First Principles Investigation of the Effects of Chemical Short-Range Ordering Clusters on the Ideal Tensile Strength and Ductility of Aluminum Alloys
Abstract
:1. Introduction
2. Computational Methods and Details
2.1. The First-Principle Calculations and Supercell Model
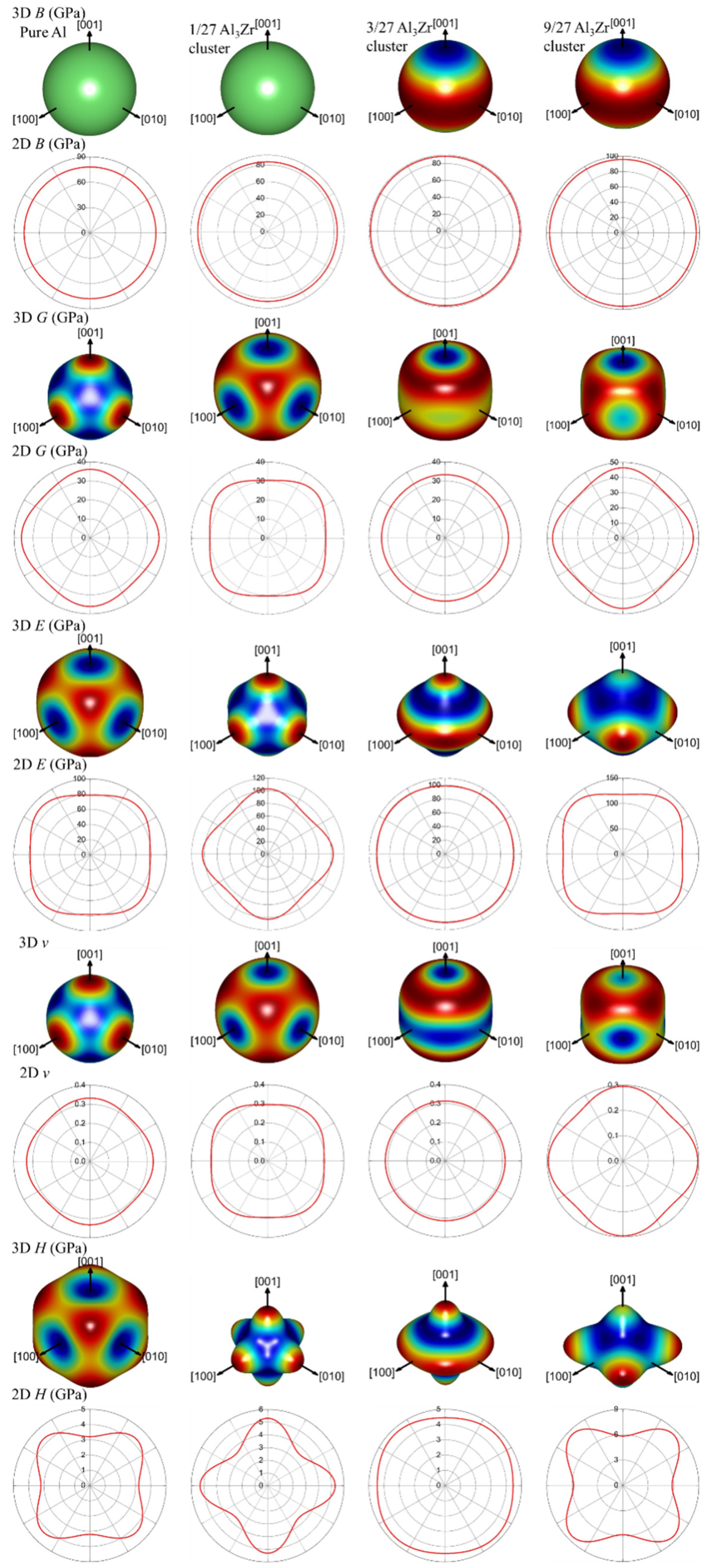
2.2. Elastic Property
2.3. Ideal Tensile Strength
2.4. The Deformation Charge Density
2.5. The Competing Fracture Mechanisms
3. Results and Discussion
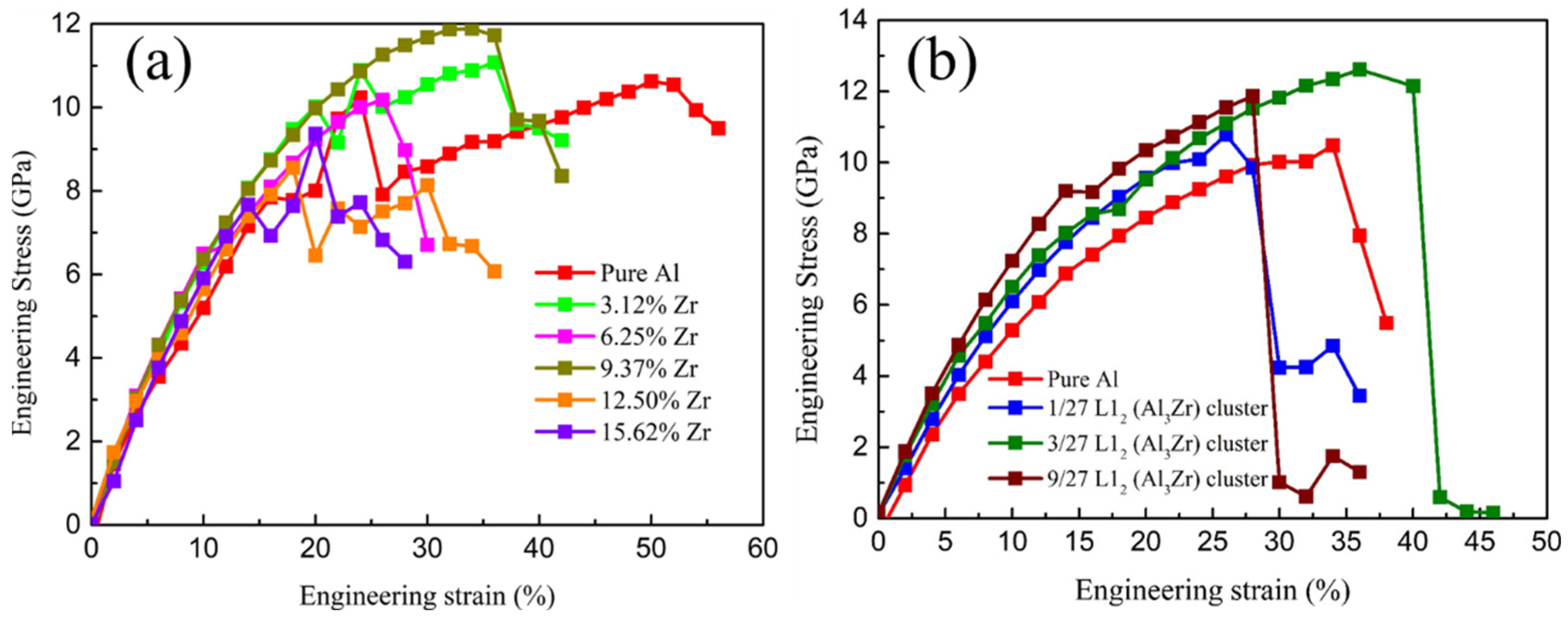
3.1. Ideal Tensile Strength
3.2. The Competing Fracture Mechanisms
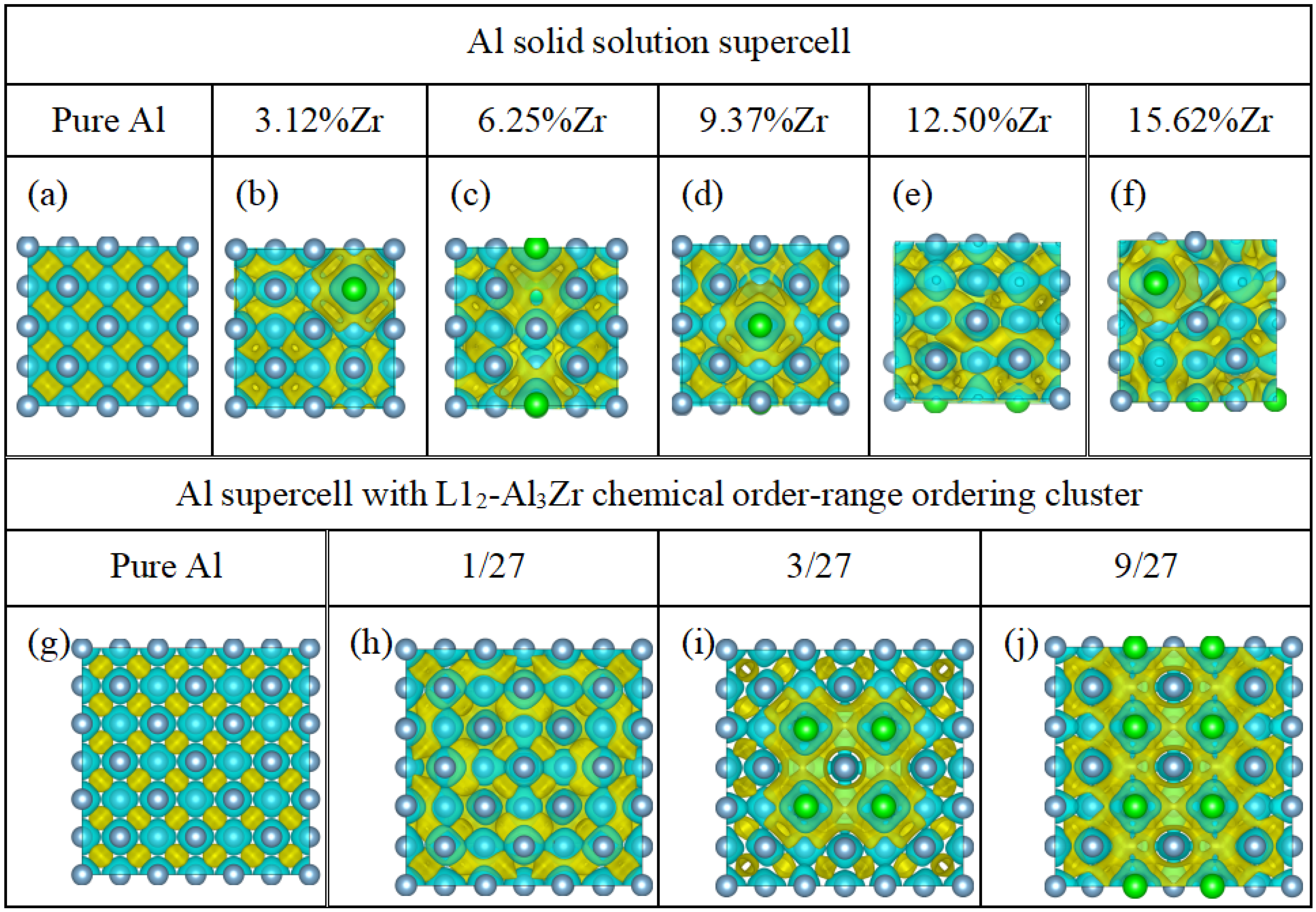
3.3. The Deformation Charge Density
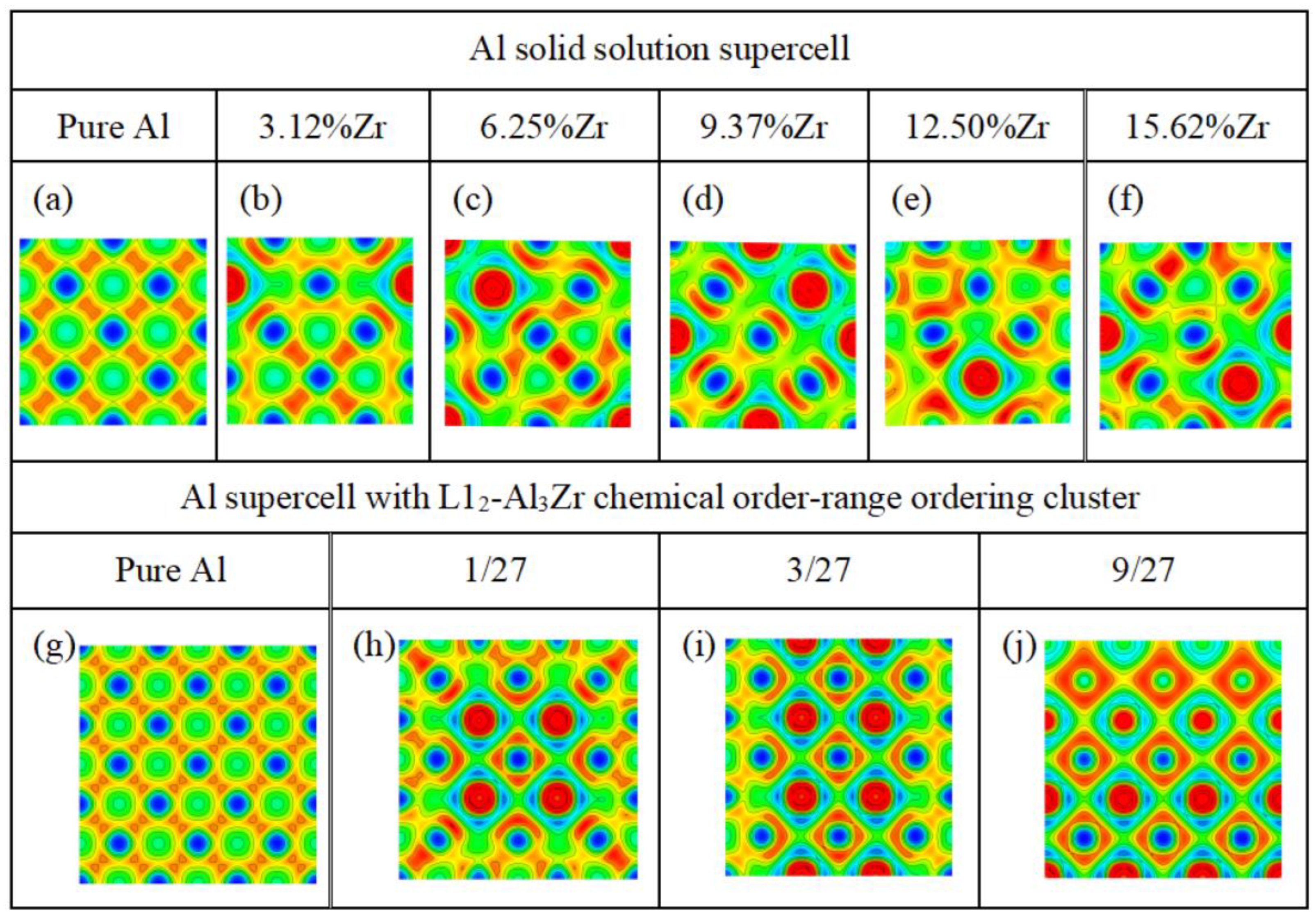
3.4. The Electron Localization Function (ELF)
3.5. The Projected Density of State (PDOS)
4. Conclusions
- (1)
- The solid solutions with varied compositions of the solute atoms Zr and the chemical short-range ordering clusters L12-Al3Zr can improve the strength of the pure Al. In particular, the chemical short-range ordering clusters L12-Al3Zr of the volume fraction of 3/27 can simultaneously improve the strength and ductility of the Al supercell. which is confirmed by the calculated elastic property such as Pugh ratio B/G and computed the ratio (Wsep/Gdisl) of the work of the separation Wsep to the work of dislocation emission Gdisl.
- (2)
