Mechanism of Layer Formation during Gas Nitriding of Remelted Ledeburitic Surface Layers on Unalloyed Cast Irons
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Parameters of the Surface Treatments
2.2. Characterization Methods
3. Results and Discussion
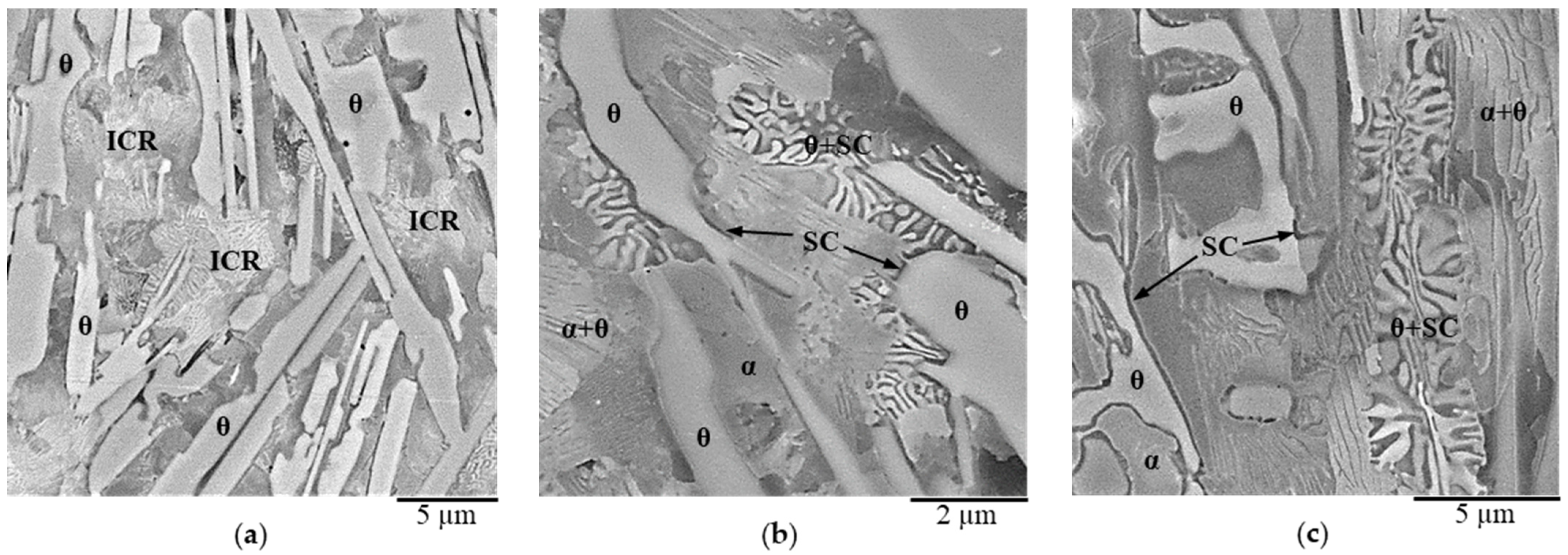
3.1. Characterization of the Initial White-Solidified Microstructures
3.2. Nitriding Mechanism of White-Solidified Microstructures
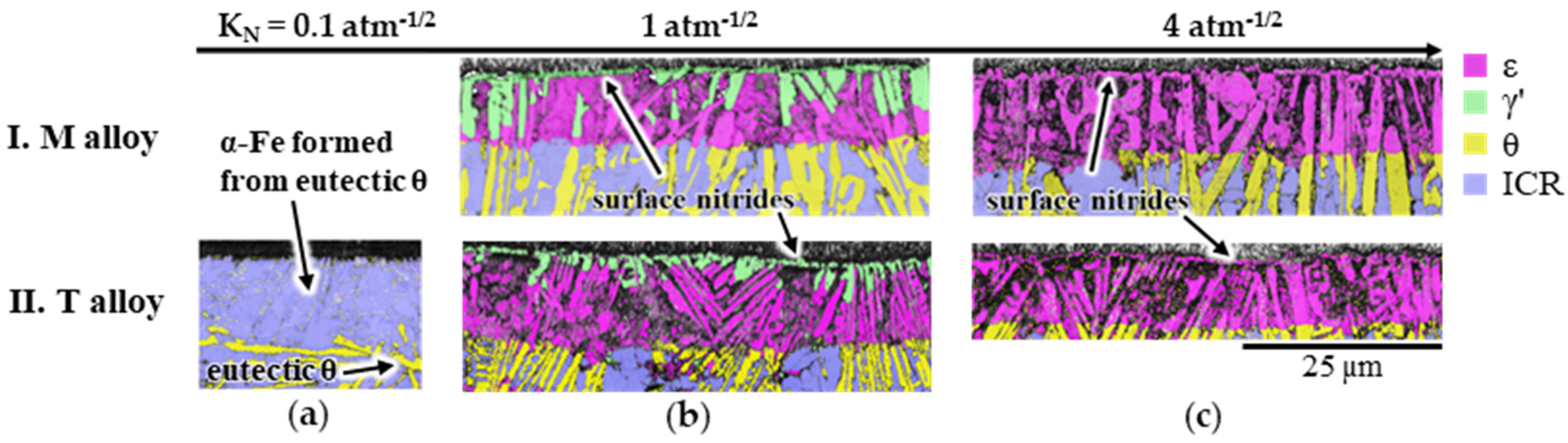
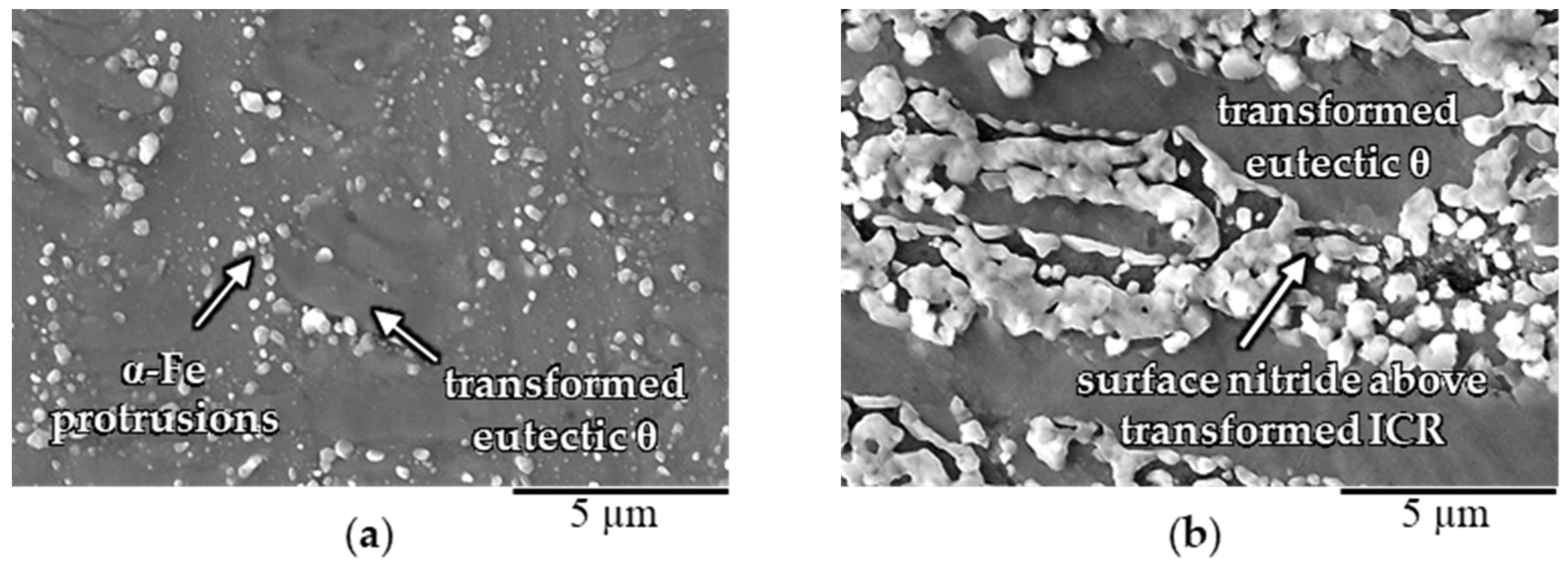
3.2.1. Influence of Silicon (Si)
3.2.2. Influence of Manganese (Mn) and Copper (Cu)
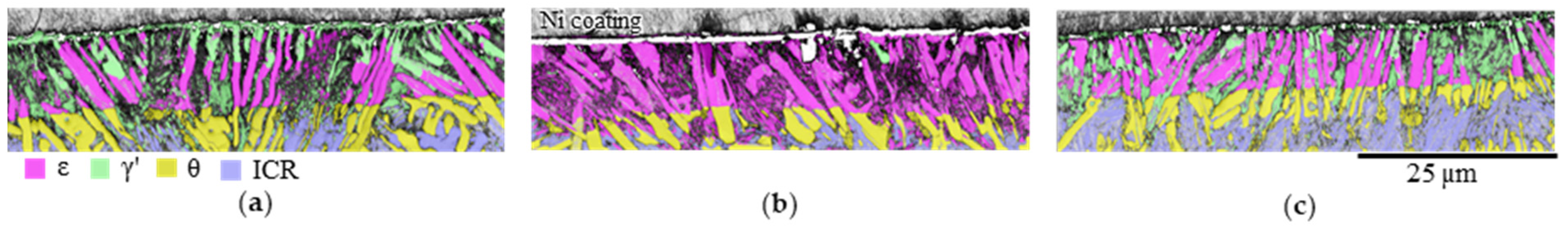
3.2.3. Influence of the Alloy Composition on the Hardness of the Compound Layer
4. Conclusions
- At least initially, Si-free eutectic cementite transformed completely to ε at KN ≥ 1 atm−1/2. At KN = 1 atm−1/2, longer nitriding times and Mn contents of <0.2 wt%, the already formed ε transformed into γ’ in the near-surface region. The hardness of this γ’ nitride formed from Si-free eutectic cementite was lower than that of the ε-nitride by ΔHIT ≈ 1.8 GPa.
- Iron silicocarbide (SC), which typically formed along eutectic cementite plates, decomposed in the presence of nitrogen in the diffusion zone into α-Fe and X (X: Si-rich nitride precipitates). During compound layer growth, the α + X phase mixture formed from the SC transformed into ε and X.
- In the intercarbidic, ferritic and pearlitic regions, silicon was enriched in α-Fe. The addition of nitrogen caused X to precipitate from α-Fe. As a result of the ε-promoting effect of the residual Si still dissolved in the α-Fe, the α-Fe transformed into ε. The lamellar Si-free pearlitic cementite transformed into ε. As a result of the Fe-Si nitrides X, the ε formed in the intercarbidic regions (ICR) exhibited a hardness that was higher by ΔHIT ≈ 1.9 GPa than the hardness of Si- and X-free ε formed from Si-free eutectic cementite.
