Modification of the Surface of 40 Kh Steel by Electrolytic Plasma Hardening
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Belkin, P.N.; Kusmanov, S.A. Plasma electrolytic hardening of steels: Review. Surf. Eng. Appl. Electrochem. 2016, 52, 531–546. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Buranich, V.V.; Satbayeva, Z.A.; Sagdoldina, Z.B.; Kozhanova, R.S.; Pogrebnjak, A.D. The cathodic electrolytic plasma hardening of the 20Cr2Ni4A chromium-nickel steel. J. Mater. Res. Technol. 2020, 9, 6969–6976. [Google Scholar] [CrossRef]
- Pantelis, D.I.; Bouyiouri, E.; Kouloumbi, N.; Vassiliou, P.; Koutsomichalis, A. Wear and corrosion resistance of laser surface hardened structural steel. Surf. Coat. Technol. 2002, 161, 125–134. [Google Scholar] [CrossRef]
- Kulka, M.; Pertek, A. Laser surface modification of carburized and borocarburized 15CrNi6 steel. Mater. Charact. 2007, 58, 461–470. [Google Scholar] [CrossRef]
- Skeeba, V.Y.; Ivancivsky, V.V.; Martyushev, N.V. Peculiarities of High-Energy Induction Heating during Surface Hardening in Hybrid Processing Conditions. Metals 2021, 11, 1354. [Google Scholar] [CrossRef]
- Mazhyn, S.; Laila, Z.; Michael, S. Influence of regimes electrolytic-plasma processing on phase structure and hardening of steel 30CrMnSi. Adv. Mater. Res. 2013, 601, 79–83. [Google Scholar] [CrossRef]
- Ramanathan, T.; Sekar, K.; Shanmugam, N. Microstructural evaluation and effect of heat generation in FSW of AA1100. Chiang Mai J. Sci. 2022, 49, 487–495. [Google Scholar] [CrossRef]
