Hydriding, Oxidation, and Ductility Evaluation of Cr-Coated Zircaloy-4 Tubing
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
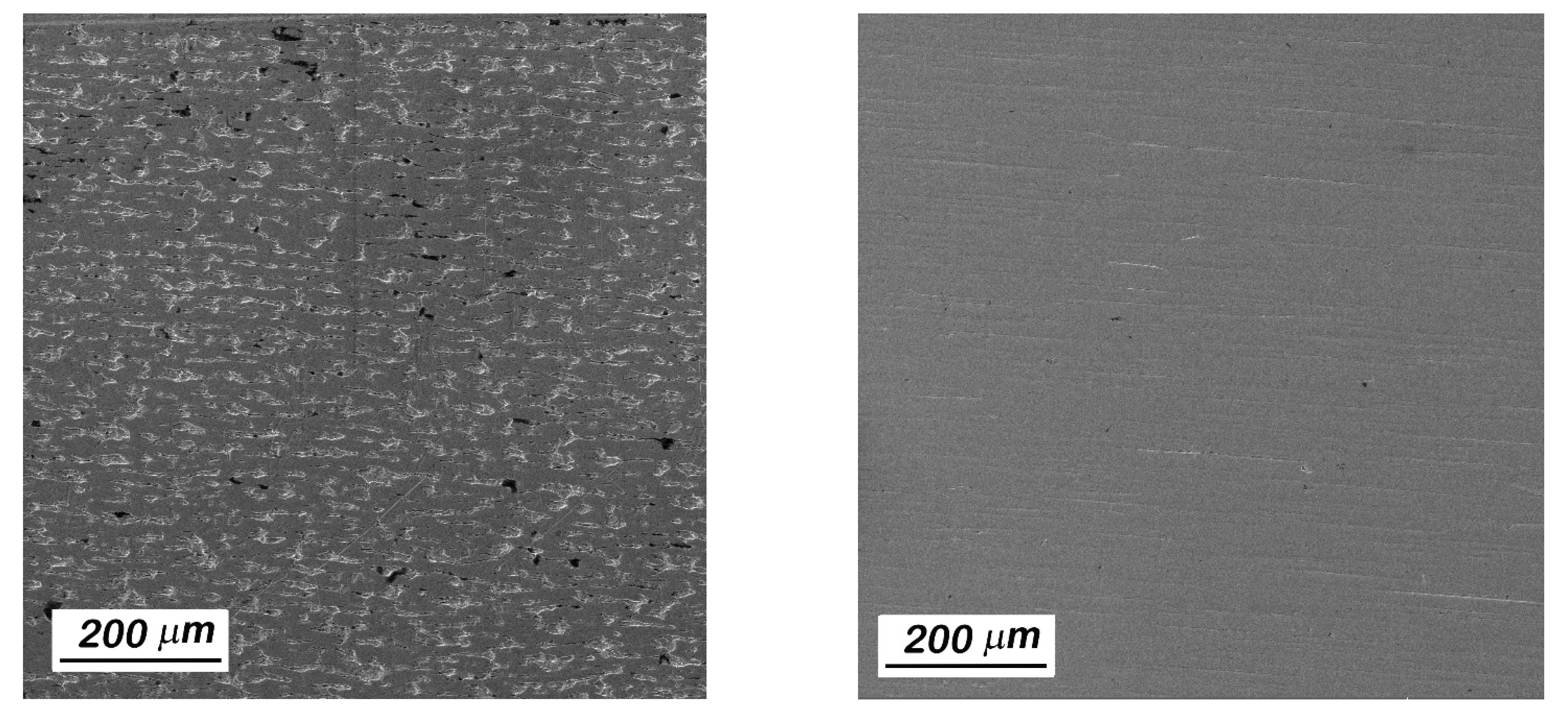
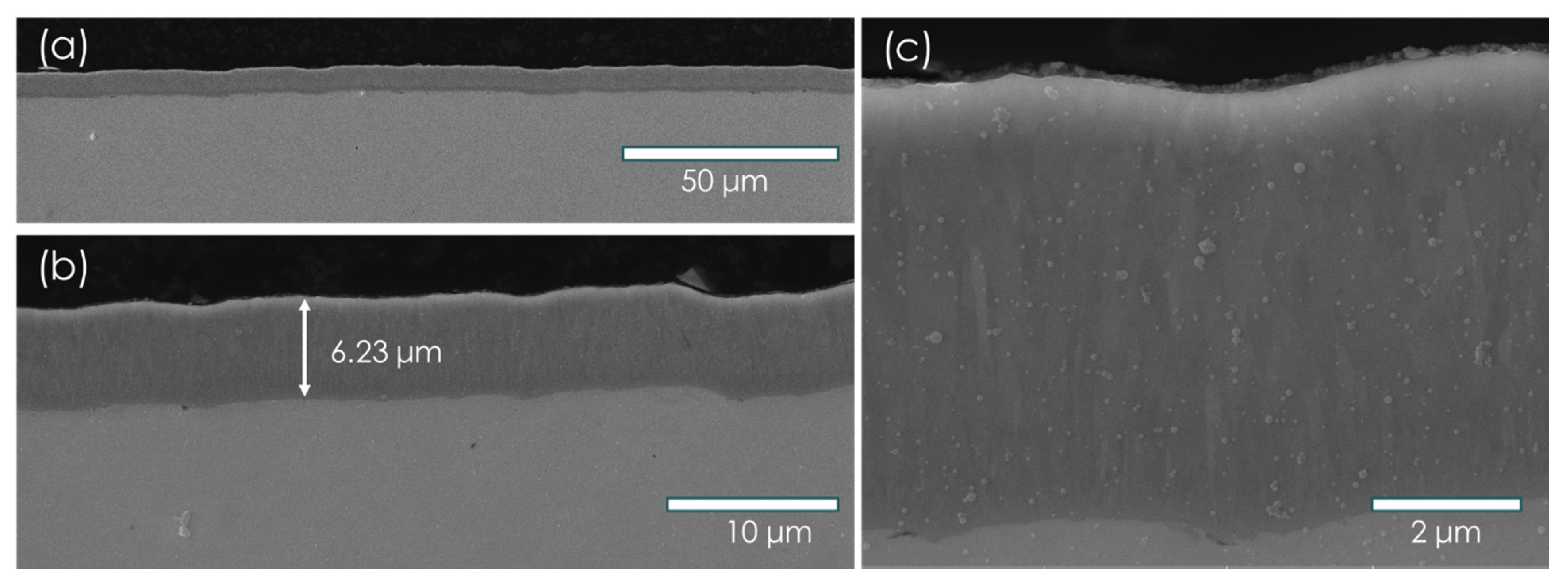
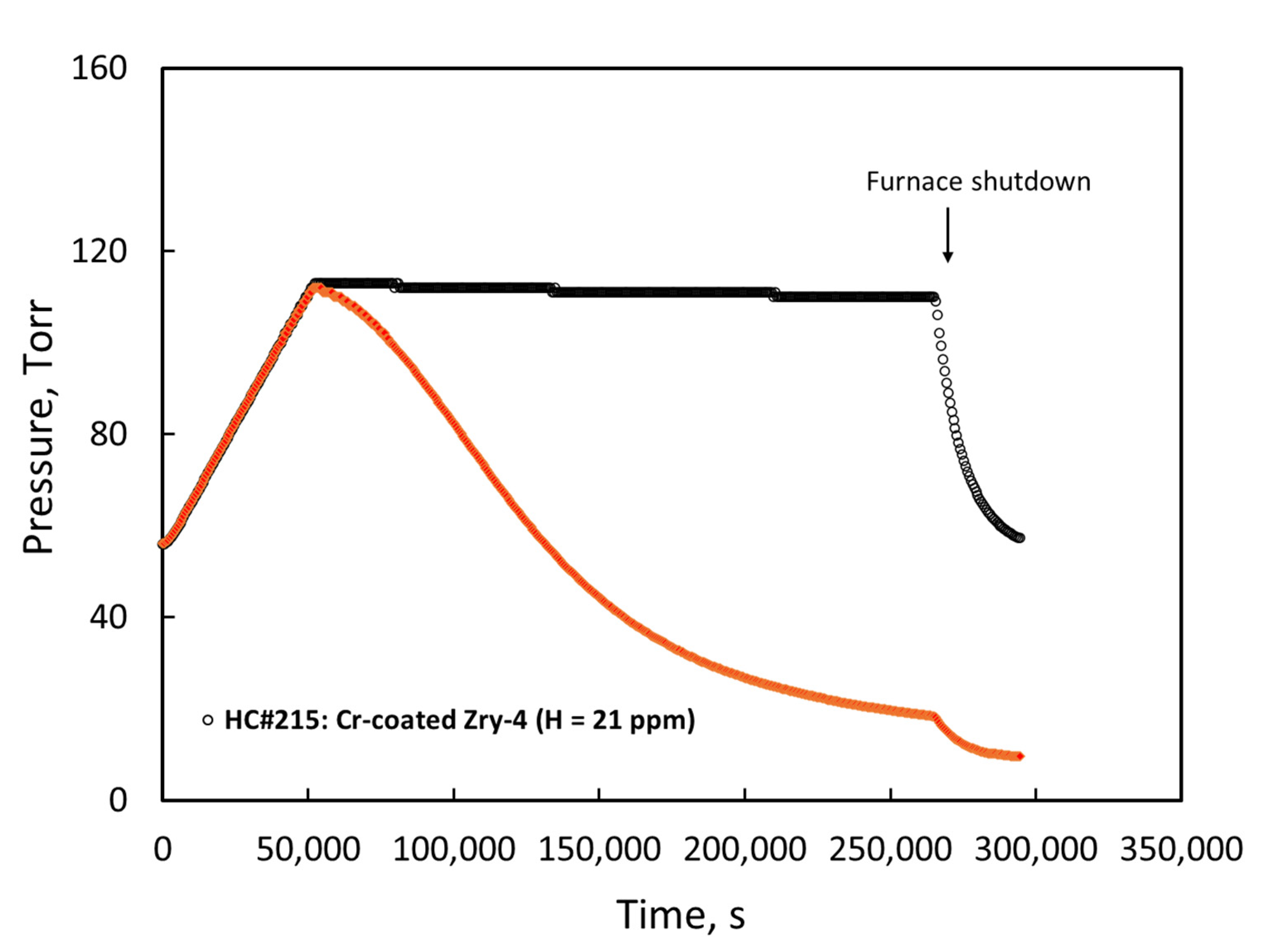
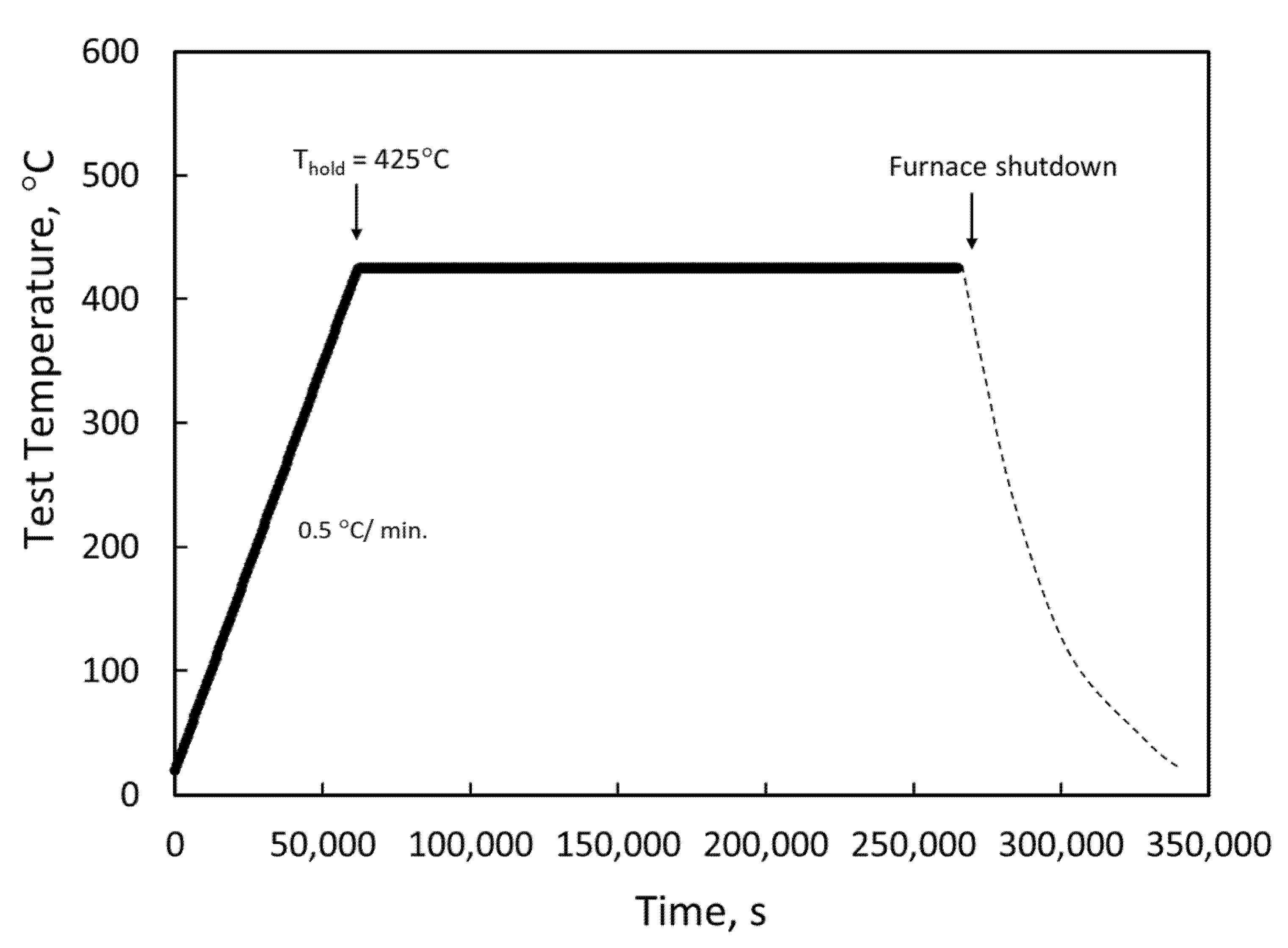
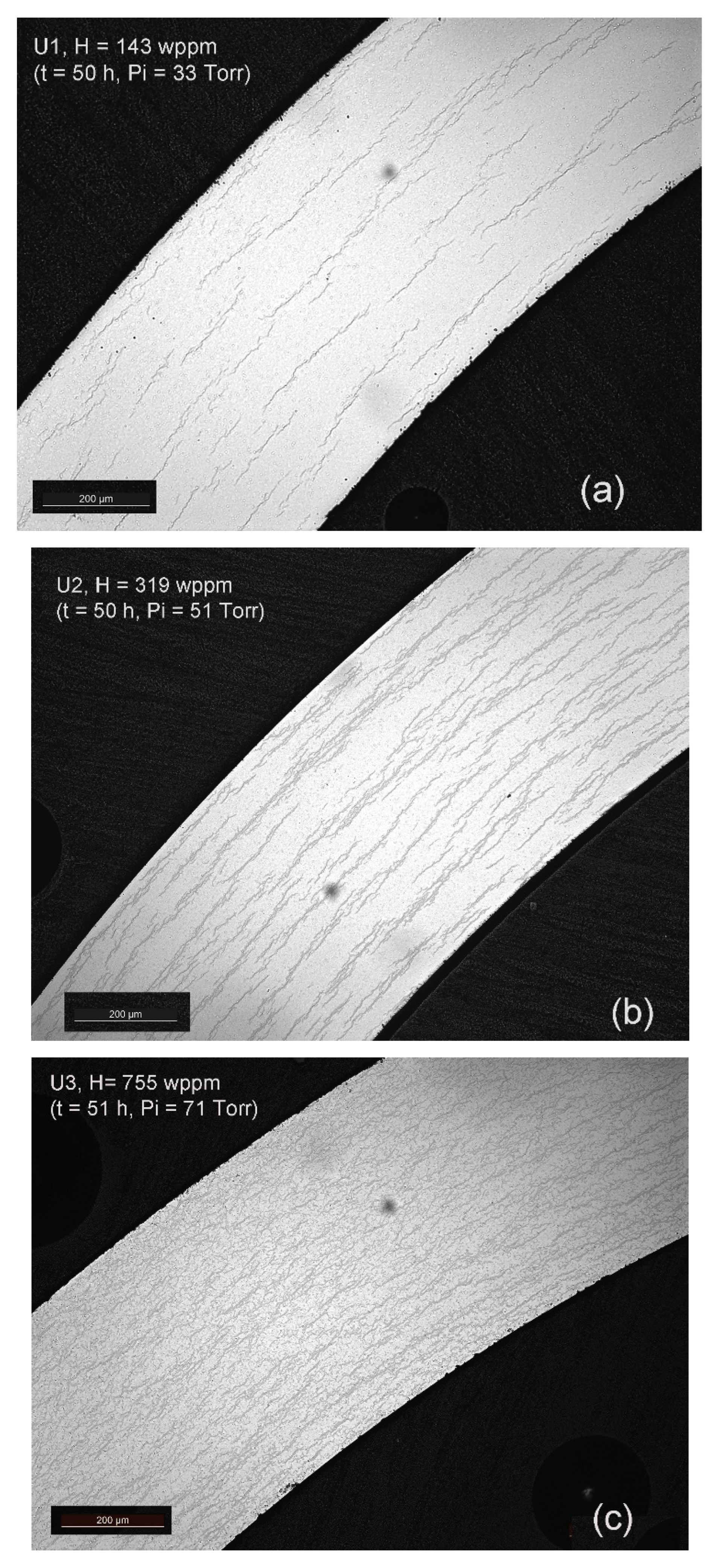
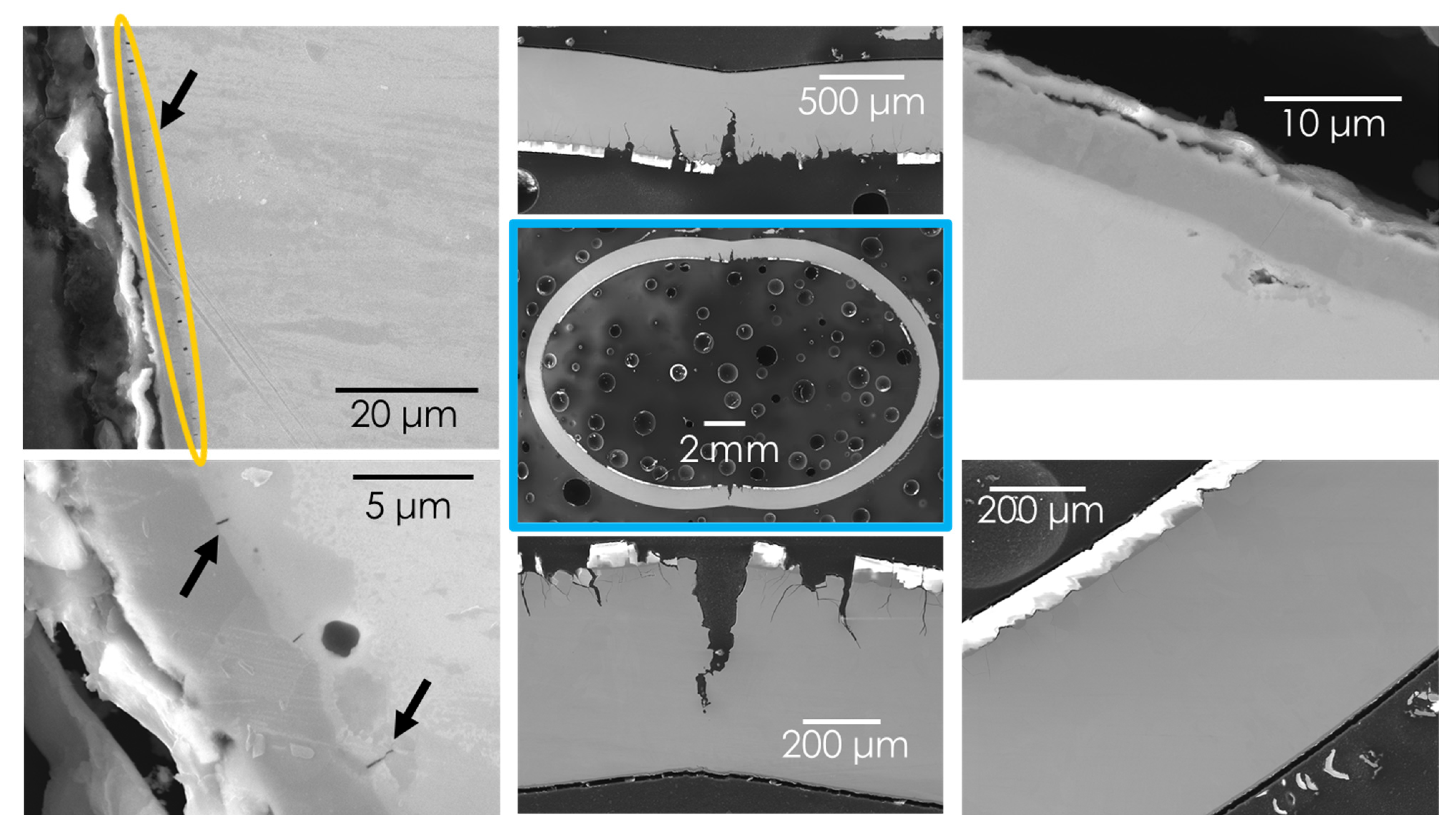
3.1. Materials Hydriding and Characterization
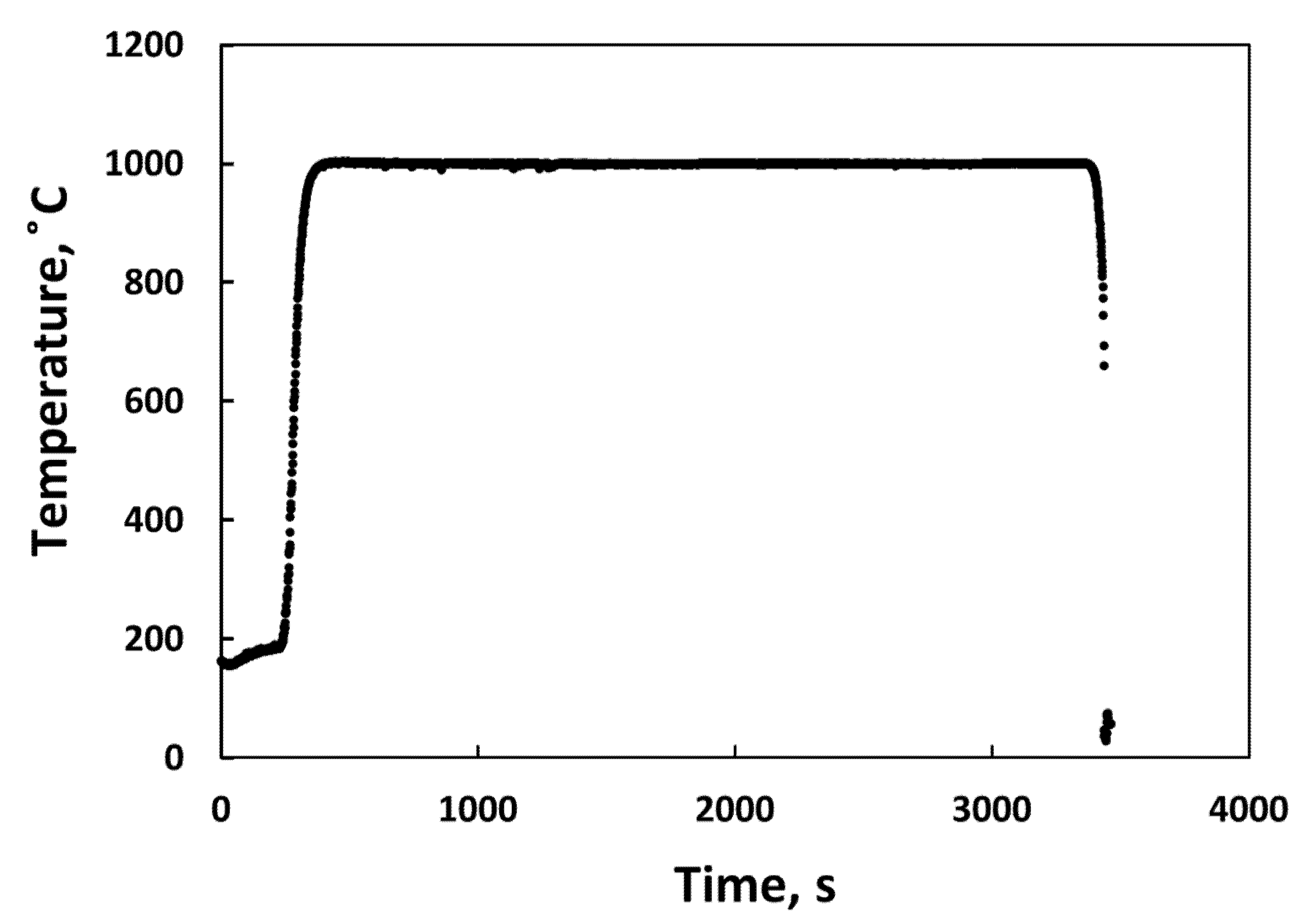
3.2. High Temperature Steam Oxidation Tests
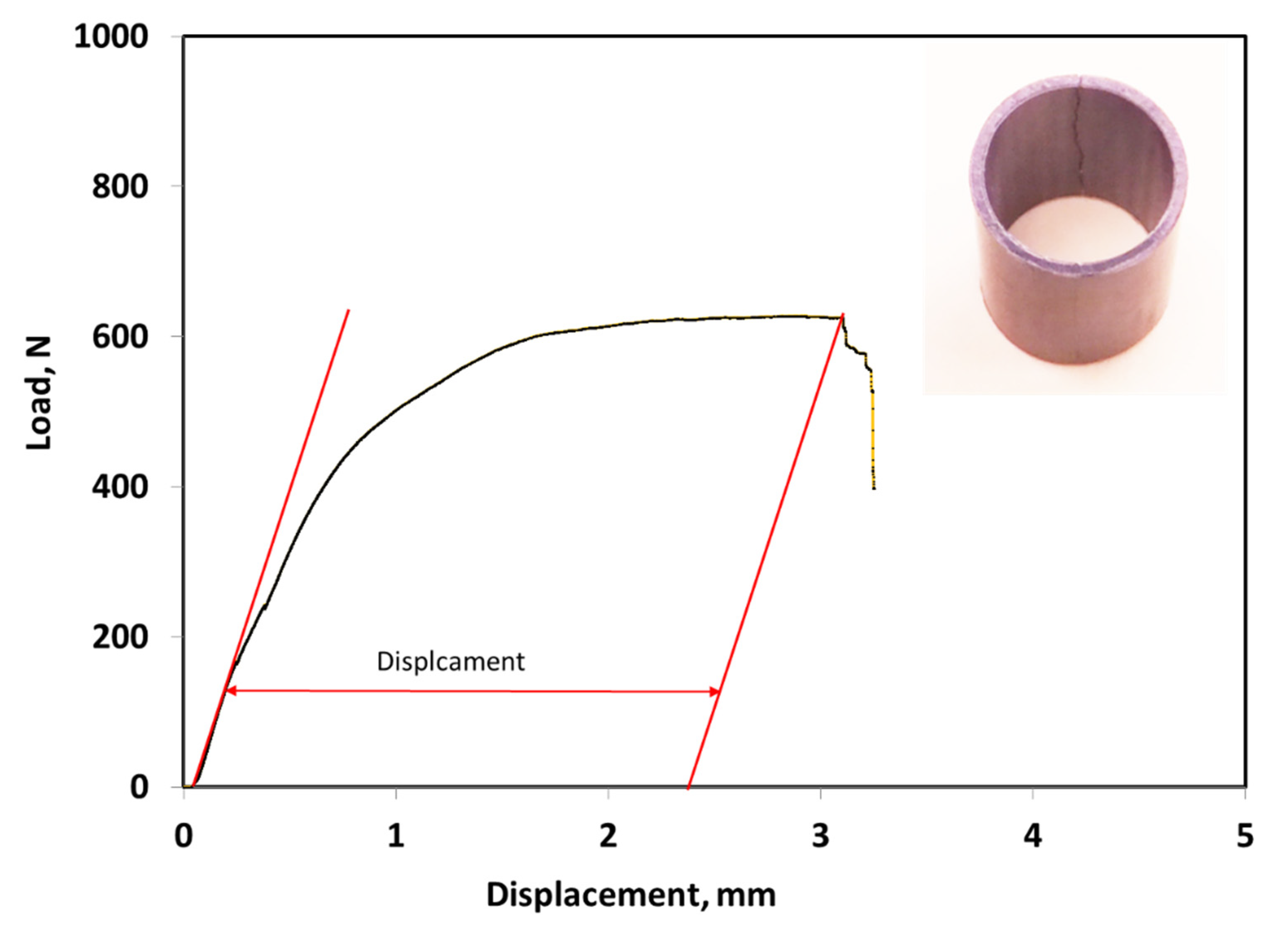
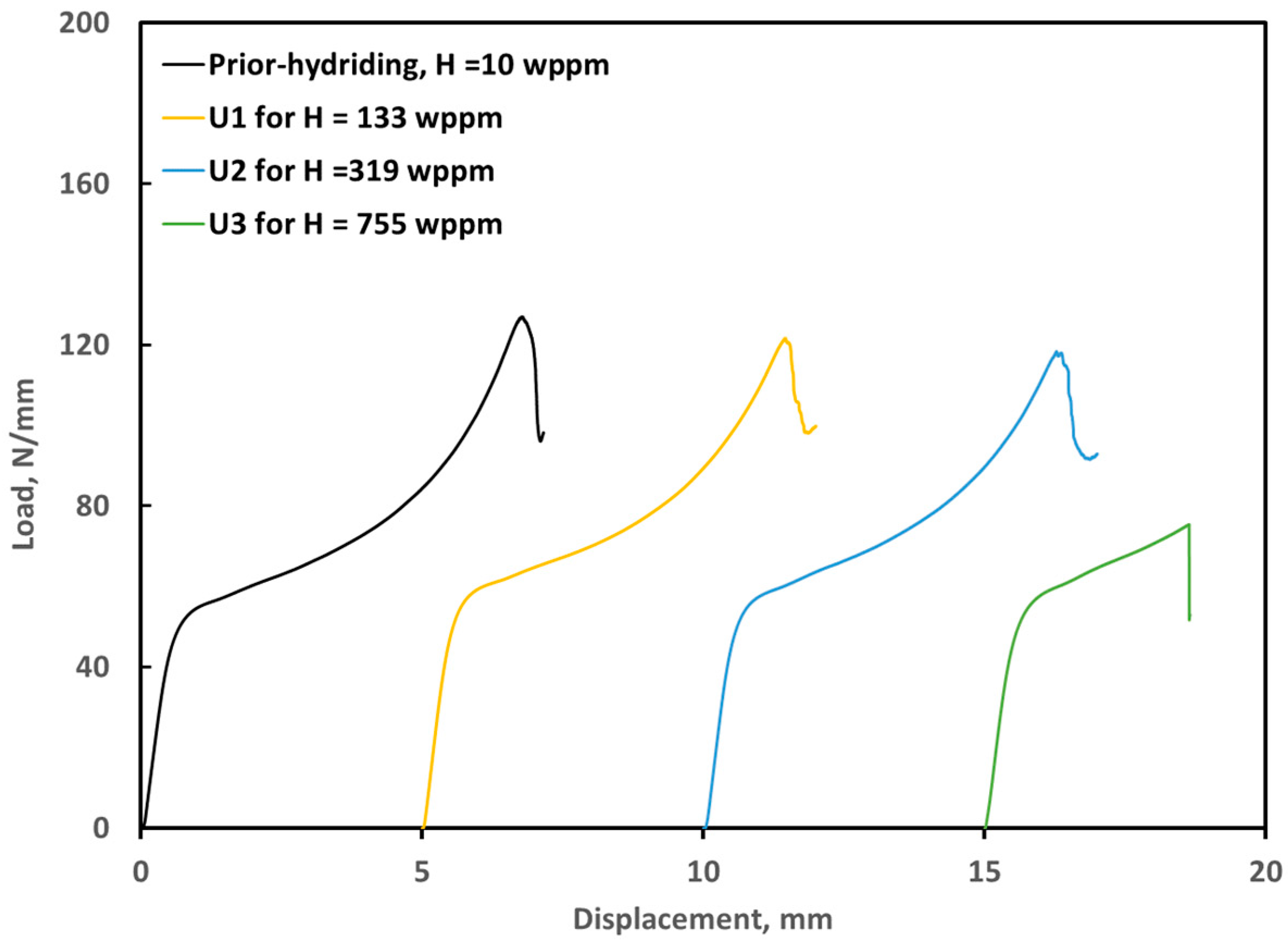
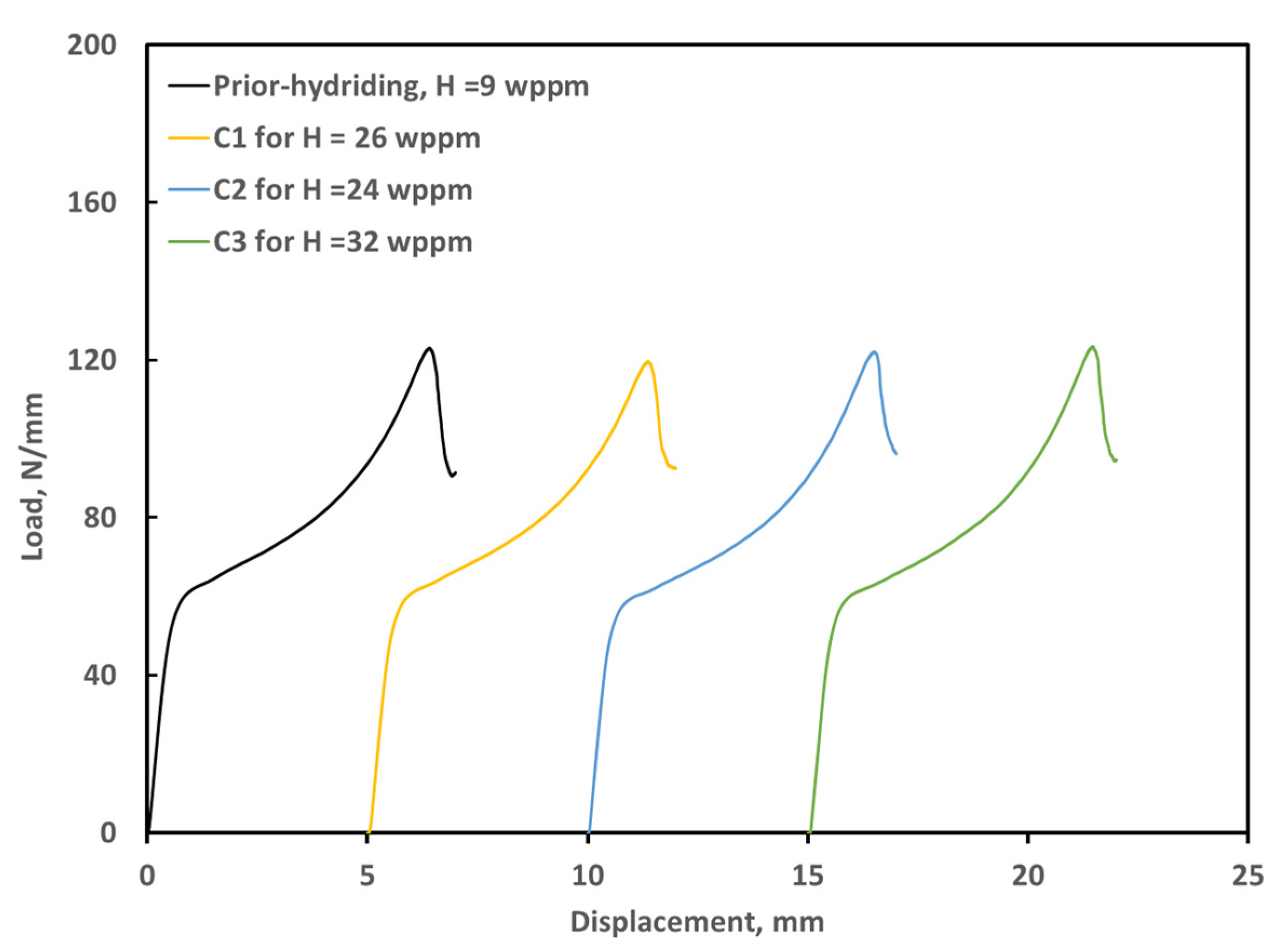
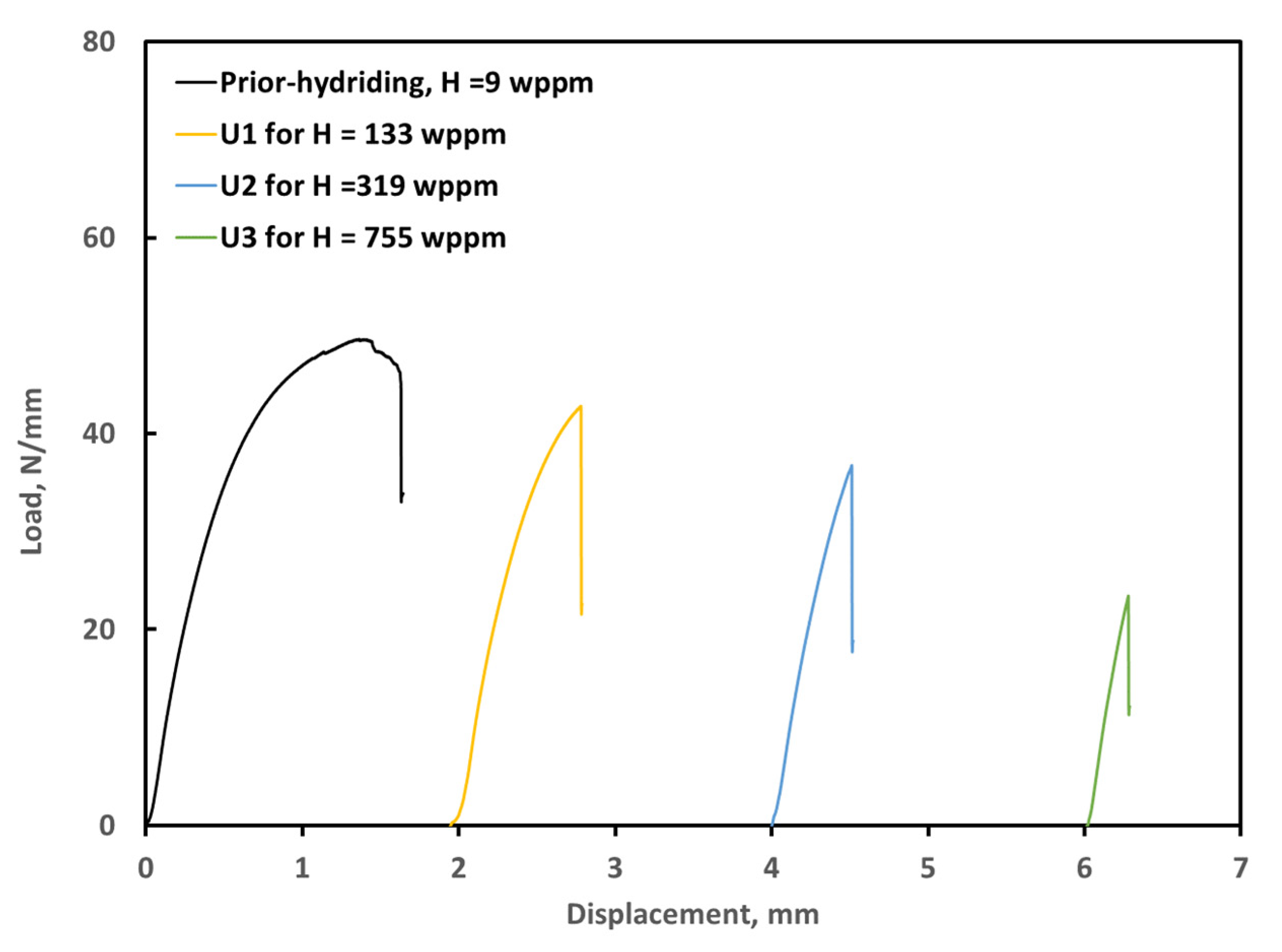
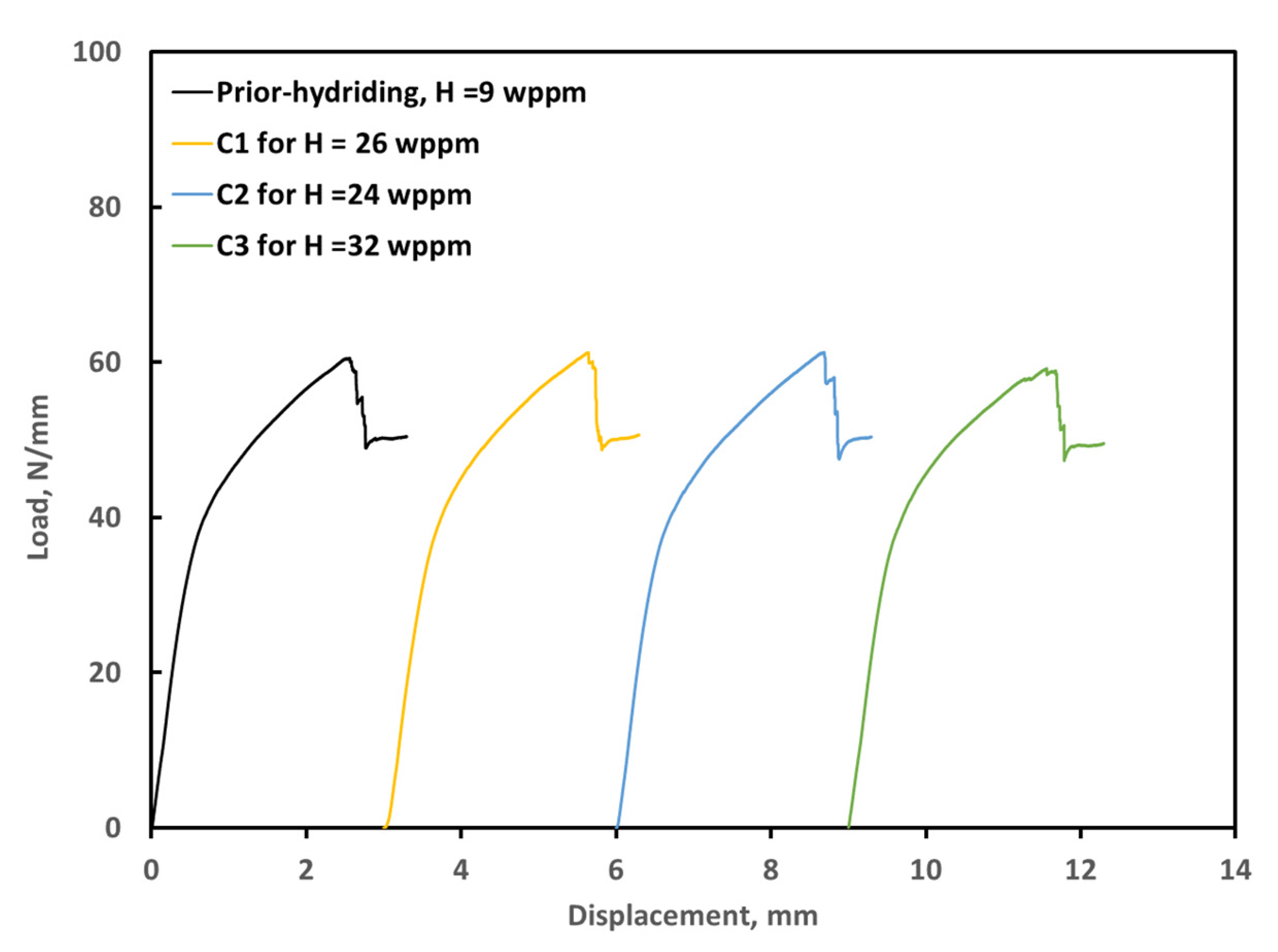
3.3. Ring Compression Testing of Hydrided and Oxidized Zircaloy-4 Specimens
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zinkle, S.J.; Terrani, K.A.; Gehin, J.C.; Ott, L.J.; Snead, L.L. Accident tolerant fuels for LWRs: A perspective. J. Nucl. Mater. 2014, 448, 374–379. [Google Scholar] [CrossRef]
- Kurata, M. Research and Development Methodology for Practical Use of Accident Tolerant Fuel in Light Water Reactors. Nucl. Eng. Technol. 2016, 48, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Stueber, M.; Seifert, H.J.; Steinbrueck, M. Protective coatings on zirconium-based alloys as accident-Tolerant fuel (ATF) claddings. Corros. Rev. 2017, 35, 141–165. [Google Scholar] [CrossRef]