- During nitriding under the “γ’ condition” (KN = 1 atm−1/2), γ’ was formed on the surface and during nitriding under the “ε condition” (KN = 4 atm−1/2), ε-nitride was formed on the surface.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spies, H.-J. Nitrieren und Nitrocarburieren, Gefüge—Eigenschaften—Kontrolliertes Nitrieren. HTM J. Heat Treatm. Mat. 2013, 68, 86–96. [Google Scholar] [CrossRef]
- Liedtke, D. Wärmebehandlung von Eisenwerkstoffen II, Nitrieren und Nitrocarburieren, 7th ed.; Expert Verlag: Tübingen, Germany, 2018. [Google Scholar]
- Buchwalder, A. Potenziale und Grenzen Kombinierter Randschichtbehandlungstechnologien in Verbindung Mit Einer Fest-/Flüssigphasen-Randschichtbehandlung Mittels Energiereicher Strahlverfahren für Gusseisenwerkstoffe, 1st ed.; Freiberger Forschungshefte, Werkstoffwissenschaft, B390; TU Bergakademie Freiberg: Freiberg, Germany, 2020. [Google Scholar]
- Buchwalder, A.; Spies, H.-J.; Klose, N.; Jung, A.; Zenker, R. Effects of Different Microstructural and Hardness Gradients Generated by Single and Combined Surface Treatments with a Nitriding Top Layer. In Proceedings of the 23rd IFHTSE Congress, Savannah, GA, USA, 18–21 April 2016. [Google Scholar]
- Kante, S.; Leineweber, A.; Holst, A.; Buchwalder, A. Low-temperature Annealing and Graphitizing of Low-alloy White Cast Irons Produced by Rapid Solidification Technique. Materialwiss. Werkstofftech. 2019, 50, 682–695. [Google Scholar] [CrossRef]
- Holst, A.; Buchwalder, A.; Hollmann, P.; Zenker, R. Influence of Cooling Rate on the Microstructural Features of a Remelted White-Solidified Cast Iron Surface and its Effects on Nitriding Behaviour. J. Mater. Process. Technol. 2019, 271, 377–383. [Google Scholar] [CrossRef]
- Kante, S. Microstructure and Nitriding of White-Solidified Fe–C–Si Alloys. Ph.D. Thesis, TU Bergakademie Freiberg, Freiberg, Germany, 2021. [Google Scholar]
- Kante, S.; Motylenko, M.; Leineweber, A. Nitriding of White-Solidified Fe–C–Si Alloys: Diffusion Path Concept Applied to Inhomogeneous Microstructures. Adv. Eng. Mater. 2022, 24, 2100833. [Google Scholar] [CrossRef]
