Magnesium Oxide Nanoparticles (MgO-NPs) Alleviate Arsenic Toxicity in Soybean by Modulating Photosynthetic Function, Nutrient Uptake and Antioxidant Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Growth Conditions
2.2. Determination of Growth Characteristics
2.3. SPAD Index Calculation
2.4. Determination of Photosynthetic Efficiency
2.5. Enzymatic Activity Assays
2.6. Lipid Peroxidation (MDA) and Hydrogen Peroxide (H2O2) Determination
2.7. Estimation of Arsenic and Protein Content
2.8. Mineral Nutrient Analysis
2.9. Statistical Analysis
3. Results
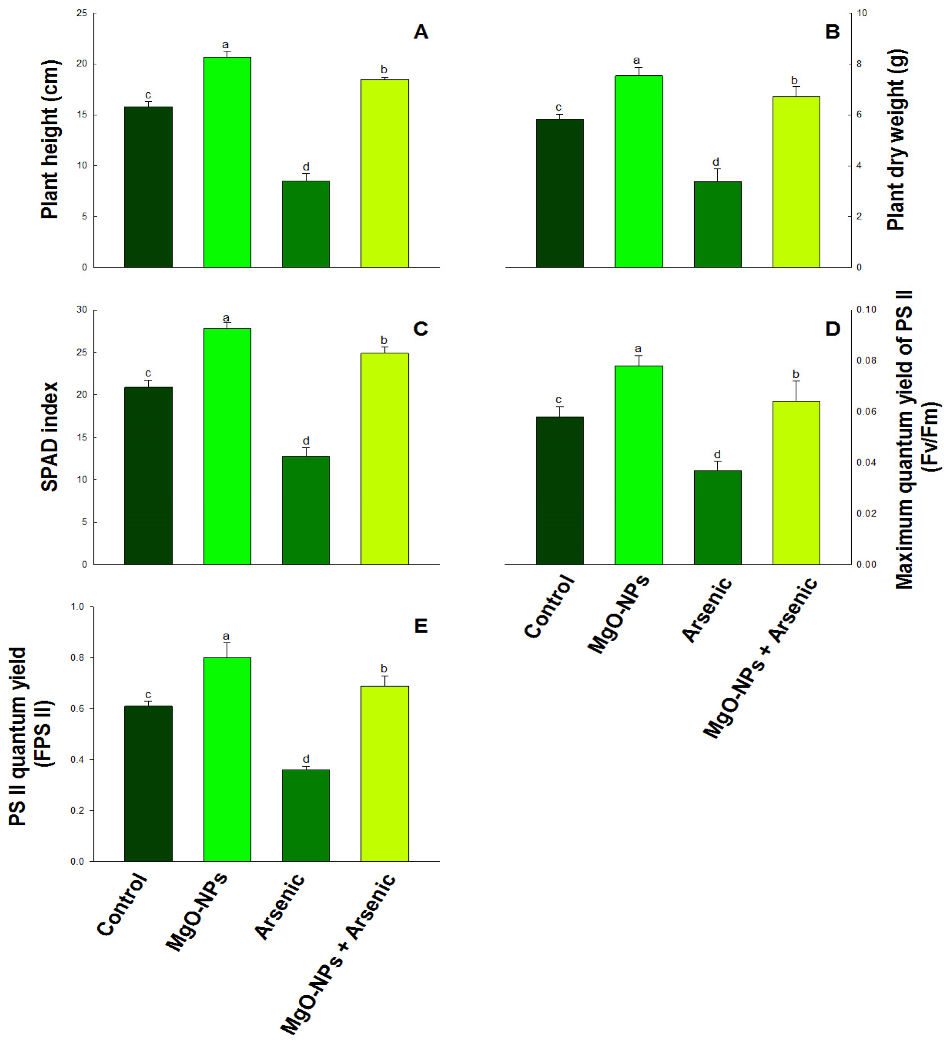
3.1. MgO-NPs Impact on Growth
3.2. Effect of MgO-NPs on Chlorophyll Content (SPAD Index)
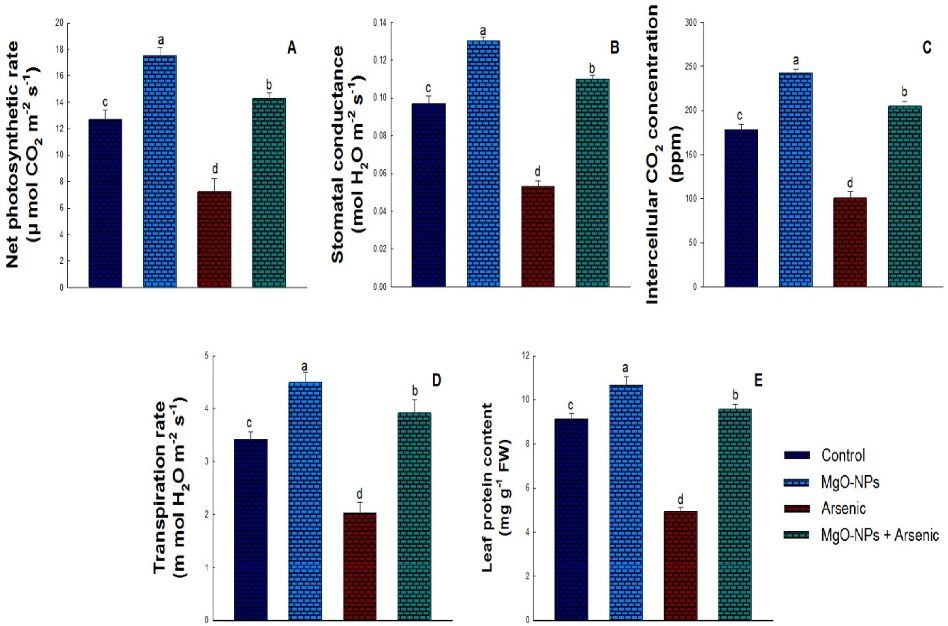
3.3. Effect of MgO-NPs on Photosynthesis
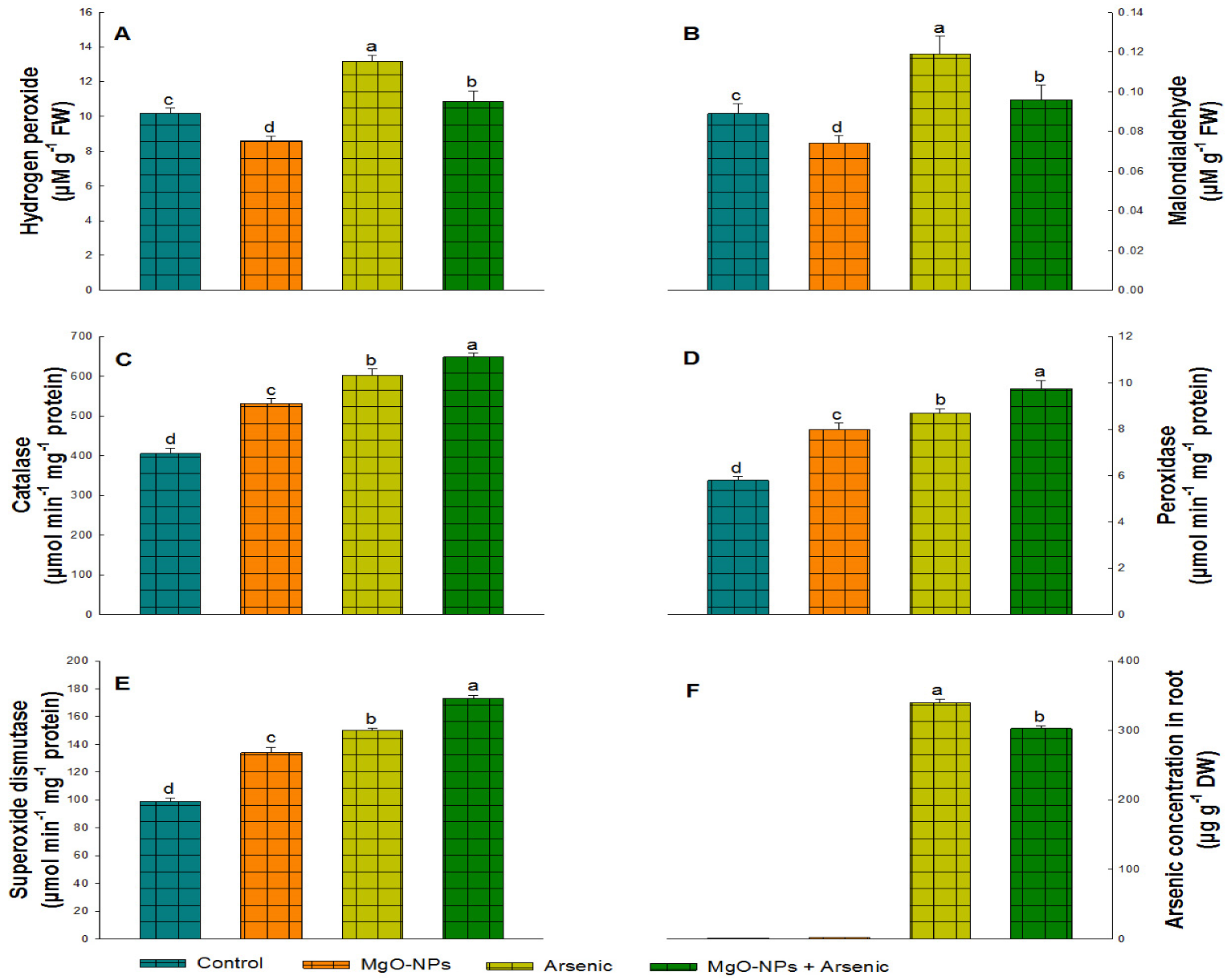
3.4. Impact of MgO-NPs on Lipid Peroxidation (MDA) and Hydrogen Peroxide (H2O2) Content
3.5. Impacts of MgO-NPs on Antioxidant Enzymes
3.6. MgO-NPs Impact on Protein and Arsenic Content
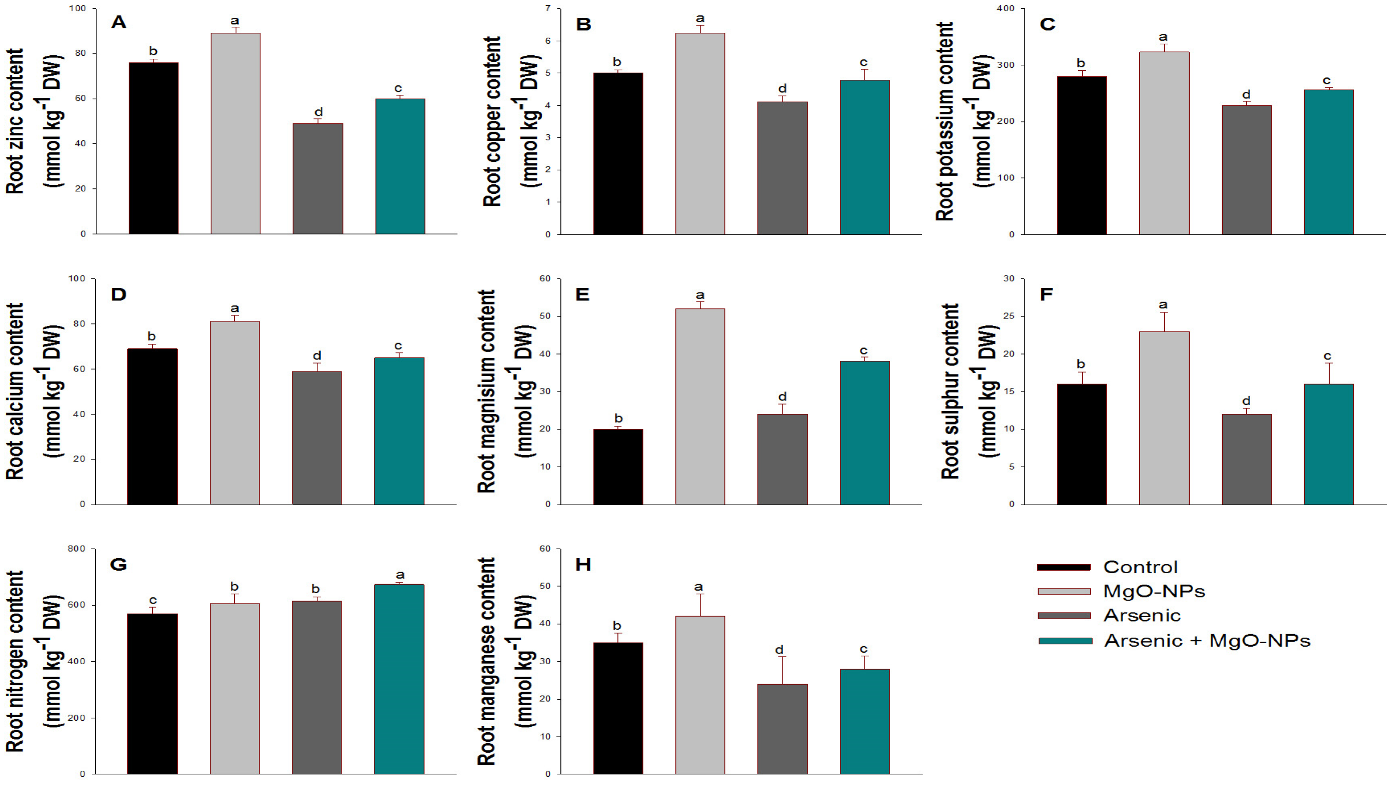
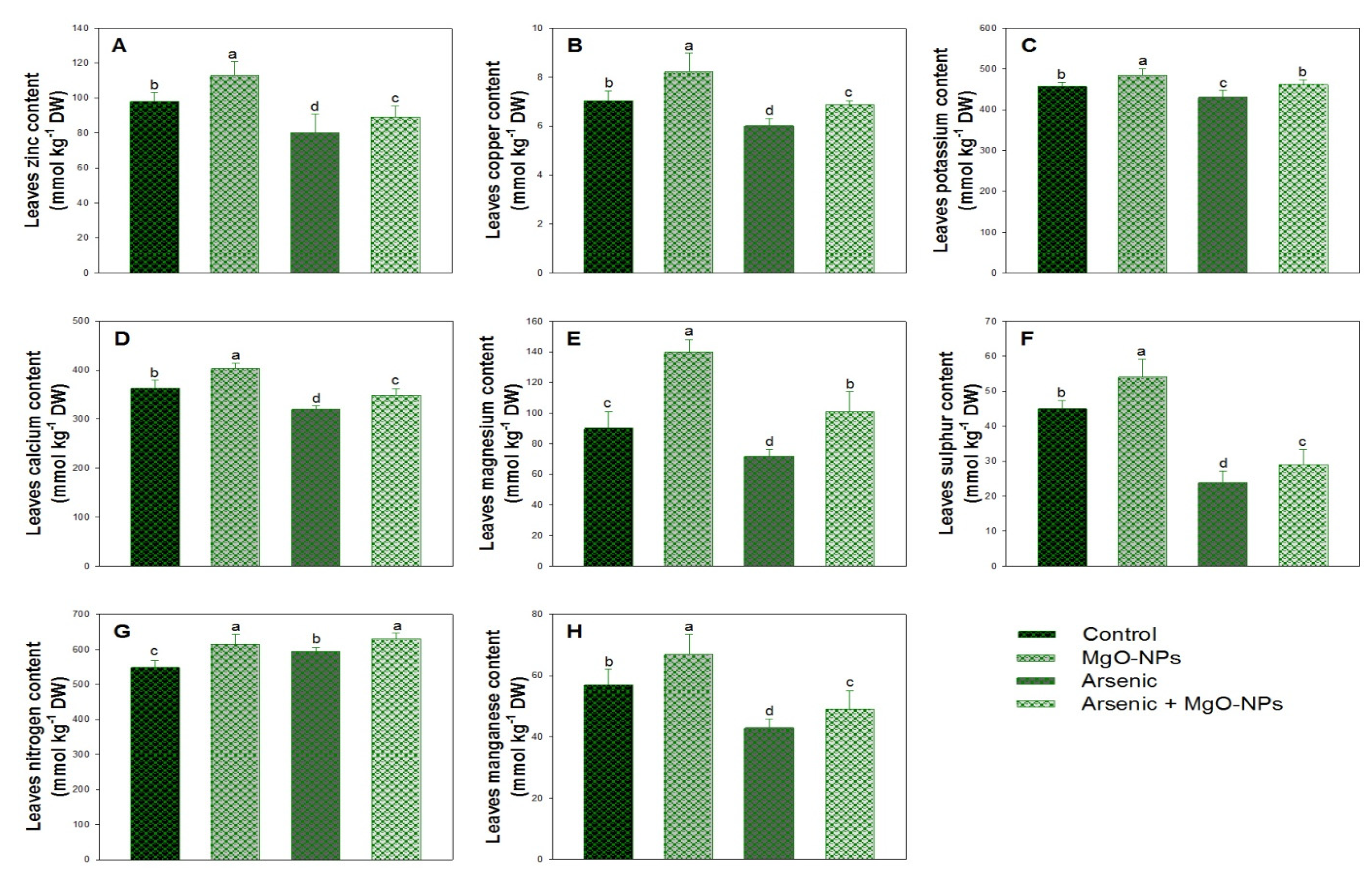
3.7. Effect of Arsenic on Mineral Nutrient Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraceto, L.F.; Grillo, R.; de Medeiros, G.A.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in agriculture: Which innovation potential does it have? Front. Environ. Sci. 2016, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Faizan, M.; Bhat, J.A.; Noureldeen, A.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles and 24-epibrassinolide alleviates Cu toxicity in tomato by regulating ROS scavenging, stomatal movement and photosynthesis. Ecotoxicol. Environ. Saf. 2021, 218, 112293. [Google Scholar] [CrossRef]
- Servin, A.D.; White, J.C. Nanotechnology in agriculture: Next steps for understanding engineered nanoparticle exposure and risk. NanoImpact 2016, 1, 9–12. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Faraz, A.; Mir, A.R.; Hayat, S. Role of zinc oxide nanoparticles in countering negative effects generated by cadmium in Lycopersicon esculentum. J. Plant Growth Regul. 