An Electrochemical Study of Gold Dissolution in Thiosulfate Solution with Cobalt–Ammonia Catalysis
Abstract
:1. Introduction
2. Experimental Work
2.1. Electrochemical Experiments
2.2. Instrumentation and Chemicals
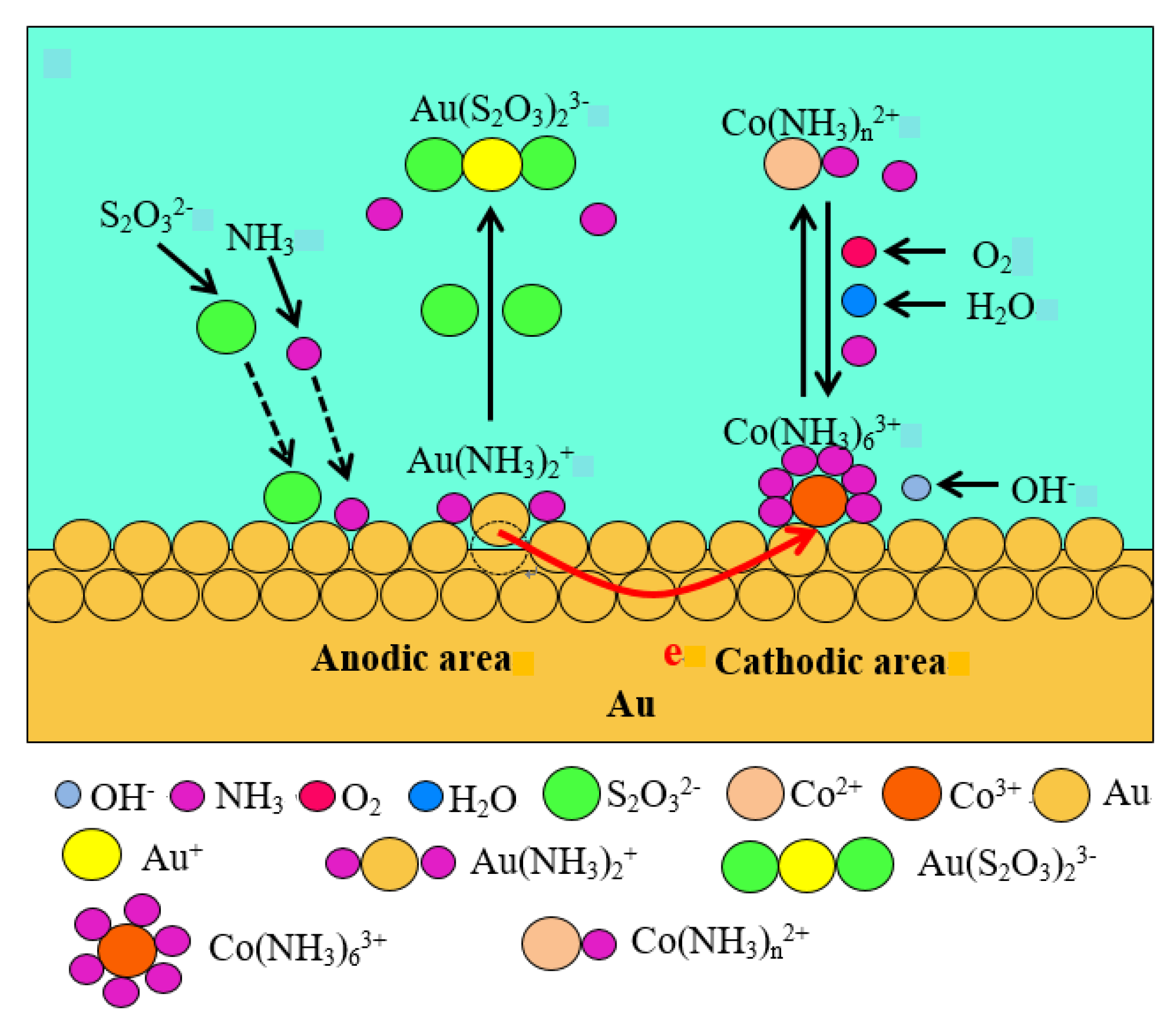
3. Results and Discussion
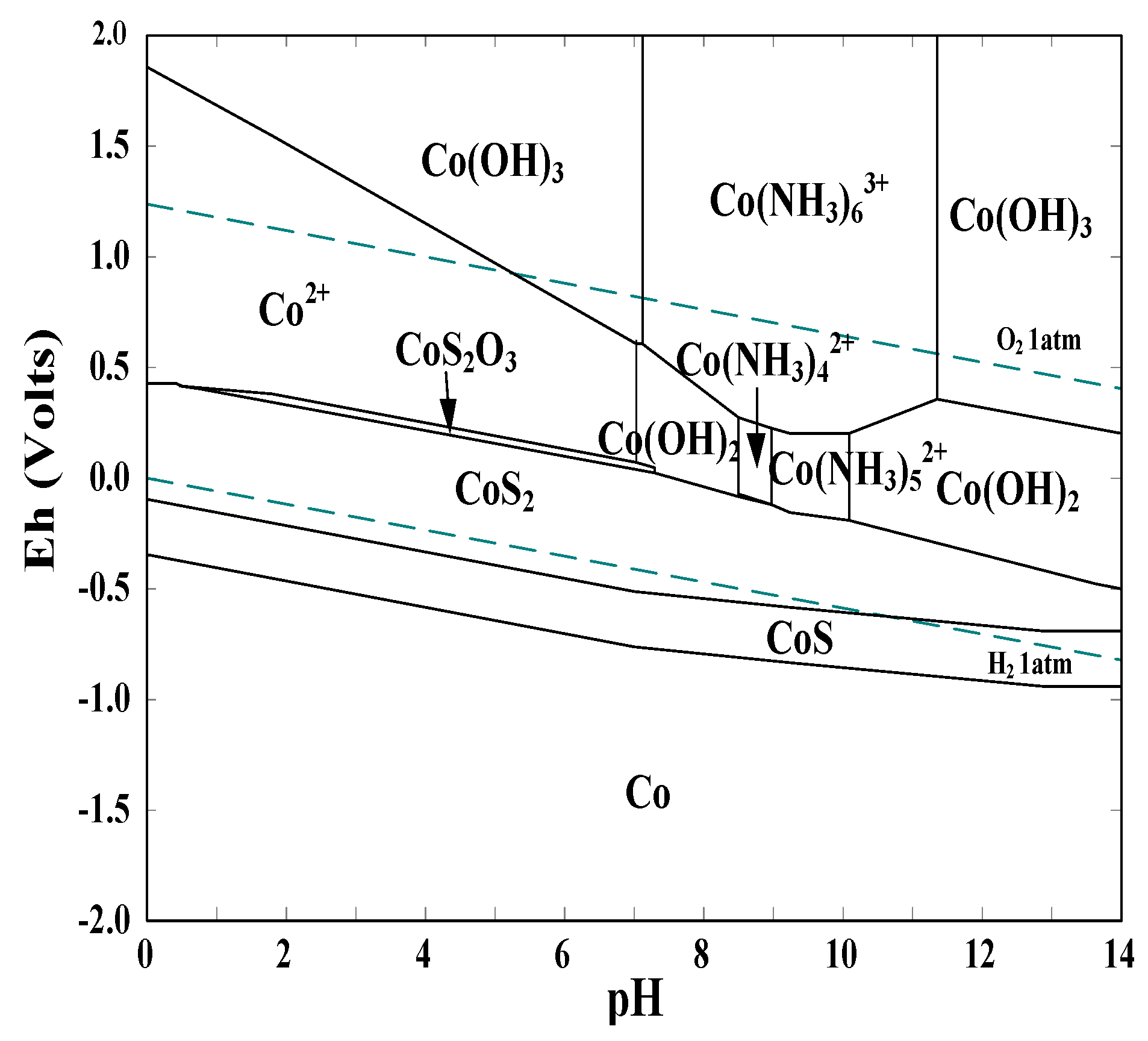
3.1. Thermodynamic Analysis
3.2. Polarization Curve
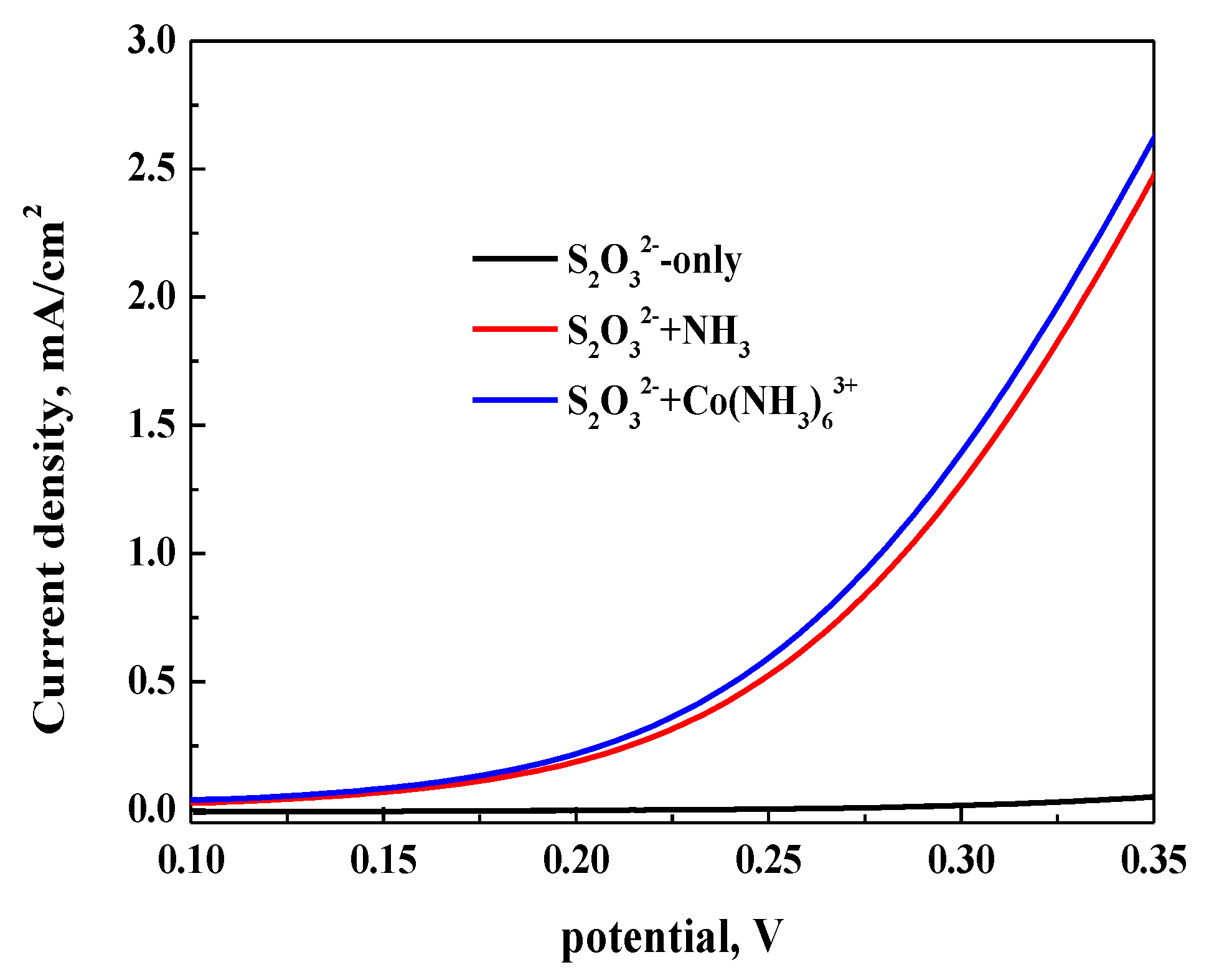
3.2.1. Anodic Polarization Curve
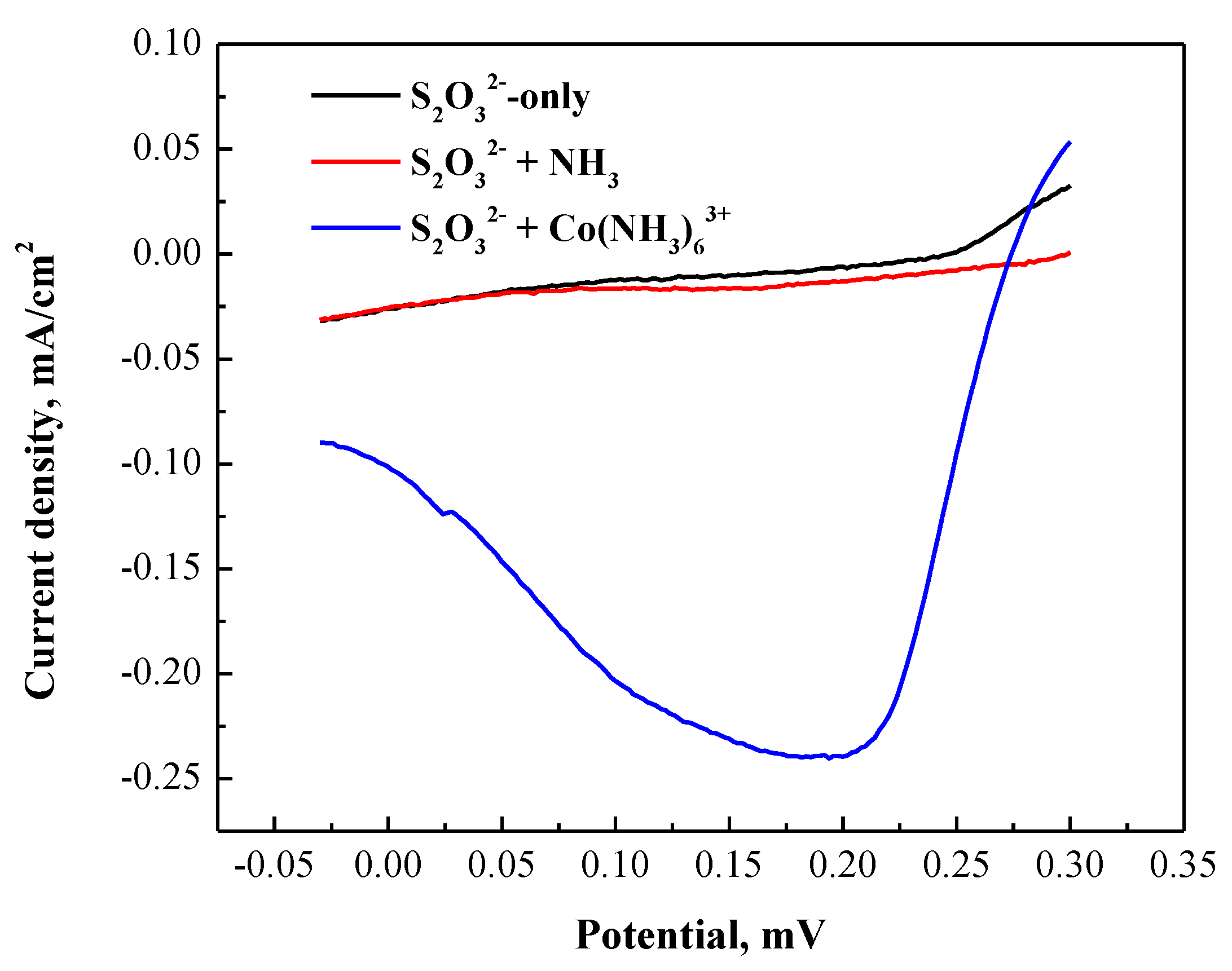
3.2.2. Cathodic Polarization Curve
3.3. Gold Dissolution in Thiosulfate Solution
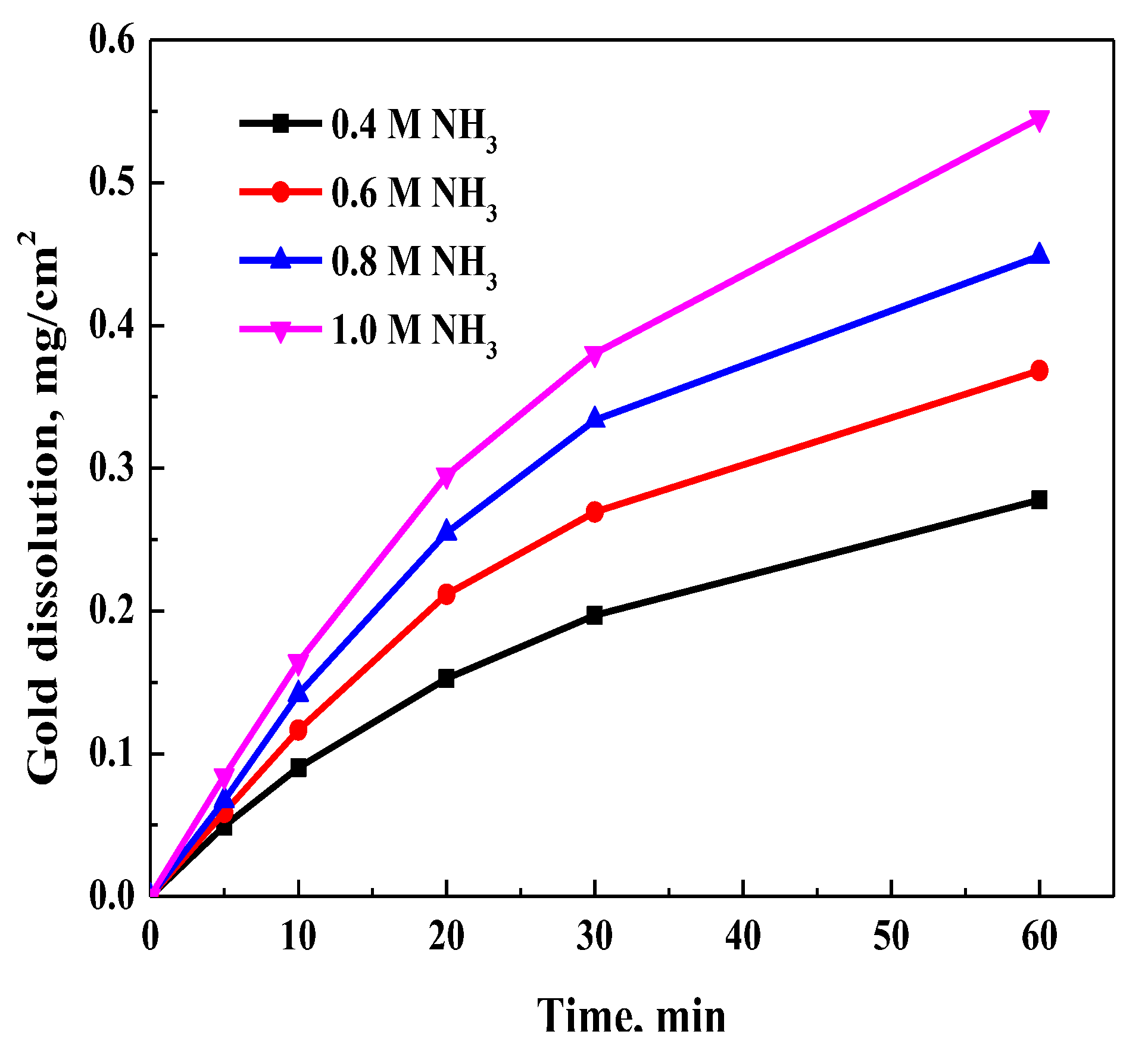
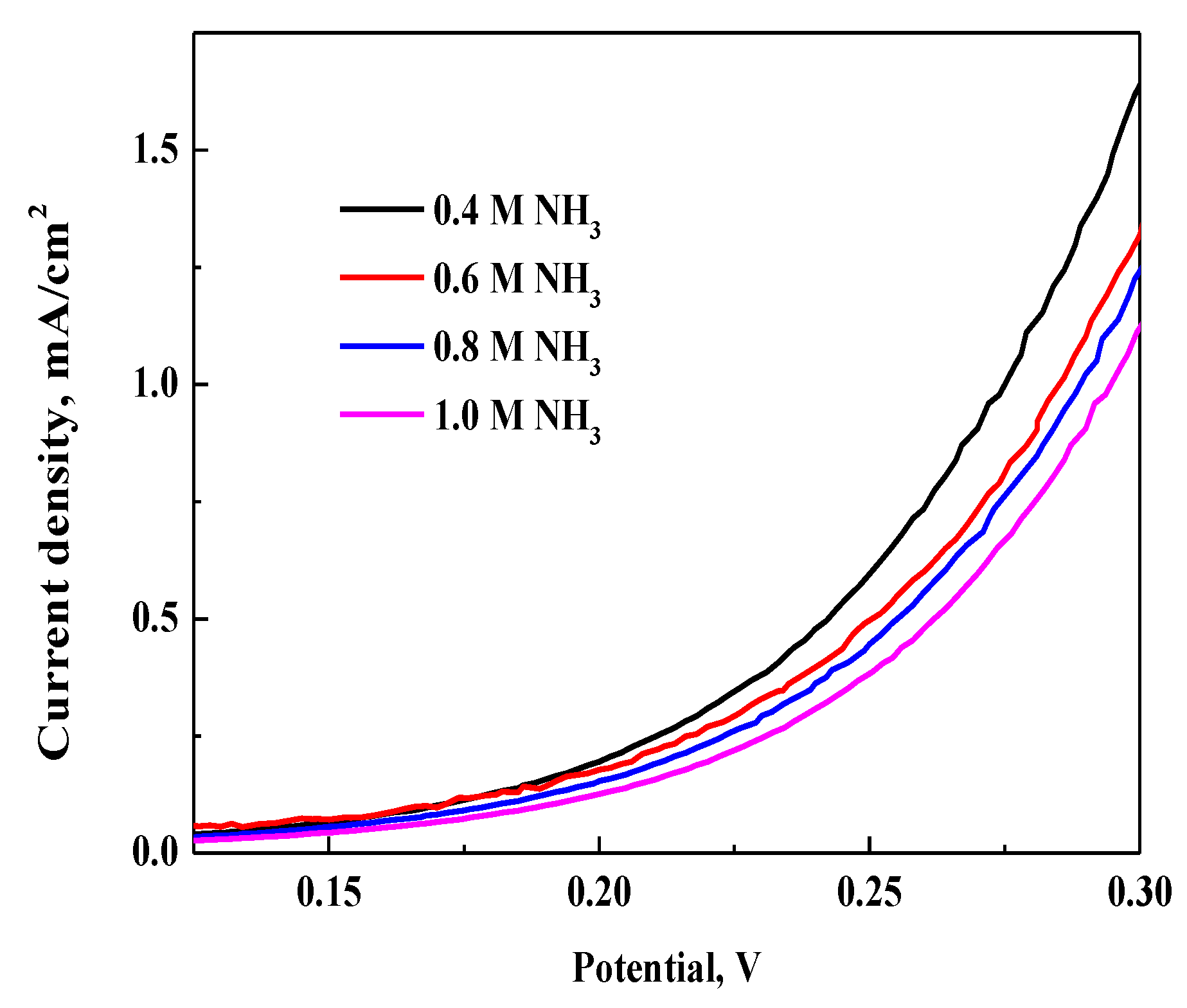
3.3.1. Effect of NH3 Concentration
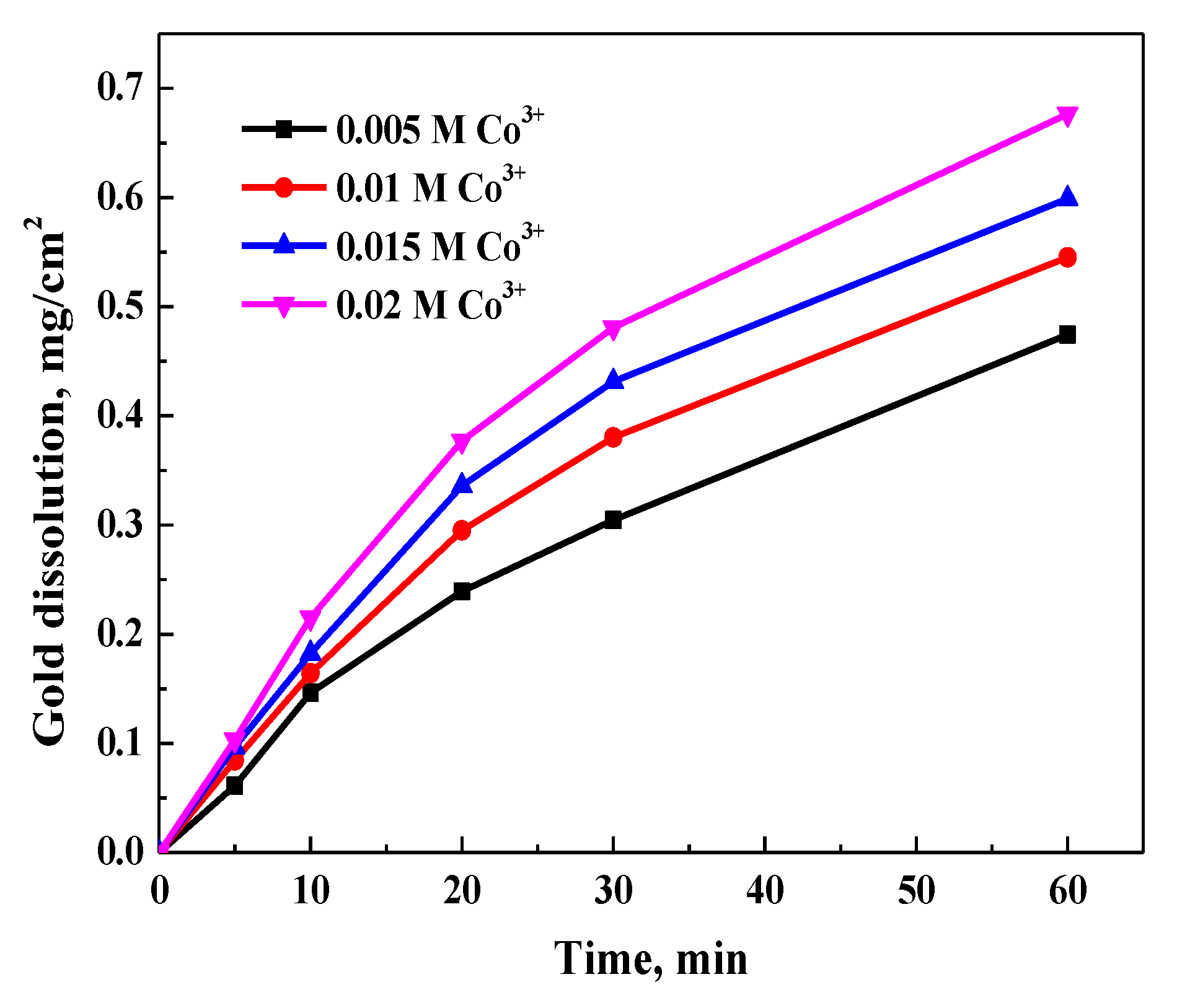
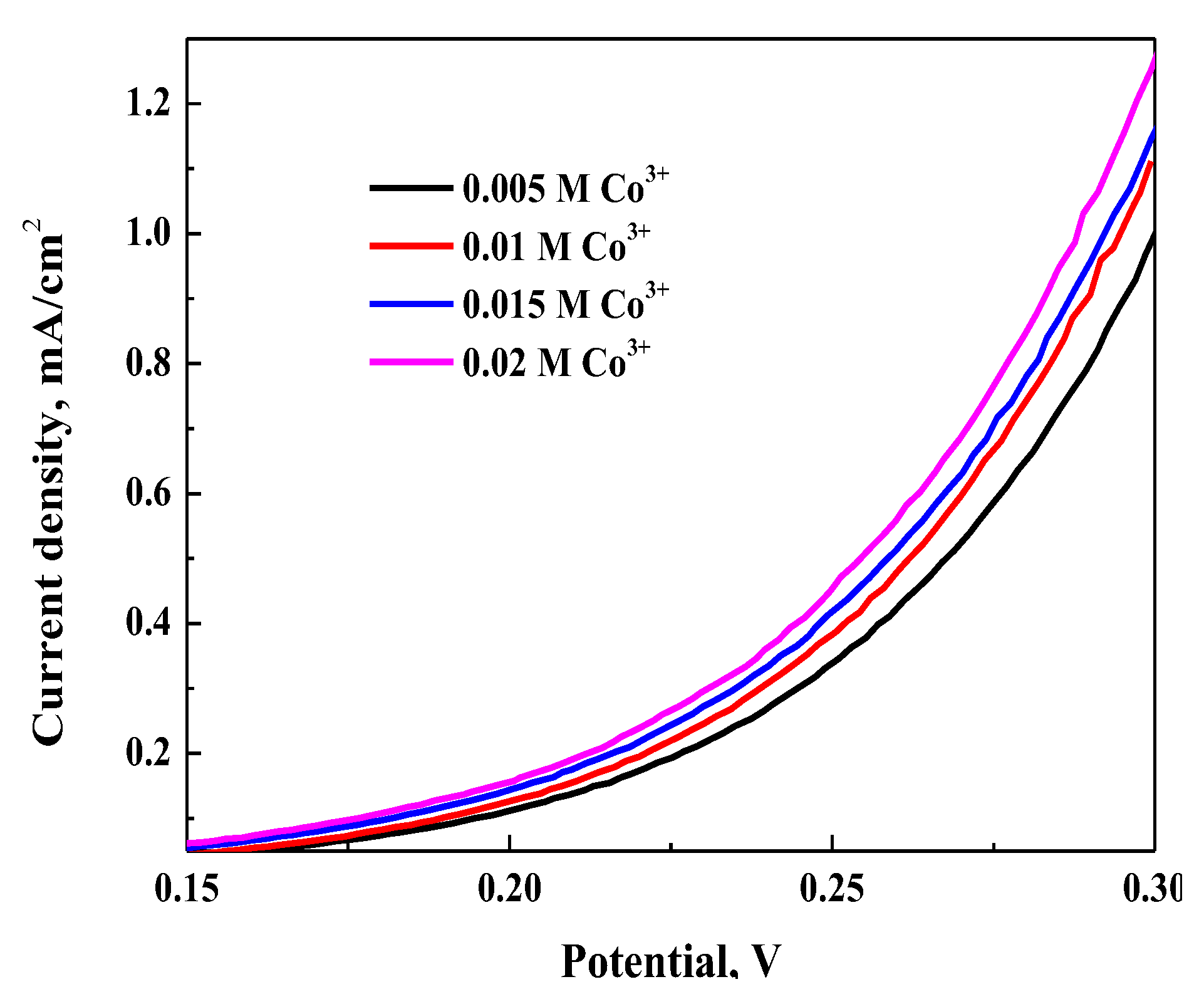
3.3.2. Effect of Cobalt(III) Concentration
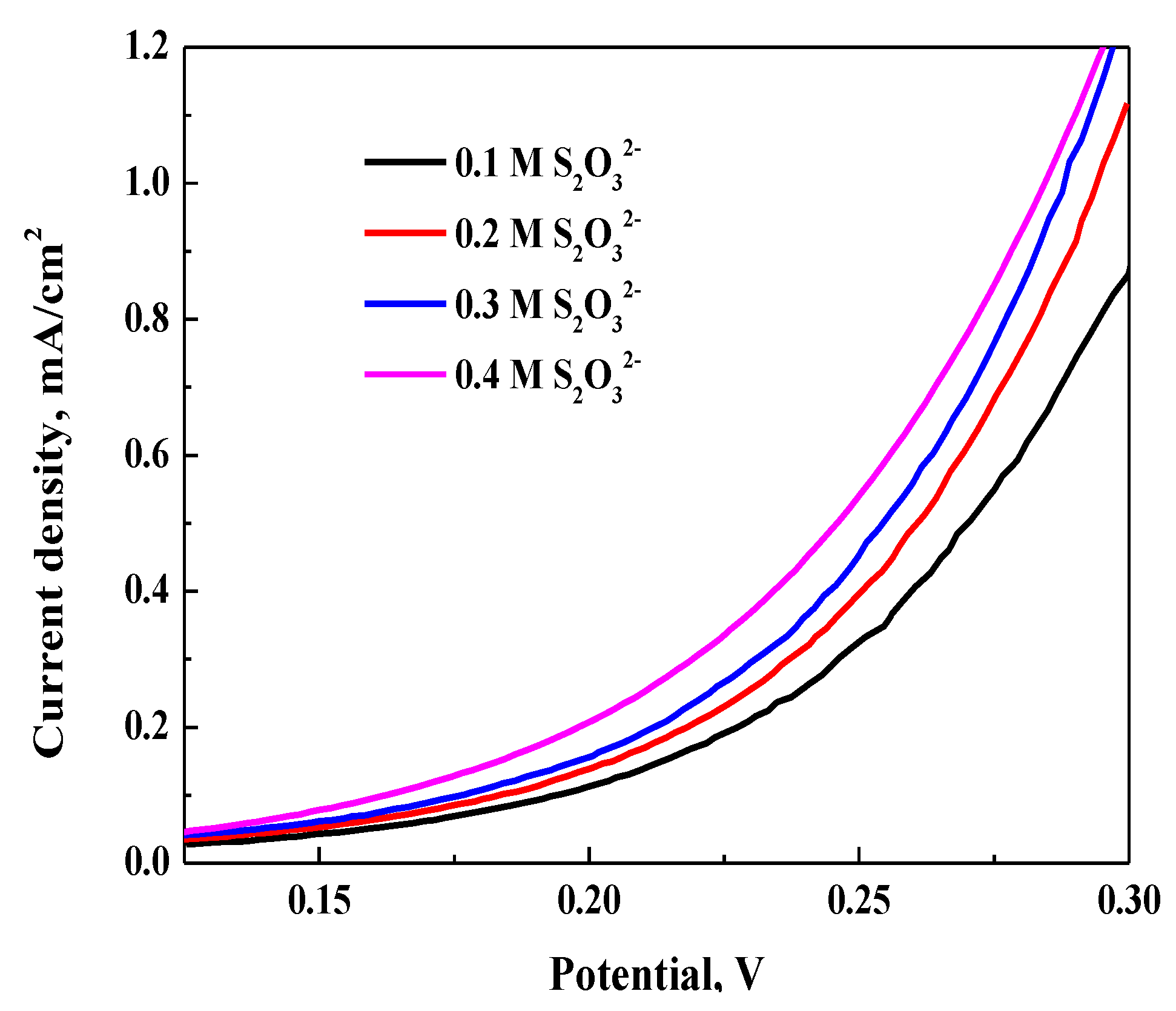
3.3.3. Effect of S2O32− Concentration
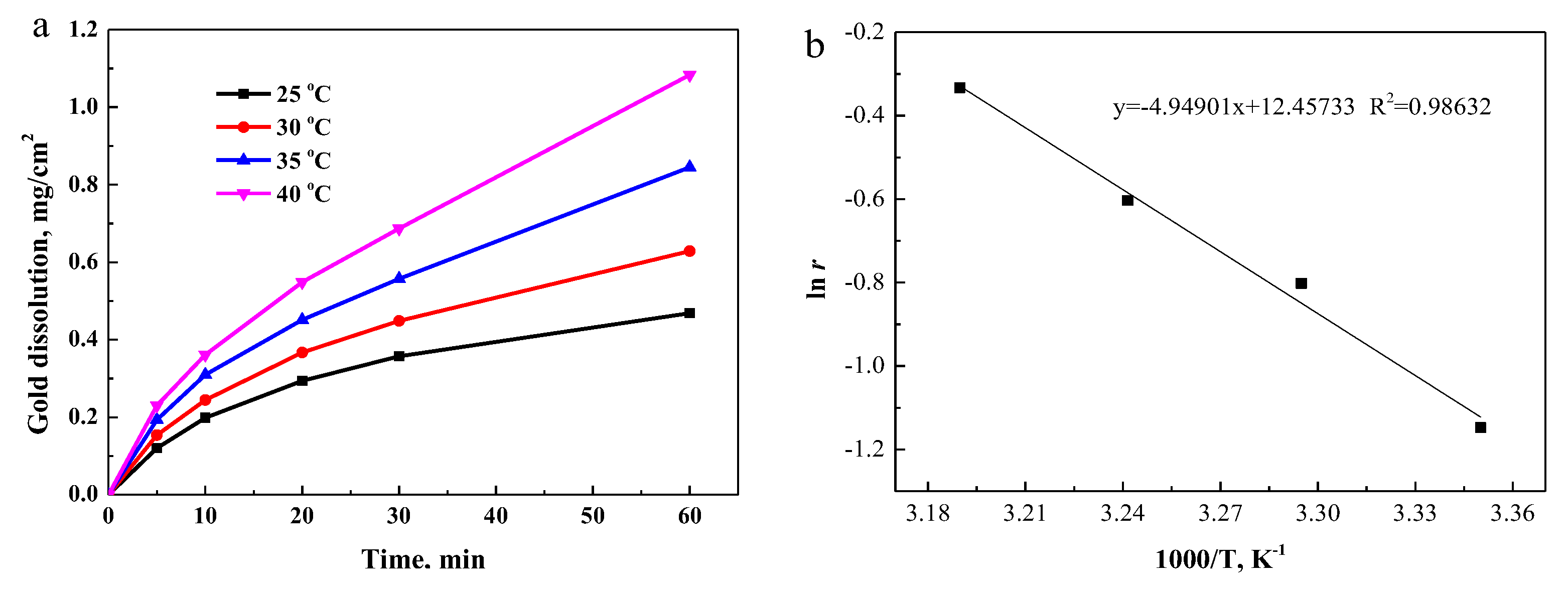
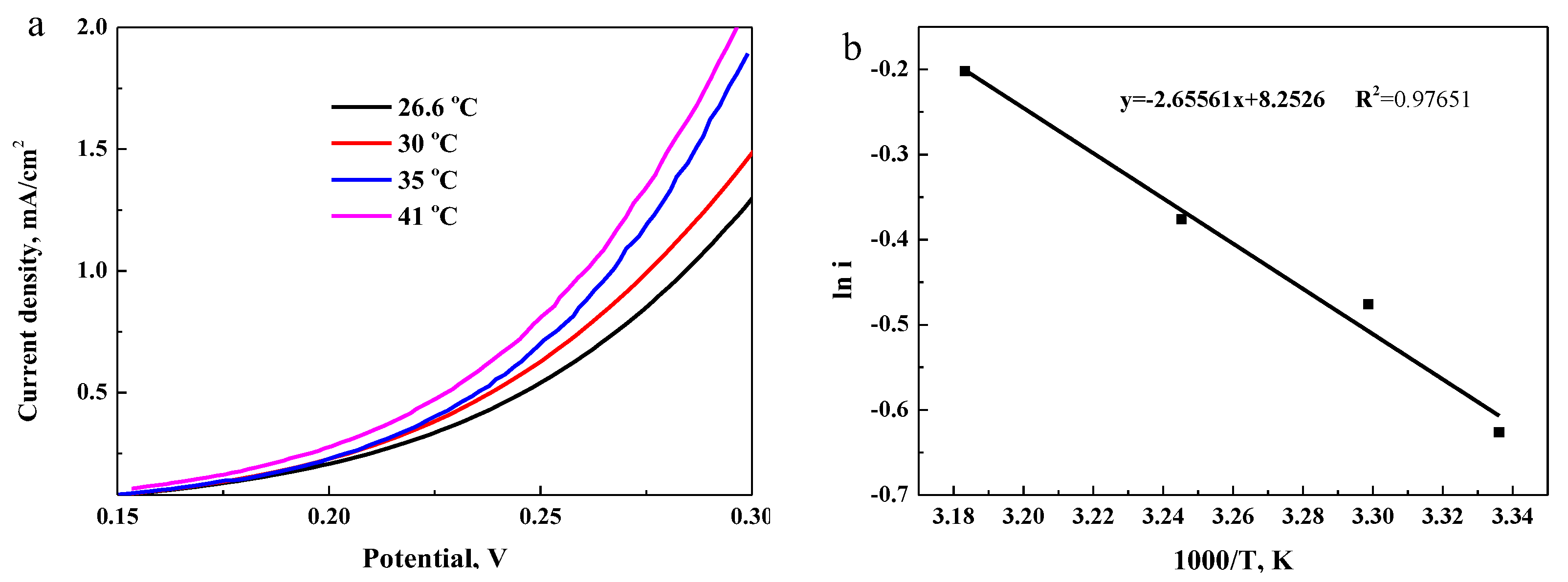
3.3.4. Effect of Temperature
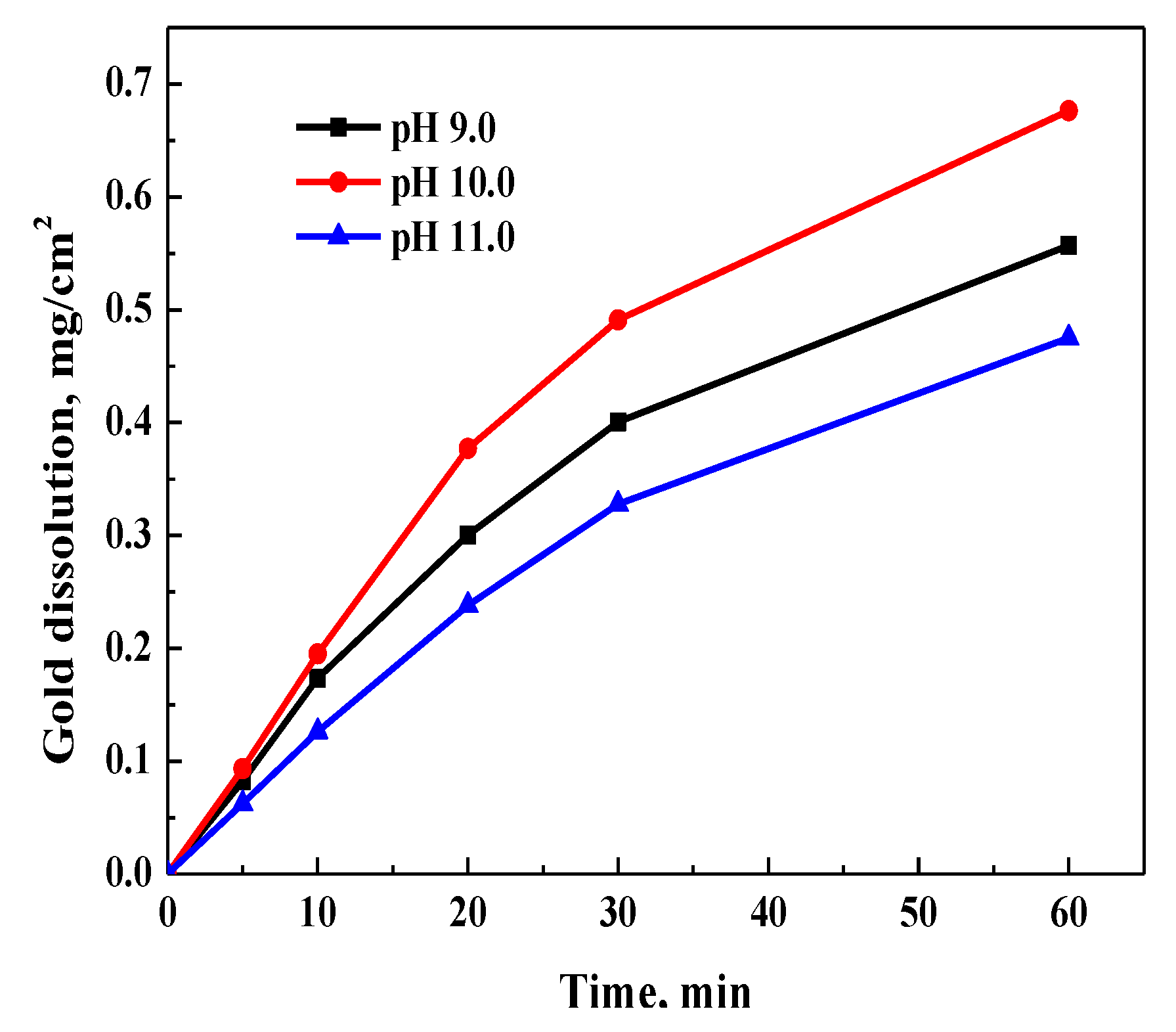
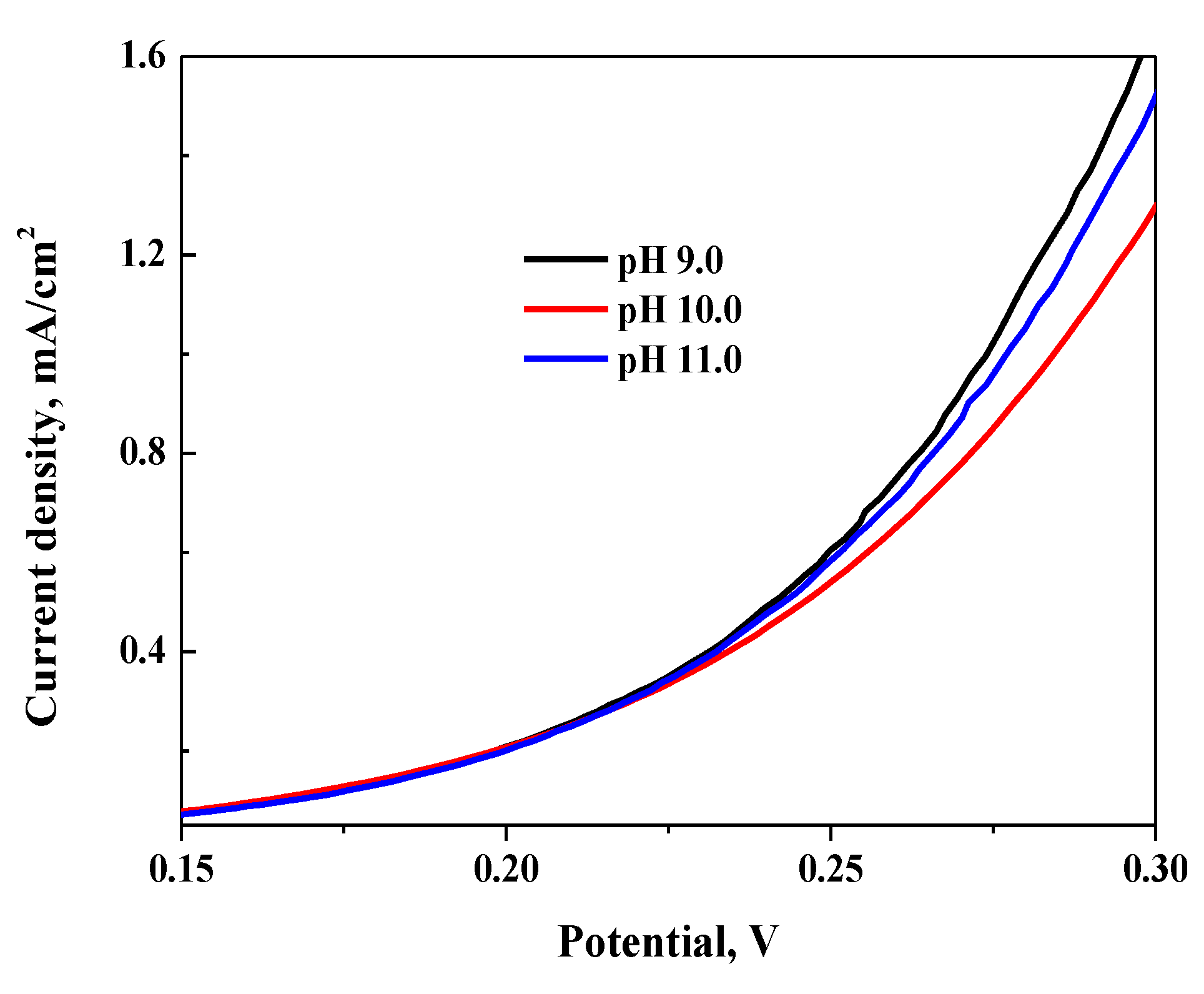
3.3.5. Effect of pH
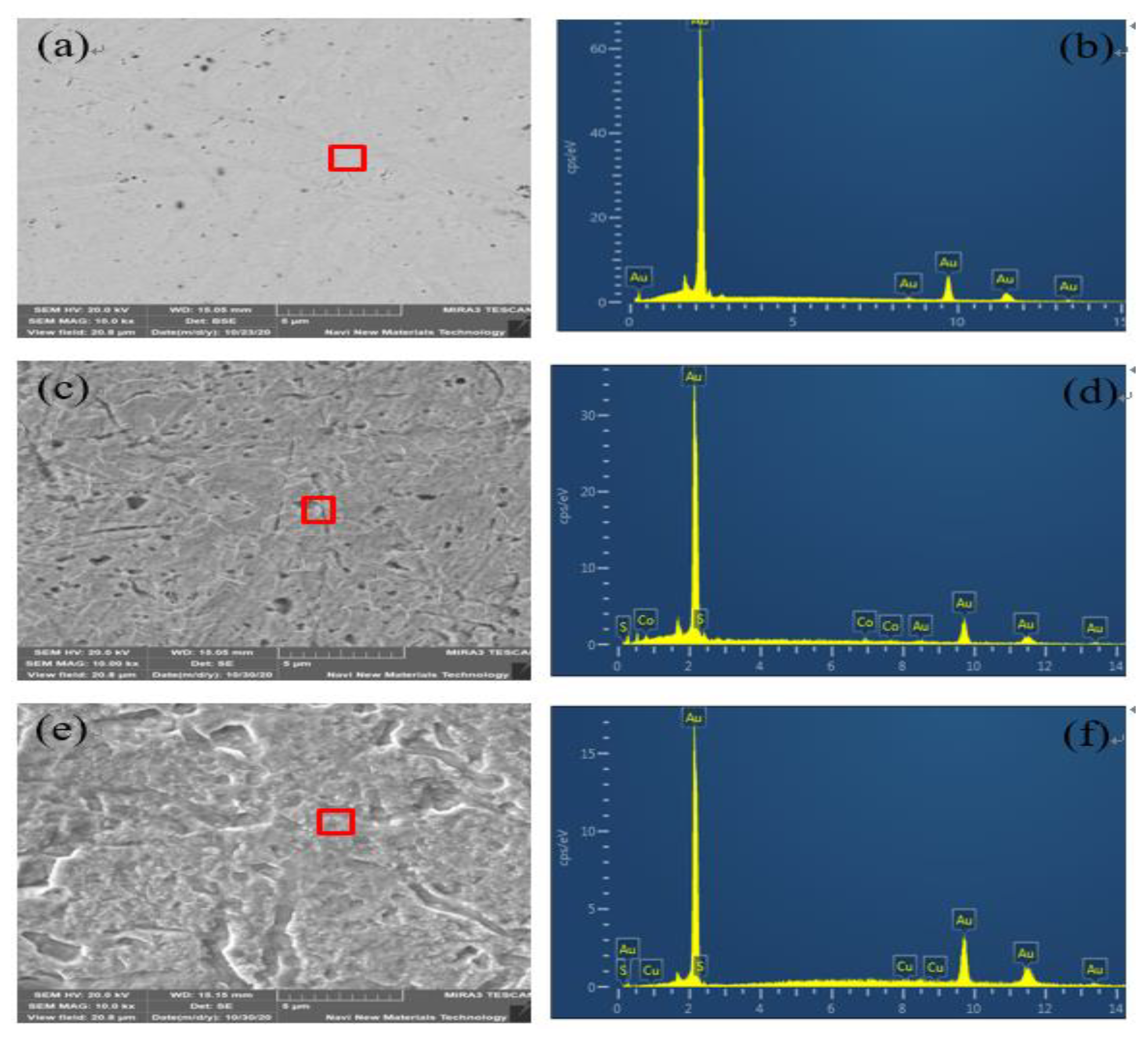
3.4. SEM-EDS and XPS Analyses of Gold Surface
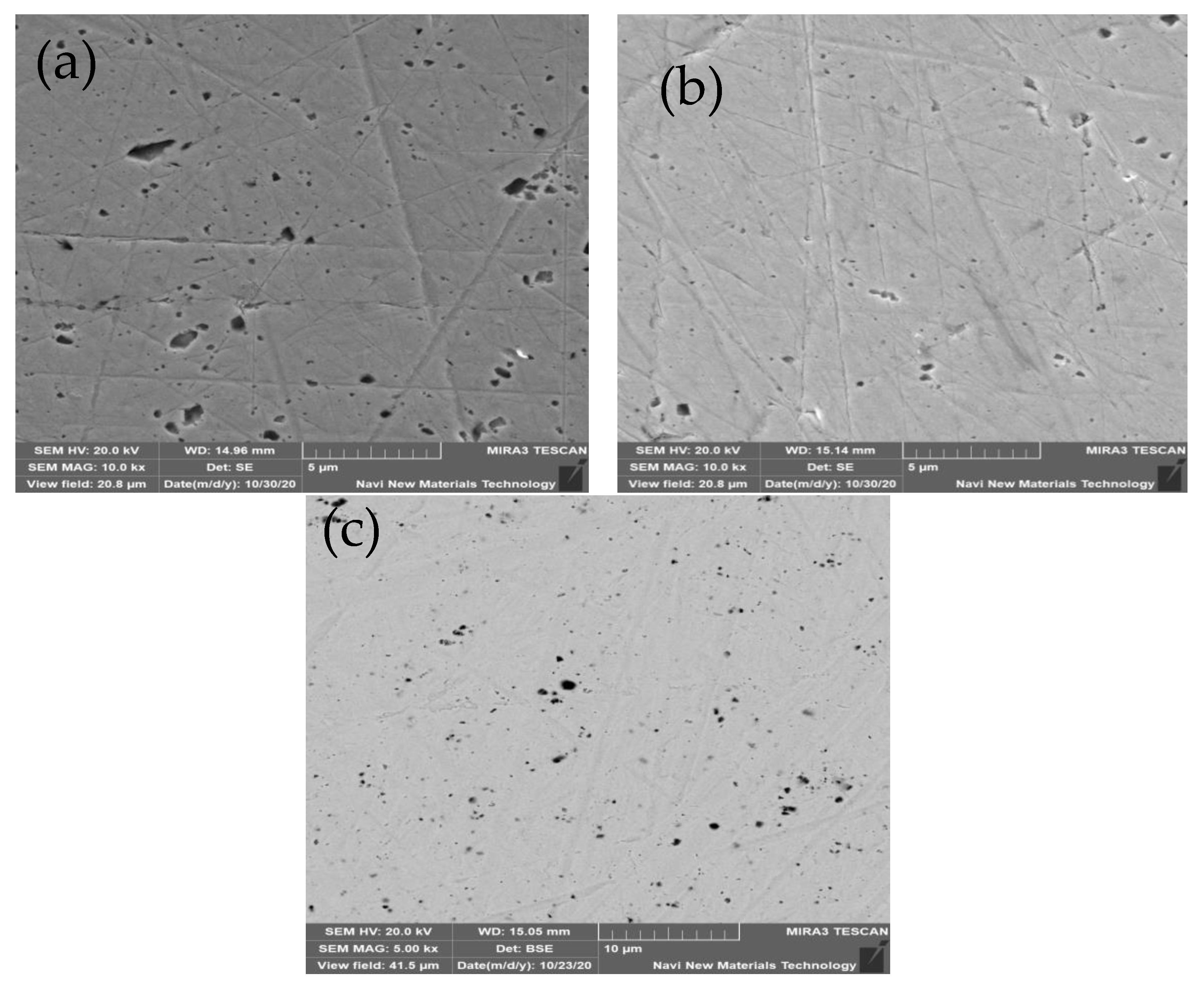
3.4.1. SEM-EDS Analysis
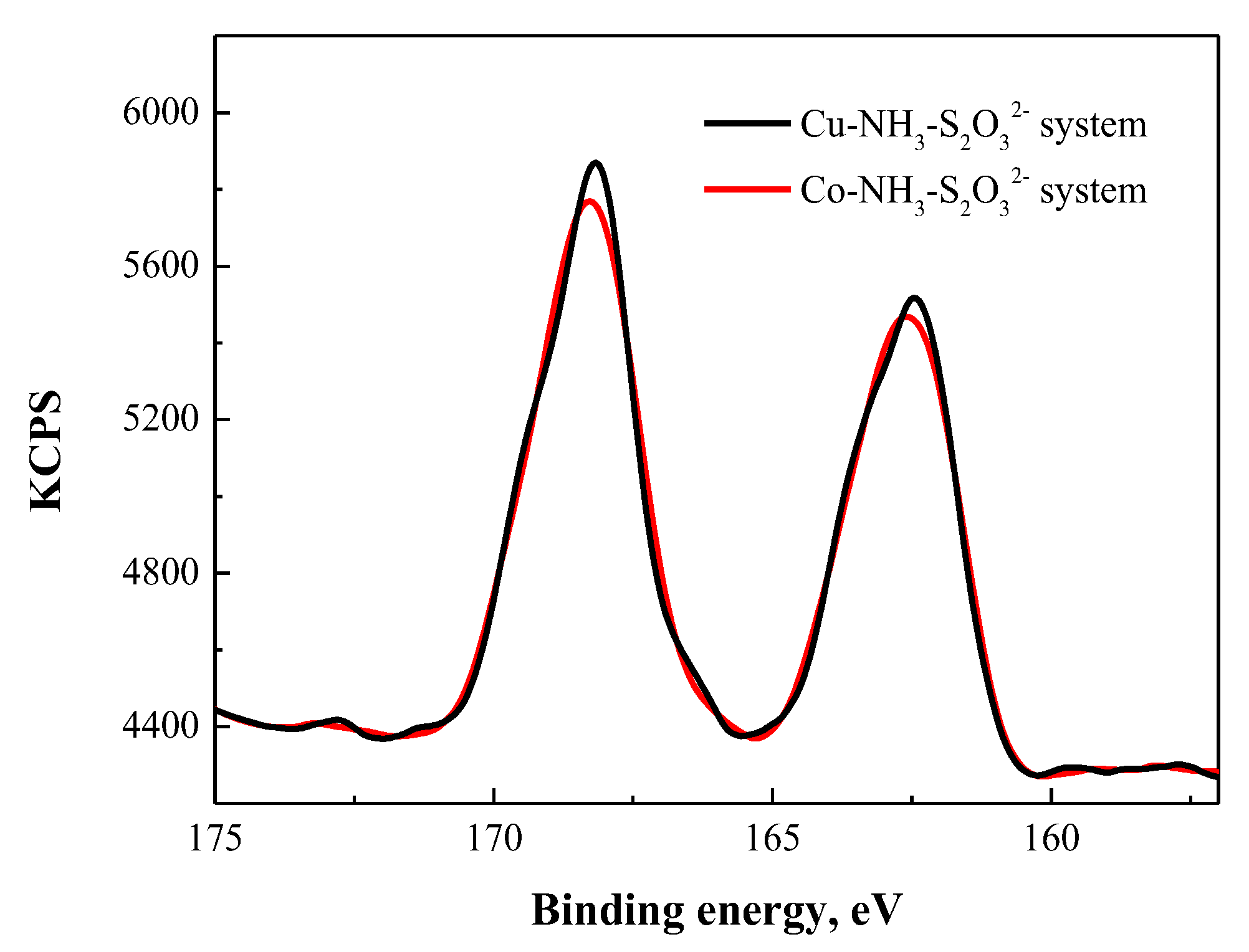
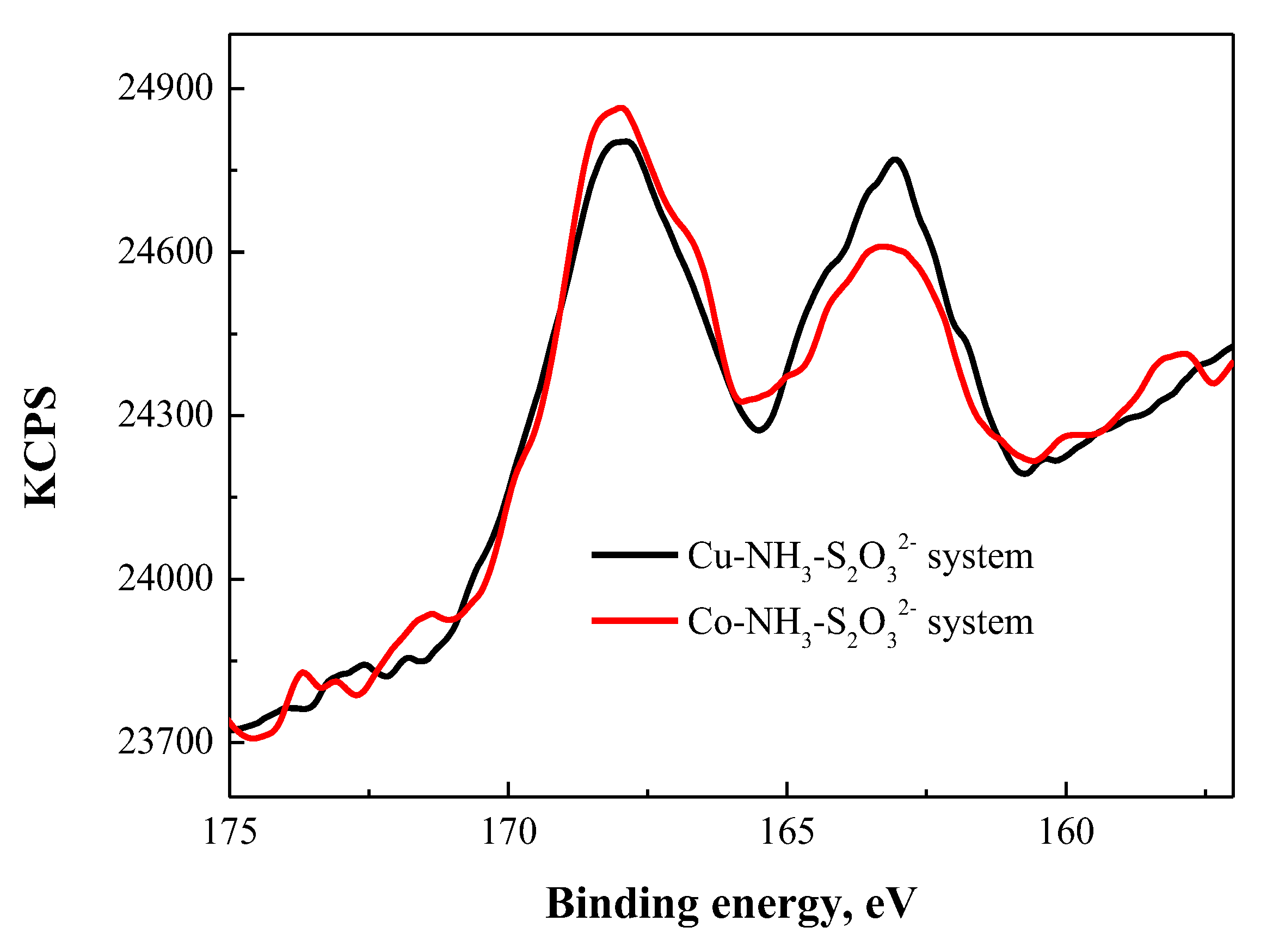
3.4.2. XPS Analysis
3.5. Decomposition of Thiosulfate
3.5.1. Effect of NH3 Concentration
3.5.2. Effect of Cobalt(III) Concentration
3.5.3. Effect of S2O32− Concentration
3.5.4. Effect of Temperature
3.5.5. Effect of pH
3.6. SEM-EDS and XPS Analyses of Platinum Surface
3.6.1. SEM-EDS Analysis
3.6.2. XPS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hilson, G.; Monhemius, A.J. Alternatives to cyanide in the gold mining industry: What prospects for the future? J. Clean. Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- Chen, C.; Lung, T.; Wan, C. A study of the leaching of gold and silver by acidothioureation. Hydrometallurgy 1980, 5, 207–212. [Google Scholar] [CrossRef]
