AM60-AlN Nanocomposite and AM60 Alloy Corrosion Activity in Simulated Marine-Coastal Ambience
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and SME Model Solution
2.2. Immersion Test and Analysis of Surface
2.3. Electrochemical Methods
3. Results and Discussion
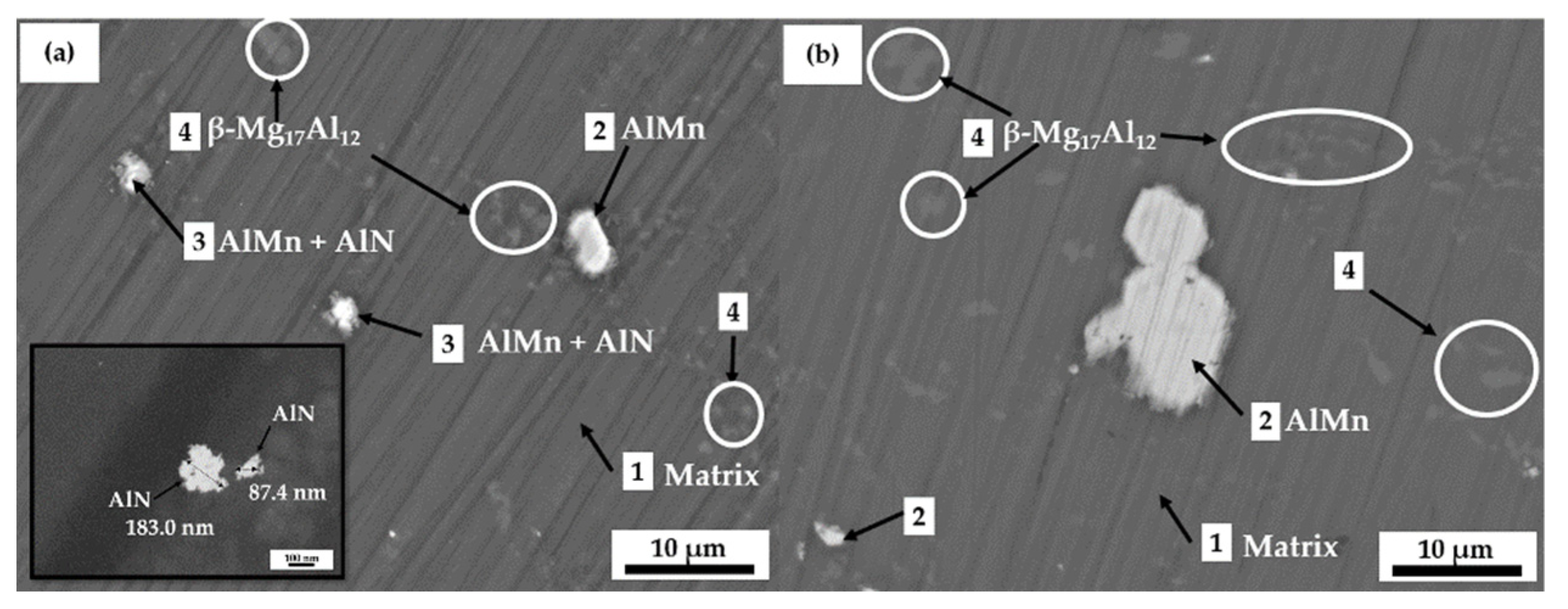
3.1. Microstructure of AM60-AlN and AM60
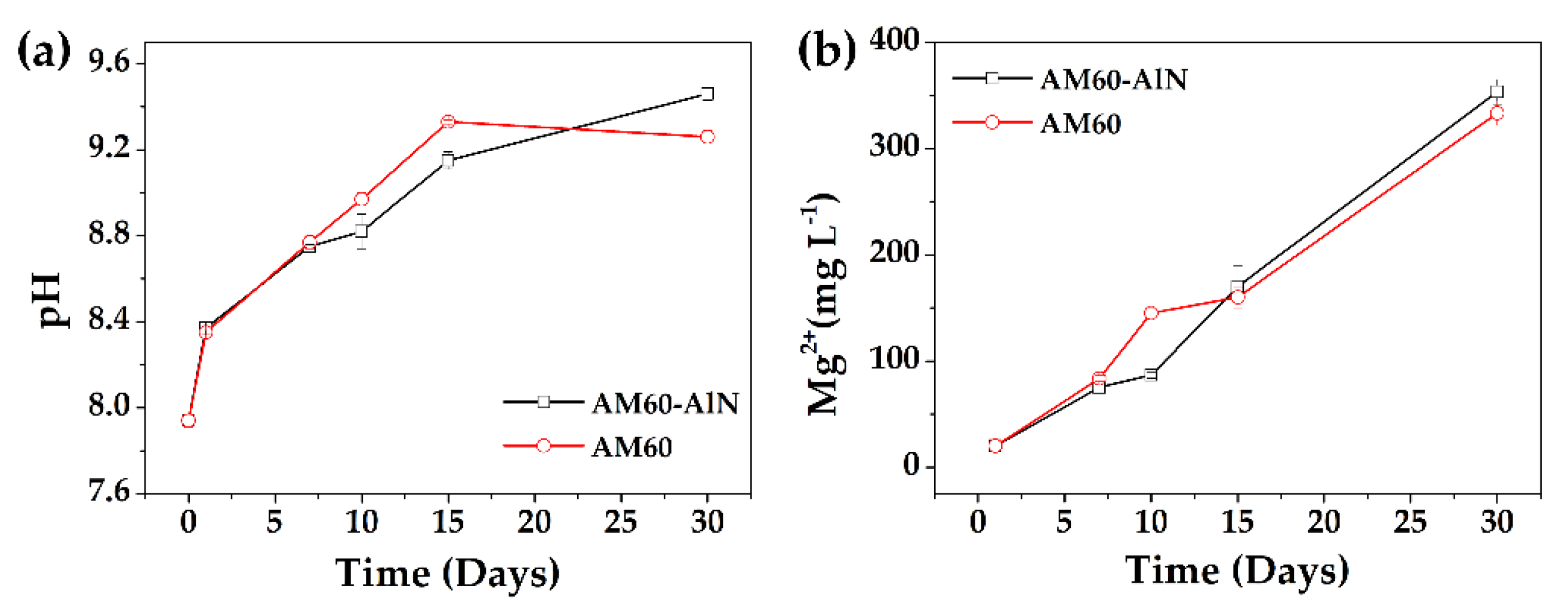
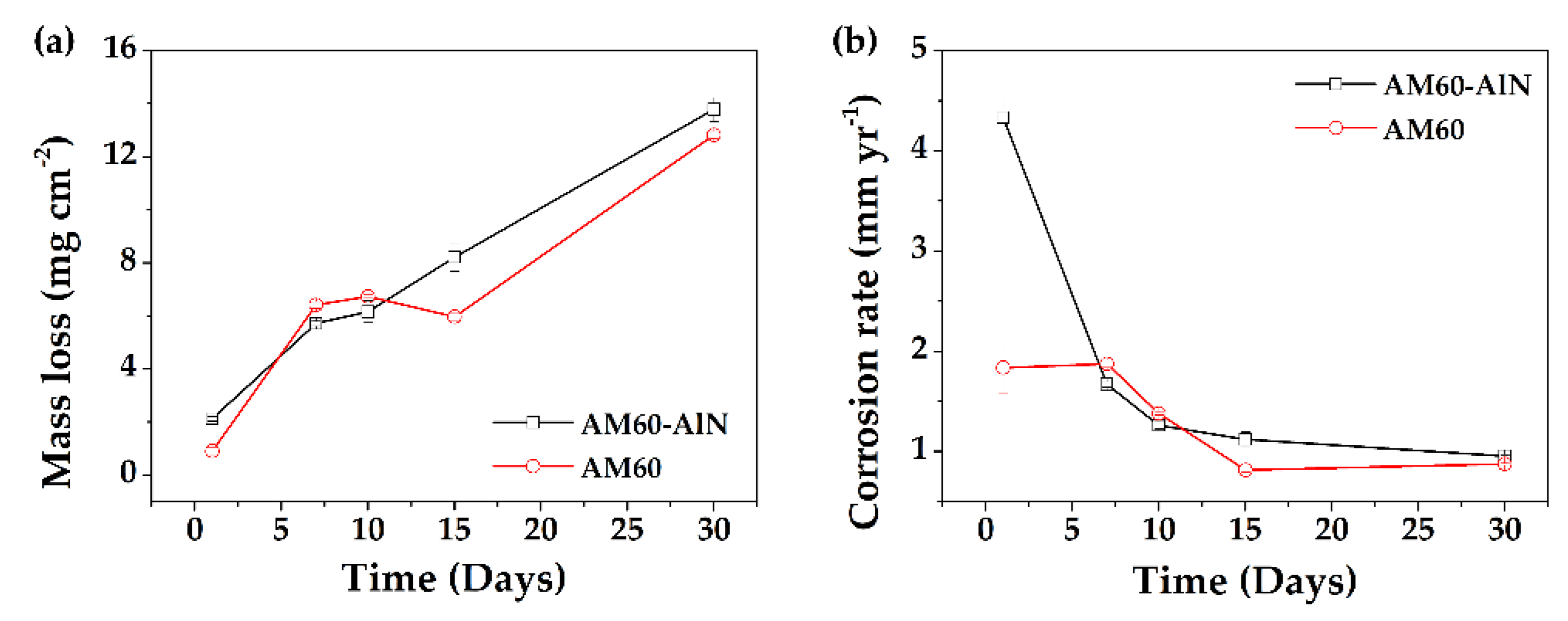
3.2. Solution Monitoring and Mass Loss
