Abstract
The presence of uranium-containing wastes from large provinces in the Republic of Kazakhstan significantly complicates the ecological situation, causing damage to the soil and hydrosphere due to the uncontrolled spread of large volumes of natural waters contaminated with radionuclides. They are usually utilized by the sorption method; however, the use of synthesized sorption materials is limited by their high price, and natural minerals are limited by low sorption characteristics. Many modification options are used in order to improve the sorption characteristics, but only a few methods have been found applied in industry. The main disadvantages include the complexity in the application and modification of reagents rarely used in industrial practice, which increases their cost, and is an obstacle to their widespread use. The authors of this research have studied the possibility of using technogenic raw materials—slags of phosphorus production—as a modifier of natural minerals. The methods of slag activation are investigated, the optimal conditions for the modification of the natural minerals zeolite and shungite by activated slag are determined, and the sorption properties of modified sorbents are studied.
1. Introduction
Kazakhstan is the world leader in uranium production [1]. Despite the fact that uranium is mined in the republic by relatively environmentally friendly methods, the method of underground borehole leaching, previously accumulated waste from uranium production, as well as large and small uranium-bearing hydrolytic provinces contribute to the contamination of natural waters with radionuclides. For the treatment of radioactively contaminated natural waters and wastewater, methods based on sorption, precipitation, and membrane processes are most often used [2,3,4,5]. The choice of method depends on the chemical and radionuclide composition of the liquid radioactive waste (LRW) and is determined by the state of the radionuclides in the solution.
The precipitation method is the most universal method for extracting radionuclides in both ionic and colloidal states. The main drawback of the method is the low degree of purification of solutions and the formation of large amounts of secondary waste. It is most expedient to use membrane methods based on micro- and ultrafiltration processes in order to purify solutions from radionuclides that are in solutions in a colloidal and coarsely dispersed state. Membrane methods are widely used for water treatment, seawater desalination, and the food industry.
The use of this method for the disposal of LRW is difficult and expensive because the process requires special equipment. One of the most effective methods for cleaning objects contaminated with radionuclides, particularly water, is the use of sorption methods. To date, methods of sorption purification using synthetic and natural sorbents are the most common and effective, and in many cases, there are no other alternatives [6,7,8].
The disadvantage of using synthetic sorbents is their high cost, while natural sorbents have low sorption characteristics, and in particular, a low sorption exchange capacity (SEC). Natural materials used to purify solutions are subjected to various kinds of modifications in order to improve their sorption properties. The literature describes many methods for modifying natural minerals in order to increase the SEC [3,4,5,6,9,10,11,12,13] but most of them have significant drawbacks such as the complexity in execution, high cost, and the use of scarce reagents. Therefore, only a few methods have been implemented in the industry; large-scale disposal of liquid radioactive waste with modified natural sorbents is ineffective. This study proposes to use the waste from the phosphorus industry for the modification of natural minerals, i.e., phosphorus slag, which is formed during the electro-thermal production of yellow phosphorus. The influence of the modifier on the sorption exchange capacity of natural minerals was studied.
2. Materials and Methods
The initial natural sorbents were zeolite and shungite, and the modifier was phosphorus slag. Shungite was used after preliminary flotation [9]. Optimal conditions for the activation of phosphorus slag were achieved, such as the concentration of carbonate or sodium chloride made up of 100–150 g/dm3, the temperature regime was 150–200 °C, the ratio was S:L = 1:5, and the process duration lasted 4–6 h. The modification of natural sorbents with activated phosphorus slag was carried out by mixing zeolite or shungite with preliminarily activated phosphorus slag in a ratio of 3:1 and further treated with a solution of polyacrylamide (20 g/dm3). Processing lasted 72 h.
The sorption of uranium was carried out at a temperature of 22 °C in a static mode, at a ratio of S:L = 1:100. The concentration of uranium in the model solution varied from 50.0 to 700 mg/dm3, and that of iron in the productive solution varied from 0.1 to 3.0 g/dm3. The process lasted for 2 h. Changes in the uranium content in solutions before and after sorption were determined using an Optima 8300DV spectrometer (ICP, PerkinElmer, Norwalk, CT, USA).
