konno, M. Synthesis of Highly Monodisperse Particles Composed of a Magnetic Core and Fluorescent Shell. Langmuir 2008, 24, 9804–9808. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Malwal, H.; Manuja, A.; Chaudhury, A. Biosynthesis of Biocompatible and Recyclable Silver/Iron and Gold/Iron Core-Shell Nanoparticles for Water Purification Technology. Biocatal. Agric. Biotechnol. 2018, 14, 189–197. [Google Scholar] [CrossRef]
- Lehner, R.; Wang, X.; Marsch, S.; Hunziker, P. Intelligent Nanomaterials for Medicine: Carrier Platforms and Targeting Strategies in the Context of Clinical Application. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 742–757. [Google Scholar] [CrossRef]
- Platania, V.; Kaldeli-Kerou, A.; Karamanidou, T.; Kouki, M.; Tsouknidas, A.; Chatzinikolaidou, M. Antibacterial Effect of Colloidal Suspensions Varying in Silver Nanoparticles and Ions Concentrations. Nanomaterials 2021, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Ntasiou, P.; Kaldeli Kerou, A.; Karamanidou, T.; Vlachou, A.; Tziros, G.T.; Tsouknidas, A.; Karaoglanidis, G.S. Synthesis and Characterization of Novel Copper Nanoparticles for the Control of Leaf Spot and Anthracnose Diseases of Olive. Nanomaterials 2021, 11, 1667. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.; Bruininks, B.M.H.; Singh, P.; dos Santos, L.; Dal Pizzol, C.; Dieamant, G.d.C.; Kruger, O.; Martin, A.A.; Marrink, S.J.; Souza, P.C.T.; et al. Complex Nanoemulsion for Vitamin Delivery: Droplet Organization and Interaction with Skin Membranes. Nanoscale 2022, 14, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R. Nanomaterials: A Membrane-Based Synthetic Approach. Science 1994, 266, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Karamanidou, T.; Tsouknidas, A. Plant-Derived Extracellular Vesicles as Therapeutic Nanocarriers. Int. J. Mol. Sci. 2021, 23, 191. [Google Scholar] [CrossRef] [PubMed]
- Cammas, S.; Suzuki, K.; Sone, C.; Sakurai, Y.; Kataoka, K.; Okano, T. Thermo-Responsive Polymer Nanoparticles with a Core-Shell Micelle Structure as Site-Specific Drug Carriers. J. Control. Release 1997, 48, 157–164. [Google Scholar] [CrossRef]
- Law, W.-C.; Yong, K.-T.; Roy, I.; Xu, G.; Ding, H.; Bergey, E.J.; Zeng, H.; Prasad, P.N. Optically and Magnetically Doped Organically Modified Silica Nanoparticles as Efficient Magnetically Guided Biomarkers for Two-Photon Imaging of Live Cancer Cells. J. Phys. Chem. C 2008, 112, 7972–7977. [Google Scholar] [CrossRef]
- Kateb, B.; Chiu, K.; Black, K.L.; Yamamoto, V.; Khalsa, B.; Ljubimova, J.Y.; Ding, H.; Patil, R.; Portilla-Arias, J.A.; Modo, M.; et al. Nanoplatforms for Constructing New Approaches to Cancer Treatment, Imaging, and Drug Delivery: What Should Be the Policy? NeuroImage 2011, 54, S106–S124. [Google Scholar] [CrossRef]
- Kayal, S.; Ramanujan, R.V. Anti-Cancer Drug Loaded Iron–Gold Core–Shell Nanoparticles (Fe@Au) for Magnetic Drug Targeting. J. Nanosci. Nanotechnol. 2010, 10, 5527–5539. [Google Scholar] [CrossRef]
- Berry, C.C.; Curtis, A.S.G. Functionalisation of Magnetic Nanoparticles for Applications in Biomedicine. J. Phys. D Appl. Phys. 2003, 36, R198–R206. [Google Scholar] [CrossRef]