2020, 40, 101–115. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Hayat, S. Effective use of zinc oxide nanoparticles through root dipping on the performance of growth, quality, photosynthesis and antioxidant system in tomato. J. Plant Biochem. Biotechnol. 2019, 29, 553–567. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.; Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Cai, L.; Chen, J.; Liu, Z.; Wang, H.; Yang, H.; Ding, W. Magnesium oxide nanoparticles: Effective agricultural antibacterial agent against Ralstonia solanacearum. Front. Microbiol. 2018, 9, 790. [Google Scholar] [CrossRef] [Green Version]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Shen, Y.; He, L.; Yang, Z.; Xiong, Y. Corrosion behavior of different coatings prepared on the surface of AZ80 magnesium alloy in simulated body fluid. J. Mater. Eng. Perform. 2020, 29, 1609–1621. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabhu, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B Biol. 2019, 190, 86–97. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.; Singh, S.; Gautam, R.; Choudhary, K.; Maurino, V.G.; Saharan, V. MgO nanoparticles biosynthesis and its effect on chlorophyll contents in the leaves of clusterbean (Cyamopsis tetragonoloba L.). Adv. Sci. Eng. Med. 2014, 6, 538–545. [Google Scholar] [CrossRef]
- Kanjana, D. Foliar application of magnesium oxide nanoparticles on nutrient element concentrations, growth, physiological, and yield parameters of cotton. J. Plant Nutr. 2020, 43, 3035–3049. [Google Scholar] [CrossRef]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.; Arellano-Mendoza, M.; Tamay-Cach, F.; Valenzuela-Limón, O.; García-Montalvo, E.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef]
- Finnegan, P.M.; Chen, W. Arsenic toxicity: The effects on plant metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef] [Green Version]
- Stoeva, N.; Berova, M.; Zlatev, Z. Effect of arsenic on some physiological parameters in bean plants. Biol. Plant. 2005, 49, 293–296. [Google Scholar] [CrossRef]