X-ray phase studies of phosphorus slag were performed on a D8 Advance diffractometer (Bruker AXS GmbH, Karlsruhe, Germany). The interpretation of diffractograms and the calculation of interplanar distances was carried out using the EVA software (HVAC version, LBS, Bhandup West, Mumbai, India), and a search/match program was used with the ASTM card database for the interpretation of samples and phase. The results of the X-ray phase method are supplemented by the data of mineralogical analysis performed using a LEICA DM 250 P microscope (Danaher, Leica microsystems, Wetzlar, Germany) in immersion liquids by selecting them in order to determine the mineral phases. The mineralogical analysis of a sample consists of the determination of minerals by appearance through an optical examination under a binocular microscope, and the verification by chemical reactions. Under a binocular microscope (Celestron, New York, NY, USA), minerals are determined by their appearance, and physical and chemical properties: the shape of grains, the habit of crystals, the nature of crystal faces, fragments, fracture, cohesion, shading, transparency, brilliance, color, hardness, and solubility in acids. The tasks of mineralogical testing include determining the mineral composition of a mineral, the composition, and properties of the most important minerals, as well as the textural and structural features of the ore. An artificial polished section, a briquette was made from the sample material for mineralogical analysis.
Infrared (IR) spectra were recorded on an Avatar 370 CsI IR-Fourier spectrometer (Nicolet Instrument Corporation, Raleigh, NC, USA) in the spectral range 4000–300 cm−1. The preparations were used in the form of a tablet, which was prepared by pressing 2 mg of the sample and 200 mg of KBr. The baseline was automatically corrected. The authors of this study used a prefix for the experiment—Transmission E.S.P. (Electronic Stability Program).
3. Results
In the course of the experimental work, phosphorus slag was preliminarily studied by physicochemical methods. The X-ray phase method identified the amorphous phase in the composition of the slag as the basis, and the mineral content of the crystal was determined as calcite—Ca(CO3)—66.85%, oxide silicate—Ca3(SiO4)O—19.64%, ankerite—Ca(Fe+2,Mg)(CO3)2—8.29%, and iron silicon—(Fe0.9 Si0.1)—5.23%.
The results of the X-ray phase analysis were confirmed and supplemented by mineralogical and IR spectroscopic methods (Bruker AXS, Billerick, MA, USA). It was established by the mineralogical method that the main phase was more than 90% represented by the amorphous phase of volostanite. There were small amounts of calcite, ankerite, and carbonates. Phosphorus was present in the form of lazulite [14,15,16,17,18,19].
According to IR spectroscopic analysis (Figure 1), the bands in the spectrum at wave numbers 1039, 922, 754, and 497 cm−1 indicate the presence of multicomponent glass in the sample [16]. In this case, Ca2+ ions, like Na+ ions, are depolymerizing cations, magnesium atoms are included in the anionic framework of glass, and Fe3+ ions are in the cationic framework built based on oxygen-containing silicon compounds [16,20].
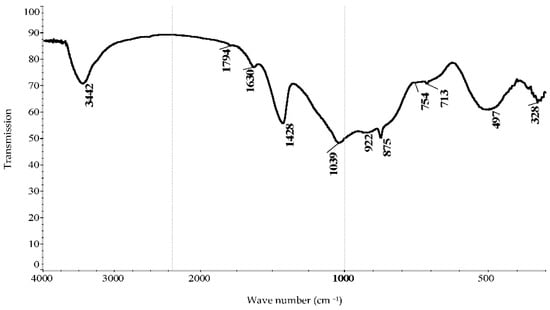
Figure 1.
Infrared spectrum of the initial sample of phosphorus slag.
Thus, IR spectroscopic analysis recorded the presence of bands in the spectrum of the slag sample at wave numbers corresponding to multicomponent glass. In the course of the research, previously identified zeolite from the Kosmurun deposit and shungite from the Koksu deposit were used [14,16]. Natural minerals were modified with phosphorus slag after activation with sodium chloride or carbonate solutions.