- Kholmogorov, A.G.; Kononova, O.N.; Pashkov, G.L.; Kononov, Y.S. Thiocyanate solutions in gold technology. Hydrometallurgy 2002, 64, 43–48. [Google Scholar] [CrossRef]
- Mentha-Biney, R.; Reid, K.J.; Hepworth, M.T. Kinetics of gold-bromide loading onto activated carbon. Min. Metall. Explor. 1997, 14, 7–13. [Google Scholar]
- Chai, L.; Okido, M.; Wei, W. Effect of Na2SO3 on electrochemical aspects of gold dissolution in alkaline thiourea solution. Hydrometallurgy 1999, 53, 255–266. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Yang, J.; Chen, Y.; Li, Q.; Yang, Y. Comprehensive recoveries of selenium, copper, gold, silver and lead from a copper anode slime with a clean and economical hydrometallurgical process. Chem. Eng. J. 2020, 393, 124762. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Y.; Dong, Z.; Jiang, T.; Zhang, B.; Liu, G.; Yang, J.; Li, Q.; Yang, Y. Eco-friendly and efficient extraction of valuable elements from copper anode mud using an integrated pyro-hydrometallurgical process. Resour. Conserv. Recycl. 2021, 164, 105195. [Google Scholar] [CrossRef]
- Alzate, A.L.; Opez, E.; Serna, C.; Gonzalez, O. Gold recovery from printed circuit boards by selective breaking of internal metallic bonds using activated persulfate solutions. J. Clean. Prod. 2017, 166, 1102–1112. [Google Scholar] [CrossRef]
- Bisceglie, F.; Civati, D.; Bonati, B.; Faraci, F.D. Reduction of potassium cyanide usage in a consolidated industrial process for gold recovery from wastes and scraps. J. Clean. Prod. 2017, 142, 1810–1818. [Google Scholar] [CrossRef]
- Lu, Y.; Song, Q.; Xu, Z. Integrated technology for recovering Au from waste memory module by chlorination process: Selective leaching, extraction, and distillation. J. Clean. Prod. 2017, 161, 30–39. [Google Scholar] [CrossRef]