- The significant charge accumulations and the electronic interactions between the Al atoms and the nearest Zr atoms bring an excellent ideal tensile strength for the Al supercells with the chemical short-range ordering clusters L12-Al3Zr. In addition, the ratio (Wsep/Gdisl) of the work of the separation Wsep to the work of dislocation emission Gdisl for Al supercells with the chemical short-range ordering clusters L12-Al3Zr of the volume fraction of 3/27 is larger than that of the pure Al supercell. This explains that the ductility of the Al supercell with the chemical short-range ordering clusters L12-Al3Zr is much larger than that of the pure Al supercell.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaufman, J.G.; Rooy, E.L. Aluminum Alloy Castings: Properties, Processes, and Applications; ASM International: Almere, The Netherlands, 2004. [Google Scholar]
- Talamantes-Silva, M.A.; Rodríguez, A.; Talamantes-Silva, J.; Valtierra, S.; Colás, R. Characterization of an Al-Cu cast alloy. Mater. Charact. 2008, 59, 1434–1439. [Google Scholar] [CrossRef]
- Elgallad, E.M.; Zhang, Z.; Chen, X.G. Effect of two-step aging on the mechanical properties of AA2219 DC cast alloy. Mater. Sci. Eng. 2015, 625, 213–220. [Google Scholar] [CrossRef]
- Polmear, I.; John, D.S.; Nie, J.-F.; Qian, M. Physical Metallurgy of Aluminium Alloys. Butterworth-Heinemann 2017, 31–107. [Google Scholar] [CrossRef]
- Mondolfo, L.F. Aluminum Alloys: Structure and Properties; Butterworths: Boston, MA, USA, 1979. [Google Scholar]
- Ilm, A.W. Physical-Metallurgical investigations of aluminum alloys containing magnesium. Metallurgie 1911, 8, 29–96. [Google Scholar]