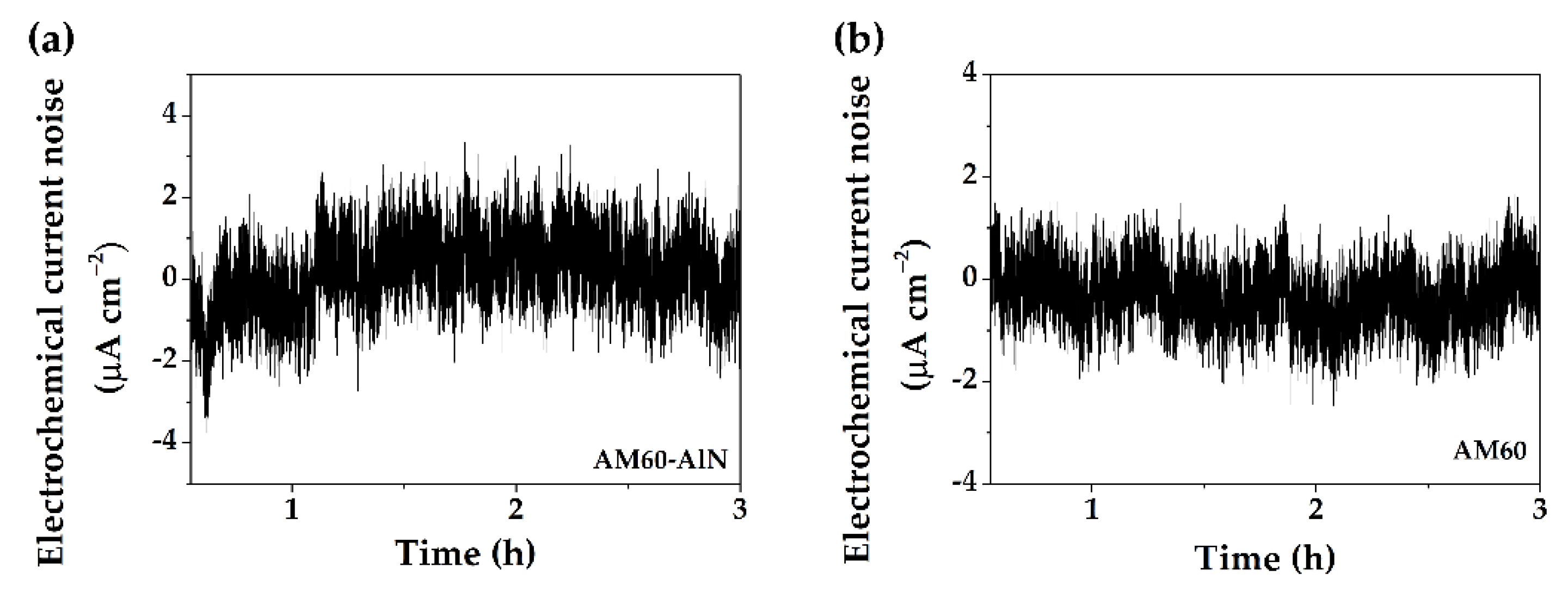
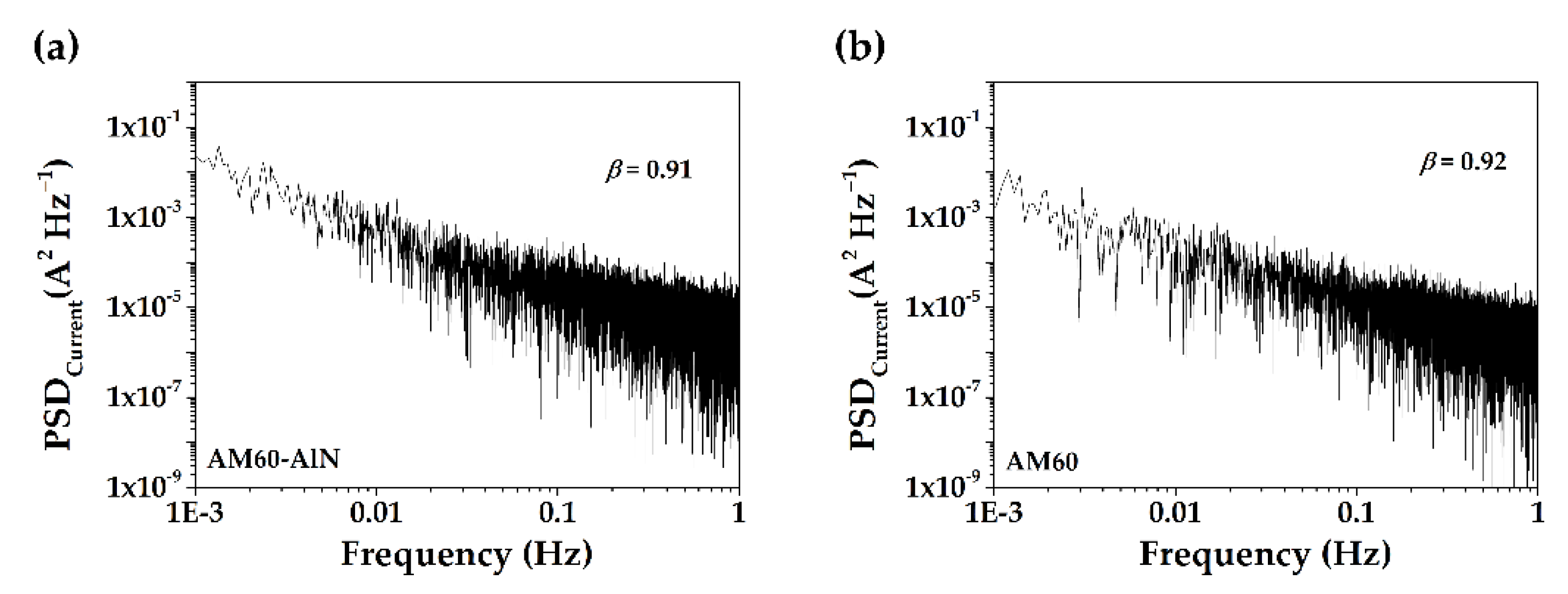
3.3. Electrochemical Measurement
3.4. Surface Characterization after Immersion in SME
3.4.1. SEM-EDS Analysis
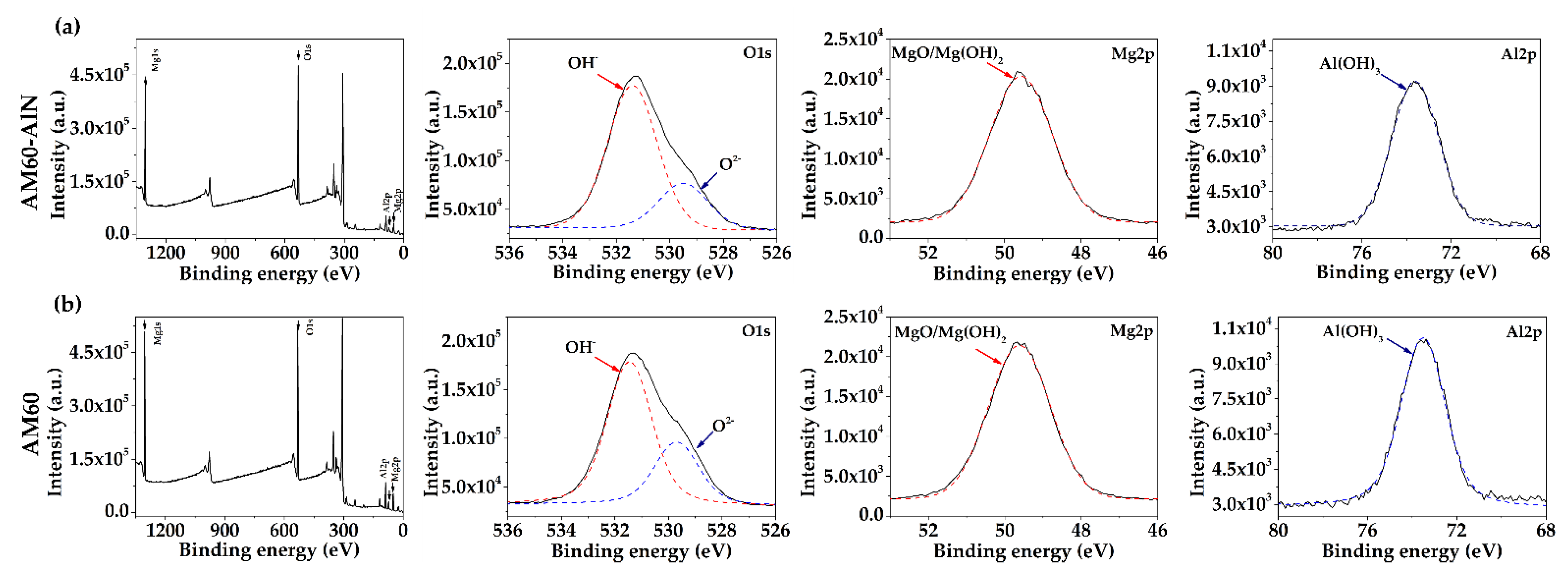