- Terrani, K.A. Accident tolerant fuel cladding development: Promise, status, and challenges. J. Nucl. Mater. 2018, 501, 13–30. [Google Scholar] [CrossRef]
- Geelhood, K.G.; Luscher, W.G. Degradation and Failure Phenomena of Accident Tolerant Fuel Concepts; PNNL-28437; Pacific Northwest National Laboratory: Richland, WA, USA, 2019. [Google Scholar]
- Krejčí, J.; Ševeče, M.; Kabátová, J.; Manoch, F.; Kočí, J.; Cvrček, L.; Málek, J.; Krum, S.; Šutta, P.; Bublíková, P.; et al. Experimental Behavior of Chromium-Based Coatings. TopFuel 2018, 2018, A0233. [Google Scholar]
- Evans, A.K.; Kelly, P.J.; Goddard, D.T.; Cole-Baker, A.; Obasi, G.; Preuss, M.; Vernon, E.P. Fabrication, characterization, and testing of Cr-coated Zr alloy nuclear fuel rod cladding for enhanced accident tolerance. In Proceedings of the Light Water Reactor Fuel Performance Conference 2019, Seattle, WA, USA, 22–27 September 2019; pp. 864–872. [Google Scholar]
- Park, D.J.; Kim, H.G.; Jung, Y.I.; Park, J.H.; Yang, J.H.; Koo, Y.H. Behavior of an improved Zr fuel cladding with oxidation resistant coating under loss-of-coolant accident condition. J. Nucl. Mater. 2016, 482, 75–82. [Google Scholar] [CrossRef]