Studies in the field of calcium silicate synthesis have shown that the most effective way to obtain synthetic calcium silicates is by the hydrothermal method [21,22,23,24,25], which proceeds at elevated temperatures and pressures and simulates the formation of minerals in the earth’s crust. The hydrothermal method affects the structure and morphology of hydrosilicates, as well as, indirectly, their sorption properties [23,24,25]. Sodium carbonates and chlorides, as well as calcium salts, are usually used as the aqueous phase.
The results of our studies showed a change in the structure during the hydrothermal activation of phosphorus slag with sodium carbonate. A transformation of the amorphous phase into a crystalline phase was observed, and it was reflected by a change in the size and shape of the particles; large particles break up into smaller particles and acquire an acicular shape (Figure 2). The process proceeded gradually within 4–6 h, where further changes are hardly noticeable. With increasing temperature, the changes were more pronounced (Table 1).
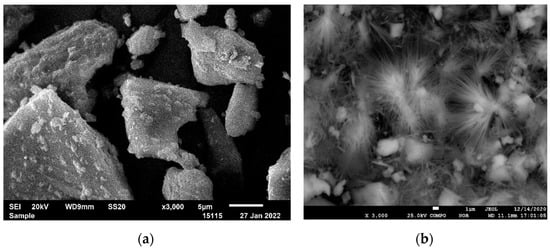
Figure 2.
The structure of phosphorus slag activated in a carbonate medium (Na2CO3—150 g/dm3, T–230 °C). Increase: (a) 750× and (b) 3000×.

Table 1.
Phase composition of activated phosphorus slag in a carbonate medium for six hours.
Thus, based on the studies performed, it can be assumed that the chemical activation of phosphorus slag in a carbonate environment causes a change in its composition and structure; mineral components partially decompose, and new active centers appear in the system, which later play a certain role in the construction of a new rationale for data structure conditions. In general, there is an active rearrangement of the structure with the formation of the first intermediate phases, and then new compounds, i.e., there is a change in the phase composition and structure.
During the hydrothermal treatment of phosphorus slag with sodium chloride, the amorphous structure of the slag was preserved, however, some changes indicated in the IR spectrum were still present. In the course of slag activation, a decrease in the content of ferric iron cations involved in the construction of the frame of multicomponent glass based on oxygen compounds of silicon, which is the basis of the matrix, was observed. Thus, in the process of hydrothermal activation of phosphorus slag with sodium chloride, the main phase, which is amorphous, is preserved, but it undergoes some changes [19].
At the next stage, the conditions for modifying natural minerals such as zeolite and shungite were established with activated phosphorus slag and activation methods. The optimal conditions for activation and modification are indicated above.
Studies have shown that in the process of modifying natural minerals with technogenic raw materials, such as phosphorus slag activated by sodium chloride, an amorphous phase of phosphorus slag is also partially detected. Figure 3 shows a feature of modified shungite (Figure 3).
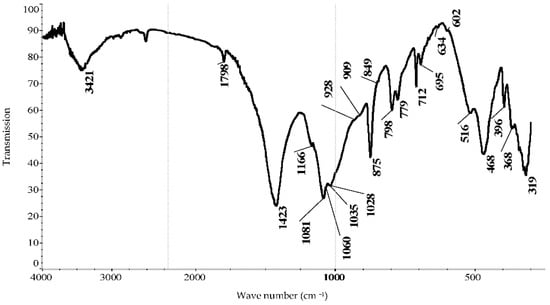
Figure 3.
IR spectrum of shungite modified with activated slag.
Using infrared spectroscopy, the following components were identified in the composition of modified shungite: calcite—CaCO3—1798, 1423, 875, 849, and 712 cm−1, quartz—SiO2—1166, 1081, 798, 779, 695, 516, 468, 396, and 368 cm−1 [26,27,28]. The 319 cm−1 wave characterizes the Ca—O bonds [29]. The 1081, 1060p, 1035, 928p, 909p, 516, and 468 cm−1 waves characterize CaSiO3 [27], 1028.928 cm−1—KAl2[(OH, F)2| AlSi3O10]—[28,29,30], and 1035.928 cm−1—multicomponent glass [20]. The 1035 cm−1 wave representing the stretching vibrations of the Si–O–Si bonds are the shoulder at 928 cm−1, corresponding to the stretching vibrations of the end bonds Si–O– [26], and 634, 602, 468 cm−1—Ca[SO4]½H2O [30].