- Gayle, F.W.; Goodway, M. Precipitation hardening in the first aerospace aluminum alloy: The wright flyer crankcase. Science 1994, 266, 1015–1017. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Wang, Z.; Chen, K.; Wang, Y. The origins of segregation behaviors of solute atoms and their effects on strength of α-Al//θ’-Al2Cu interfaces in Al-Cu alloys. Phys. Chem. Chem. Phys. 2022, 24, 18370–18392. [Google Scholar] [CrossRef]
- Antillon, E.; Woodward, C.; Rao, S.I.; Akdim, B. Chemical short range order strengthening in BCC complex concentrated alloys. Acta Mater. 2021, 215, 117012. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, S.; Ding, J.; Chong, Y.; Jia, T.; Ophus, C.; Asta, M.; Ritchie, R.O.; Minor, A.M. Short-range order and its impact on the CrCoNi medium-entropy alloy. Nature 2020, 581, 283–287. [Google Scholar] [CrossRef]
- Song, Z.Z.; Niu, R.M.; Cui, X.Y.; Bobruk, E.V.; Murashkin, M.; Enikeev, N.A.; Valiev, R.Z.; Ringer, S.P.; Liao, X.Z. Room-temperature-deformation-induced chemical short-range ordering in a supersaturated ultrafine-grained Al-Zn alloy. Scr. Mater. 2022, 210, 114423. [Google Scholar] [CrossRef]
- Pei, R.; KyuWoo, S.; Yi, S.; Al-Samman, T. Effect of solute clusters on plastic instability in magnesium alloys. Mater. Sci. Eng. A 2022, 835, 142685. [Google Scholar] [CrossRef]
- Chen, K.; Chen, X.; Ding, D.; Shi, G.; Wan, Z. Crystallographic features of iron-rich nanoparticles in cast Cu-10Sn-2Zn-1.5Fe-0.5Co alloy. Mater Charact. 2016, 113, 34–42. [Google Scholar] [CrossRef]