3.4.2. XPS Spectra Analysis
4. Conclusions
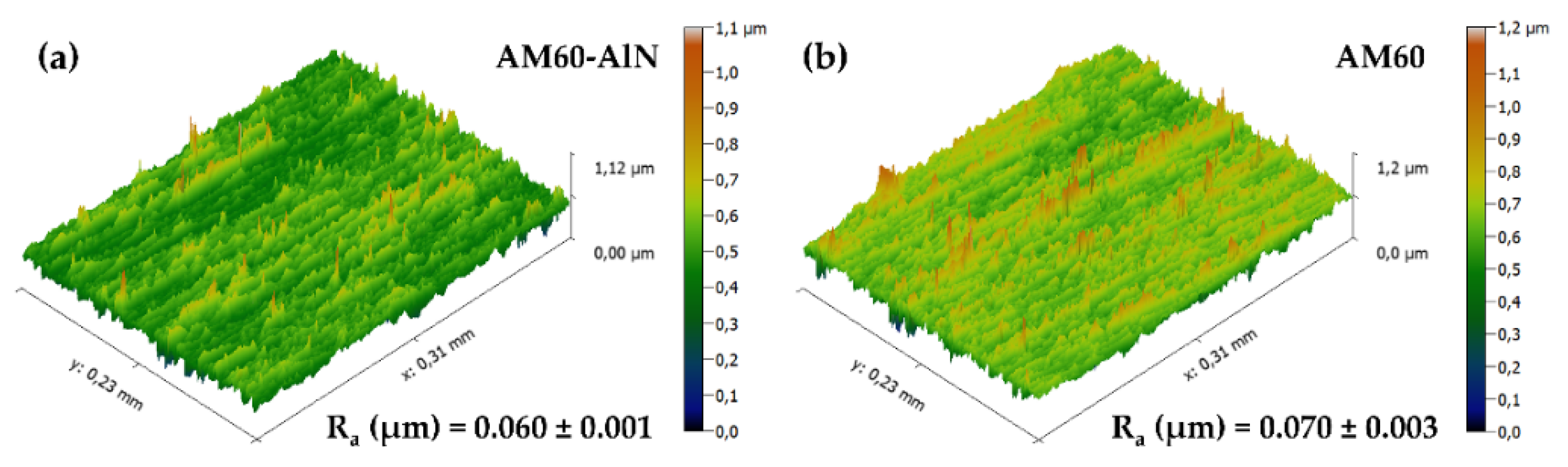
- The nanocomposite presented ≈15% decrease in the roughness, attributed to the incorporation of the AlN nanoparticles that allowed the diminishing in grain size.