- Garg, N.; Singla, P. Arsenic toxicity in crop plants: Physiological effects and tolerance mechanisms. Environ. Chem. Lett. 2011, 9, 303–321. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Mechanisms to cope with arsenic or cadmium excess in plants. Curr. Opin. Plant Biol. 2009, 12, 364–372. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-I.; Lee, J.; Chae, M.-J.; Kong, M.-S.; Lee, C.-H.; Kang, S.-S.; Kim, Y.-H. Growth-inhibition patterns and transfer-factor profiles in arsenic-stressed rice (Oryza sativa L.). Environ. Monit. Assess. 2017, 189, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedin, M.J.; Feldmann, J.; Meharg, A.A. Uptake kinetics of arsenic species in rice plants. Plant Physiol. 2002, 128, 1120–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiani, A.; Fumagalli, P.; Nguyen Van, T.; Gentili, R.; Citterio, S. The combined toxic and genotoxic effects of Cd and As to plant bioindicator Trifolium repens L. PLoS ONE 2014, 9, e99239. [Google Scholar] [CrossRef] [Green Version]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [Green Version]
- Babaeian, M.; Esmaeilian, Y.; Tavassoli, A.; Asgharzade, A. Efficacy of different iron, zinc and magnesium fertilizers on yield and yield components of barley. Afr. J. Microbiol. Res. 2012, 6, 5754–5756. [Google Scholar]
- Kashinath, B.; Murthy, A.G.; Senthivel, T.; Pitchai, G.J.; Sadashiva, A. Effect of applied magnesium on yield and quality of tomato in Alfisols of Karnataka. J. Hortic. Sci. 2013, 8, 55–59. [Google Scholar]
- Ozenc, N.; Ozenc, D.B. Effect of magnesium fertilization on some plant nutrient interactions and nut quality properties in Turkish hazelnut (Corylus avellana L.). Sci. Res. Essays 2015, 10, 465–470. [Google Scholar]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 1–10. [Google Scholar] [CrossRef]
- Faiz, S.; Yasin, N.A.; Khan, W.U.; Shah, A.A.; Akram, W.; Ahmad, A.; Ali, A.; Naveed, N.H.; Riaz, L. Role of magnesium oxide nanoparticles in the mitigation of lead-induced stress in Daucus carota: Modulation in polyamines and antioxidant enzymes. Int. J. Phytoremediation 2022, 24, 364–372. [Google Scholar] [CrossRef]