- Yeom, H.; Maier, B.; Johnson, G.; Dabney, T.; Lenling, M.; Sridharan, K. High temperature oxidation and microstructural evolution of cold spray chromium coatings on Zircaloy-4 in steam environments. J. Nucl. Mater. 2019, 526, 151737. [Google Scholar] [CrossRef]
- Yook, H.; Shirvan, K.; Phillips, B.; Lee, Y. Post-LOCA ductility of Cr-coated cladding and its embrittlement limit. J. Nucl. Mater. 2022, 558, 153354. [Google Scholar] [CrossRef]
- Malgin, A.; Sheleppov, I.; Markelov, V.; Eremin, I.; Vorobev, E.; Novikov, V.; Karpyuk, L. Corrosion and high teemperature oxidation resistance of chromium-coated zirconium based caldding for accident tolerant fuel. In Proceedings of the TopFuel 2021, Santander, Spain, 24–28 October 2021. [Google Scholar]
- Ma, H.; Yan, J.; Zhao, Y.; Liu, T.; Ren, Q.; Liao, Y.; Zuo, J.; Liu, G.; Yao, M. Oxidation behavior of Cr-coated zirconium alloy cladding in high-temperature steam above 1200 °C. Mater. Degrad. 2021, 5, 7. [Google Scholar] [CrossRef]
- Bell, S.B.; Graening, T.; Evans, A.; Kelly, P.; Pint, B.A.; Kane, K.A. Burst and oxidation behavior of Cr-coated Zirlo during simulated LOCA testing. J. Nucl. Mater. 2022, 564, 153679. [Google Scholar] [CrossRef]
