Impact of Copper, Tin and Titanium Addition on Bending-Induced Damage of Intermetallic Phases in Hot Dip Galvanizing
Abstract
:1. Introduction
- a barrier effect that physically separates the metal material from the aggressive environment; [2]
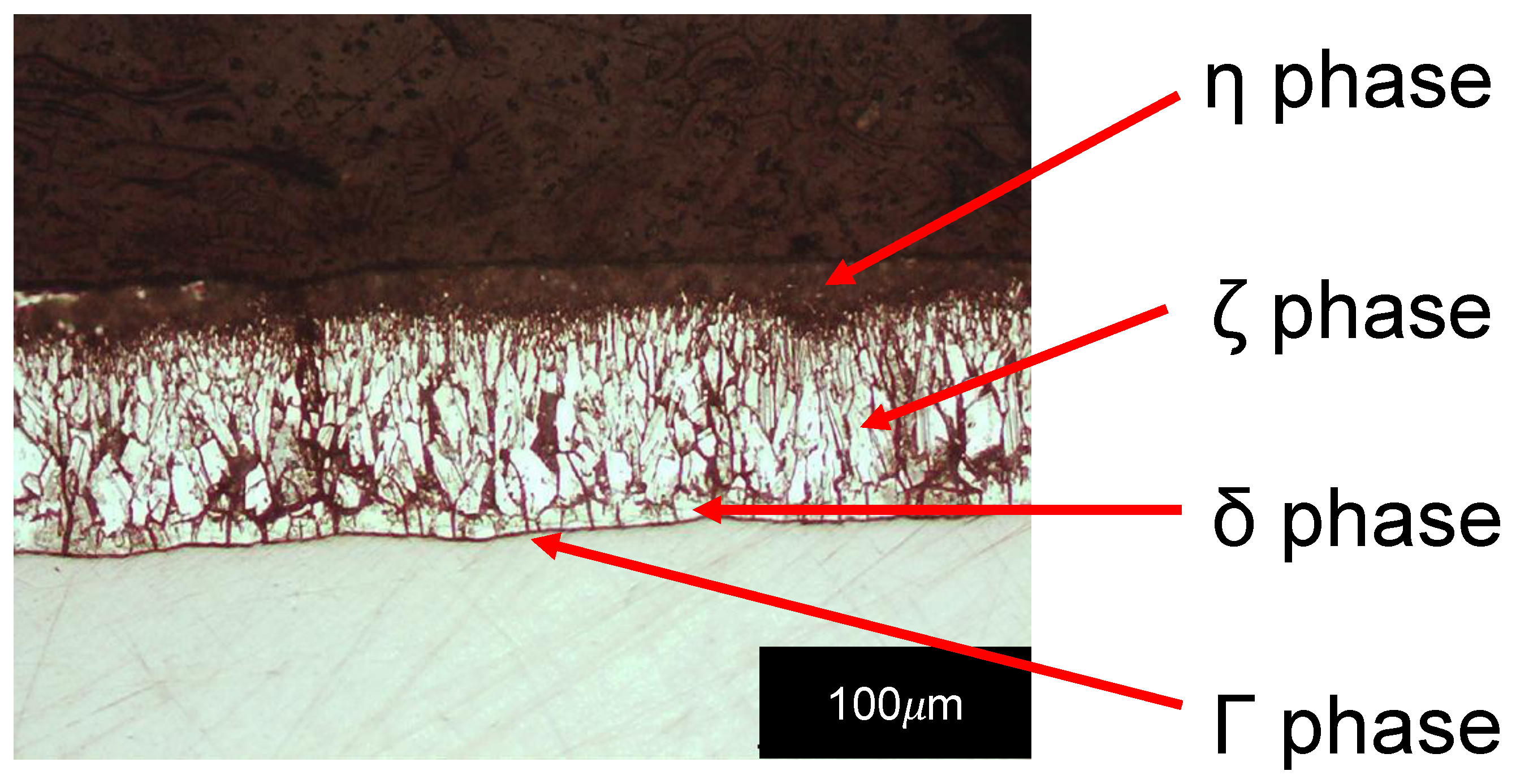
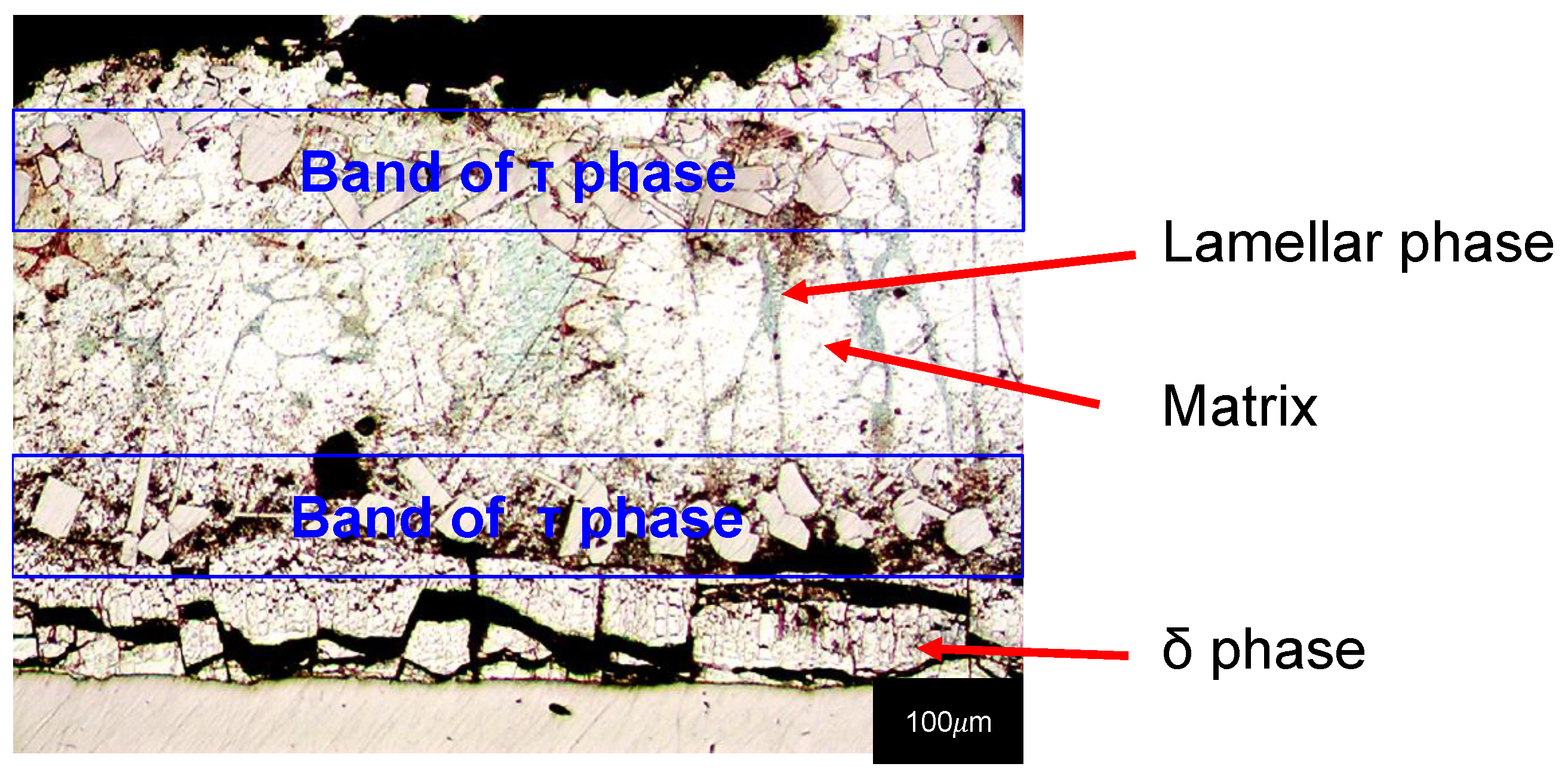
- The phase in contact with the substrate to be protected is the Γ phase, which includes all the phases with an iron content between 17% and 28% by weight. It is characterized by a high degree of brittleness and its depth can be occasionally neglected in comparison to the depths of the other ones.
- The phase immediately external to phase Γ is phase δ, which presents an iron quantity between 7% and 11.5% by weight. It has a compact morphology, it is characterized by a brittle mechanical behaviour and by a hardness higher than that of the steel used as a support to be protected, which generally consists of low carbon steels.
- The phase in contact with the δ phase is the ζ phase and is characterized by an iron amount between 5% and 6% by weight. Usually, its morphology is columnar due to its growth by directional diffusion in the solid phase of iron atoms from the δ phase and zinc atoms from the molten bath. With prolonged stays at high temperatures (e.g., in 460 °C baths for periods of more than 6 min), it can collapse into an undirected microstructure.
- The η phase is present in the outermost part of the coating and, therefore, constitutes its surface. The iron content of this phase is very low; in fact, its chemical composition is comparable with that of the galvanizing bath because it is generated by the solidification of the molten zinc layer obtained by wettability during extraction from the bath. Compared to the other intermetallic phases, its hardness is quite low and its toughness is higher due to the reduced iron content.
2. Materials and Methods
- Pure zinc,
- Zinc with 3% by weight of tin,
- Zinc with 0.5% by weight of copper,
- Zinc with 0.5% by weight of titanium,
- Zinc with 5% by weight of aluminium.
3. Results
- The phase δ is the closest one to the surface of the steel specimen in all specimens, except for Figure 9c were a poorly visible Γ phase is also present. The metallography of Figure 9c shows that δ phase is affected by radial cracks as a result of the variable thermal expansion during cooling, but there are other radial cracks due to the bending tests also in the baths containing tin and copper. All the cracks present in δ go through the phase, propagating in the ζ phase or stopping at the δ-ζ interface.
- The ζ phases are all with a well-developed columnar morphology, in all specimens, even those obtained at high immersion times. Radial cracks are also present in this phase, but they all come from the δ phase. These cracks go through the ζ phase or stop at the ζ-η interface. For this reason, the ζ phase is less fragile than the δ phase.
- Outside, the η phase can be observed in all images, which is even more ductile than the ζ phase as it is not crossed by any crack, as well explained by other authors [1].
4. Conclusions
- The production of the coating phases is significantly influenced by the chemical composition of the galvanizing bath, where in addition to the iron-rich phase, a new phase rich in iron and titanium is formed.