The data of the IR spectroscopic analysis are supplemented by the results of the X-ray phase analysis (Table 2).

Table 2.
Phase analysis of modified shungite.
The indicator of modification is the sorption process. Figure 4 and Figure 5 show isotherms of the sorption of uranium and iron on shungite modified with phosphorus slag. Shungite is preliminarily subjected to flotation [9,31,32], and phosphorus slag is activated in a chloride and carbonate environment.
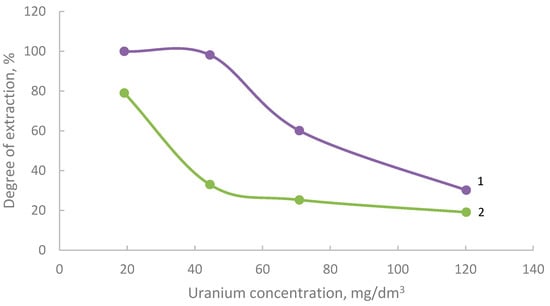
Figure 4.
Isotherms of the sorption of uranium by modified shungite, previously activated in a chloride (1) and carbonate (2) environment.
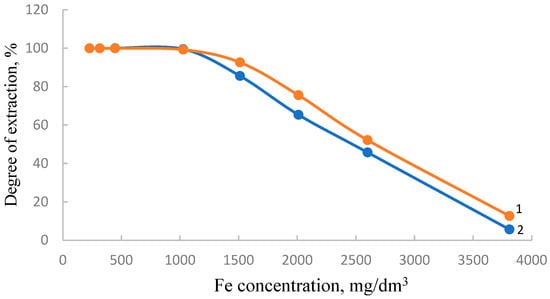
Figure 5.
Isotherms of iron sorption by modified shungite, previously activated in a carbonate (1) and chloride (2) environment.
As follows from the obtained results, uranium is mostly sorbed by modified shungite. The modifier is phosphorus slag, which was preactivated with sodium chloride. This effect can be explained by the filling of “voids” formed during the activation of the slag with sodium chloride-sorbed ions.
The degree of iron sorption on modified shungite activated in a carbonate and chloride environment differs slightly. Alkaline earth carbonates have enhanced sorption properties due to the adsorption of ions on a developed surface.
In Figure 4 and Figure 5, the separation level decreases with increasing concentration because each sorbent has a certain value of sorption capacity. At a low initial concentration of the sorbed component, the degree of sorption relative to the initial concentration is maximum; with an increase in the initial concentration, the ratio of the amount of the sorbed ion, determined by the value of the sorption capacity, to the initial concentration decreases.
In the course of the research, the authors determined the sorption capacity of natural zeolite and shungite for uranium. A model solution was used as the initial solution. The sorption capacity of zeolite was 10.2 mg/g of sorbent, and for shungite (after flotation), 12.8 mg/g. They also determined the sorption capacity of the modified zeolite and shungite for uranium and iron in total. The initial, productive solution contained uranium and iron. For the modified zeolite, the total sorption capacity for uranium + iron was 78.9 mg/g, and for shungite (after flotation), it was 91.1 mg/g.
The mechanical strength of sorbents modified in various ways was investigated in this study. The mechanical strength of the material is characterized by the ability to resist various external mechanical influences. The mechanical strength of the material is characterized by strength limits:
(1) during compression; (2) in tension; (3) in bending; and (4) abrasion resistance.
As a rule, the mechanical strength of a material is determined by testing standard samples made from the same material. To determine the effect of activators on mechanical strength, six samples were made in the form of pressed briquettes (Figure 6) of premodified natural sorbents (zeolite and shungite):

Figure 6.
Pressed natural sorbents zeolite and shungite.
- Initial.
- Sorbent modified with phosphorus slag preliminarily activated in a chloride medium.