- Hegyi, G.; Szigeti, G.P.; Szász, A. Hyperthermia versus Oncothermia: Cellular Effects in Complementary Cancer Therapy. Evid.-Based Complement. Altern. Med. 2013, 2013, 672873. [Google Scholar] [CrossRef]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective Inductive Heating of Lymph Nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Tajabadi, M.; Rahmani, I.; Mirkazemi, S.M.; Goran Orimi, H. Insights into the Synthesis Optimization of Fe@SiO2 Core-Shell Nanostructure as a Highly Efficient Nano-Heater for Magnetic Hyperthermia Treatment. Adv. Powder Technol. 2022, 33, 103366. [Google Scholar] [CrossRef]
- Chen, Z.; Song, S.; Ma, J.; da Ling, S.; Wang, Y.D.; Kong, T.T.; Xu, J.H. Fabrication of Magnetic Core/Shell Hydrogels via Microfluidics for Controlled Drug Delivery. Chem. Eng. Sci. 2022, 248, 117216. [Google Scholar] [CrossRef]
- Etemadi, H.; Plieger, P.G. Magnetic Fluid Hyperthermia Based on Magnetic Nanoparticles: Physical Characteristics, Historical Perspective, Clinical Trials, Technological Challenges, and Recent Advances. Adv. Ther. 2020, 3, 2000061. [Google Scholar] [CrossRef]

| Core | Shell | ||||||
|---|---|---|---|---|---|---|---|
| Core/Shell | Shape | Average Size | Method | Basic Reagent | Method | Basic Reagent | Ref. |
| Fe/C | irregular spherical | 16–41 nm | thermal decomposition | FeCl3·6H2O | fructose, glucose, and sucrose | [26] | |
| Fe/C | spherical | 25 nm | DC plasma arc discharge method | Fe powder (high purity) | carbon powder | [27] | |
| Fe/C | spherical | 200 nm | reduction with CVD | Fe3O4 | carbon powder | [28] | |
| Fe/C | spherical | 50–60 nm | thermal decomposition | Ferrocene, silicon wafer | thermal decomposition | heavy oil | [29] |
| Fe/SiO2 | spherical agglomerates | 97 nm | gas-phase hybridization | Fe powder | gas-phase hybridization | SiO2 powder | [30] |
| Fe/SiO2 | spherical | 10–40 nm | reduction by KBH4 | FeCl2·4H2O | hydrolysis, condensation polymerization | TEOS | [31] |
| Fe/SiO2 | spherical | 10–90 nm | arc discharge | Fe powder | SiO2 powder | [32] | |
| Fe/FeO, Fe3O4 | spherical | 30–80 nm | solar vapor phase condensation | Fe powder | solar vapor phase condensation | Fe powder, Fe3O4 powder | [33] |
| Fe/Ag | spherical | 33 nm | reduction by NaBH4 | FeSO4·7H2O | AgNO3 | [34] | |
| Fe/Ag | spherical | 10 nm | reduction by LiBEt3H | FeCl2 | reduction in organic media | AgNO3 | [35] |
| Ag/Fe | spherical | 30 nm | reduction via phytochemicals | Fe(NO3)3 | AgNO3 | [36] | |
| Fe/Pt | spherical | ≈2 nm | reduction by NaBH4 | FeSO4 | reduction transmetalation | K2PtCl4 | [37] |
| Fe/Pt | spherical | 3 nm | reduction | C15H21FeO6 | reduction | PtCl4 | [38] |
| Fe/PIB | spherical | 20–100 nm | thermal decomposition | Fe(CO)5 | PIB | [39] | |
| Fe/PS | spherical | 20–100 nm | thermal decomposition | Fe(CO)5 | polystyrene (PS) | [39] | |
| Fe-CBC/SdC | nanoparticles on fibers | <100 nm fiber diameter (NPs << 50 nm) | cellulose-assisted hydrolysis, polymer wrapping | FeCl3, BC hydrogels | carbonization | PEDOT, EDOT | [40] |