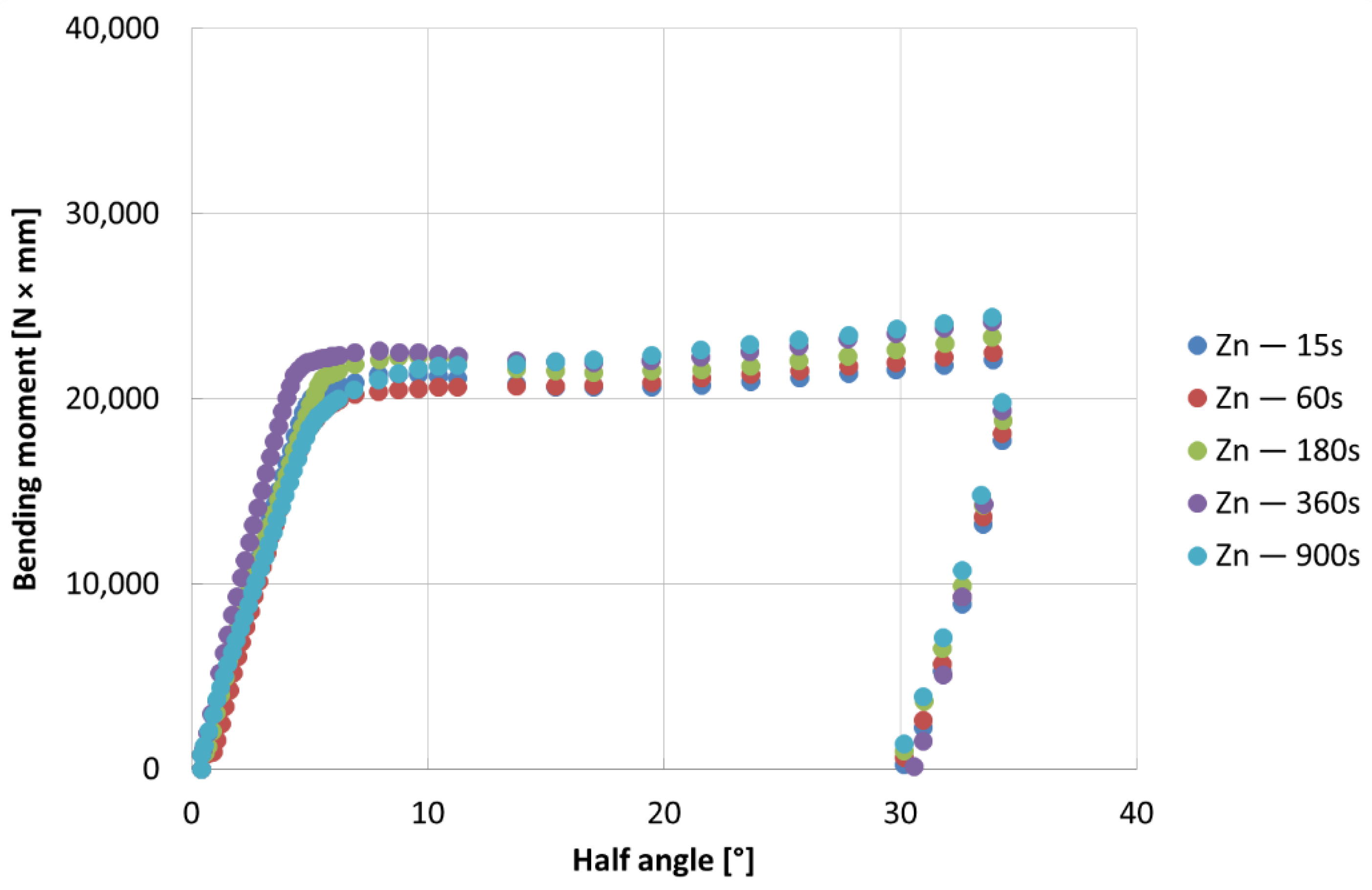
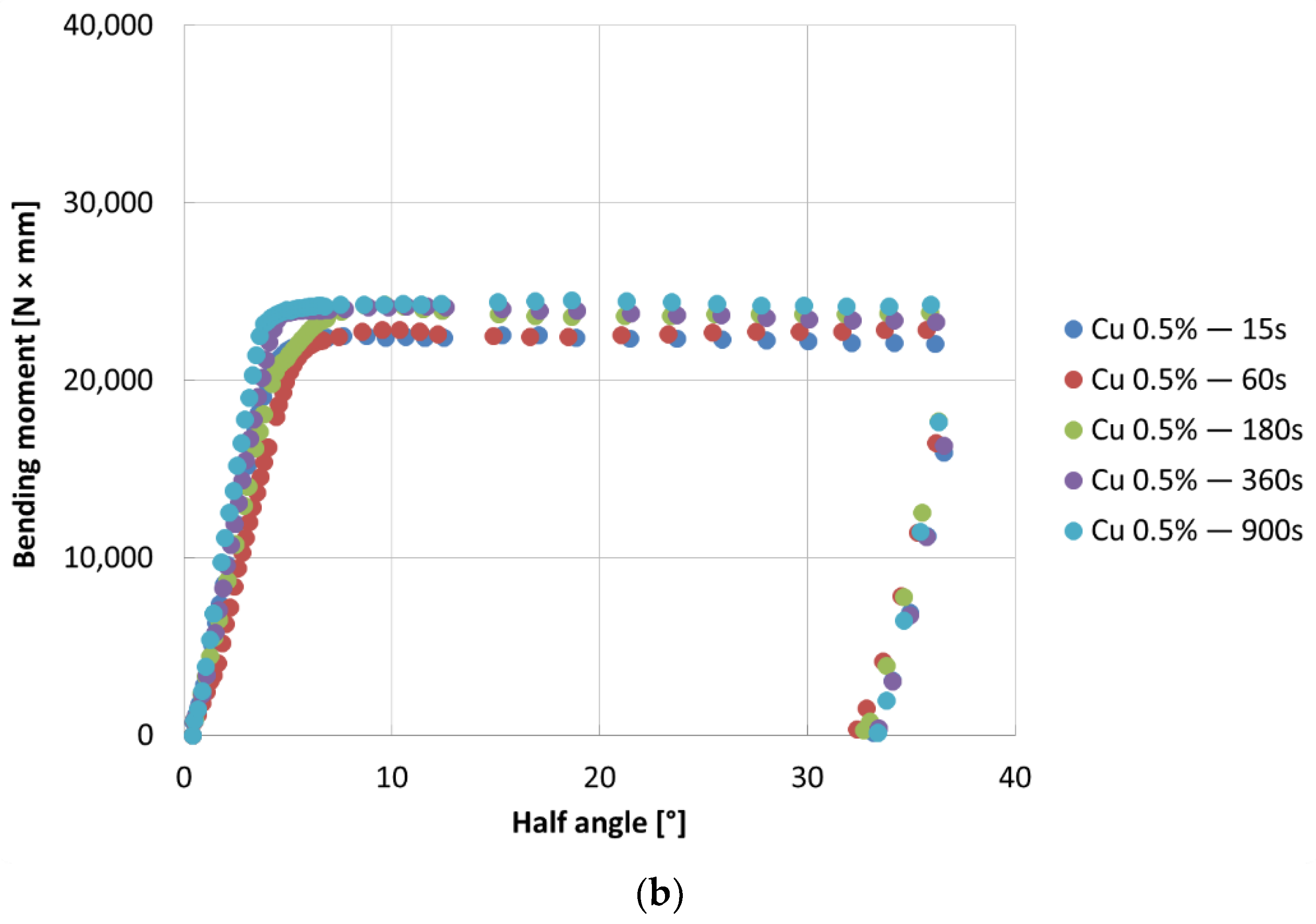
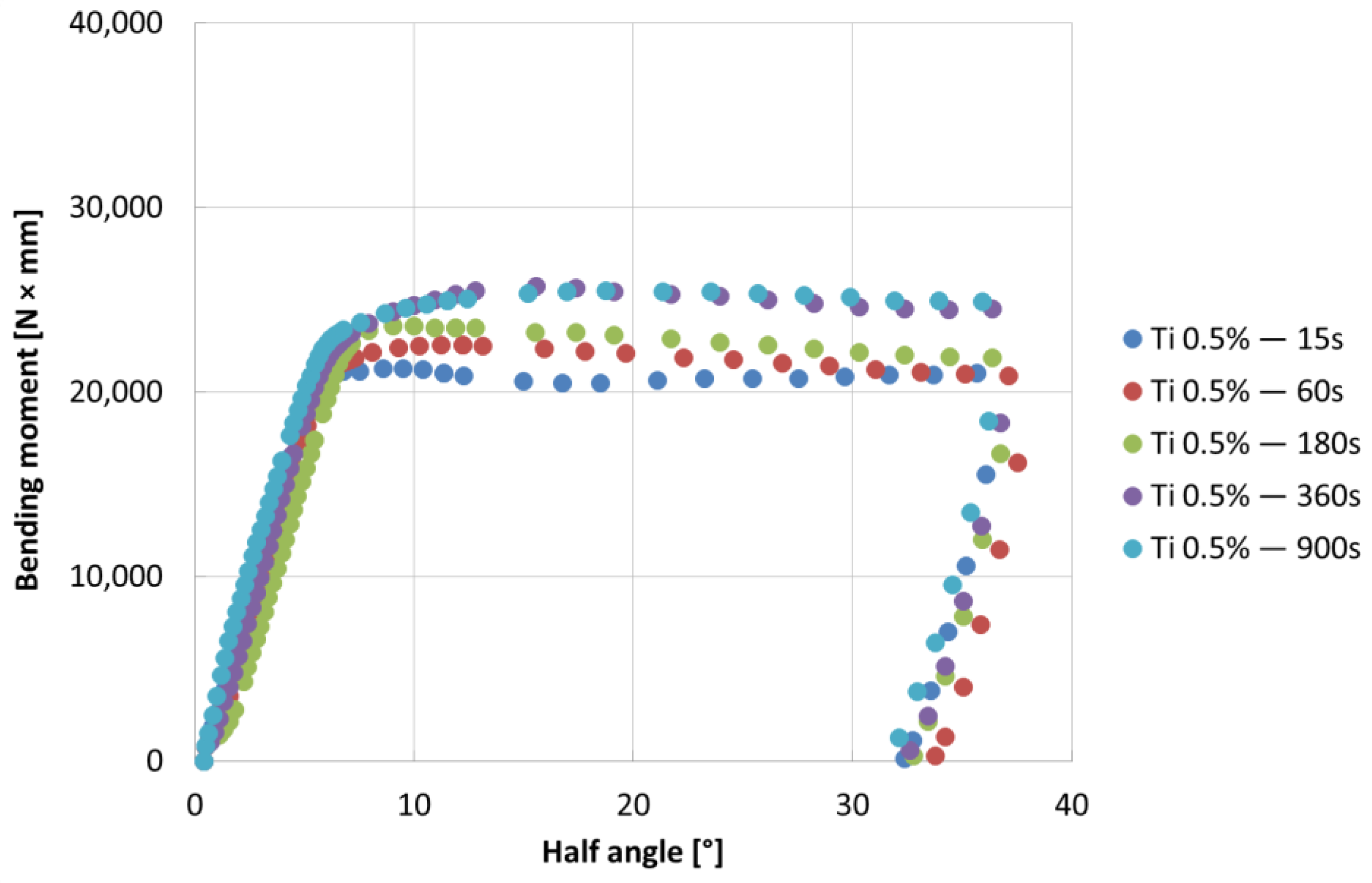
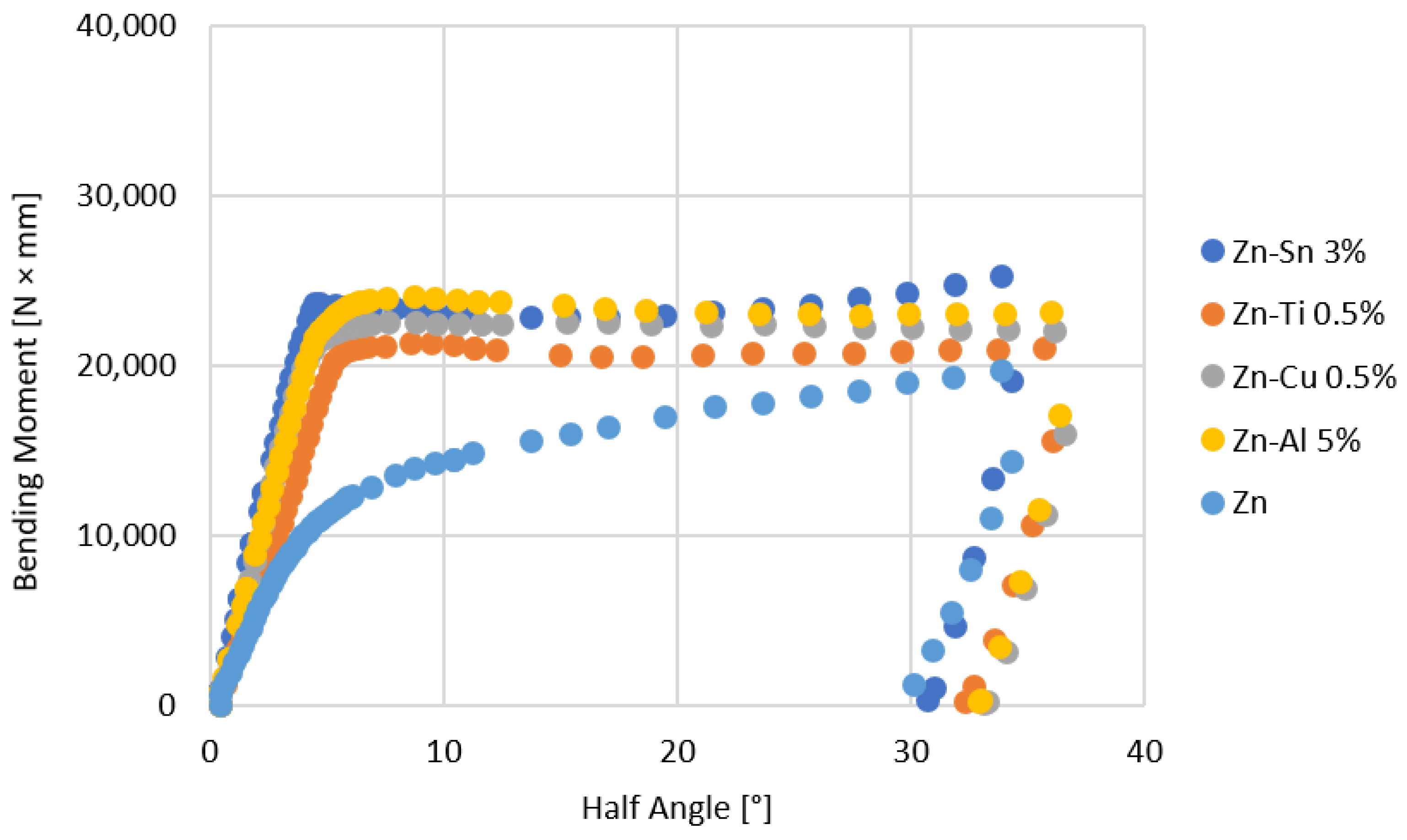
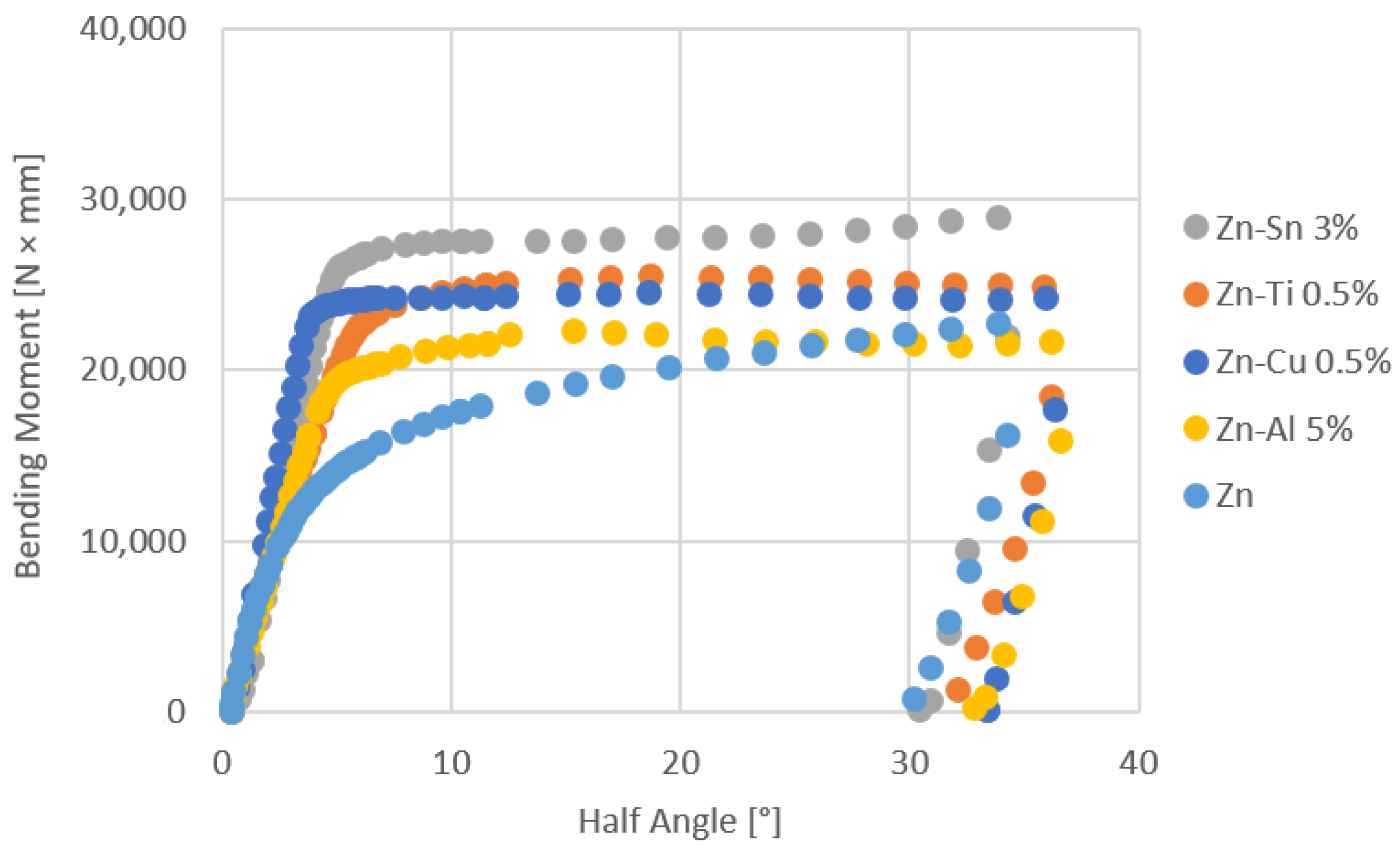
- The bending tests demonstrate that the phases mostly affect the specimens’ resistance at high deformation levels; the higher bending moment values are due to the thickness of the coatings, which can be observed for longer immersion times (immersion time correlates with greater thickness, as well reported in the literature [24])
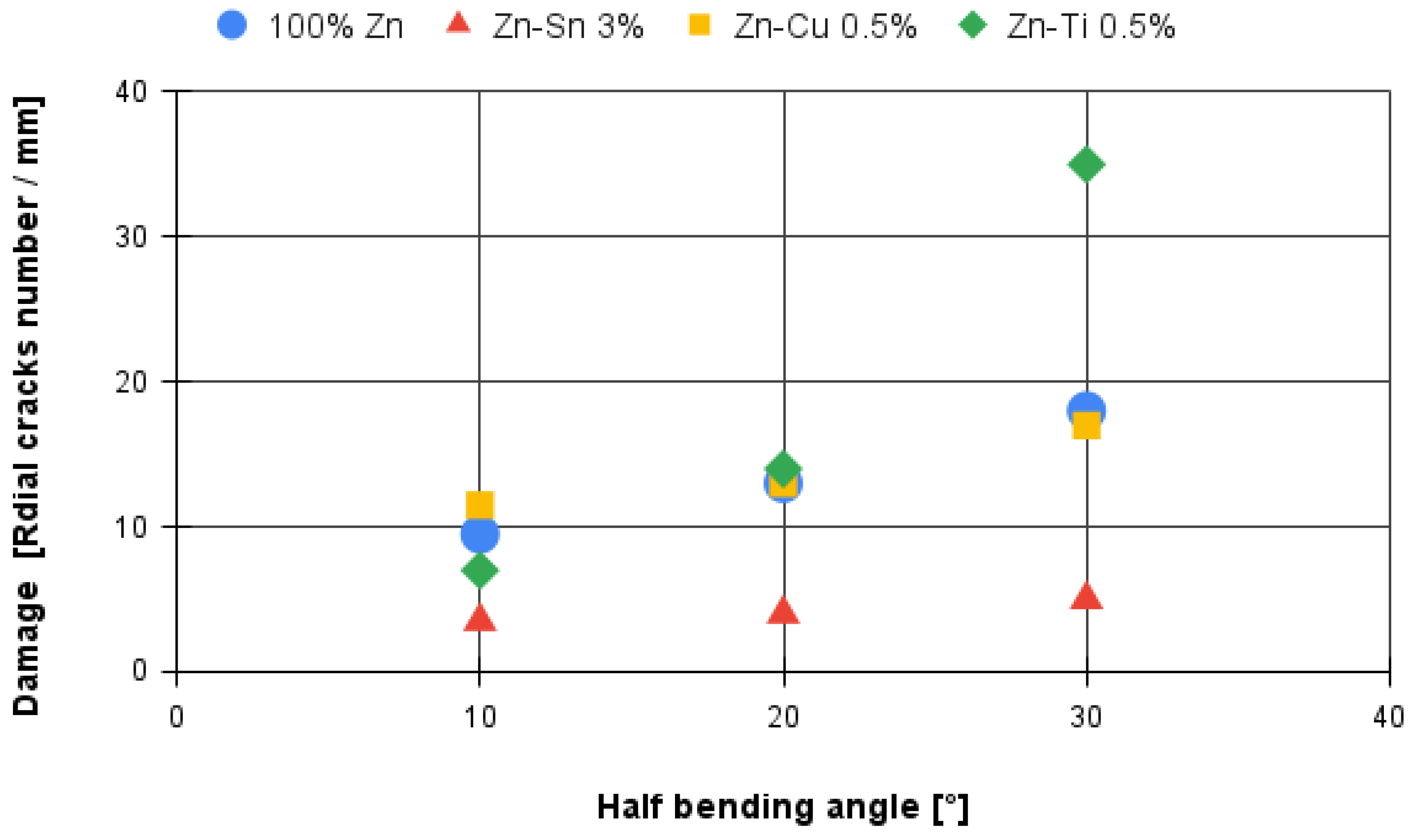
- For all the investigated coating conditions, radial cracks are observed. In conventional coatings, the cracks are originated at the substrate-coating interface, then they grow through the δ phase, and finally they stop in the ζ phase or at the δ-ζ interface, which demonstrate that δ is a brittle phase as well reported in literature [25].
- In the coatings characterized by the addition of Ti, the δ phase is characterized by the presence of radial cracks. Sometimes, delaminations may arise between the δ phase and the three-phase zone, while the τ phase is characterized by intergranular cracks.