- Hurtado-Delgado, E.; Huerta-Larumbe, L.; Miranda-Pérez, A.; Aguirre-Sánchez, Á. Microcracks reduction in laser hardened layers of ductile iron. Coatings 2021, 11, 368. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Miniyazov, A.Z.; Skakov, M.K.; Sagdoldina, Z.B.; Tulenbergenov, T.R.; Sapataev, E.E. Structural modification and erosion of plasma-irradiated tungsten and molybdenum surfaces. Tech. Phys. 2020, 65, 382–391. [Google Scholar] [CrossRef]
- Dudkina, N.G.; Arisova, V.N. Surface layer of 40Kh steel after electromechanical treatment with dynamic force impact. Izv. Ferr. Metall. 2021, 64, 259–265. [Google Scholar] [CrossRef]
- Zhu, X. Effect of fast cooling on mechanical properties of continuous annealed cold rolled ultra high strength steel. Cailiao Rechuli Xuebao/Trans. Mater. Heat Treat. 2006, 27, 49–52. [Google Scholar]
- Rakhadilov, B.K.; Kozhanova, R.S.; Baizhan, D.; Zhurerova, L.G.; Yerbolatova, G.U.; Kalitova, A.A.; Zhanuzakova, L.N. Influence of plasma electrolytic hardening modes on the structure and properties of 65G steel. Eurasian J. Phys. Funct. Mater. 2021, 5, 209–221. [Google Scholar] [CrossRef]
- Zhurerova, L.G.; Rakhadilov, B.K.; Popova, N.A.; Kylyshkanov, M.K.; Buranich, V.V.; Pogrebnjak, A.D. Effect of the PEN/C surface layer modification on the microstructure, mechanical and tribological properties of the 30CrMnSiA mild-carbon steel. J. Mater. Res. Technol. 2020, 9, 291–300. [Google Scholar] [CrossRef]
- Ayday, A.; Durman, M. Effect of different surface-heat-treatment methods on the surface properties of AISI 4140 steel. Mater. Tehnol. 2014, 48, 787–790. [Google Scholar]