- Faizan, M.; Rajput, V.D.; Al-Khuraif, A.A.; Arshad, M.; Minkina, T.; Sushkova, S.; Yu, F. Effect of foliar fertigation of chitosan nanoparticles on cadmium accumulation and toxicity in Solanum lycopersicum. Biology 2021, 10, 666. [Google Scholar] [CrossRef]
- Jhansi, K.; Jayarambabu, N.; Reddy, K.P.; Reddy, N.M.; Suvarna, R.P.; Rao, K.V.; Kumar, V.R.; Rajendar, V. Biosynthesis of MgO nanoparticles using mushroom extract: Effect on peanut (Arachis hypogaea L.) seed germination. 3 Biotech 2017, 7, 263. [Google Scholar] [CrossRef]
- Juhel, G.; Batisse, E.; Hugues, Q.; Daly, D.; van Pelt, F.N.; O’Halloran, J.; Jansen, M.A. Alumina nanoparticles enhance growth of Lemna minor. Aquat. Toxicol. 2011, 105, 328–336. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.; Ramachandran, R.; Abirami, S.; Mohan, N.; Kalaichelvan, P. Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochem. 2012, 47, 651–658. [Google Scholar] [CrossRef]
- Adhikari, T. Magnesium oxide nano particles effects on utilization of soil phosphorus by maize (Zea mays L.) plant. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 410–419. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, S.; Yang, D.; Zou, Z.; Li, J. Physiological effects of MgO and ZnO nanoparticles on the Citrus maxima. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2019, 34, 243–253. [Google Scholar] [CrossRef]
- Samborska, I.A.; Kalaji, H.M.; Sieczko, L.; Goltsev, V.; Borucki, W.; Jajoo, A. Structural and functional disorder in the photosynthetic apparatus of radish plants under magnesium deficiency. Funct. Plant Biol. 2018, 45, 668–679. [Google Scholar] [CrossRef]
- Tränkner, M.; Jákli, B.; Tavakol, E.; Geilfus, C.-M.; Cakmak, I.; Dittert, K.; Senbayram, M. Magnesium deficiency decreases biomass water-use efficiency and increases leaf water-use efficiency and oxidative stress in barley plants. Plant Soil 2016, 406, 409–423. [Google Scholar] [CrossRef] [Green Version]
- Faizan, M.; Yu, F.; Chen, C.; Faraz, A.; Hayat, S. Zinc oxide nanoparticles help to enhance plant growth and alleviate abiotic stress: A review. Curr. Protein Pept. Sci. 2020, 22, 362–375. [Google Scholar] [CrossRef]