- The coatings obtained from a bath with 5% aluminium never present radial cracks as well as the η phases in all investigated conditions. In addition, the thickness of the coating is very thin when compared with other coatings.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marder, A.R. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Meena, B.N.; Remya, R. A review on recent approaches in the field of hot dip zinc galvanizing process. Surf. Coat. Technol. 2015, 262, 210–215. [Google Scholar] [CrossRef]
- Proskurkin, E.V.; Gorbunov, N.S. Diffusion Zinc Coatings; Metallurgiya Press: Moscow, Russia, 1972. [Google Scholar]
- Mackowiak, J.; Short, N.R. Metallurgy of galvanized coatings. Int. Mater. Rev. 1979, 1, 1–19. [Google Scholar] [CrossRef]
- Tzimas, E.; Papadimitriou, G. Cracking mechanisms in high temperature hot-dip galvanized coatings. Surf. Coat. Technol. 2001, 145, 176–185. [Google Scholar] [CrossRef]
- Natali, S.; Iacoviello, F.; Di Cocco, V. Damaging micromechanisms in hot-dip galvanizing Zn based coatings. Theor. Appl. Fract. Mech. 2014, 70, 91–98. [Google Scholar] [CrossRef]
- Massalski, T.B.; Okamoto, H.L.; Subramanian, P.R.; Kacprzak, L. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1996. [Google Scholar]