- Muir, D.M.; Aylmore, M.G. Thiosulphate as an alternative to cyanide for gold processing-issues and impediments. Miner. Processing Extr. Metall. 2004, 113, 2–12. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. A review of current progress of recycling technologies for metals from waste electrical and electronic equipment. J. Clean. Prod. 2016, 127, 19–36. [Google Scholar] [CrossRef]
- Ter-Arakelyan, K.A.; Bagdasaryan, K.A.; Oganyan, A.G.; Mkrtchyan, R.T.; Babayan, G.G. On technological expediency of sodium thiosulphate usage for gold extraction from raw material. Izv. VUZ Tsvetn. Metall. 1984, 5, 72–76. [Google Scholar]
- Abbruzzese, C.; Fornari, P.; Massidda, R.; Vegli, O.F.; Ubaldini, S. Thiosulphate leaching for gold hydrometallurgy. Hydrometallurgy 1995, 39, 265–276. [Google Scholar] [CrossRef]
- Breuer, P.L.; Jeffrey, M.I. Thiosulfate leaching kinetics of gold in the presence of copper and ammonia. Miner. Eng. 2000, 13, 1071–1081. [Google Scholar] [CrossRef]
- Chen, J.; Deng, T.; Zhu, G.C.; Zhao, J. Leaching and recovery of gold in thiosulfate based system: A research summary at ICM. Tran. Indian Inst. Met. 1996, 49, 841–849. [Google Scholar]
- Jeffrey, M.I. Kinetic aspects of gold and silver leaching in ammonia-thiosulfate solutions. Hydrometallurgy 2001, 60, 7–16. [Google Scholar] [CrossRef]
- Zipperian, D.; Raghavan, S.; Wilson, J.P. Gold and silver extraction by ammoniacal thiosulfate leaching from a rhyolite ore. Hydrometallurgy 1988, 19, 361–375. [Google Scholar] [CrossRef]
- Melashvili, M.; Fleming, C.; Dymov, I.; Matthews, D.; Dreisinger, D. Equation for thiosulphate yield during pyrite oxidation. Miner. Eng. 2015, 74, 105–111. [Google Scholar] [CrossRef]
- Breuer, P.L.; Jeffrey, M.I. The reduction of copper(II) and the oxidation of thiosulphate and oxysulphur anions in gold leaching solutions. Hydrometallurgy 2003, 70, 163–173. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J.V. Leaching behaviour of sulphides in ammoniacal thiosulphate systems. Hydrometallurgy 2002, 63, 189–200. [Google Scholar] [CrossRef]