- Sorbent modified with phosphorus slag preliminarily activated in a carbonate medium.
In order to determine the properties of the material under study, it is of great importance to determine the strength of the material using instruments and fixtures that provide sufficient measurement accuracy. For this purpose, the authors used the universal floor testing machine Autograph AG-X 100 kN, Shimadzu GmbH (Figure 7), Kyoto, Japan. Pressed briquettes were made using a PSU-10 hydraulic press designed for the static compression testing of standard samples of building materials.

Figure 7.
Floor testing machine Autograph AG-X 100 kN, Shimadzu GmbH.
In the first case, all samples were pressed at a load of 200 kg/dm3, liquid glass was used as a binder, and cylinder-shaped briquettes (r = 8, h = 16) were obtained, which were thoroughly dried and compressed to the first crack. A compression speed of 0.1 mm/sec was used. The results obtained are presented in Table 3, from which it follows that shungite has a mechanical strength much higher than zeolite.

Table 3.
Influence of activators on the mechanical strength of natural sorbents (binder—liquid glass).
Liquid glass was used as a binder.
Figure 8 shows the dependence of the degree of deformation of shungite and zeolite samples modified with phosphorus slag on stress. Based on the conducted studies, it can be concluded that the ability to resist external mechanical influences (in our case, compression) decreases when both zeolite and shungite are modified with phosphorus slag which has been previously activated in a carbonate medium. The degree of deformation of shungite modified in a chloride medium increases, while that of zeolite decreases (Figure 8).
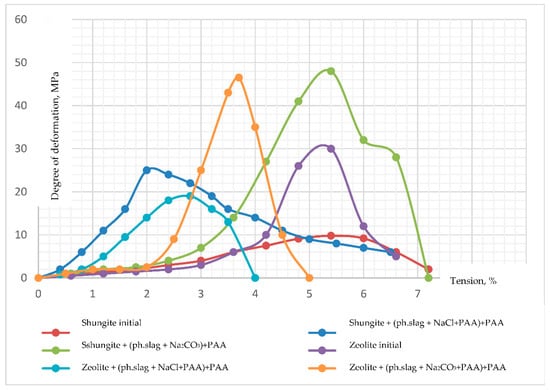
Figure 8.
Graph of the degree of deformation of shungite and zeolite from voltage.
Thus, if phosphorus slag is preliminarily activated in a chloride surrounding, the modified sorbent actively extracts uranium and iron, and in a carbonated surrounding, iron is removed to a greater extent. This property can be used to separate them.
4. Conclusions
During the research, it was found that natural sorbent modified by phosphorus slag and preliminarily activated in a chloride surrounding, actively extracts both uranium and iron, while in a carbonated surrounding, it extracts iron to a greater extent. This property can be used to separate them.
Physical and chemical studies of phosphorus slag showed that its basis is a glass phase based on volostanite, calcite, and calcium, and iron silicates are included in a small amount. Phosphorus is present in the form of lazulite. When phosphorus slag is activated by the hydrothermal method in a carbonated surrounding, and in a wide temperature range, its phase composition, structure, and morphology of particles change when treated with sodium chloride. The main amorphous phase is preserved but it undergoes some changes. Conditions for the activation of natural minerals such as zeolite and shungite preactivated by phosphorus slag were developed in the course of this research. It has been established that when modifying a natural mineral with activated sodium chloride phosphorus slag, the sorbent actively extracts uranium and iron, and it extracts iron to a greater extent by sodium carbonate. This property can be used to separate uranium from iron.
Author Contributions
Conceptualization, T.S.; methodology, L.A.; software, B.A., B.M.; validation, B.K., T.S.; formal analysis, B.K., T.S., B.M.; investigation, T.S.; resources, A.B.; data curation, A.B.; writing—original draft preparation, T.S., B.A.; writing—review and editing, T.S., B.A.; visualization, D.Y.; supervision, B.K.; project administration, B.K.; funding acquisition, B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan (Grant No. AP08856246).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data analyzed in this study were a re-analysis of existing data, which are openly available at locations cited in the reference section, available via DOI or URL links. All data presented are under open access of the journal “Metals” and can be reused by correct citation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- OECD-NEA; IAEA. Uranium 2020: Resources, Production and Demand (‘Red Book’) World Nuclear Association, The Nuclear Fuel Report 2015, 2017, 2019 & 2021. Available online: https://www.world-nuclear.org/information-library/country-profiles/countries-g-n/kazakhstan.aspx (accessed on 27 September 2022).