- Yan, Y.; Qian, S.; Littrell, K.; Parish, C.M.; Plummer, L.K. Fast, Quantitative, and Nondestructive Evaluation of Hydrided LWR Fuel Cladding by Small Angle Incoherent Neutron Scattering of Hydrogen. J. Nucl. Mater. 2015, 460, 114–121. [Google Scholar] [CrossRef]
- US Nuclear Regulatroy Commission. NRC Draft Regulatory Guideline DG-1263: Establishing Analytical Limits for Zirconium-Based Alloy Cladding; US Nuclear Regulatroy Commission: Rockville, MD, USA, 2014.
- Yan, Y.; Garrison, B.E.; Howell, M.; Bell, G.L. High-Temperature Oxidation Kinetics of Sponge-Based E110 Cladding Alloy. J. Nucl. Mater. 2018, 499, 596. [Google Scholar] [CrossRef]
- Yan, Y.; Garrison, B.E.; Nelson, A.T.; Lutz, D. Correlations of the Steam Oxidation Rate Constant of BWR Alloy Zircaloy-2 at 800–1400 °C. Oxid. Met. 2022, 97, 227–239. [Google Scholar] [CrossRef]
- Billone, M.; Yan, Y.; Burtseva, T.; Daum, R. Cladding Embrittlement during Postulated Loss-of-Coolant Accidents; NUREG/CR-6967, ANL-07/04; Argonne National Lab. (ANL): Argonne, IL, USA, 2008. [Google Scholar]
- Cathcart, J.V.; Pawel, R.E.; McKee, R.A.; Druschel, R.E.; Yurek, G.J.; Cambell, J.J.; Jury, S.H. Zirconium Metal-Water Oxidation Kinetics IV; Reaction Rate Studies, ORNL/NUREG-17; Oak Ridge National Laboratory: Oak Riidge, TN, USA, 1977.
- Stein, F.; Leineweber, A. Laves phases: A review of their functional and structural applications and an improved fundamental understanding of stability and properties. J. Mater. Sci. 2021, 56, 5321–5427. [Google Scholar] [CrossRef]