- Rakhadilov, B.; Seitkhanova, A.; Satbayeva, Z.; Yerbolatova, G.; Icheva, Y.; Sagdoldina, Z. Investigation of the structural, mechanical and tribological properties of plasma electrolytic hardened chromium-nickel steel. Lubricants 2021, 9, 11. [Google Scholar] [CrossRef]
- Aravindkumar, D.; Thirumalai, R. Investigations on microstructural characteristics and mechanical properties of 316 L stainless steel welded joints using nickel coated filler material by gas tungsten arc welding. Mater. Res. Express 2021, 8, 046513. [Google Scholar] [CrossRef]
- Kumruoğlu, L.C.; Özel, A. Surface Modification of AISI 4140 Steel Using Electrolytic Plasma Thermocyclic Treatment. Mater. Manuf. Process. 2010, 25, 923–931. [Google Scholar] [CrossRef]
- Tabiyeva, Y.Y.; Rakhadilov, B.K.; Uazyrkhanova, G.K.; Zhurerova, L.G.; Maulit, A.; Baizhan, D. Surface modification of steel mark 2 electrolytic-plasma exposure. Eurasian J. Phys. Funct. Mater. 2019, 3, 355–362. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Kozhanova, R.S.; Kowalewski, P.; Baizhan, D.R.; Sagdoldina, Z.B.; Zhurerova, L.G.; Yerbolatova, G.U. Impact of Volume and Surface Heat Treatment on the Structure and Properties of Steel 30HGSA. Bull. Univ. Karaganda-Phys. 2021, 4, 16–24. [Google Scholar] [CrossRef]
- Rahadilov, B.K.; Zhurerova, L.G.; Sagdoldina, Z.B.; Kenesbekov, A.B.; Bayatanova, L.B. Morphological changes in the dislocation structure of structural steel 20GL after electrolytic-plasma hardening of the surface. J. Surf. Investig. 2021, 15, 408–413. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Satbayeva, Z.A.; Wieleba, W.; Kylyshkanov, M.K.; Baizhan, D.R. Changes in structure and properties of structural chromonickel steels after plasma electrolyte hardening. News Natl. Acad. Sci. Repub. Kazakhstan 2021, 4, 76–82. [Google Scholar] [CrossRef]