- Jin, M.; Xiao, X.; Qin, L.; Geng, W.; Gao, Y.; Li, L.; Xue, J. Physiological and morphological responses and tolerance mechanisms of Isochrysis galbana to Cr (VI) stress. Bioresour. Technol. 2020, 302, 122860. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Alam, P.; Balawi, T.H.; Altalayan, F.H.; Ahanger, M.A.; Ashraf, M. Sodium nitroprusside (SNP) improves tolerance to arsenic (As) toxicity in Vicia faba through the modifications of biochemical attributes, antioxidants, ascorbate-glutathione cycle and glyoxalase cycle. Chemosphere 2020, 244, 125480. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; Hessini, K.; Yu, F.; Ahmad, P. Zinc oxide nanoparticles alleviates the adverse effects of cadmium stress on Oryza sativa via modulation of the photosynthesis and antioxidant defense system. Ecotoxicol. Environ. Saf. 2021, 220, 112401. [Google Scholar] [CrossRef] [PubMed]
- Inupakutika, M.A.; Sengupta, S.; Devireddy, A.R.; Azad, R.K.; Mittler, R. The evolution of reactive oxygen species metabolism. J. Exp. Bot. 2016, 67, 5933–5943. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Adamakis, I.D.S.; Sperdouli, I.; Eleftheriou, E.P.; Moustakas, M. Hydrogen peroxide production by the spot-like mode action of bisphenol A. Front. Plant Sci. 2020, 11, 1196. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Sperdouli, I.; Andreadis, S.; Moustaka, J.; Panteris, E.; Tsaballa, A.; Moustakas, M. Changes in light energy utilization in photosystem II and reactive oxygen species generation in potato leaves by the pinworm Tuta absoluta. Molecules 2021, 26, 2984. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Moustakas, M. Plant photochemistry, reactive oxygen species, and photoprotection. Photochem 2022, 2, 5–8. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Terrón-Camero, L.C.; Peláez-Vico, M.Á.; Molina-Moya, E.; Sandalio, L.M. An update on redox signals in plant responses to biotic and abiotic stress crosstalk: Insights from cadmium and fungal pathogen interactions. J. Exp. Bot. 2021, 72, 5857–5875. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox signaling in plants. Antioxid. Redox Signal. 2013, 18, 2087–2090. [Google Scholar] [CrossRef]