- Grosse, A.C.; Dicinoski, G.W.; Shaw, M.J.; Haddad, P.R. Leaching and recovery of gold using ammoniacal thiosulfate leach liquors (a review). Hydrometallurgy 2003, 69, 1–21. [Google Scholar] [CrossRef]
- Senanayake, G. Analysis of reaction kinetics, speciation and mechanism of gold leaching and thiosulphate oxidation by ammoniacal copper(II) solutions. Hydrometallurgy 2004, 75, 55–75. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Yang, Y.; Li, Q. An eco-friendly and efficient process of low potential thiosulfate leaching-resin adsorption recovery for extracting gold from a roasted gold concentrate. J. Clean. Prod. 2019, 229, 387–398. [Google Scholar] [CrossRef]
- Xu, B.; Li, K.; Zhong, Q.; Li, Q.; Yang, Y.; Jiang, T. Study on the oxygen pressure alkaline leaching of gold with generated thiosulfate from sulfur oxidation. Hydrometallurgy 2018, 177, 178–186. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, J.; Xu, S. Electrochemistry and mechanism of leaching gold with ammoniacal thiosulphate. In Proceedings of the XVIII International Mineral Processing Congress, Sydney, Australia, 23–28 May 1993; pp. 1141–1146. [Google Scholar]
- Yang, Y.; Gao, W.; Xu, B.; Li, Q.; Jiang, T. Study on oxygen pressure thiosulfate leaching of gold without the catalysis of copper and ammonia. Hydrometallurgy 2019, 187, 71–80. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Zhang, X.; Wang, D. Improved thiosulfate leaching of a refractory gold concentrate calcine with additives. Hydrometallurgy 2015, 152, 87–94. [Google Scholar] [CrossRef]
- Jeffrey, M.I.; Watling, K.; Hope, G.A.; Woods, R. Identification of surface species that inhibit and passivate thiosulfate leaching of gold. Miner. Eng. 2008, 21, 443–452. [Google Scholar] [CrossRef]