- Nikiforov, A.S.; Kulichenko, V.V.; Zhikharev, M.I. Neutralization of Liquid Radioactive Waste; Energoatomizdat: Moscow, Russia, 1985; 28p. [Google Scholar]
- Korostelev, D.P. Treatment of Radioactive Water and Gases at Nuclear Power Plants; Energoatomizdat: Moscow, Russia, 1988; 152p. [Google Scholar]
- Treatment Technologies for Low and Intermediate Level Waste from Nuclear Applications. Final Report of a Coordinated Research Program 1991–1996; IAEA-TECDOC-0929; International Atomic Energy Agency: Vienna, Austria, 1997; 207p.
- Innovative Waste Treatment and Conditioning Technologies at Nuclear Power Plants; IAEA-TECDOC-1504; International Atomic Energy Agency: Vienna, Austria, 2006; 57p.
- Piontkovskaya, M.A. Physicochemical, Adsorption and Catalytic Properties of Modified Faujasites; Naukova Dumka: Moscow, Russia, 1978; 200p. [Google Scholar]
- Kadirbekov, K.A. The Use of Natural Zeolites in the Cracking of Heavy Hydrocarbon Raw Materials; Khim. Zhurnal of Kazakhstan: Almaty, Kazakhstan, 2010; pp. 103–120. [Google Scholar]
- Ergozhin, E.E.; Akimbaeva, A.M. Organo-Mineral Sorbents and Semi-Functional Systems Based on Natural Aluminosilicate and Coal-Mineral Raw Materials; Print-S: Almaty, Kazakhstan, 2007; 373p. [Google Scholar]
- Kenzhaliyev, B.K.; Surkova, T.Y.; Berkinbayeva, A.N.; Dosymbayeva, Z.D.; Mukhanova, A.A.; Abdikerim, B.E. Development of a method of modifying a natural sorbent for uranium extraction. J. Chem. Technol. Metall. 2020, 55, 1041–1046. [Google Scholar]
- Myasoedova, G.V.; Nikashina, V.A.; Molochnikova, N.P.; Lileeva, L.V. Properties of new types of fibrous sorbents with ami-doxime and hydrazine groups. J. Anal. Chem. 2000, 55, 611–615. [Google Scholar] [CrossRef]
- Druzhinina, T.V.; Smolenskaya, L.M.; Struganova, M.A. Sorption of heavy metals from model solutions by amine-containing chemisorption polyamide fiber. J. Appl. Chem. 2003, 76, 1976–1980. [Google Scholar] [CrossRef]
- Myasoedova, V.G.; Nikashina, V.A. Sorption materials for the extraction of radionuclides from aqueous media. Russ. Chem. J. 2006, 50, 55. [Google Scholar]
- Kenzhaliyev, B.K.; Surkova, T.Y.; Berkinbayeva, A.N.; Dosymbayeva, Z.D.; Abdikerim, B.E. Revisiting the Kazakhstan natural sorbents modification. Metalurgija 2020, 1, 117–120. [Google Scholar]
- Surkova, T.; Abdikerim, B.; Berkinbayeva, A.; Azlan, M.; Baltabekova, Z. Obtaining modified sorbents based on natural raw materials of Kazakhstan and research of their properties. Complex Use Miner. Resour. 2022, 322, 23–32. [Google Scholar] [CrossRef]
- Kolesova, V.A. Comparative study of the IR absorption spectra of alkali-free and Na2O-containing calcium and magnesium silicate glasses. Bulletin of the Academy of Sciences of the USSR. Inorg. Mater. 1966, 2, 1497–1504. [Google Scholar]
- Abdikerim, B.; Kenzhaliyev, B.; Surkova, T.; Didik, N.; Berkinbayeva, A.; Dosymbayeva, Z.; Umirbekova, N. Uranium extraction with modified sorbents. Use Miner. Resour. 2020, 314, 84–90. [Google Scholar] [CrossRef]