- Moustakas, M. The role of metal ions in biology, biochemistry and medicine. Materials 2021, 14, 549. [Google Scholar] [CrossRef]
- Sperdouli, I.; Adamakis, I.D.S.; Dobrikova, A.; Apostolova, E.; Hanć, A.; Moustakas, M. Excess zinc supply reduces cadmium uptake and mitigates cadmium toxicity effects on chloroplast structure, oxidative stress, and photosystem II photochemical efficiency in Salvia sclarea plants. Toxics 2022, 10, 36. [Google Scholar] [CrossRef]
- Moustakas, M.; Moustaka, J.; Sperdouli, I. Hormesis in photosystem II: A mechanistic approach. Curr. Opin. Toxicol. 2022, 29, 57–64. [Google Scholar] [CrossRef]
- Adamakis, I.-D.S.; Sperdouli, I.; Hanć, A.; Dobrikova, A.; Apostolova, E.; Moustakas, M. Rapid hormetic responses of photosystem II photochemistry of clary sage to cadmium exposure. Int. J. Mol. Sci. 2021, 22, 41. [Google Scholar] [CrossRef]
- Małkowski, E.; Sitko, K.; Szopiński, M.; Gieroń, Z.; Pogrzeba, M.; Kalaji, H.M.; Zieléznik-Rusinowska, P. Hormesis in plants: The role of oxidative stress, auxins and photosynthesis in corn treated with Cd or Pb. Int. J. Mol. Sci. 2020, 21, 2099. [Google Scholar] [CrossRef] [Green Version]
- Marschner, P. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Beale, S.I. Enzymes of chlorophyll biosynthesis. Photosynth. Res. 1999, 60, 43–73. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Moustakas, M.; Dobrikova, A.; Sperdouli, I.; Hanć, A.; Adamakis, I.-D.S.; Moustaka, J.; Apostolova, E. A hormetic spatiotemporal photosystem II response mechanism of Salvia to excess zinc exposure. Int. J. Mol. Sci. 2022, 23, 11232. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Zeng, F.; Song, P.; Sun, B.; Wang, Q.; Wang, J. Effects of reduced chlorophyll content on photosystem functions and photosynthetic electron transport rate in rice leaves. J. Plant Physiol. 2022, 272, 153669. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.-D.S.; Moustaka, J.; İşgören, S.; Şaş, B. Harnessing the role of foliar applied salicylic acid in decreasing chlorophyll content to reassess photosystem II photoprotection in crop plants. Int. J. Mol. Sci. 2022, 23, 7038. [Google Scholar] [CrossRef] [PubMed]