- During the immersion of the studied alloys, the initial pH value (7.94) of the SME solution shifted to more alkaline values (>9), which lead to an instablity of the AlN, followed by a slight rise in the Mg-matrix corrosion and release of Mg-ions over time, influenced by the presence of Cl-ions in the SME solution.
- According to SEM-EDS and XPS analysis, the main corrosion products of the Mg-matrix was , accompanied by , as well as also by a low content of corrosion product, attributed to the Al-Mn phase and AlN particles corrosion at pH >9, in the presence of chloride ions.
- The corrosion layers on the alloy surfaces showed cracks, which may favor the chloride ion penetration to the Mg-matrix. After removal of the corrosion layers, the Al-Mn intermetallic particles remained on the alloy surfaces and were considered as efficient local cathodes, accelerating in their vicinity the corrosion attack of the Mg-matrix.
- The electrochemical noise resistance , as an equivalent to the polarization resistance, showed that AM60-AlN nanocomposite value was lower at 30 days than that of AM60 alloy, which in fact suggested that the progress in the corrosion of the nanocomposite occurred with less difficulty.
- The corrosion current fluctuations were classified as electrochemical noise (EN) and their power spectral density (PSD) plots, exponent values, suggested that the corrosion process is weakly persistent localized, dominated by fractional Gaussian noise (fGn).
- Cross-sectional SEM images revealed higher depths of localized corrosion attacks on the AM60-AlN nanocomposite surface. The β-Mg17Al12 secondary phase was considered as surface locations for corrosion pits.
- The results of this study suggest that the surfaces of the studied alloys will need a posterior treatment and/or application of protective coatings to improve the corrosion resistance in the presence of chloride ions, which are characteristics of the marine-coastal environment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, T.; Shao, Y.; Meng, G.; Wang, F. Electrochemical noise analysis of the corrosion of AZ91D magnesium alloy in alkaline chloride solution. Electrochim. Acta 2007, 53, 561–568. [Google Scholar] [CrossRef]
- Schumann, S.; Friedrich, H.E. Engineering Requirements, Strategies and Examplaes. In Magnesium Technology: Metallurgy, Design Data, Applications; Friedrich, H.E., Mordike, B.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 499–632. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Hou, J.; Du, W. A review on thermal conductivity of magnesium and its alloys. J. Magnes. Alloy. 2020, 8, 78–90. [Google Scholar] [CrossRef]
- Watarai, H. Trend of research and development for magnesium alloys-Reducing the weight of structural materials in motor vehicles. Sci. Technol. Trends. 2006, 18, 84–97. [Google Scholar]
- Mordike, B.L.; Ebert, T. Magnesium: Properties—Applications—Potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Arrabal, R.; Feliú, S., Jr. Influence of microstructure and composition on the corrosion behaviour of Mg/Al alloys in chloride media. Electrochim. Acta 2008, 53, 7890–7902. [Google Scholar] [CrossRef]
- Brady, M.P.; Joost, W.J.; Warren, C.D. Insights from a recent meeting: Current status and future directions in magnesium corrosion research. Corrosion 2017, 73, 452–462. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbillis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Nguyen, Q.B.; Sim, Y.H.M.; Gupta, M.; Lim, C.Y.J. Tribology characteristics of magnesium alloy AZ31B and its composites. Tribol. Int. 2015, 82, 464–471. [Google Scholar] [CrossRef]
- Makar, G.; Kruger, J. Corrosion of magnesium. Int. Mater. Rev. 1993, 38, 138–153. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; National Assoc Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Cui, Z.Y.; Li, X.G.; Dong, C.F.; Liu, Z.Y.; Wang, L.W. Pitting corrosion behaviour of AZ31 magnesium in tropical marine atmosphere. Corros. Eng. Sci. Technol. 2014, 49, 363–371. [Google Scholar] [CrossRef]
- Haynes, W.M. Properties of the elements and inorganic compounds. In CRC Handbook of Chemistry and Physics, 97th ed.; Haynes, W.M., Ed.; CRC Press Technology: Boca Raton, FL, USA, 2017; pp. 4–71. [Google Scholar]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–13. [Google Scholar] [CrossRef]