- Sagdoldina, Z.; Rakhadilov, B.; Kurbanbekov, S.; Kozhanova, R.; Kengesbekov, A. Effect of Irradiation with Si+ Ions on Phase Transformations in Ti–Al System during Thermal Annealing. Coatings 2021, 11, 205. [Google Scholar] [CrossRef]
- Bayati, M.R.; Molaei, R.; Janghorban, K. Surface alloying of carbon steels from electrolytic plasma. Met. Sci. Heat Treat. 2011, 53, 91–94. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Kengesbekov, A.; Zhurerova, L.; Kozhanova, R.; Sagdoldina, Z. Impact of Electronic Radiation on the Morphology of the Fine Structure of the Surface Layer of R6M5 Steel. Machines 2021, 9, 24. [Google Scholar] [CrossRef]
- Ayday, A.; Kırsever, D.; Demirkıran, A.Ş. The effects of overlapping in electrolytic plasma hardening on wear behavior of carbon steel. Trans. Indian Inst. Met. 2022, 75, 27–33. [Google Scholar] [CrossRef]
- Dayança, A.; Karaca, B.; Kumruoǧlu, L.C. The cathodic electrolytic plasma hardening of steel and cast iron based automotive camshafts. Acta Phys. Pol. A 2017, 131, 374–378. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Tabiyeva, Y.Y.; Uazyrkhanova, G.K.; Zhurerova, L.G.; Baizhan, D. Influence of electrolytic-plasma surface quenching on the structure and strength properties of ferritic-pearlite class wheel steel. Eurasian J. Phys. Funct. Mater. 2020, 4, 167–173. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Baizhan, D. Creation of bioceramic coatings on the surface of Ti–6Al–4V alloy by plasma electrolytic oxidation followed by gas detonation spraying. Coatings 2021, 11, 1433. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Satbayeva, Z.; Baizhan, D. Effect of electrolytic-plasma surface strengthening on the structure and properties of steel 40KhN. In Proceedings of the 28th International Conference on Metallurgy and Materials, METAL, Brno, Czech Republic, 22–24 May 2019; pp. 950–955. [Google Scholar]
- Rakhadilov, B.K.; Baizhan, D.R.; Sagdoldina, Z.B.; Buitkenov, D.B.; Maulet, M. Phase composition and structure of composite Ti/HA coatings synthesized by detonation spraying. AIP Conf. Proc. 2020, 2297, 020022. [Google Scholar] [CrossRef]
- Oliver, W.; Pharr, G. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- ArslanHafeez, M.; Usman, M.; Arshad, M.A.; AdeelUmer, M. Nanoindentation-Based Micro-Mechanical and Electrochemical Properties of Quench-Hardened, Tempered Low-Carbon Steel. Crystals 2020, 10, 508. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Silkin, S.A.; Smirnov, A.A.; Belkin, P.N. Possibilities of increasing wear resistance of steel surface by plasma electrolytic treatment. Wear 2017, 386–387, 239–246. [Google Scholar] [CrossRef]
- Kozlov, E.; Popova, N.; Zhurerova, L.; Nikonenko, E.; Kalashnikov, M.; Skakov, M. Structural and phase transformations in 0.3C-1Cr-1Mn-1Si-fe steel after electrolytic plasma treatment. Pap. Present. AIP Conf. Proc. 2016, 1783, 020112. [Google Scholar] [CrossRef]