- Chen, K.; Chen, X.; Wang, Z.; Mao, H.; Sandström, R. Optimization of deformation properties in as-cast copper by microstructural engineering. Part I. microstructure. J. Alloys Compd. 2018, 763, 592–605. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Wang, Q.; Shih, I.; Xu, J. Fabrication of a nanocomposite from in situ iron nanoparticle reinforced copper alloy. Nanotech 2009, 20, 075605. [Google Scholar] [CrossRef]
- Zhu, S.; Shih, H.; Cui, X.; Yu, C.; Ringer, S.P. Design of solute clustering during thermomechanical processing of AA6016 Al-Mg-Si alloy. Acta Mater. 2021, 203, 116455. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, K.; Bian, J.; Zhang, J.; Peng, Y.; Liu, G.; Deschamps, A.; Sun, J. Solute cluster evolution during deformation and high strain hardening capability in naturally aged Al-Zn-Mg alloy. Acta Mater. 2021, 207, 116682. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, S.; Ophus, C.; Deng, Y.; Vachhani, S.J.; Ozdol, B.; Traylor, R.; Bustillo, K.C.; Morris, J.W., Jr.; Chrzan, D.C.; et al. Direct imaging of short-range order and its impact on deformation in Ti-6Al. Sci. Adv. 2019, 5, 2799. [Google Scholar] [CrossRef] [Green Version]
- Antillona, E.; Woodward, C.; Rao, S.I.; Akdim, B.; Parthasarathy, T.A. Chemical short range order strengthening in a model FCC high entropy alloy. Acta Mater. 2020, 190, 29–42. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Y.; Gault, B.; Makineni, S.K.; Ponge, D.; Raabe, D. (Al, Zn)3Zr dispersoids assisted η′ precipitation in anAl-Zn-Mg-Cu-Zr alloy. Materialia 2020, 10, 100641. [Google Scholar] [CrossRef]