- Mirza, J.; Smith, S.R.; Baron, J.Y.; Choi, Y.; Lipkowski, J. A SERS characterization of the stability of polythionates at the gold-electrolyte interface. Surf. Sci. 2015, 631, 196–206. [Google Scholar] [CrossRef]
- Jeffrey, M.I.; Hewitt, D.M.; Dai, X.; Brunt, S.D. Ion exchange adsorption and elution for recovering gold thiosulfate from leach solutions. Hydrometallurgy 2010, 100, 136–143. [Google Scholar] [CrossRef]
- Navarro, P.; Vargas, C.; Alonso, M.; Alguacil, F.J. Towards a more environmentally friendly process for gold: Models on gold adsorption onto activated carbon from ammoniacal thiosulfate solutions. Desalination 2007, 211, 58–63. [Google Scholar] [CrossRef]
- Fotoohi, B.; Mercier, L. Recovery of precious metals from ammoniacal thiosulfate solutions by hybrid mesoporous silica: 1-Factors affecting gold adsorption. Sep. Purif. Technol. 2014, 127, 84–96. [Google Scholar] [CrossRef]
- Zhao, J.I.N.; Wu, Z.; Chen, J.C.Y. Separation of gold from other metals in thiosulfate solutions by solvent extraction. Sep. Sci. Technol. 1999, 34, 2061–2068. [Google Scholar] [CrossRef]
- Sullivan, A.M.; Kohl, P.A. Electrochemical study of the gold thiosulfate reduction. J. Electrochem. Soc. 1997, 144, 1686–1690. [Google Scholar] [CrossRef]
- Choo, W.L.; Jeffrey, M.I. An electrochemical study of copper cementation of gold(I) thiosulfate. Hydrometallurgy 2004, 71, 351–362. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Yang, Y.; Li, Q. Recovery of gold from pregnant thiosulfate solutions by the resin adsorption technique. Metals 2017, 7, 555. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.G.; Dreisinger, D.B. The recovery of gold from ammonical thiosulfate solutions containing copper using ion exchange resin columns. Hydrometallurgy 2004, 72, 225–234. [Google Scholar] [CrossRef]