- Carpinteri, A.; Di Cocco, V.; Fortese, G.; Iacoviello, F.; Natali, S.; Ronchei, C.; Scorza, D.; Vantadori, S. Kinetics of Intermetallic Phases and Mechanical Behavior of ZnSn3% Hot-Dip Galvanization Coatings. Adv. Eng. Mater. 2016, 12, 2088–2094. [Google Scholar] [CrossRef]
- Vantadori, S.; Carpinteri, A.; Di Cocco, V.; Fortese, G.; Iacoviello, F.; Natali, S.; Ronchei, C.; Scorza, D.; Zanichelli, A. Novel zinc-based alloys used to improve the corrosion protection of metallic substrates. Eng. Fail. Anal. 2017, 82, 327–339. [Google Scholar] [CrossRef]
- Nairin, J.A.; Kim, S.R. A fracture mechanics analysis of multiple cracking in coatings. Eng. Fract. Mech. 1992, 42, 195–208. [Google Scholar] [CrossRef]
- Kim, S.R.; Narin, J.A. Fracture mechanics analysis of coating/substrate systems: Part I: Analysis of tensile and bending experiments. Eng. Fract. Mech. 2000, 65, 573–593. [Google Scholar] [CrossRef]
- Vantadori, S.; Zanichelli, A.; Ronchei, C.; Scorza, D.; Di Cocco, V.; Iacoviello, F. Numerical Simulation of Traditional and Technological Zinc-Based Coatings: Part I. Adv. Eng. Mater. 2022, 24, 2101619. [Google Scholar] [CrossRef]
- Al-Negheimish, A.; Hussain, R.R.; Alhozaimy, A.; Singh, D.D.N. Corrosion performance of hot-dip galvanized zinc-aluminum coated steel rebars in comparison to the conventional pure zinc coated rebars in concrete environment. Constr. Build. Mater. 2021, 274, 121921. [Google Scholar] [CrossRef]