- Liu, S.; Li, K.; Lu, J.; Sha, G.; Wang, J.; Yang, M.; Ji, G.; Song, M.; Wang, J.; Du, Y. On the atomic model of Guinier-Preston zones in Al-Mg-Si-Cu alloys. J. Alloys Compd. 2018, 745, 644–650. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Cheng, Z.; Zhu, M.; Zhou, H.; Jiang, P.; Zhou, L.; Xue, Q.; Yuan, F.; Zhu, J.; et al. Direct observation of chemical short-range order in a medium-entropy alloy. Nature 2021, 592, 712–716. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Q.; Wang, J.; Chen, X.F.; Jiang, P.; Yuan, F.P.; Cheng, Z.Y.; Ma, E.; Wu, X.L. Chemical short-range order in Fe50Mn30Co10Cr10 high-entropy alloy. Acta Mater. 2021, 16, 100139. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Liao, X.Z.; Zhu, Y.T. Enhanced mechanical properties in ultrafine grained 7075 Al alloy. J. Mater. Res. 2005, 20, 288–291. [Google Scholar] [CrossRef]
- Wang, Y.B.; Liao, X.Z.; Zhao, Y.H.; Cooley, J.C.; Horita, Z.; Zhu, Y.T. Elemental separation in nanocrystalline Cu-Al alloys. Appl. Phys. Lett. 2013, 102, 23192. [Google Scholar] [CrossRef] [Green Version]
- Bobruk, E.V.; Sauvage, X.; Enikeev, N.A.; Valiev, R.Z. Influence of fine scale features on room temperature superplastic behaviour of an ultrafine-grained Al-30Zn alloy. Mater. Lett. 2019, 254, 329–331. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, J.; Cui, X.; Zhang, B.; Zhen, L.; Ringer, S.P. Correlation between precipitates evolution and mechanical properties of Al-Sc-Zr alloy with Er additions. J. Mater. Sci. Technol. 