- Elsheery, N.I.; Helaly, M.N.; El-Hefnawy, S.F.; Elhamahmy, M.M.; Abdelrazik, E.M.; Sardarov, Y.B.; Ahmad, P.; Zivcak, M.; Brestic, M.; Allakhverdiev, S.I. 5-Aminolevulinic acid (ALA) reduces arsenic toxicity stress in wheat (Triticum aestivum L.). J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Rafiq, M.; Bakhat, H.F.; Imran, M.; Abbas, T.; Bibi, I.; Dumat, C. Arsenic behavior in soil plant system: Biochemical reactions, chemical special ion influences. In Enhancing Cleanup of Environmental Pollutants; Springer: Berlin/Heidelberg, Germany, 2017; pp. 97–140. [Google Scholar]
- Poddar, R.; Acharjee, P.U.; Bhattacharyya, K.; Patra, S.K. Mitigation of arsenic in broccoli through consumptive use of ground water and pond water as sources for irrigation. Arch. Agron. Soil Sci. 2022. [Google Scholar] [CrossRef]
- Verma, G.; Srivastava, D.; Narayan, S.; Shirke, P.A.; Chakrabarty, D. Exogenous application of methyl jasmonate alleviates arsenic toxicity by modulating its uptake and translocation in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2020, 201, 110735. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Niknejad, Y.; Fallah, H.; Tari, D.B. Methyl jasmonate alleviates arsenic toxicity in rice. Plant Cell Rep. 2020, 39, 1041–1060. [Google Scholar] [CrossRef]
- Ghorbani, A.; Pishkar, L.; Roodbari, N.; Tavakoli, S.A.; Jahromi, M.E.; Wu, C. Nitrate reductase is needed for methyl jasmonate-mediated arsenic toxicity tolerance of rice by modulating the antioxidant defense system, glyoxalase system and arsenic sequestration mechanism. J. Plant Growth Regul. 2022; ahead of print. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Tania, S.S.; Imran, S.; Rauf, F.; Kibria, M.G.; Ye, W.; Hasanuzzaman, M.; Murata, Y. Seed priming with nanoparticles: An emerging technique for improving plant growth, development, and abiotic stress tolerance. J. Soil Sci. Plant Nutr. 2022; ahead of print. [Google Scholar] [CrossRef]
