Effect of Chloride and Iodide on the Corrosion Behavior of 13Cr Stainless Steel
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Material
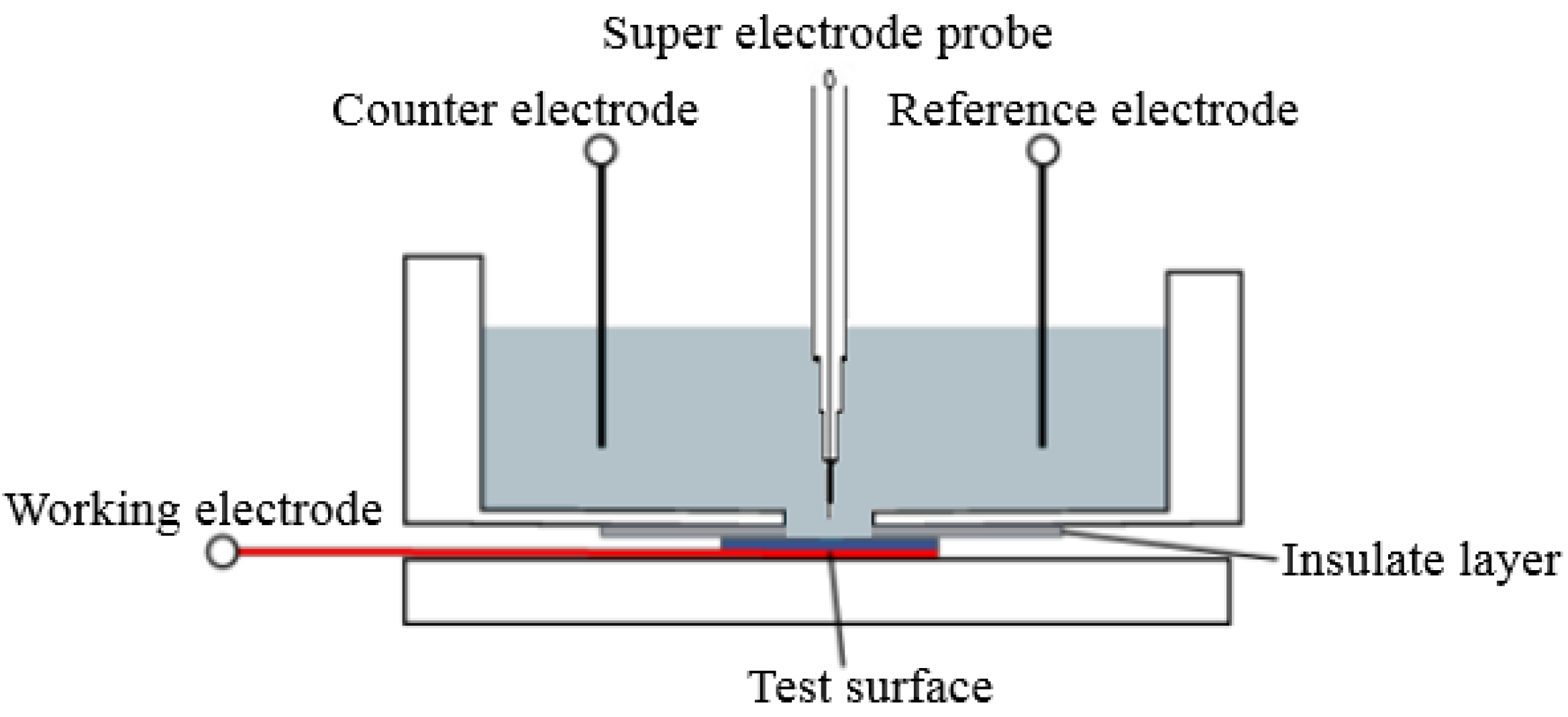
2.2. Methods and Techniques
3. Results and Discussion
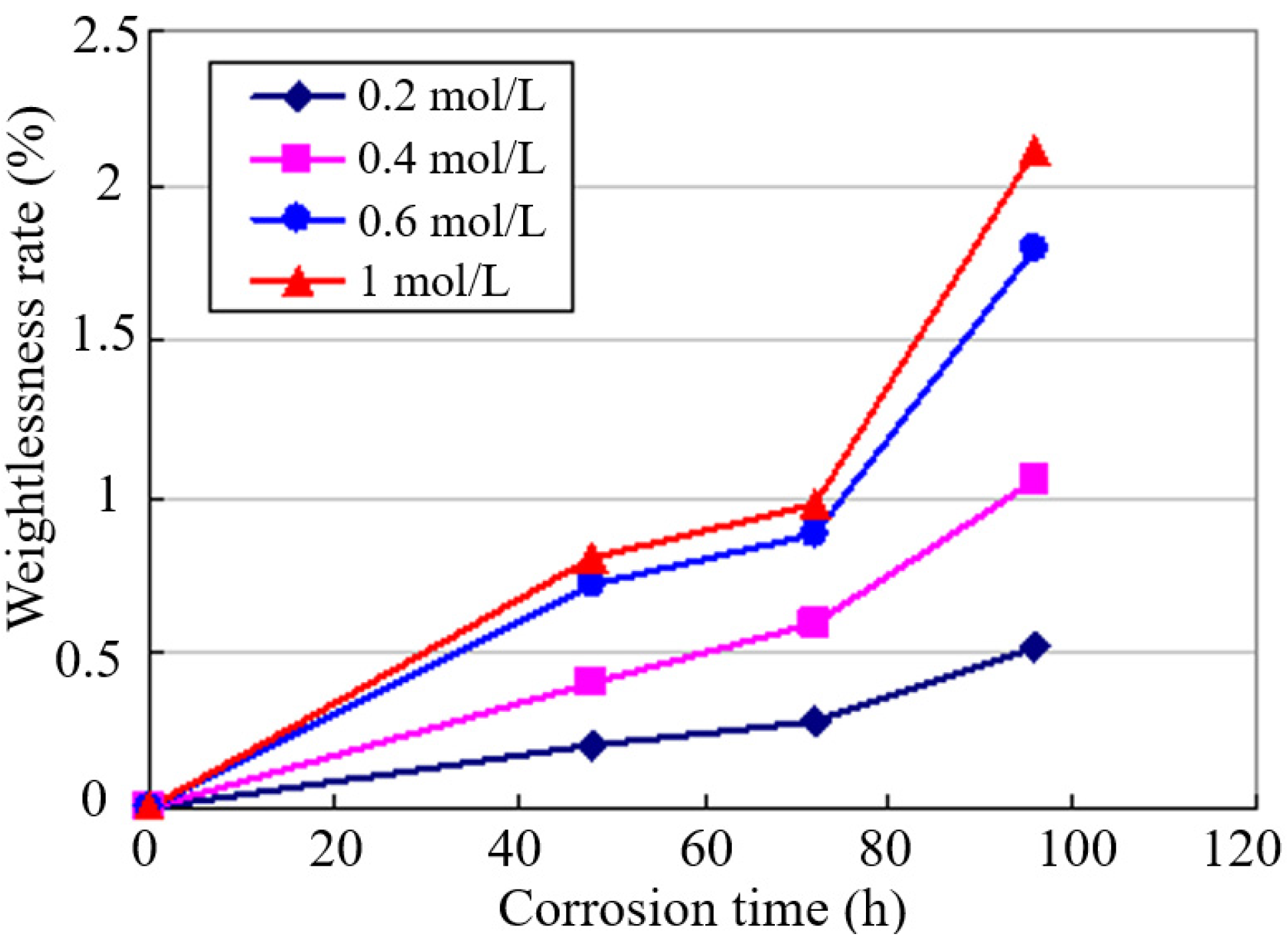
3.1. Weight Loss Test