2022, 99, 61–72. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmueller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1759. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Zunger, A.; Wei, S.-H.; Ferreira, L.G.; Bernard, J.E. Special quasirandom structures. Phys. Rev. Lett. 1990, 65, 353–356. [Google Scholar] [CrossRef]
- Shin, D.; van de Walle, A.; Wang, Y.; Liu, Z.-K. First-principles study of ternary fcc solution phases from special quasirandom structures. Phys. Rev. B 2007, 76, 144204. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.; Arróyave, R.; Liu, Z.-K.; van de Walle, A. Thermodynamic properties of binary hcp solution phases from special quasirandom structures. Phys. Rev. B 2006, 74, 24204. [Google Scholar] [CrossRef] [Green Version]
- Slater, J.C. Atomic Radii in Crystals. J. Chem. Phys. 1964, 39, 3199. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond, 3rd ed.; Cornell University: Ithaca, NY, USA, 1960. [Google Scholar]
- Gordy, W.; Thomas, W.J.O. Electronegativities of the Elements. J. Chem. Phys. 1956, 24, 439–444. [Google Scholar] [CrossRef]
- Voigt, W. Lehrbuch der kristallphysik; Vieweg+Teubner Verlag: Wiesbaden, Germany, 1928; p. 962. [Google Scholar]
- Reuss, A.; Angew, Z. Berechnung der fließgrenze von mischkristallen auf grund der plastizitätsbedingung für einkristalle. Math. Mech. 1929, 9, 49. [Google Scholar] [CrossRef]