- Meng, F.; Miyamoto, G.; Furuhara, T. Effects of Alloying Elements on Microstructure, Hardness and Growth Rate of Compound Layer in Gaseous-Nitrided Ferritic Alloys. Mater. Trans. 2021, 62, 596–602. [Google Scholar] [CrossRef]
- Leineweber, A.; Fischer, M.; Kante, S.; Martin, S. Effect of Cu on Nitriding of α-Fe. Metals 2022, 12, 619. [Google Scholar] [CrossRef]
- Kante, S.; Leineweber, A. Interaction of N with White-solidified Cast Iron Model Alloys: The Effect of Mn and Cu on the Formation of Fe and Si Nitrides. JCME 2021, 5, 66–70. [Google Scholar] [CrossRef]
- Buchwalder, A.; Zenker, R.; Rüthrich, K.; Nagel, K.; Griesbach, W.; Hartwig, S.; Siedler, J. Eine neue kombinierte Randschichttechnologie für hochbeanspruchte Gusseisenwerkstoffe. HTM J. Heat Treatm. Mat. 2014, 69, 138–147. [Google Scholar] [CrossRef]
- Lehrer, E. Über das Eisen-Wasserstoff-Ammoniak-Gleichgewicht. Z. Elektrochem. Angew. Phys. Chem. 1930, 36, 383–392. [Google Scholar]
- Bachmann, F.; Hielscher, R.; Schaeben, H. Texture Analysis with MTEX—Free and Open Source Software Toolbox. SSP 2010, 160, 63–68. [Google Scholar] [CrossRef]
- Krakow, R.; Bennett, R.J.; Johnstone, D.N.; Vukmanovic, Z.; Solano-Alvarez, W.; Lainé, S.J.; Einsle, J.F.; Midgley, P.A.; Rae, C.M.F.; Hielscher, R. On three-dimensional misorientation spaces. Proc. R. Soc. A 2017, 473, 20170274. [Google Scholar] [CrossRef] [PubMed]
- Kante, S.; Kürnsteiner, P.; Motylenko, M.; Gault, B.; Leineweber, A. Eutectoid growth of nanoscale amorphous Fe-Si nitride upon nitriding. Acta Mater. 2021, 209, 116774. [Google Scholar] [CrossRef]
- Kante, S.; Leineweber, A. The iron silicocarbide in cast irons revisited. J. Alloys Compd. 2020, 815, 152468. [Google Scholar] [CrossRef]
- Spinat, P.; Senateur, J.P.; Fruchart, R.; Herpin, P. Caractérisation des deux phases isotypes «Mn8Si2C» et «Fe8Si2C». C. R. Acad. Sci. Paris Sér. C 1972, 274, 1159–1162. [Google Scholar]
- Marles, D. The Carbides in Iron-Carbon-Silicon Alloys and Cast Irons. J. Iron Steel Inst. Lond. 1948, 158, 433–436. [Google Scholar]