- Jiang, J.; Du, M.; Pan, Z.; Yuan, M.; Ma, X.; Wang, B. Effects of oxidation and inter-diffusion on the fracture mechanisms of Cr-coated Zry-4 alloys: An in situ three-point bending study. Mater. Des. 2021, 212, 110168. [Google Scholar] [CrossRef]
- Hu, X.; Dong, C.; Wang, Q.; Chen, B.; Yang, H.; Wei, T.; Zhang, R.; Gu, W.; Chen, D. High-temperature oxidation of thick Cr coating prepared by arc deposition for accident tolerant fuel claddings. J. Nucl. Mater. 2019, 519, 145–156. [Google Scholar] [CrossRef]
- Han, X.; Chen, C.; Tan, Y.; Feng, W.; Peng, S.; Zhang, H. A systematic study of the oxidation behavior of Cr coatings on Zry4 substrates in high temperature steam environment. Corros. Sci. 2020, 174, 108826. [Google Scholar] [CrossRef]


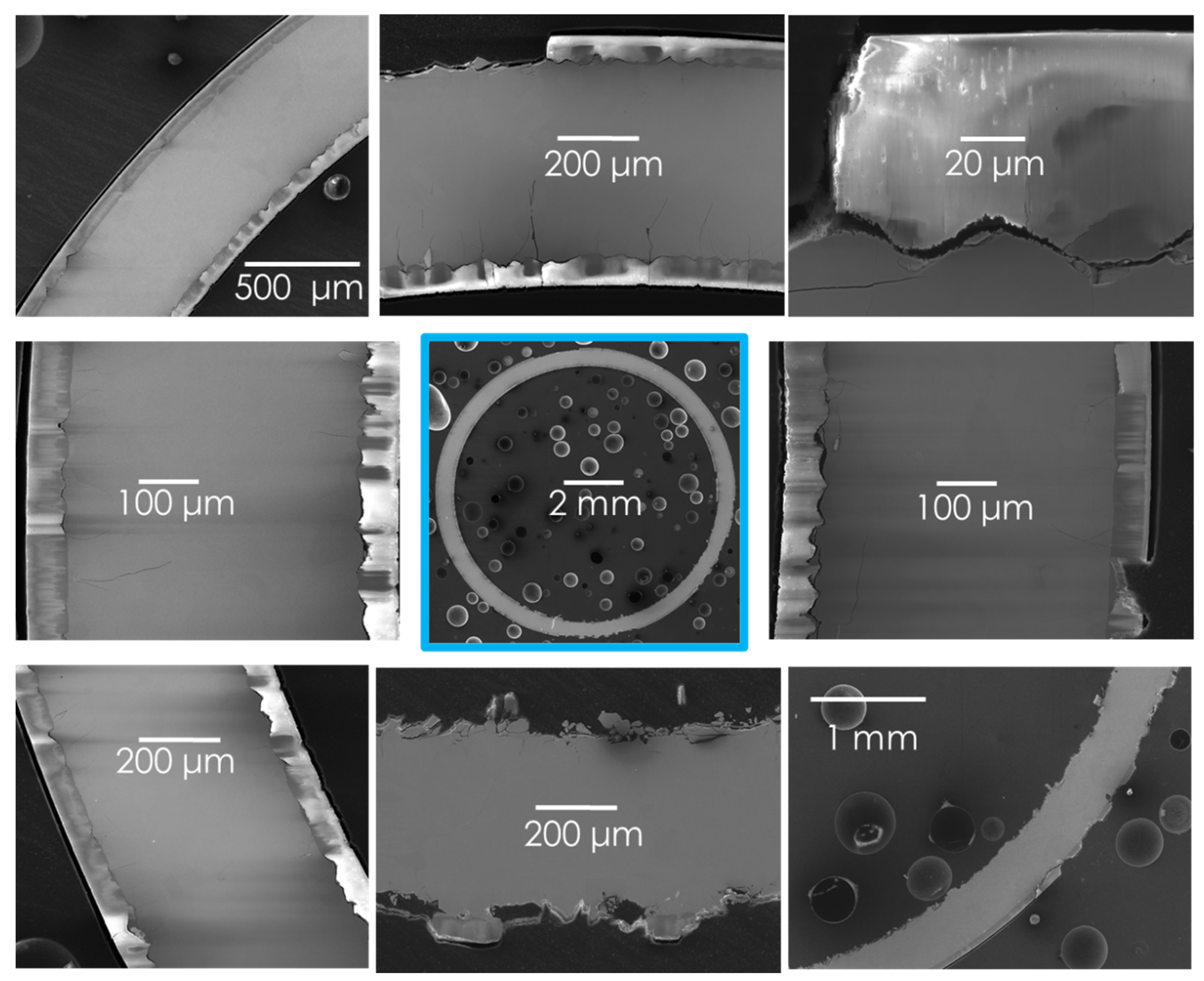
| Parameter | ORNL Zircaloy-4 |
|---|---|
| Zr (wt %) | Balance |
| Sn (wt %) | 1.29–1.37 |
| Fe (wt %) | 0.30–0.34 |
| Cr (wt %) | 0.10 |
| Ni (wt %) | - |
| H (wppm) | 4–6 |
| Sample | Materials | Hydriding Temp. (°C) | Pressure (Torr) | Time (h) | Measured H (wppm) | Comments |
|---|---|---|---|---|---|---|
| U0 | Uncoated | N/A | N/A | N/A | 10 | As-polished |
| C0 | Coated | N/A | N/A | N/A | 9 | As-coated |