- Popova, N.A.; Zhurerova, L.G.; Nikonenko, E.L.; Skakov, M.K. Effect of plasma electrolytic nitrocarburizing on phase composition of 0.3C-1Mn-1Si-fe steel. Inorg. Mater. Appl. Res. 2017, 8, 130–135. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Sun, W.; Zhang, T. Nanoindentation-Induced Pile-Up in the Residual Impression of Crystalline Cu with Different Grain Size. Crystals 2018, 8, 9. [Google Scholar] [CrossRef]
- Li, J.; Cao, Z.; Liu, L.; Liu, X.; Peng, J. Effect of microstructure on hardness and wear properties of 45 steel after induction hardening. Steel Res. Int. 2021, 92, 2000540. [Google Scholar] [CrossRef]
- Wen, E.; Song, R.; Xiong, W. Effect of Tempering Temperature on Microstructures and Wear Behavior of a 500 HB Grade Wear-Resistant Steel. Metals 2019, 9, 45. [Google Scholar] [CrossRef]
- Baizhan, D.; Rakhadilov, B.; Zhurerova, L.; Tyurin, Y.; Sagdoldina, Z.; Adilkanova, M.; Kozhanova, R. Investigation of Changes in the Structural-Phase State and the Efficiency of Hardening of 30CrMnSiA Steel by the Method of Electrolytic Plasma Thermocyclic Surface Treatment. Coatings 2022, 12, 1696. [Google Scholar] [CrossRef]
- Kumruoglu, L.C.; Becerik, D.A.; Ozel, A.; Mimaroglu, A. Surface modification of medium carbon steel by using electrolytic plasma thermocyclic treatment. Mater. Manuf. Process. 2009, 24, 781–785. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Skakov, M.; Miniyzov, A.; Kenesbekov, A. Hydrogen and deuterium storage in tungsten when irradiation with plasma beam. In Proceedings of the 27th International Conference on Metallurgy and Materials, METAL, Brno, Czech Republic, 23–25 May 2018; pp. 1216–1221. [Google Scholar]