- Aliev, S.; Omarbekov, Y. Influence of the “pumping wells” technology on the indicators of in situ leaching of uranium. Complex Use Miner. Resour. 2021, 317, 30–36. [Google Scholar] [CrossRef]
- Duisebayeva, T.; Arbuz, A. Prospects for use of chlorine-containing leaching solutions for extraction of associated useful components from spent ores of uranium deposits. Complex Use Miner. Resour. 2021, 319, 25–31. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.K.; Surkova, T.Y.; Abdikerim, B.E.; Berkinbayeva, A.N.; Abikak, Y.B.; Yessimova, D.M. Research on sorption properties of phosphoric production slag-waste. Metalurgija 2022, 61, 209–212. [Google Scholar]
- Kruchinin, Y.D. Lapshinova II Glass formation and properties of glasses in the CaO*Fe2O3—SiO system. Glass Phys. Chem. 1983, 9, 275–283. [Google Scholar]
- Golikova, E.V.; Goloudina, S.I. Chemical Methods of Obtaining Ceramic and Polymer Nanomaterials from the Liquid Phase. 2013. 218p. Available online: https://elibrary.ru/item.asp?id=26665772 (accessed on 27 September 2022).
- Eliseev, A.A.; Lukashin, A.V. Functional Nanomaterials; M. FIZMATLIT: Moscow, Russia, 2010; 456p, ISBN 978-5-92211-120-1. [Google Scholar]
- Ilyukhin, V.V.; Kuznetsov, V.A.; Lobachev, A.N.; Bakshutov, V.S. Calcium Hydrosilicates. Single Crystal Synthesis and Crystal Chemistry; Nauka: Moscow, Russia, 1979; 184p. [Google Scholar]
- Udalov, Y.P.; German, A.M.; Zhabreev, V.A.; Kazakov, V.G.; Molchanov, S.A.; Soloveichik, E.Y. Technology of Inorganic Powder Materials and Coatings for Functional Purposes/Textbook; Manual for Universities: St. Petersburg, Russia, 2001; 428p, ISBN 5-92-760009-3. [Google Scholar]
- Krzheminsky, S.A.; Sudina, N.K.; Kroychuk, L.A.; Varlamov, V.P. Autoclave Treatment of Silicate Products; M. Stroyizdat: Ufa, Russia, 1974; 160p. [Google Scholar]
- Lazarev, A.N. Vibrational Spectra and Structure of Silicates; Springer: New York, NY, USA, 1968; 348p. [Google Scholar]
- Moenke, H. Mineralspektren; AkademieVerlag: Berlin, Germany, 1962; 394p. [Google Scholar]
- Thermo Electron Corporation for Nicolet FT-IR; US Geological Survey Minerals library; U.S. Geological Survey: Reston, VA, USA, 2004.
- Solntseva, L.S.; Sidorenko, G.A.; Solntsev, B.P. Application of IR spectroscopy to the study of the nature of the bond and coor-dination of cations by oxygen and halogens in minerals. Const. Prop. Miner. 1972, 6, 30–46. [Google Scholar]
- Farmer, V.C. The Infrared Spectra of Minerals; Mineralogical Society: London, UK, 1974; 539p. [Google Scholar]
- Kenzhaliev, B.K.; Mironov, V.G.; Shilov, G.T.; Ilmaliyev, Z.B.; Yermekov, D.K. The study of the effect of chromium boron (CrB2) hardening additive on the developed pg-z40 surfacing powder. Bulg. Chem. Commun. 2018, 50, 140–145. [Google Scholar]
- Kenzhaliev, B.K.; Surkova, T.Y.; Berkinbayeva, A.N.; Dosymbayeva, Z.D.; Chukmanova, M.T. To the question of recovery of uranium from raw materials. Ser. Geol. Technol. Sci. 2019, 1, 112–119. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).