| Ni/C | spherical | 14 nm | reduction with CVD | Ni(acac)2 | annealing | [41] | |
| Ni/C | 15–35 nm | thermal decomposition | Ni(C5H7O2)2 | thermal decomposition | Ni(C5H7O2)2 | [29] | |
| SiO2/Ni | spherical | 150 nm | Stöber method | TEOS | deposition, precipitation | Ni(NO3)2·6H2O | [42] |
| Ni/SiO2 | spherical | 80 nm | WEE technique | Ni wire | Stöber method | TEOS | [43] |
| Ni/SiO2 | elliptic and spherical | ≈300 nm (20–80 nm shell) | reduction by citric acid | NiCl2·6H2O | Stöber method | TEOS | [44] |
| Ag/Ni | spherical | 50–100 nm 95–165 nm | reduction by NaBH4 in microemulsion | Ni(NO3)2 | reduction by NaBH4 in microemulsion | AgNO3 | [45] |
| Ag/Ni | nanowire (and spherical) | <300 nm (50–150 nm shell) | hydrothermal method | AgNO3 | hydrothermal method | Ni(AC)2 | [46] |
| Ni/Pt | irregular spherical agglomerated | <10 nm | reduction by NaBH4 | Ni(CH3COO)2 | reduction by NaBH4 and N2H4 | H2PtCl4·6H2O | [47] |
| Co/CoO@FeNC | spherical core onion-like shells | 50 nm | microemulsion, pyrolysis | FeCl3, CoCl2·6H2O, RuO2, Pt/C, cyclohexane | [48] | ||
| Co/SiO2 | spherical | ≈60 nm | cryogenic melting method | Co bulk metal | Stöber method | TEOS | [49] |
| Co/CdSe | spherical | ≈15 nm | high-temperature thermal decomposition | Co2(CO)8 | precipitation | Cd(CH3)2, Se | [50] |
| CoNi/SiO2/TiO2 | spherical | 1.35 µm/100 nm shell | solvothermal | C4H6NiO4 4H2O, C4H6CoO4 4H2O | Stöber method, solvothermal | TEOS, TIP | [51] |
| CoNi@Air@TiO2 | Spherical yolk type | 1.35 µm/200 nm shell | solvothermal | C4H6NiO4 4H2O, C4H6CoO4 4H2O | Stöber method, solvothermal, hydrothermal via NaOH | TEOS, TIP | [51] |
| Au/Co | spherical | 65 nm | reduction by sodium citrate | HAuCl4 | reduction by N2H4, H2O | CoCl2 | [52] |
| Au/Ni | spherical | 65 nm | reduction by sodium citrate | HAuCl4 | reduction by N2H4, H2O | NiCl2 | [52] |
| Co/Au, Pd, Pt, Cu | spherical | ≈6.5 nm | Hydrothermal decomposition | Co2(CO)8 | Reduction transmetalation | Au precursor Pd(hfac)2, Pt(hfac)2, Cu(hfac)2 | [53] |
| ZnCo2O4/C | nanowire arrays | 100 nm | hydrothermal, annealing | Zn(NO3)2·6H2O, Co(NO3)2·6H2O, urea | CVD | C2H2 | [54] |
| FeNi/C | spherical | 50 nm | precipitation, reduction with NaOH | FeCl2, NiCl2, aniline, hydrochloric acid, formaldehyde | pyrolysis | [55] | |
| FeNi/SiO2 | spherical | ≈60 nm | cryogenic melting method | Fe, Ni bulk metal | Stöber method | TEOS | [49] |
| Fe, Cu/ Au, Pt, Pd, Ag | cubical, spherical | 5–50 nm, 50–60 nm | reduction by vitamin C | Fe(NO3)3 9H2O, CuCl2 | reduction by vitamin C | Na2PtCl6·6H2O, HAuCl4·3H2O, AgNO3, PdCl2 | [56] |
| FePt/CdS | spherical | <10 nm | thermal decomposition with reduction | Fe(CO)5, Pt(C5H7O2)2 | precipitation | sulfur, Cd(C5H7O2)2 | [57] |
| Fe58Pt42/Fe3O4 | spherical | 4–7 nm | reduction with thermal decomposition | Pt(C5H7O2)2, Fe(CO)5 | thermal decomposition | 1,2-hexadecanediol, oleic acid, Fe(C5H7O2)3 , oleylamine | [58] |
| MnFe2O4/TEHA-co-PDLLA @PTX | spherical, irregularly shaped particles | <160 nm | solvothermal | acetylacetonate iron (III), manganese (II), Fe(acac)3, Mn(acac)2 | emulsion | TEHA-co-PDLLA block copolymer, Paclitaxel | [2] |