- Lin, K.L.; Chue, C.H.; Ching Kou, B. Deformation and corrosion of hot dip galvanized coatings. Mater. Chem. Phys. 1997, 50, 82–87. [Google Scholar] [CrossRef]
- Di Cocco, V. Sn and Ti influences on intermetallic phases damage in hot dip galvanizing. Fract. Struct. Integr. 2012, 22, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Duncan, J.L.; Ding, S.C.; Jiang, V.L. Moment-curvature measurement in thin sheet—Part I: Equipment. Int. J. Mech. Sci. 1999, 41, 249–260. [Google Scholar] [CrossRef]
- Lu, X. Correlation between Microstructural Evolution and Mechanical Properties of 2000 MPa Cold-Drawn Pearlitic Steel Wires during Galvanizing Simulated Annealing. Metals 2019, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- García-Martino, Á.; García, C.; Prieto, M.M.; Díaz, J. Prediction of Mechanical Properties for High Strength Low Alloyed Steels in a Commercial Hot Dip Galvanizing Line without Soaking Section. Metals 2020, 10, 561. [Google Scholar] [CrossRef]
- Colla, V.; Cateni, S.; Maddaloni, A.; Vignali, A. A Modular Machine-Learning-Based Approach to Improve Tensile Properties Uniformity Along Hot Dip Galvanized Steel Strips for Automotive Applications. Metals 2020, 10, 923. [Google Scholar] [CrossRef]