- Xu, B.; Kong, W.; Li, Q.; Yang, Y.; Jiang, T. A review of thiosulfate leaching of gold: Focus on thiosulfate consumption and gold recovery from pregnant solution. Metals 2017, 7, 222. [Google Scholar] [CrossRef]
- Chandra, I.; Jeffrey, M.I. A fundamental study of ferric oxalate for dissolving gold in thiosulfate solutions. Hydrometallurgy 2005, 77, 191–201. [Google Scholar] [CrossRef]
- Heath, J.A.; Jeffrey, M.I.; Zhang, H.G.; Rumball, J.A. Anaerobic thiosulfate leaching: Development of in situ gold leaching systems. Miner. Eng. 2008, 21, 424–433. [Google Scholar] [CrossRef]
- Yu, H.; Zi, F.; Hu, X.; Zhong, J.; Nie, Y.; Xiang, P. The copper-ethanediamine-thiosulphate leaching of gold ore containing limonite with cetyltrimethyl ammonium bromide as the synergist. Hydrometallurgy 2014, 150, 178–183. [Google Scholar] [CrossRef]
- Feng, D.; Deventer, J.S.J. The role of heavy metal ions in gold dissolution in the ammoniacal thiosulphate system. Hydrometallurgy 2002, 64, 231–246. [Google Scholar] [CrossRef]
- Liu, X.; Xu, B.; Jiang, T.; Yang, Y.; Li, Q.; He, Y. Thermodynamic analysis of ammoniacal thiosulphate leaching of gold catalysed by Co(III)/Co(II) using Eh-pH and speciation diagrams. Hydrometallurgy 2018, 178, 240–249. [Google Scholar] [CrossRef]
- Xu, B.; Li, K.; Li, Q.; Yang, Y.; Liu, X.; Jiang, T. Kinetic studies of gold leaching by thiosulfate with cobalt-ammonia catalysis and gold recovery by resin adsorption from its pregnant solution. Sep. Purif. Technol. 2019, 213, 368–377. [Google Scholar] [CrossRef]
- Bard, A.J.; Ketelaar, J.A. Encyclopedia of Electrochemistry of the Elements. J. Electrochem. Soc. 1975, 122, 139C. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Muir, D.M. Thiosulfate leaching of gold—A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Breuer, P.L.; Jeffrey, M.I. An electrochemical study of gold leaching in thiosulfate solutions containing copper and ammonia. Hydrometallurgy 2002, 65, 145–157. [Google Scholar] [CrossRef]