- Hill, R. Proceedings of the Physical Society. Section A 1952, 65, 349. [Google Scholar]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Sun, Y.; Beltz, G.E.; Rice, J.R. Estimates from atomic models of tension-shear coupling in dislocation nucleation from a crack tip. Mater. Sci. Eng. 1993, 170, 67–85. [Google Scholar] [CrossRef]
- Griffith, A.A. The Phenomena of Rupture and Flow in Solids. Philos. Trans. R. Soc. Lond. A 1921, 221, 163–198. [Google Scholar]
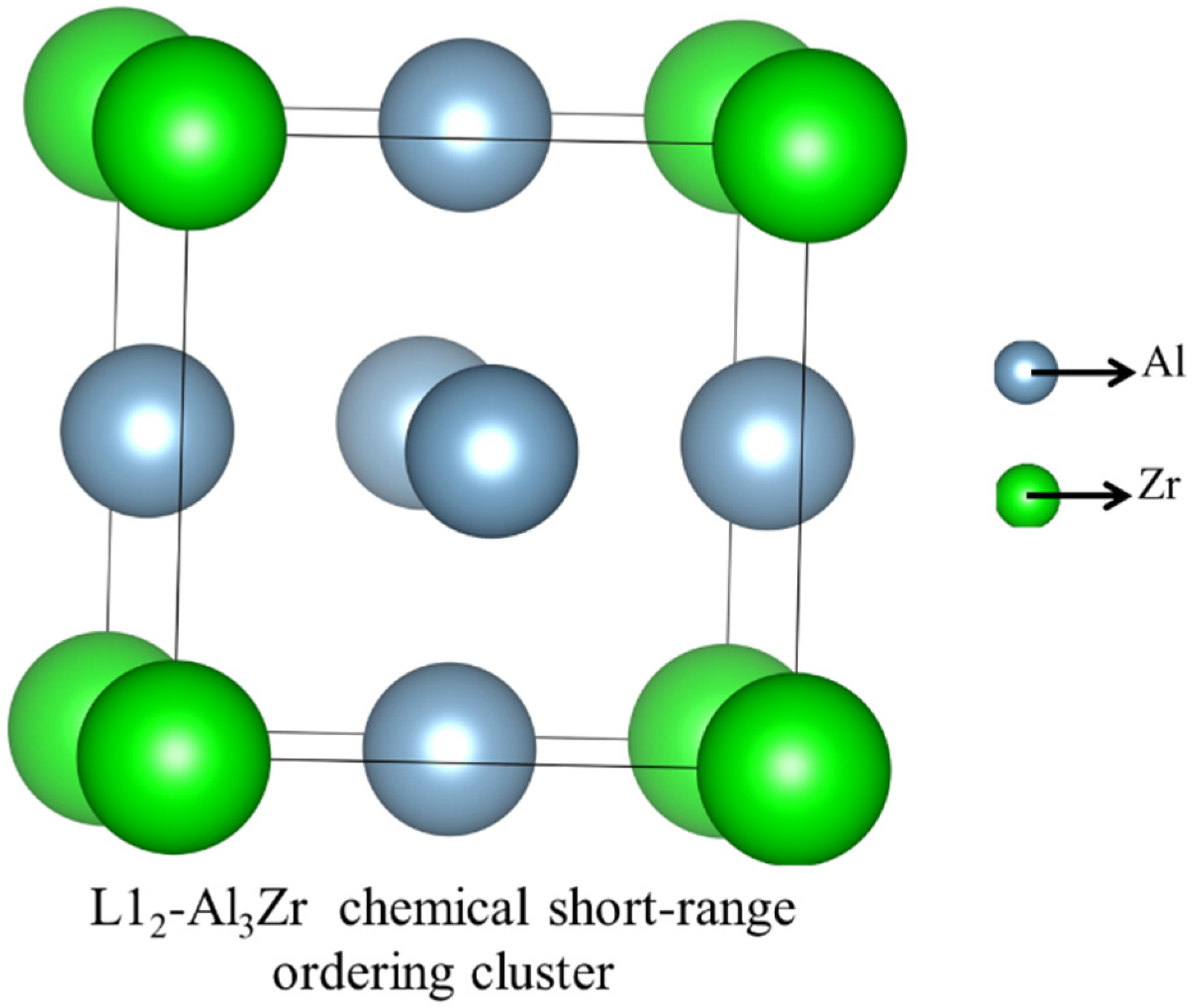
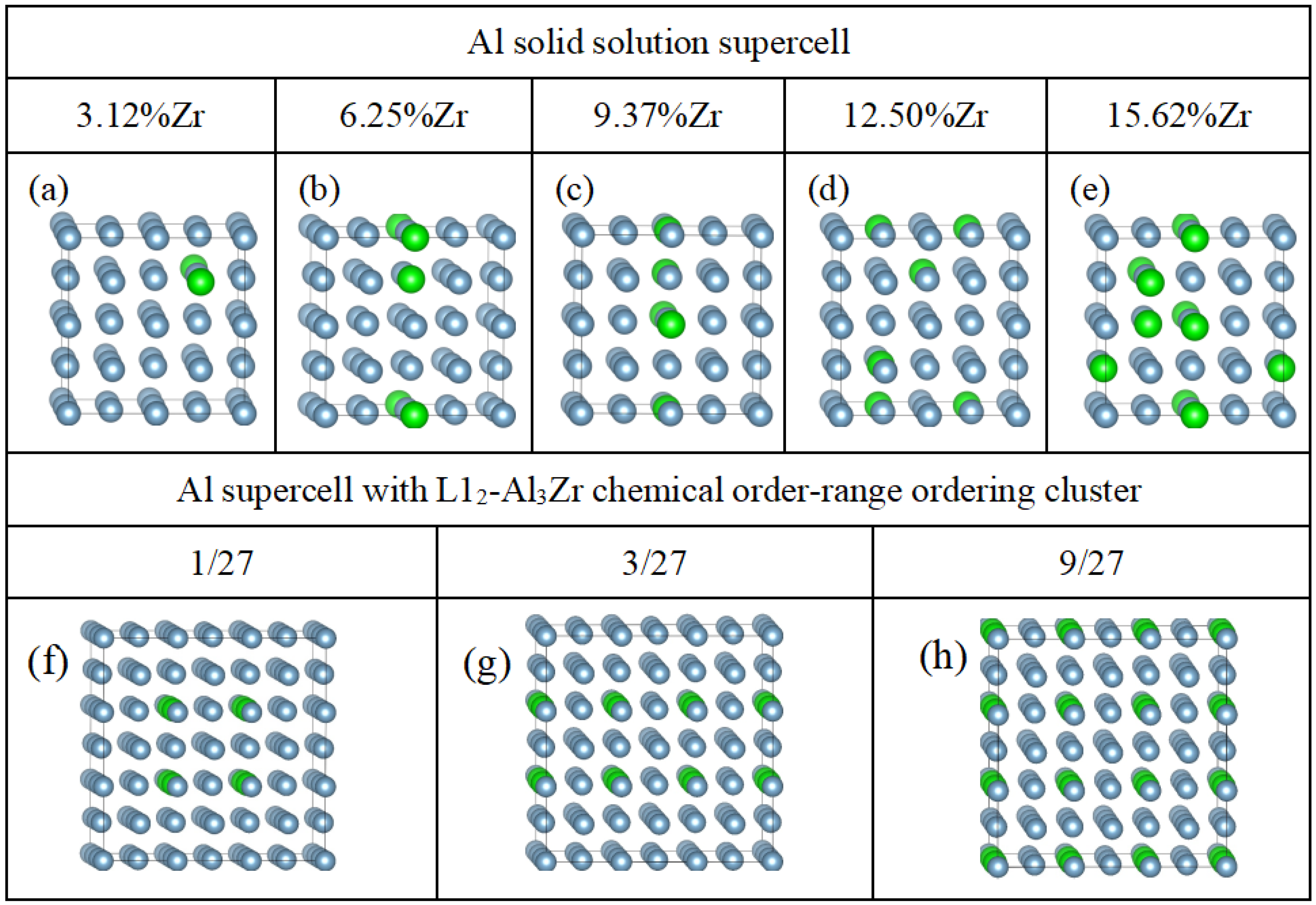



| Zr | Al | |
|---|---|---|
| Size (Å) | 1.60 | 1.25 |
| Size mismatch | 28.00% | – |
| Electronegativity | 1.33 | 1.61 |
| Electronegativity differences | 17.39% | – |
| Supercell | B (GPa) | G (GPa) | E (GPa) | ν | H (GPa) | B/G |
|---|---|---|---|---|---|---|
| Al | 77.964 | 33.283 | 87.411 | 0.313 | 4.180 | 2.340 |
| 1/27Al3Zr | 83.763 | 33.830 | 89.448 | 0.322 | 3.970 | 2.476 |
| 3/27Al3Zr | 87.610 | 34.053 | 90.442 | 0.328 | 3.819 | 2.573 |
| 9/27Al3Zr | 93.568 | 44.370 | 114.942 | 0.295 | 5.774 | 2.109 |
| Supercell | B (GPa) | G (GPa) | E (GPa) | ν |
|---|---|---|---|---|
| Al | 1.000 | 1.228 | 1.197 | 1.592 |
| 1/27Al3Zr | 1.000 | 1.310 | 1.268 | 1.680 |
| 3/27Al3Zr | 1.027 | 1.345 | 1.235 | 1.723 |
| 9/27Al3Zr | 1.073 | 1.548 | 1.355 | 2.226 |
| Solid Solution | Chemical Short-Range Ordering Cluster | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pure Al | 3.12%Zr | 6.25%Zr | 9.37%Zr | 12.50%Zr | 15.62%Zr | Pure Al | 1/27 Al3Zr | 3/27 Al3Zr | 9/27 Al3Zr | |