- Giorgi, M.L.; Guillot, J.B.; Nicolle, R. Theoretical model of the interfacial reactions between solid iron and liquid zinc-aluminium alloy. J. Mater. Sci. 2005, 40, 2263–2268. [Google Scholar] [CrossRef]
- Min, T.; Gao, Y.; Huang, X.; Gong, Z.; Li, K.; Ma, S. Effects of aluminum concentration on the formation of inhibition layer during hot-dip galvanizing. Int. J. Heat Mass Tran. 2018, 127, 394–402. [Google Scholar] [CrossRef]
- Di Cocco, V.; Iacoviello, F.; D’Agostino, L.; Natali, S. Damage micromechanisms in a hot dip galvanized steel. Proc. Struct. Integr. 2017, 3, 231–236. [Google Scholar] [CrossRef]
- Sjoukes, F. Chemical reactions in fluxes for hot dip galvanizing. Anti-Corros. Methods Mater. 1990, 37, 12–14. [Google Scholar] [CrossRef]
- Salles, P.; Pinto, D.; Hantanasirisakul, K.; Maleski, K.; Shuck, C.E.; Gogotsi, Y. Electrochromic effect in titanium carbide MXene thin films produced by dip-coating. Adv. Funct. Mater. 2019, 29, 1809223. [Google Scholar] [CrossRef]
- Manna, M.; Naidu, G.; Rani, N.; Bandyopadhyay, N. Characterisation of coating on rebar surface using hot-dip Zn and Zn-4.9 Al-0.1 misch metal bath. Surf. Coat. Technol. 2008, 202, 1510–1516. [Google Scholar] [CrossRef]