| Fe3O4/SiO2 | spherical | 30 nm | co-precipitation | FeCl2⋅4H2O, FeCl3⋅6H2O | Stöber method | TEOS | [59] |
| Fe3O4/SiO2 | spherical | 30 nm | thermal decomposition | Fe(C5H7O2)3 | sol-gel | TEOS | [60] |
| Fe3O4/SiO2 | acicular | <5 nm | chemical reaction in microemulsion | FeCl3, FeSO4 | sol-gel reaction in microemulsion | TEOS | [61] |
| Fe3O4/SiO2 | spherical | 15–20 nm | wet chemical reaction | FeCl3, FeSO4 | hydrolysis | Na2SiO3 | [62] |
| Fe3O4/SiO2 | spherical | 7–12 nm | wet chemical reaction | FeCl3, N2H4 | sol-gel | TEOS | [63] |
| Fe3O4/SiO2 | spherical | 80 nm | co-precipitation | FeCl2·4H2O, FeCl3·6H2O | sonication, hydrolysis | TEOS | [64] |
| Fe3O4/SiO2 | stellate | 100 nm | thermal decomposition | FeSt3 | sol-gel | TEOS, CTATos | [65] |
| Fe3O4/Polyaniline/Au | irregular spherical | 200 nm (4 nm Au NPs as shell) | solvothermal | FeCl3·6H2O, sodium acrylate, CH3COONa | reduction by Na3C6H5O7, NaBH4 | HAuCl4 | [66] |
| Fe3O4/Au | octahedral | 100–150 nm | physical grinding | commercial Fe3O4 | calcination | HAuCl4 | [67] |
| Fe3O4/Au | spherical | 7 nm | solvothermal | Fe(C5H7O2)3, phenyl ether, oleic acid, oleylamine | hydrolysis with thermal decomposition | Au(OOCCH3)3, 1,2-hexadecanediol, oleic acid, oleylamine | [68] |
| Fe3O4/Au | spherical | 8 nm | wet chemical reaction | FeCl3 | reduction by NaBH4 | HAuCl4 | [69] |
| Fe3O4/Ag | octahedral | 100–150 nm | physical grinding | commercial Fe3O4 | calcination | AgNO3 | [67] |
| Fe3O4/C | spherical | ~20 nm | co-precipitation | FeCl3·6H2O, FeCl2·4H2O | hydrothermal | glucose | [70] |
| Fe3O4/C | rod, diamond, spindle | 2 μm, 3 μm, 600 nm | MOF-derived method and solvothermal | FeCl3·6H2O, fumaric acid, dimethyl formamide | pyrolysis | [71] | |
| Fe3O4/C | spherical | 52, 80, and 110 nm | High-temperature solution-phase reaction | Fe(acac)3, oleic acid, dibenzyl ether, 1-Octadecene, hexane | carbonization | [72] | |
| Fe7C3/FexOy/C | spherical | 12–45 nm | thermal decomposition | Fe(C5H5)2 | thermal decomposition | [73] | |
| FexOy/C | spherical | 7 nm @6 um spheres | wet chemical reaction | Fe(NO3)3·6H2O | thermal decomposition | glucose | [74] |
| Fe3O4/TiO2 | spherical | 20 nm | wet chemical reaction | FeCl3·6H2O, FeCl2·4H2O | precipitation | Ti(SO4)2, CO(NH2)2 | [75] |
| Fe3O4/SiO2/TiO2 | irregular spherical | ≈30 nm | wet chemical reaction | FeCl3, FeSO4 | sol-gel | TEOS, TBOT | [62] |
| Fe3O4/SiO2/Al2O3 | irregular spherical | 20–25 nm | wet chemical reaction | FeCl3, FeSO4 | sol-gel with precipitation | TEOS | [62] |
| Fe3O4, γ-Fe2O3/NaYF4:Yb, Er | spherical | 68 nm | co-precipitation | FeCl2, FeCl3 | co-precipitation | NaF, YCl3, YbCl3, ErCl3, EDTA | [76] |
| Fe3O4/PEG | spherical | 10–40 nm | wet chemical reaction | FeCl2·4H2O, FeCl3·6H2O | PEG-400 | [77] | |
| Fe3O4/PEG | spherical | 2–10 nm | co-precipitation | FeCl2·4H2O, FeCl3·6H2O | co-precipitation, sonication | PEG-400 | [78] |
| Fe3O4/PEG | irregular spherical | 12 nm | co-precipitation | FeCl3 | PEG (mol wt. 4000) | [79] | |
| Fe3O4/PLA | spherical | 10 nm | co-precipitation | FeCl3·6H2O, FeCl2·4H2O | ring-opening polymerization | L-lactide | [80] |