- Zelinsky, A.G.; Novgorodtseva, O.N. EQCM study of the dissolution of gold in thiosulfate solutions. Hydrometallurgy 2013, 138, 79–83. [Google Scholar] [CrossRef]
- Zhang, S.; Nicol, M.J. An electrochemical study of the dissolution of gold in thiosulfate solutions Part I: Alkaline solutions. J. Appl. Electrochem. 2003, 33, 767–775. [Google Scholar] [CrossRef]
- Jiang, T. Chemistry of Extractive Metallurgy of Gold; Hunan Science & Technology Press: Changsha, China, 1998. [Google Scholar]
- Chanut, J.; Lagorce, A.; Lequin, S.; Gougeon, R.D.; Karbowiak, T. Fast manometric method for determining the effective oxygen diffusion coefficient through wine stopper. Polym. Test. 2021, 93, 106924. [Google Scholar] [CrossRef]
- Falina, I.V.; Berezina, N.P. Diffusion of solutions in the course of the matrix synthesis of composite membranes mf-4sc—Polyaniline and their transport properties. Polym. Sci. 2010, 52, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Puente-Siller, D.M.; Fuentes-Aceituno, J.C.; Nava-Alonso, F. A kinetic-thermodynamic study of silver leaching in thiosulfate-copper-ammonia-EDTA solutions. Hydrometallurgy 2013, 134, 124–131. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. Ammoniacal thiosulphate leaching of gold in the presence of pyrite. Hydrometallurgy 2006, 82, 126–132. [Google Scholar] [CrossRef]
- Nava, D.; Ignacio, G.; Dietmar, L.; Ramos-Barrado, J.R. Surface characterization by X-ray photoelectron spectroscopy and cyclic voltammetry of products formed during the potentiostatic reduction of chalcopyrite. Electrochim. Acta 2008, 53, 4889–4899. [Google Scholar] [CrossRef]
- Wagner, C.D. Photoelectron and Auger energies and Auger parameters: A data set. In Practical Surface Analysis: By Auger and X-ray Photoelectron Spectroscopy; Briggs, D., Seah, M.P., Eds.; Wiley: Hoboken, NJ, USA, 1990. [Google Scholar]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Zhang, X.; Li, G. Effect of common associated sulfide minerals on thiosulfate leaching of gold and the role of humic acid additive. Hydrometallurgy 2017, 171, 44–52. [Google Scholar] [CrossRef]