| Stress (GPa) | 10.63 | 11.08 | 10.18 | 11.88 | 8.56 | 9.37 | 10.48 | 10.78 | 12.15 | 11.87 |
| Strain (%) | 50.00 | 36.00 | 26.00 | 34.00 | 18.00 | 20.00 | 34.00 | 26.00 | 40.00 | 28.00 |
| Al3Zr (1/27) | Al3Zr (3/27) | Pure Al Matrix | |
|---|---|---|---|
| Wsep (J/m2) | 2.76 | 2.82 | 2.50 |
| Gdis (J/m2) | 0.97 | 0.95 | 1.46 |
| Wsep/Gdisl | 2.85 | 2.97 | 1.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Chen, X.; Wang, Z.; Wang, Y.; Chen, K.; Zhu, Y. First Principles Investigation of the Effects of Chemical Short-Range Ordering Clusters on the Ideal Tensile Strength and Ductility of Aluminum Alloys. Metals 2022, 12, 2143. https://doi.org/10.3390/met12122143
Chen X, Chen X, Wang Z, Wang Y, Chen K, Zhu Y. First Principles Investigation of the Effects of Chemical Short-Range Ordering Clusters on the Ideal Tensile Strength and Ductility of Aluminum Alloys. Metals. 2022; 12(12):2143. https://doi.org/10.3390/met12122143
Chicago/Turabian StyleChen, Xiangkai, Xiaohua Chen, Zidong Wang, Yanlin Wang, Kaixuan Chen, and Yuzhi Zhu. 2022. "First Principles Investigation of the Effects of Chemical Short-Range Ordering Clusters on the Ideal Tensile Strength and Ductility of Aluminum Alloys" Metals 12, no. 12: 2143. https://doi.org/10.3390/met12122143
APA StyleChen, X., Chen, X., Wang, Z., Wang, Y., Chen, K., & Zhu, Y. (2022). First Principles Investigation of the Effects of Chemical Short-Range Ordering Clusters on the Ideal Tensile Strength and Ductility of Aluminum Alloys. Metals, 12(12), 2143. https://doi.org/10.3390/met12122143