| wt% C | wt% Si | wt% Mn | wt% P | wt% S | wt% N |
|---|---|---|---|---|---|
| 0.090 | 0.167 | 0.540 | 0.010 | 0.004 | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, C.; Di Cocco, V.; Iacoviello, F.; Mocanu, L.P. Impact of Copper, Tin and Titanium Addition on Bending-Induced Damage of Intermetallic Phases in Hot Dip Galvanizing. Metals 2022, 12, 2035. https://doi.org/10.3390/met12122035
Bellini C, Di Cocco V, Iacoviello F, Mocanu LP. Impact of Copper, Tin and Titanium Addition on Bending-Induced Damage of Intermetallic Phases in Hot Dip Galvanizing. Metals. 2022; 12(12):2035. https://doi.org/10.3390/met12122035
Chicago/Turabian StyleBellini, Costanzo, Vittorio Di Cocco, Francesco Iacoviello, and Larisa Patricia Mocanu. 2022. "Impact of Copper, Tin and Titanium Addition on Bending-Induced Damage of Intermetallic Phases in Hot Dip Galvanizing" Metals 12, no. 12: 2035. https://doi.org/10.3390/met12122035
APA StyleBellini, C., Di Cocco, V., Iacoviello, F., & Mocanu, L. P. (2022). Impact of Copper, Tin and Titanium Addition on Bending-Induced Damage of Intermetallic Phases in Hot Dip Galvanizing. Metals, 12(12), 2035. https://doi.org/10.3390/met12122035