- Liu, X.; Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Zhang, X.; Zhang, Y. Effect of galena on thiosulfate leaching of gold. Hydrometallurgy 2017, 171, 157–164. [Google Scholar] [CrossRef]
- Liu, X. A Fundamental Investigation into Thiosulphate Leaching of Gold Catalysed by the Cobalt-Ammonia System. Ph.D. Thesis, Central South University, Changsha, China, 2019. [Google Scholar]
- Nie, Y.; Yu, Q.; Hu, X.; Zi, F.; Yu, H. The effect of ammonia on the anodic process of gold in copper-free thiosulfate solution. J. Electrochem. Soc. 2016, 163, 123–129. [Google Scholar] [CrossRef]
- Zhang, X.; Senanayake, G.; Nicol, M.J. A study of the gold colloid dissolution kinetics in oxygenated ammoniacal thiosulfate solutions. Hydrometallurgy 2004, 74, 243–257. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Li, Q.; Xu, B.; Yang, Y.; Jiang, T. An Electrochemical Study of Gold Dissolution in Thiosulfate Solution with Cobalt–Ammonia Catalysis. Metals 2022, 12, 317. https://doi.org/10.3390/met12020317
Li K, Li Q, Xu B, Yang Y, Jiang T. An Electrochemical Study of Gold Dissolution in Thiosulfate Solution with Cobalt–Ammonia Catalysis. Metals. 2022; 12(2):317. https://doi.org/10.3390/met12020317
Chicago/Turabian StyleLi, Ke, Qian Li, Bin Xu, Yongbin Yang, and Tao Jiang. 2022. "An Electrochemical Study of Gold Dissolution in Thiosulfate Solution with Cobalt–Ammonia Catalysis" Metals 12, no. 2: 317. https://doi.org/10.3390/met12020317
APA StyleLi, K., Li, Q., Xu, B., Yang, Y., & Jiang, T. (2022). An Electrochemical Study of Gold Dissolution in Thiosulfate Solution with Cobalt–Ammonia Catalysis. Metals, 12(2), 317. https://doi.org/10.3390/met12020317