- Polmear, I.; StJhon, D.; Nie, J.-F.; Qian, M. Magnesium alloys. In Light Alloys: Metallurgy of the Light Metals, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 287–367. [Google Scholar]
- Cheng, Y.-L.; Qin, T.-W.; Wang, H.-M.; Zhang, Z. Comparison of corrosion behaviors of AZ31, AZ91, AM60 and ZK60 magnesium alloys. Trans. Nonferrous Met. Soc. China 2009, 19, 517–524. [Google Scholar] [CrossRef]
- Jönsson, M.; Persson, D.; Leygraf, C. Atmospheric corrosion of field-exposed magnesium alloy AZ91D. Corros. Sci. 2008, 50, 1406–1413. [Google Scholar] [CrossRef]
- Lindström, R.; Svensson, J.-E.; Johansson, L.-G. The influence of carbon dioxide on the atmospheric corrosion of some magnesium alloys in the presence of NaCl. J. Electrochem. Soc. 2002, 149, B103–B107. [Google Scholar] [CrossRef]
- LeBozec, N.; Jönsson, M.; Thierry, D. Atmospheric Corrosion of Magnesium Alloys: Influence of Temperature, Relative Humidity. Corrosion 2004, 60, 356–361. [Google Scholar] [CrossRef]
- Danaie, M.; Asmussen, R.M.; Jakupi, P.; Shoesmith, D.W.; Botton, G.A. The role of aluminum distribution on the local corrosion resistance of the microstructure in a sand-cast AM50 alloy. Corros. Sci. 2013, 77, 151–163. [Google Scholar] [CrossRef]
- Cao, F.; Zhao, C.; You, J.; Hu, J.; Zheng, D.; Song, G.-L. The inhibitive effect of artificial seawater on magnesium corrosion. Adv. Eng. Mater. 2019, 21, 1900363–1900376. [Google Scholar] [CrossRef]
- Cuevas, A.C.; Bercerril, E.B.; Martínez, M.S.; Ruiz, J.L. Metal Matrix Composites; Springer Nature: Basel, Switzerland, 2018; p. 4. [Google Scholar] [CrossRef]
- Chawla, K.K. Metal matrix composite. In Composite Materials: Science and Engineering, 3rd ed.; Chawla, K.K., Ed.; Springer: Birmingham, AL, USA, 2012; pp. 197–248. [Google Scholar] [CrossRef]
- Dey, A.; Pandey, K.M. Magnesium Metal Matrix Composites—A Review. Rev. Adv. Mater. Sci. 2015, 42, 58–67. [Google Scholar]
- Malaki, M.; Xu, W.; Kasar, A.K.; Menezes, P.L.; Dieringa, H.; Varma, R.S.; Gupta, M. Advanced metal matrix nanocomposites. Metals 2019, 9, 330. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Deepakaravind, V.; Gopal, P.; Azhagiri, P. Analysis the mechanical properties and material characterization on Magnesium Metal Matrix Nano composites through stir casting process. Mater. Today: Proc. 2021, 46, 7436–7441. [Google Scholar] [CrossRef]
- Haghshenas, M. Metal–matrix composites. Ref. Mod. Mater. Sci. Mater. Eng. 2016, 03950–03953. [Google Scholar] [CrossRef]
- Lee, C.J.; Huang, J.C.; Hsieh, P.J. Mg based nano-composites fabricated by friction stir processing. Scr. Mater. 2006, 54, 1415–1420. [Google Scholar] [CrossRef]
- Hassan, S.F. Creation of New Magnesium-Based Material Using Different Types of Reinforcements. Ph.D Thesis, National University of Singapore, Singapore, 2006. [Google Scholar]
- Tun, K.S. Development and Characterization of New Magnesium Based Nanocomposites. Ph.D. Thesis, National University of Singapore, Singapore, 2009. [Google Scholar]
- Choi, H.; Alba-Baena, N.; Nimityongskul, S.; Jones, M.; Wood, T.; Sahoo, M.; Lakes, R.; Kou, S.; Li, X. Characterization of hot extruded Mg/SiC nanocomposites fabricated by casting. J. Mater. Sci. 2011, 46, 2991–2997. [Google Scholar] [CrossRef]
- Nie, K.B.; Wang, X.J.; Wu, K.; Hu, X.S.; Zheng, M.Y. Development of SiCp/AZ91 magnesium matrix nanocomposites using ultrasonic vibration. Mater. Sci. Eng. A 2012, 540, 123–129. [Google Scholar] [CrossRef]
- Dieringa, H.; Katsarou, L.; Buzolin, R.; Szakácks, G.; Horstmann, M.; Wolff, M.; Mendis, C.; Vorozhtsov, S.; StJhon, D. Ultrasound assisted casting of an AM60 based metal matrix nanocomposite, its properties, and recyclability. Metals 2017, 7, 388. [Google Scholar] [CrossRef]
- Bedolla, E.; Lemus-Ruiz, J.; Contreras, A. Synthesis and characterization of Mg-AZ91/AlN composites. Mater. Des. 2012, 38, 91–98. [Google Scholar] [CrossRef]
- Cao, G.; Choi, H.; Oportus, J.; Konishi, H.; Li, X. Study on tensile properties and microstructure of cast AZ91D/AlN nanocomposites. Mater. Sci. Eng. A 2008, 494, 127–131. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Habibi, M.K.; Jayalakshmi, S.; Jia Ai, K.; Almajida, A.; Gupta, M. Nano-AlN particle reinforced Mg composites: Microstructural and mechanical properties. Mater. Sci. Technol. 2015, 31, 1122–1131. [Google Scholar] [CrossRef]