| U1 | Uncoated | 425 | 33 | 50 | 143 | Target: 150 wppm |
| C1 | Coated | 425 | 33 | 50 | 26 | Repeat Test U1 |
| U2 | Uncoated | 425 | 51 | 50 | 319 | Target: 300 wppm |
| C2 | Coated | 425 | 51 | 50 | 24 | Repeat Test U2 |
| U3 | Uncoated | 425 | 71 | 51 | 755 | Target: 750 wppm |
| C3 | Coated | 425 | 71 | 51 | 32 | Repeat Test U3 |
| Sample | Measured H * (wppm) | Oxidation Temp. (°C) | Hold Time at Target Temp. (s) | Measured Weight Gain (mg/cm2) | Comments |
|---|---|---|---|---|---|
| ox1205-U0 | 10 | 1205 | 100 | 8.7 | As-polished |
| ox1205-C0 | 9 | 1205 | 100 | 4.5 | As-coated |
| ox1205-U1 | 143 | 1205 | 100 | 8.6 | Hydrided for 50 h, Pi = 32.7 Torr |
| ox1205-C1 | 26 | 1205 | 100 | 4.5 | Hydrided for 50 h, Pi = 32.8 Torr |
| ox1205-U2 | 319 | 1205 | 100 | 8.9 | Hydrided for 50 h, Pi = 51.1 Torr |
| ox1205-C2 | 24 | 1205 | 100 | 4.6 | Hydrided for 50 h, Pi = 51.0 Torr |
| ox1205-U3 | 755 | 1205 | 100 | 8.6 | Hydrided for 51 h, Pi = 71.3 Torr |
| ox1205-C3 | 32 | 1205 | 100 | 4.5 | Hydrided for 51 h, Pi = 71.3 Torr |
| Sample | Measured H * (wppm) | Oxidation Temp. (°C) | Hold Time at Target Temp. (s) | Measured Weight Gain (mg/cm2) | Comments |
|---|---|---|---|---|---|
| ox1000-U0 | 10 | 1000 | 3000 | 12.0 | As-polished |
| ox1000-C0 | 9 | 1000 | 3000 | 5.9 | As-coated |