- Malinochka, Y.N.; Osada, N.G.; Koval’chuk, G.Z. A new structural constituent in Fe-C-Si alloys. Met. Sci. Heat Treat. 1961, 3, 299–305. [Google Scholar] [CrossRef]
- Kagawa, A.; Okamoto, T. Coefficients for equilibrium partition of a third element between solid and liquid in iron-carbon base ternary alloys and their relation to graphitization during iron-carbon eutectic solidification. J. Mater. Sci. 1984, 19, 2306–2318. [Google Scholar] [CrossRef]
- Ande, C.K.; Sluiter, M.H.F. First-principles prediction of partitioning of alloying elements between cementite and ferrite. Acta Mater. 2010, 58, 6276–6281. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Cementite. Int. Mater. Rev. 2020, 65, 1–27. [Google Scholar] [CrossRef]
- Al-Salman, S.A.; Lorimer, G.W.; Ridley, N. Partitioning of silicon during pearlite growth in a eutectoid steel. Acta Metall. 1979, 27, 1391–1400. [Google Scholar] [CrossRef]
- Spinat, P.; Brouty, C.; Whuler, A.; Herpin, P. Etude Structurale de la Phase ‘Mn8Si2C’. Acta Crystallogr. 1975, B31, 541–547. [Google Scholar] [CrossRef]
- Steiner, T.; Mittemeijer, E.J. Alloying Element Nitride Development in Ferritic Fe-Based Materials Upon Nitriding: A Review. J. Mater. Eng. Perform. 2016, 25, 2091–2102. [Google Scholar] [CrossRef]
- Mittemeijer, E.J.; Biglari, M.H.; Böttger, A.J.; van der Pers, N.M.; Sloof, W.G.; Tichelaar, F.D. Amorphous precipitates in a crystalline matrix; precipitation of amorphous Si3N4 in α-Fe. Scr. Mater. 1999, 41, 625–630. [Google Scholar] [CrossRef]
- van Landeghem, H.P.; Gouné, M.; Bordère, S.; Danoix, F.; Redjaïmia, A. Competitive precipitation of amorphous and crystalline silicon nitride in ferrite: Interaction between structure, morphology, and stress relaxation. Acta Mater. 2015, 93, 218–234. [Google Scholar] [CrossRef]
- Meka, S.R.; Jung, K.S.; Bischoff, E.; Mittemeijer, E.J. Unusual precipitation of amorphous silicon nitride upon nitriding Fe–2at.%Si alloy. Philos. Mag. 2012, 92, 1435–1455. [Google Scholar] [CrossRef]
- Meka, S.R.; Mittemeijer, E.J. Abnormal nitride morphologies upon nitriding iron-based substrates. JOM 2013, 65, 769–775. [Google Scholar] [CrossRef]