- Saboori, A.; Padovano, E.; Pavese, M.; Badini, C. Novel magnesium Elektron21-AlN nanocomposites produced by ultrasound-assisted casting; microstructure, thermal and electrical conductivity. Materials 2018, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Huang, Y.; Gavras, S.; Kainer, K.U.; Hort, N.; Dieringa, H. Influences of AlN/Al Nanoparticles on the Creep Properties of Elektron21 Prepared by High Shear Dispersion Technology. JOM 2019, 71, 2245–2252. [Google Scholar] [CrossRef]
- Giannopoulou, D.; Dieringa, H.; Bohlen, J. Influence of AlN nanoparticle addition on microstructure and mechanical properties of extruded pure magnesium and an aluminum-free Mg-Zn-Y alloy. Metals 2019, 9, 667. [Google Scholar] [CrossRef]
- Yang, H.; Zander, D.; Huang, Y.; Kainer, K.U.; Dieringa, H. Individual/synergistic effects of Al and AlN on the microstructural evolution and creep resistance of Elektron21 alloy. Mater. Sci. Eng. A 2020, 777, 139072–139086. [Google Scholar] [CrossRef]
- Svedberg, L.M.; Arndt, K.C.; Cima, M.J. Corrosion of aluminum nitride (AlN) in aqueous cleaning solutions. J. Am. Ceram. Soc. 2000, 83, 41–46. [Google Scholar] [CrossRef]
- Fu, H.M.; Zhang, M.-X.; Qiu, D.; Kelly, P.M.; Taylor, J.A. Grain refinement by AlN particles in Mg–Al based alloys. J. Alloys Comp. 2009, 478, 809–812. [Google Scholar] [CrossRef]
- Lee, Y.C.; Dahle, A.K.; StJohn, D.H. Grain refinement of magnesium. In Essential Readings in Magnesium Technology, 1st ed.; Mathaudu, S.N., Luo, A.A., Neelameggham, N.R., Nyberg, E.A., Sillekens, W.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 247–254. [Google Scholar] [CrossRef]
- Chávez, L.; Veleva, L.; Feliu, S.; Giannopoulou, D.; Dieringa, H. Corrosion Behavior of Extruded AM60-AlN Metal Matrix Nanocomposite and AM60 Alloy Exposed to Simulated Acid Rain Environment. Metals 2021, 11, 990. [Google Scholar] [CrossRef]
- Lerner, M.I.; Glazkova, E.A.; Lozhkomoev, A.S.; Svarovskaya, N.V.; Bakina, O.V.; Pervikov, A.V.; Psakhie, S.G. Synthesis of Al nanoparticles and Al/AlN composite nanoparticles by electrical explosion of aluminum wires in argon and nitrogen. Powder Technol. 2016, 295, 307–314. [Google Scholar] [CrossRef]
- Xia, D.H.; Deng, C.; Macdonald, D.; Jamali, S.; Mills, D.; Luo, J.L.; Strebi, M.G.; Amiri, M.; Jin, W.; Song, S.; et al. Electrochemical measurements used for assessment of corrosion and protection of metallic materials in the field: A critical review. J. Mater. Sci. Technol. 2022, 112, 151–183. [Google Scholar] [CrossRef]
- ASTM-NACE/ASTM G31-12a; Standard Guide for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- ASTM G1-03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- ASTM G199-09; Standard Guide for Electrochemical Noise Measurement. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- Eke, A.; Herman, P.; Bassingthwaighte, J.; Raymond, G.; Percival, D.; Cannon, M.; Balla, I.; Ikrényi, C. Physiological time series: Distinguishing fractal noises from motions. Pflügers Archiv. 2000, 439, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Delignieres, D.; Ramdani, S.; Lemoine, L.; Torre, K.; Fortes, M.; Ninot, G. Fractal analyses for ‘short’ time series: A re-assessment of classical methods. J. Math. Psychol. 2006, 50, 525–544. [Google Scholar] [CrossRef]
- Cao, P.; Qian, M.; StJohn, D.H. Effect of iron on grain refinement of high-purity Mg–Al alloys. Scr. Mater. 2004, 51, 125–129. [Google Scholar] [CrossRef]
- Song, G.-L. Corrosion electrochemistry of magnesium (Mg) and its alloys. In Corrosion of Magnesium Alloys, 1st ed.; Song, G.L., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 3–65. [Google Scholar] [CrossRef]
- Heakal, F.E.-T.; Fekry, A.M.; Fatayerji, M.Z. Influence of halides on the dissolution and passivation behavior of AZ91D magnesium alloy in aqueous solutions. Electrochim. Acta 2009, 54, 1545–1557. [Google Scholar] [CrossRef]
- Botana, P.J.; Bárcena, M.M.; Villero, Á.A. Ruido Electroquímico: Métodos de Análisis; Septem Ediciones: Cadiz, Spain, 2002; pp. 50–70. [Google Scholar]
- He, L.; Jiang, Y.; Guo, Y.; Wu, X. Electrochemical noise analysis of duplex stainless steel 2101 exposed to different corrosive solutions. Corros Eng. Sci. Techn. 2016, 51, 187–194. [Google Scholar] [CrossRef]