| ox1000-U1 | 143 | 1000 | 3000 | 11.8 | Hydrided for 50 h, Pi = 32.7 Torr |
| ox1000-C1 | 26 | 1000 | 100 | 5.9 | Hydrided for 50 h, Pi = 32.8 Torr |
| ox1000-U2 | 319 | 1000 | 3000 | 12.1 | Hydrided for 50 h, Pi = 51.1 Torr |
| ox1000-C2 | 24 | 1000 | 3000 | 6.1 | Hydrided for 50 h, Pi = 51.0 Torr |
| ox1000-U3 | 755 | 1000 | 3000 | 12.3 | Hydrided for 51 h, Pi = 71.3 Torr |
| ox1000-C3 | 32 | 1000 | 3000 | 6.0 | Hydrided for 51 h, Pi = 71.3 Torr |
| Sample | Measured H * (wppm) | Weight Gain (mg/cm2) | Offset Displacement(mm) | Offset Strain (%) | Comments |
|---|---|---|---|---|---|
| ox1205-U0 | 10 | 8.7 | 0.92 | 9.7 | As-polished |
| ox1205-C0 | 9 | 4.5 | 1.88 | 19.7 | As-coated |
| ox1205-U1 | 143 | 8.6 | 0.18 | 1.8 | Hydrided for 50 h, Pi = 32.7 Torr |
| ox1205-C1 | 26 | 4.5 | 1.85 | 19.5 | Hydrided for 50 h, Pi = 32.8 Torr |
| ox1205-U2 | 319 | 8.9 | 0.14 | 1.4 | Hydrided for 50 h, Pi = 51.1 Torr |
| ox1205-C2 | 24 | 4.6 | 1.88 | 19.7 | Hydrided for 50 h, Pi = 51.0 Torr |
| ox1205-U3 | 755 | 8.6 | 0.04 | 0.4 | Hydrided for 51 h, Pi = 71.3 Torr |
| ox1205-C3 | 32 | 4.5 | 1.86 | 19.6 | Hydrided for 51 h, Pi = 71.3 Torr |
| Sample | Measured H * (wppm) | Weight Gain (mg/cm2) | Offset Displacement (mm) | Offset Strain (%) | Comments |
|---|---|---|---|---|---|
| ox1000-U0 | 10 | 12.0 | 0.4 | 4.2 | As-polished |