- Rakhadilov, B.; Satbayeva, Z.; Ramankulov, S.; Shektibayev, N.; Zhurerova, L.; Popova, N.; Uazyrkhanova, G.; Sagdoldina, Z. Change of 0.34Cr-1Ni-Mo-Fe steel dislocation structure in plasma electrolyte hardening. Materials 2021, 14, 1928. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Stepanova, O.A.; Sagdoldina, Z.B.; Satbayeva, Z.A. Method for Hardening Steel Products. KZ Utility Model Patent No. 4891; Application No. 18.09.2018, 28 April 2020. [Google Scholar]
- Rahadilov, B.K.; Skakov, M.K.; Tulenbergenov, T.R. Tungsten surface erosion by hydrogen plasma irradiation. Key Eng. Mater. 2017, 736, 46–51. [Google Scholar] [CrossRef]
- Mazhyn, S.; Bauyrzhan, R.; Erlan, B.; Michael, S. Change of structure and mechanical properties of R6M5 steel surface layer at electrolytic-plasma nitriding. Adv. Mater. Res. 2014, 1040, 753–758. [Google Scholar] [CrossRef]
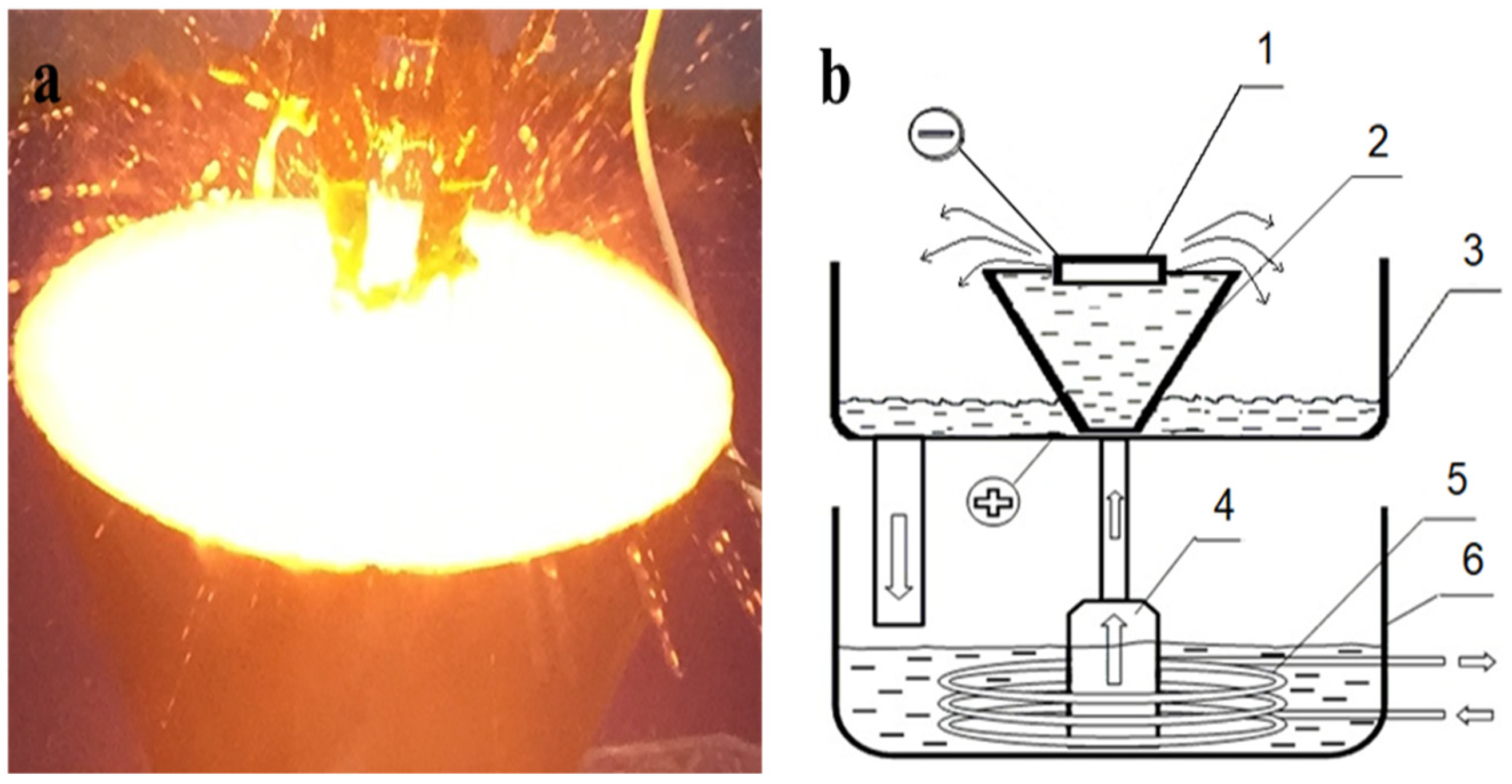
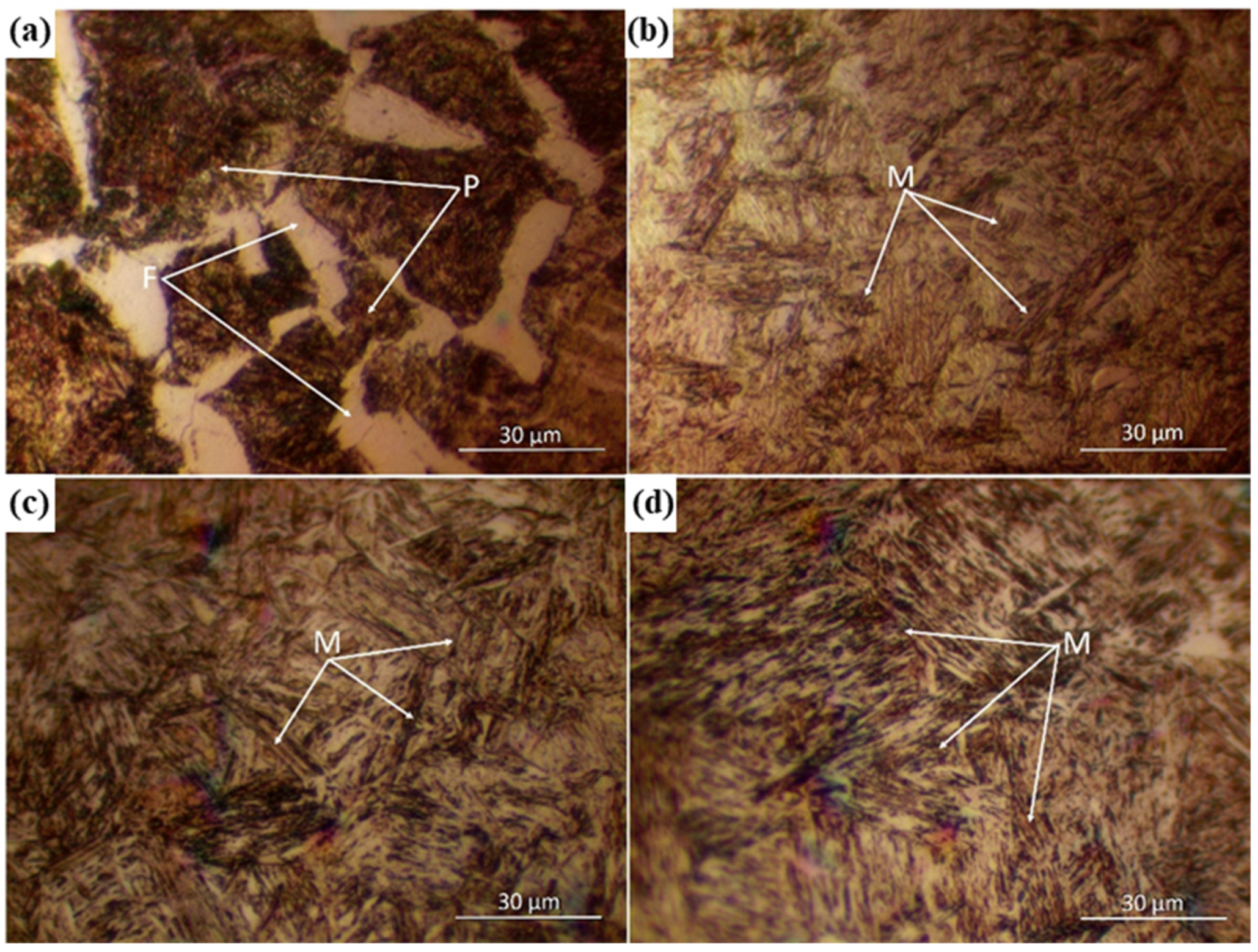
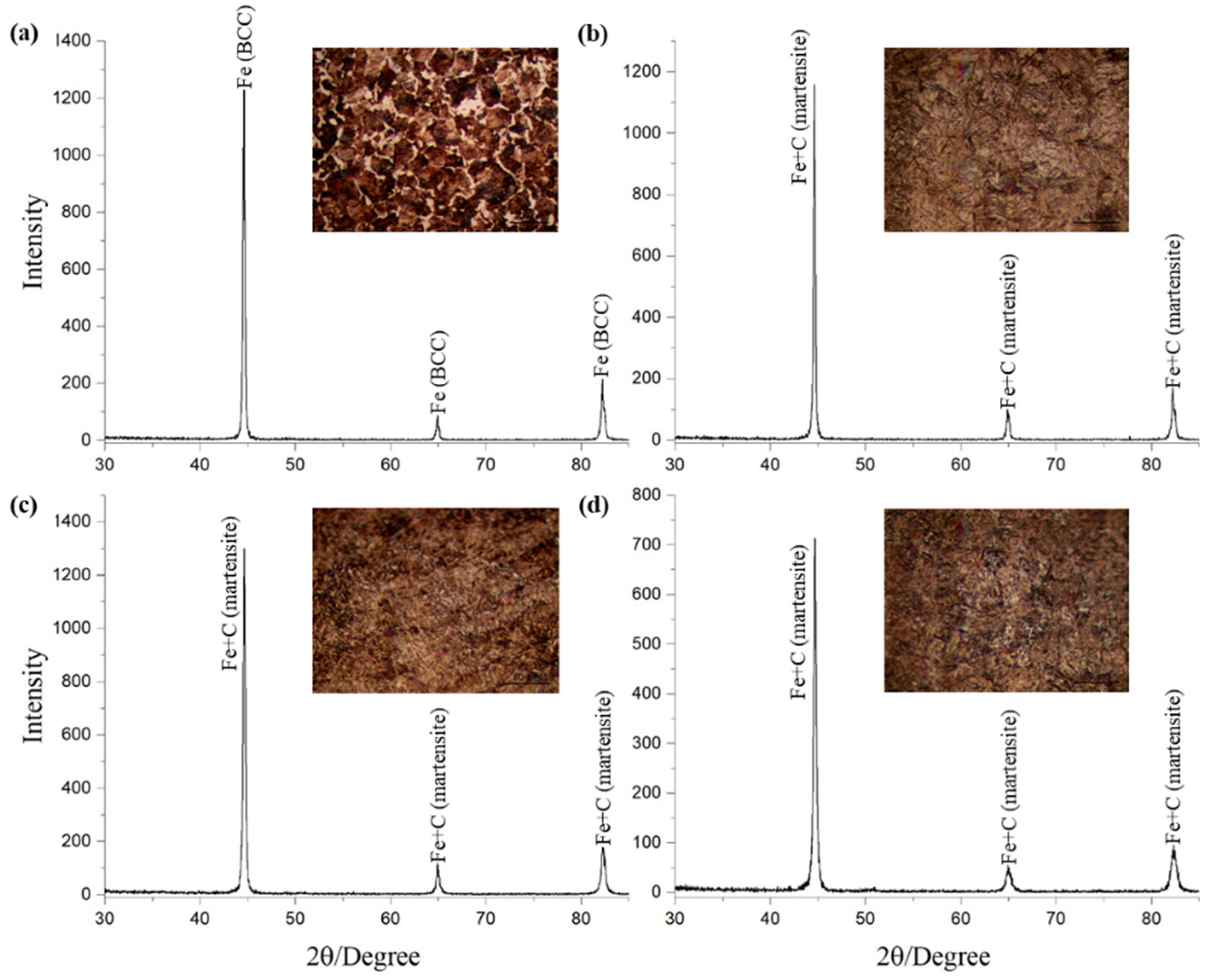
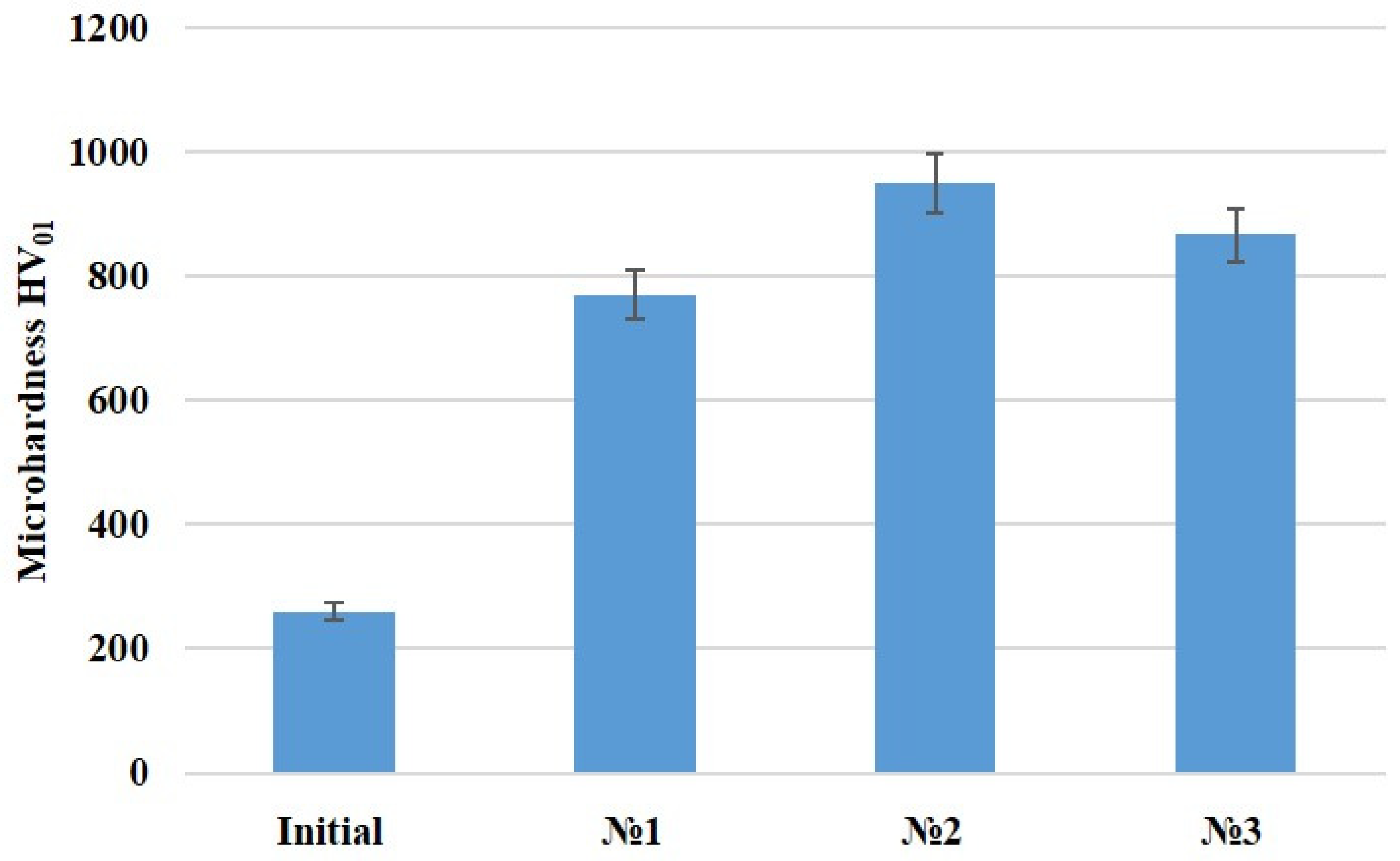


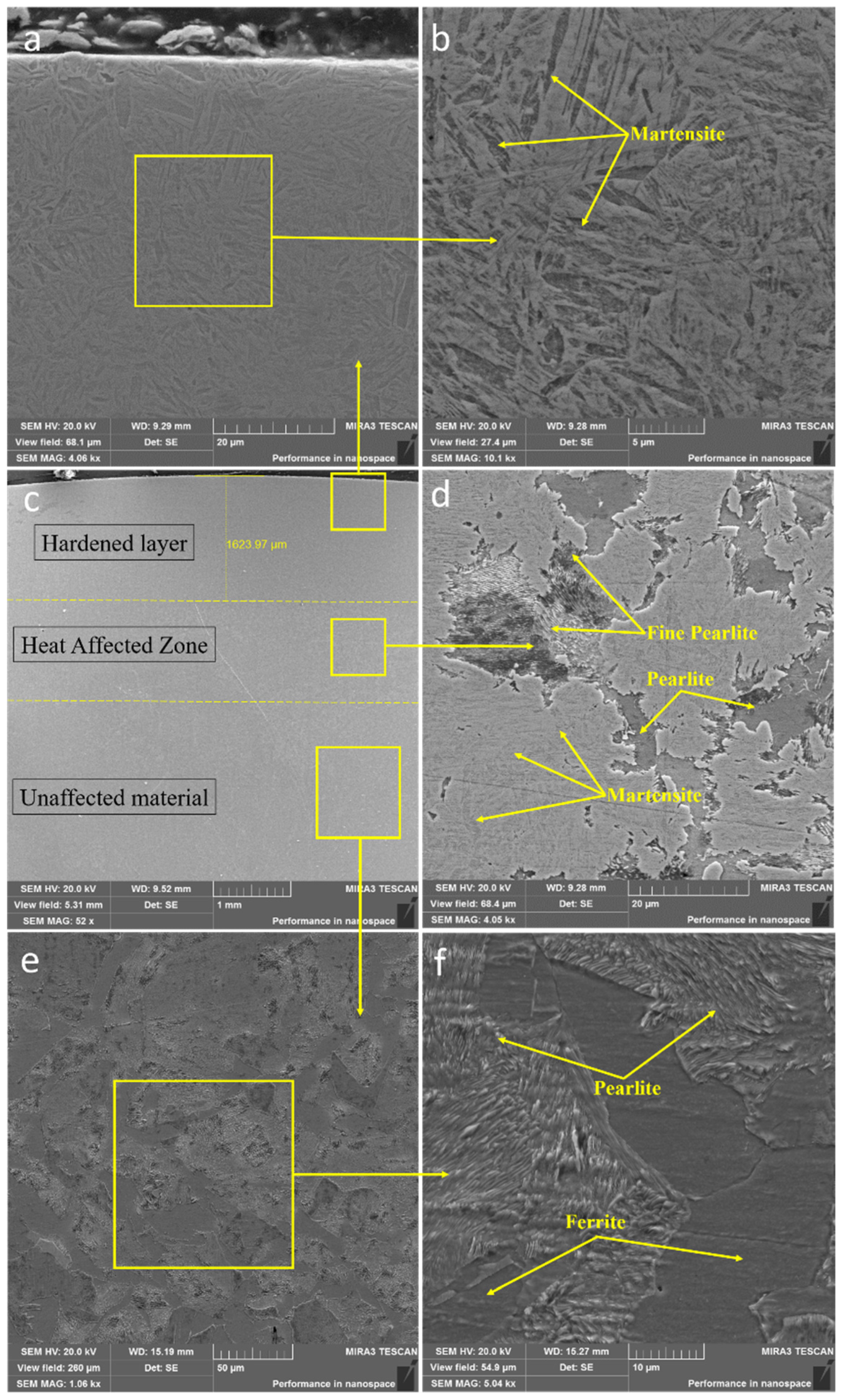
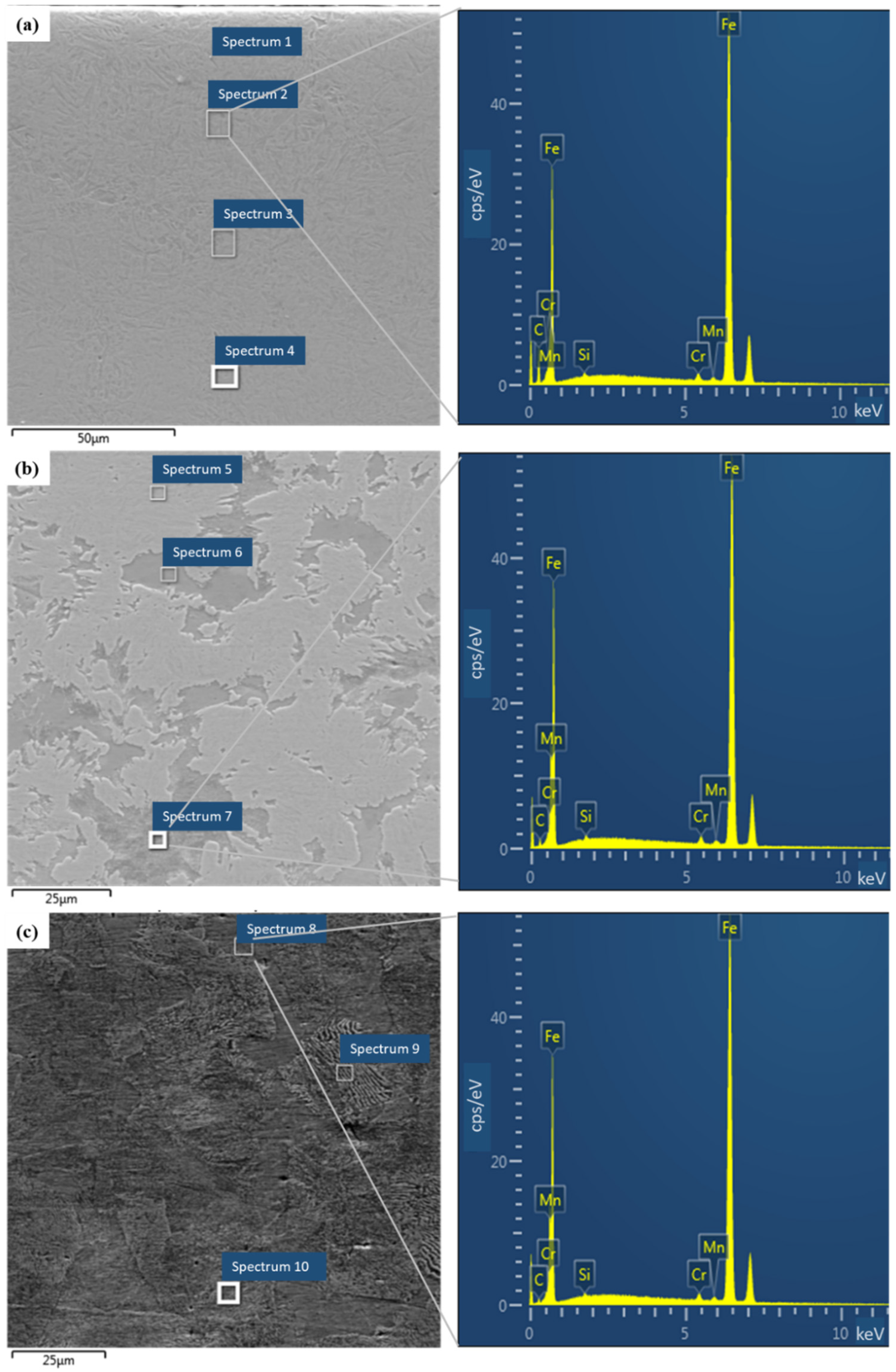

| Cycle | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | U1, V | t1, s | U2, V | t2, s | U3, V | t3, s | U4, V | t4, s | U5, V | t5, s | U6, V | t6, s | U7, V | t7, s |
| No. 1 | 320 | 1 | 50 | 7 | 320 | 1 | - | - | - | - | - | - | - | - |
| No. 2 | 320 | 2 | - | - | - | - | - | - | - | - | - | - | - | - |
| No. 3 | 320 | 1 | 250 | 3 | 50 | 5 | 320 | 1 | 250 | 2 | 50 | 5 | 320 | 1 |
| Mode | Cycle | Thickness of the Hardened Layer, mm |
|---|---|---|
| No. 1 | 3 | 1.1 |
| No. 2 | 1 | 1.6 |
| No. 3 | 7 | 4.7 |