| Fe3O4/Dextran | spherical | ≈35 nm | Fe3O4 powder | vibration ball milling | Dextran 60000 | [81] | |
| Fe3O4/MPEG | spherical | 7.8 nm | wet chemical reaction | FeCl3·6H2O, FeCl2·4H2O | MPEG (mol wt. 5000) | [69] | |
| Fe3O4/PEGMA | quasi spherical | 15 nm | co-precipitation | FeCl3·6H2O, FeCl2·4H2O | RAFT | PEGMA | [82] |
| PEGMA/PS/Fe3O4 | quasi spherical | 3.7 um (90 nm Fe3O4 NPs) | polymerization | GMA, EDMA | co-precipitation | FeCl3, NaNO2 | [83] |
| Fe3O4/PHEMA-g-PCL | spherical | 4 nm | Hydrothermal degradation | Fe(OCOCH3)3 | polymerization | pentamethyldiethylenetriamine | [84] |
| Fe3O4/PEO-PPO-PEO | spherical | 45–20 nm | reduction | FeCl3 | block polymer PEO-PPO-PEO | [85] | |
| CoFe2O4/DTPA-CS | spherical | 70 nm | low temperature solid-state method | CoCl2·6H2O, FeCl3·6H2O, NaCl | emulsion cross-linking polymerization | chitosan, DTPA | [86] |
| Fe3O4/Fe2O3 | nanorod | 30−50 nm (diameter), 0.8−1.2 μm (length) | hydrothermal | FeCl3·6H2O, Na2SO4 | electrodeposition | FeOOH, FeCl2 | [87] |
| CaO/Fe2O3 | irregular | 9–16 nm | bulk CaO | thermal decomposition | Fe(C5H7O2)3 | [88] | |
| MgO/Fe2O3 | irregular | 6–9 nm | aerogel/hypercritical drying/ dehydration | Mg(OCH3)2 | thermal decomposition | Fe(C5H7O2)3 | [88] |
| Fe2O3/Au | spindle | 80 nm × 500 nm | hydrolysis in KH2PO4 solution | FeCl3 6H2O | reduction by formaldehyde | HAuCl4 | [89] |
| Fe2O3/POS | spherical | 15–26 nm | co-precipitation | FeCl2, FeCl3 | polycondensation | TMMS, DEDMS, ClBz-T | [90] |
| γ-Fe2O3 /polyMAOETIB | spherical | ≈56 nm | precipitation | gelatin, iron oxide | emulsion polymerization | 2-MAOETIB | [91] |
| γ-Fe2O3/PEI+PEO-PGA | Irregular spherical | ≈45 nm | co-precipitation | FeCl2, FeCl3 | suspension polymerization | PEO-PGA | [92] |
| iron oxide-SiO2 composite/PS | spherical | ≈268 nm | Massart with Stöber method | FeCl2, FeCl3, TEOS | polymerization | styrene | [93] |
| FeO/Ag | spherical | 20–30 nm | wet chemical reaction with peel extract (PEP) | FeCl3 | AgNO3 | [94] | |
| FeO/Au | spherical | <100 nm | wet chemical reaction with peel extract (PEP) | FeCl3 | AuCl3 | [94] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsamos, D.; Krestou, A.; Papagiannaki, M.; Maropoulos, S. An Overview of the Production of Magnetic Core-Shell Nanoparticles and Their Biomedical Applications. Metals 2022, 12, 605. https://doi.org/10.3390/met12040605
Tsamos D, Krestou A, Papagiannaki M, Maropoulos S. An Overview of the Production of Magnetic Core-Shell Nanoparticles and Their Biomedical Applications. Metals. 2022; 12(4):605. https://doi.org/10.3390/met12040605
Chicago/Turabian StyleTsamos, Dimitris, Athina Krestou, Maria Papagiannaki, and Stergios Maropoulos. 2022. "An Overview of the Production of Magnetic Core-Shell Nanoparticles and Their Biomedical Applications" Metals 12, no. 4: 605. https://doi.org/10.3390/met12040605
APA StyleTsamos, D., Krestou, A., Papagiannaki, M., & Maropoulos, S. (2022). An Overview of the Production of Magnetic Core-Shell Nanoparticles and Their Biomedical Applications. Metals, 12(4), 605. https://doi.org/10.3390/met12040605