| ox1000-C0 | 9 | 5.9 | 2.5 | 26.4 | As-coated |
| ox1000-U1 | 143 | 11.8 | 0.27 | 2.8 | Hydrided for 50 h, Pi = 32.7 Torr |
| ox1000-C1 | 26 | 5.9 | 2.43 | 25.6 | Hydrided for 50 h, Pi = 32.8 Torr |
| ox1000-U2 | 319 | 12.1 | 0.15 | 1.6 | Hydrided for 50 h, Pi = 51.1 Torr |
| ox1000-C2 | 24 | 6.1 | 2.28 | 24.0 | Hydrided for 50 h, Pi = 51.0 Torr |
| ox1000-U3 | 755 | 12.3 | 0.03 | 0.3 | Hydrided for 51 h, Pi = 71.3 Torr |
| ox1000-C3 | 32 | 6.0 | 2.51 | 26.4 | Hydrided for 51 h, Pi = 71.3 Torr |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Graening, T.; Nelson, A.T. Hydriding, Oxidation, and Ductility Evaluation of Cr-Coated Zircaloy-4 Tubing. Metals 2022, 12, 1998. https://doi.org/10.3390/met12121998
Yan Y, Graening T, Nelson AT. Hydriding, Oxidation, and Ductility Evaluation of Cr-Coated Zircaloy-4 Tubing. Metals. 2022; 12(12):1998. https://doi.org/10.3390/met12121998
Chicago/Turabian StyleYan, Yong, Tim Graening, and Andrew T. Nelson. 2022. "Hydriding, Oxidation, and Ductility Evaluation of Cr-Coated Zircaloy-4 Tubing" Metals 12, no. 12: 1998. https://doi.org/10.3390/met12121998
APA StyleYan, Y., Graening, T., & Nelson, A. T. (2022). Hydriding, Oxidation, and Ductility Evaluation of Cr-Coated Zircaloy-4 Tubing. Metals, 12(12), 1998. https://doi.org/10.3390/met12121998