- Antonoglou, O.; Moustaka, J.; Adamakis, I.D.; Sperdouli, I.; Pantazaki, A.; Moustakas, M.; Dendrinou-Samara, C. Nanobrass CuZn nanoparticles as foliar spray non phytotoxic fungicides. ACS Appl. Mater. Interfaces 2018, 10, 4450–4461. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustaka, J.; Antonoglou, O.; Adamakis, I.-D.S.; Dendrinou-Samara, C.; Moustakas, M. Leaf age-dependent effects of foliar-sprayed CuZn nanoparticles on photosynthetic efficiency and ROS generation in Arabidopsis thaliana. Materials 2019, 12, 2498. [Google Scholar] [CrossRef]
- Castiglione, M.R.; Cremonini, R. Nanoparticles and higher plants. Caryologia 2009, 62, 161–165. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faizan, M.; Bhat, J.A.; El-Serehy, H.A.; Moustakas, M.; Ahmad, P. Magnesium Oxide Nanoparticles (MgO-NPs) Alleviate Arsenic Toxicity in Soybean by Modulating Photosynthetic Function, Nutrient Uptake and Antioxidant Potential. Metals 2022, 12, 2030. https://doi.org/10.3390/met12122030
Faizan M, Bhat JA, El-Serehy HA, Moustakas M, Ahmad P. Magnesium Oxide Nanoparticles (MgO-NPs) Alleviate Arsenic Toxicity in Soybean by Modulating Photosynthetic Function, Nutrient Uptake and Antioxidant Potential. Metals. 2022; 12(12):2030. https://doi.org/10.3390/met12122030
Chicago/Turabian StyleFaizan, Mohammad, Javaid Akhtar Bhat, Hamed A. El-Serehy, Michael Moustakas, and Parvaiz Ahmad. 2022. "Magnesium Oxide Nanoparticles (MgO-NPs) Alleviate Arsenic Toxicity in Soybean by Modulating Photosynthetic Function, Nutrient Uptake and Antioxidant Potential" Metals 12, no. 12: 2030. https://doi.org/10.3390/met12122030
APA StyleFaizan, M., Bhat, J. A., El-Serehy, H. A., Moustakas, M., & Ahmad, P. (2022). Magnesium Oxide Nanoparticles (MgO-NPs) Alleviate Arsenic Toxicity in Soybean by Modulating Photosynthetic Function, Nutrient Uptake and Antioxidant Potential. Metals, 12(12), 2030. https://doi.org/10.3390/met12122030