- Xia, D.-H.; Song, S.; Behnamian, Y.; Hu, W.; Cheng, Y.F.; Lu, J.-L.; Huet, F. Review-Electrochemical noise applied in corrosion science: Theoretical and mathematical models towards quantitative analysis. J. Electrochem. Soc. 2020, 167, 081507–081521. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Nieves-Mendonza, D.; Maldonado-Bandala, E.; Olguín-Coca, J.; López-Léon, L.D.; Flores-De los Rios, J.P.; Almeraya-Calderón, F. Electrochemical noise analysis of the corrosion of titanium alloys in NaCl and H2SO4 solutions. Metals 2021, 11, 105. [Google Scholar] [CrossRef]
- Cottis, R.A. Interpretation of electrochemical noise data. Corrosion 2001, 57, 265–285. [Google Scholar] [CrossRef]
- Bertocci, U.; Gabrielli, C.; Huet, F.; Keddam, M. Noise resistance applied to corrosion measurements: I. Theoretical analysis. J. Electrochem. Soc. 1997, 144, 31–37. [Google Scholar] [CrossRef]
- Bertocci, U.; Gabrielli, C.; Huet, F.; Keddam, M. Noise resistance applied to corrosion measurements: II. Experimental tests. J. Electrochem. Soc. 1997, 144, 37–43. [Google Scholar] [CrossRef]
- Malamud, B.D.; Turcotte, D.L. Self-affine time series: Measures of weak and strong persistence. J. Stat. Plam. Inference 1999, 80, 173–196. [Google Scholar] [CrossRef]
- Lunder, O.; Nordien, J.H.; Nisancioglu, K. Corrosion resistance of cast Mg-Al alloys. Corros. Rev. 1997, 15, 439–470. [Google Scholar] [CrossRef]
- Davoodi, A.; Pan, J.; Leygraf, C.; Norgren, S. The role of intermetallic particles in localized corrosion of aluminium alloy studied by SKPFM and integrated AFM/SECM. J. Electrochem. Soc. 2008, 155, C211–C218. [Google Scholar] [CrossRef]
- Pawar, S.; Zhou, X.; Thompson, G.E.; Scamans, G.; Fan, Z. The role of intermetallics on corrosion initiation of twin roll cast AZ31 Mg Alloy. J. Electrochem. Soc. 2015, 162, C442–C448. [Google Scholar] [CrossRef]
- Asmussen, R.M.; Binns, W.J.; Parfov-Nia, R.; Jakupi, P.; Shoesmith, D.W. The stability of aluminium-manganese intermetallic phases under the microgalvanic coupling conditions. Mater. Corros. 2016, 67, 39–50. [Google Scholar] [CrossRef]
- Koç, E. Corrosion behavior of as cast β-Mg17Al12 phase in 3.5 wt % NaCl solution. Acta. Phys. Pol. A 2019, 135, 833–881. [Google Scholar] [CrossRef]
- Santamaria, M.; Di Quarto, F.; Zanna, S.; Marcus, P. Initial surface film on magnesium metal: A characterization by X-ray photoelectron spectroscopy (XPS) and photocurrent spectroscopy (PCS). Electrochim. Acta 2007, 53, 1314–1324. [Google Scholar] [CrossRef]
- Fournier, V.; Marcus, P.; Olefjord, I. Oxidation of magnesium. Surf. Inter. Anal. 2002, 34, 494–497. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Physical Electronics Inc.: Chanhassen, MN, USA, 1992. [Google Scholar]
- Liao, J.; Hotta, M. Corrosion products of field-exposed Mg-Al series magnesium alloys. Corros. Sci. 2016, 112, 276–288. [Google Scholar] [CrossRef]

| OCP (V vs. SCE) | ||||||
|---|---|---|---|---|---|---|
| Days | Initial | 1 | 7 | 10 | 15 | 30 |
| AM60-AlN | −1.61 ± 0.05 | −1.58 ± 0.02 | −1.53 ± 0.01 | −1.54 ± 0.01 | −1.53 ± 0.02 | −1.51 ± 0.01 |
| AM60 | −1.61 ± 0.07 | −1.58 ± 0.02 | −1.54 ± 0.01 | −1.53 ± 0.01 | −1.52 ± 0.02 | −1.52 ± 0.01 |
| Days | Initial | 1 | 7 | 10 | 15 | 30 |
|---|---|---|---|---|---|---|
| AM60-AlN | 2.33 | 2.42 | 1.48 | 1.52 | 1.63 | 0.84 |
| AM60 | 1.72 | 2.09 | 1.19 | 1.00 | 2.09 | 1.06 |
| Zone | C | O | Na | Mg | Al | Si | S | Cl | Mn |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.75 | 21.86 | - | 7.64 | 22.17 | 0.55 | - | - | 45.04 |
| 2 | 4.72 | 59.97 | 0.32 | 27.31 | 6.01 | - | 0.80 | 0.65 | 0.61 |
| 3 | 2.55 | 30.42 | - | 61.08 | 5.76 | - | 0.2 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez, L.; Veleva, L.; Sánchez, G.; Dieringa, H. AM60-AlN Nanocomposite and AM60 Alloy Corrosion Activity in Simulated Marine-Coastal Ambience. Metals 2022, 12, 1997. https://doi.org/10.3390/met12121997
Chávez L, Veleva L, Sánchez G, Dieringa H. AM60-AlN Nanocomposite and AM60 Alloy Corrosion Activity in Simulated Marine-Coastal Ambience. Metals. 2022; 12(12):1997. https://doi.org/10.3390/met12121997
Chicago/Turabian StyleChávez, Luis, Lucien Veleva, Gerardo Sánchez, and Hajo Dieringa. 2022. "AM60-AlN Nanocomposite and AM60 Alloy Corrosion Activity in Simulated Marine-Coastal Ambience" Metals 12, no. 12: 1997. https://doi.org/10.3390/met12121997
APA StyleChávez, L., Veleva, L., Sánchez, G., & Dieringa, H. (2022). AM60-AlN Nanocomposite and AM60 Alloy Corrosion Activity in Simulated Marine-Coastal Ambience. Metals, 12(12), 1997. https://doi.org/10.3390/met12121997