| Spectrum | Fe (Weight %) | Cr (Weight %) | Mn (Weight %) | Si (Weight %) | C (Weight %) |
|---|---|---|---|---|---|
| Spectrum 1 | 97.4 | 1.0 | 0.6 | 0.3 | 0.7 |
| Spectrum 2 | 97.4 | 1.0 | 0.6 | 0.4 | 0.7 |
| Spectrum 3 | 97.3 | 0.9 | 0.8 | 0.3 | 0.6 |
| Spectrum 4 | 97.3 | 1.1 | 0.7 | 0.2 | 0.7 |
| Spectrum 5 | 97.7 | 0.9 | 0.7 | 0.3 | 0.4 |
| Spectrum 6 | 97.6 | 1.0 | 0.6 | 0.3 | 0.5 |
| Spectrum 7 | 97.5 | 1.1 | 0.6 | 0.3 | 0.4 |
| Spectrum 8 | 97.7 | 1.0 | 0.7 | 0.3 | 0.3 |
| Spectrum 9 | 97.8 | 0.9 | 0.7 | 0.3 | 0.3 |
| Spectrum 10 | 97.5 | 1.1 | 0.8 | 0.3 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagdoldina, Z.; Zhurerova, L.; Tyurin, Y.; Baizhan, D.; Kuykabayeba, A.; Abildinova, S.; Kozhanova, R. Modification of the Surface of 40 Kh Steel by Electrolytic Plasma Hardening. Metals 2022, 12, 2071. https://doi.org/10.3390/met12122071
Sagdoldina Z, Zhurerova L, Tyurin Y, Baizhan D, Kuykabayeba A, Abildinova S, Kozhanova R. Modification of the Surface of 40 Kh Steel by Electrolytic Plasma Hardening. Metals. 2022; 12(12):2071. https://doi.org/10.3390/met12122071
Chicago/Turabian StyleSagdoldina, Zhuldyz, Laila Zhurerova, Yuri Tyurin, Daryn Baizhan, Aizhan Kuykabayeba, Saule Abildinova, and Rauan Kozhanova. 2022. "Modification of the Surface of 40 Kh Steel by Electrolytic Plasma Hardening" Metals 12, no. 12: 2071. https://doi.org/10.3390/met12122071
APA StyleSagdoldina, Z., Zhurerova, L., Tyurin, Y., Baizhan, D., Kuykabayeba, A., Abildinova, S., & Kozhanova, R. (2022). Modification of the Surface of 40 Kh Steel by Electrolytic Plasma Hardening. Metals, 12(12), 2071. https://doi.org/10.3390/met12122071