- Holst, A.; Buchwalder, A.; Zenker, R. Influence of gas nitriding conditions on layer structure formation on grey- and white-solidified cast irons (part I). Metall. Ital. 2020, 112, 28–39. [Google Scholar]
- Kante, S.; Leineweber, A. Two-phase and three-phase crystallographic relationships in white-solidified and nitrided Fe-C-Si cast iron. Acta Mater. 2019, 170, 240–252. [Google Scholar] [CrossRef]
- Meka, S.R.; Schubert, A.; Bischoff, E.; Mittemeijer, E.J. Unusual Iron Nitride Formation Upon Nitriding Fe-Si Alloy. Metall. Mater. Trans. A 2020, 51, 3154–3166. [Google Scholar] [CrossRef]
- Kante, S.; Leineweber, A. Fe Nitride Formation in Fe–Si Alloys: Crystallographic and Thermodynamic Aspects. Metall. Mater. Trans. A 2021, 52, 4957–4973. [Google Scholar] [CrossRef]
- Spies, H.-J.; Biermann, H.; Fischer, A. Nitriding behaviour of the intermetallic alloy FeAl. Z. Metallkd. 2005, 96, 781–786. [Google Scholar] [CrossRef]
- Kodentsov, A.A.; van Dal, M.J.H.; Cserháti, C.; Daróczi, L.; van Loo, F.J.J. Permeation of Nitrogen in Solid Nickel and Deformation Phenomena Accompanying Internal Nitridation. Acta Mater. 1999, 47, 3169–3180. [Google Scholar] [CrossRef]
- Klemm-Toole, J.; Burnett, M.; Clarke, A.J.; Speer, J.G.; Findley, K.O. Influences of Vanadium and Silicon on Case Hardness and Residual Stress of Nitrided Medium Carbon Steels. Metall. Mater. Trans. A 2021, 52, 462–482. [Google Scholar] [CrossRef]






| Designation | C | Si | Mn | Cu | P | S | Mg |
|---|---|---|---|---|---|---|---|
| T-2.3Si-0.1Mn-0Cu | 3.72 | 2.32 | 0.12 | - | 0.016 | 0.009 | 0.04 |
| T-2.5Si-0.2Mn-0.1Cu | 3.44 | 2.52 | 0.15 | 0.06 | 0.011 | 0.009 | - |
| T-2.2Si-0.1Mn-0.7Cu | 3.51 | 2.18 | 0.14 | 0.73 | 0.014 | 0.009 | 0.03 |
| T-2.3Si-0.3Mn-0.7Cu | 3.55 | 2.32 | 0.34 | 0.65 | 0.024 | 0.008 | 0.04 |
| T-1.8Si-0.6Mn-0.7Cu 1 | 3.37 | 1.80 | 0.59 | 0.71 | 0.043 | 0.008 | - |
| M-3.5C | 3.57 | <0.01 | - | - | - | - | - |
| M-3.5C-1.5Si | 3.61 | 1.6 | - | - | - | - | - |
| M-3.5C-3Si | 3.60 | 3.0 | - | - | - | - | - |
| M-3.5C-3Si-1Mn | 3.61 | 2.65 | 0.86 | - | - | - | - |
| M-3.5C-3Si-1Cu | 3.58 | 2.98 | - | 0.87 | - | - | - |
| M-3.5C-3Si-1Mn-1Cu | 3.47 | 2.66 | 0.96 | 0.99 | - | - | - |
| Phase | Space Group | Typical Lattice Parameters |
|---|---|---|
| α-Fe | Imm | a = 2.87 Å |
| γ′-Fe4(N, C) | Pmm | a = 3.79 Å |
| ε-Fe3(N, C)1+x | P6322 | a = 4.72 Å, c = 4.42 Å |
| θ-Fe3C1−z | Pnma | a = 5.09 Å, b = 6.74 Å, c = 4.53 Å |
| KN [atm−1/2] | 1 | 4 | |||||
|---|---|---|---|---|---|---|---|
| MicrostructureAlloy | Cementite | ICR | Surface | Cementite | ICR | Surface | |
| Alloy | |||||||
| T-2.3Si-0.1Mn-0Cu | γ′/ε | ε | γ′ | ε | ε | ε | |
| T-2.5Si-0.2Mn-0.1Cu | γ′/ε | (γ′+) ε | γ′ | ε | ε | ε | |
| T-2.2Si-0.1Mn-0.7Cu | γ′/ε | γ′ | γ′ | ε | γ′ (+ε) | ε | |
| T-1.8Si-0.6Mn-0.7Cu | (γ′)/ε | γ′ | γ′ | ε | γ′ (+ε) | ε | |
| M-3.5C-3Si | γ′/ε | ε | γ′ | ε | ε | ε | |
| M-3.5C-3Si-1Mn | ε | ε | γ′ | - | - | - | |
| M-3.5C-3Si-1Cu | γ′/ε | γ′ | γ′ | - | - | - | |
| M-3.5C-3Si-1Mn-1Cu | (γ′)/ε | γ′ | γ′ | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holst, A.; Kante, S.; Leineweber, A.; Buchwalder, A. Mechanism of Layer Formation during Gas Nitriding of Remelted Ledeburitic Surface Layers on Unalloyed Cast Irons. Metals 2023, 13, 156. https://doi.org/10.3390/met13010156
Holst A, Kante S, Leineweber A, Buchwalder A. Mechanism of Layer Formation during Gas Nitriding of Remelted Ledeburitic Surface Layers on Unalloyed Cast Irons. Metals. 2023; 13(1):156. https://doi.org/10.3390/met13010156
Chicago/Turabian StyleHolst, Anja, Stefan Kante, Andreas Leineweber, and Anja Buchwalder. 2023. "Mechanism of Layer Formation during Gas Nitriding of Remelted Ledeburitic Surface Layers on Unalloyed Cast Irons" Metals 13, no. 1: 156. https://doi.org/10.3390/met13010156
APA StyleHolst, A., Kante, S., Leineweber, A., & Buchwalder, A. (2023). Mechanism of Layer Formation during Gas Nitriding of Remelted Ledeburitic Surface Layers on Unalloyed Cast Irons. Metals, 13(1), 156. https://doi.org/10.3390/met13010156