3.2. Surface SEM Morphology of the Inclusions
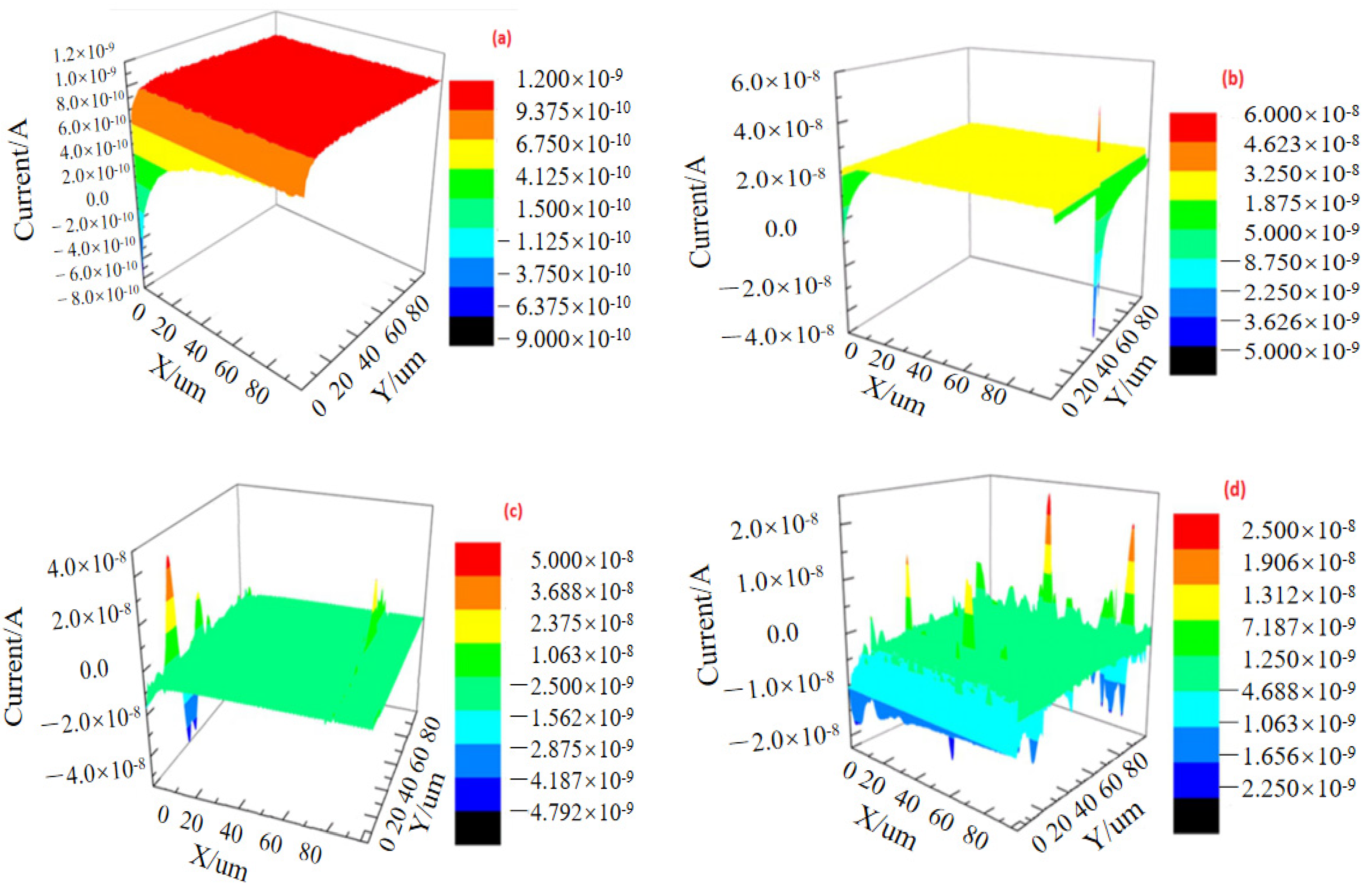
3.3. SECM Corrosion Behavior
3.4. SEM Morphology
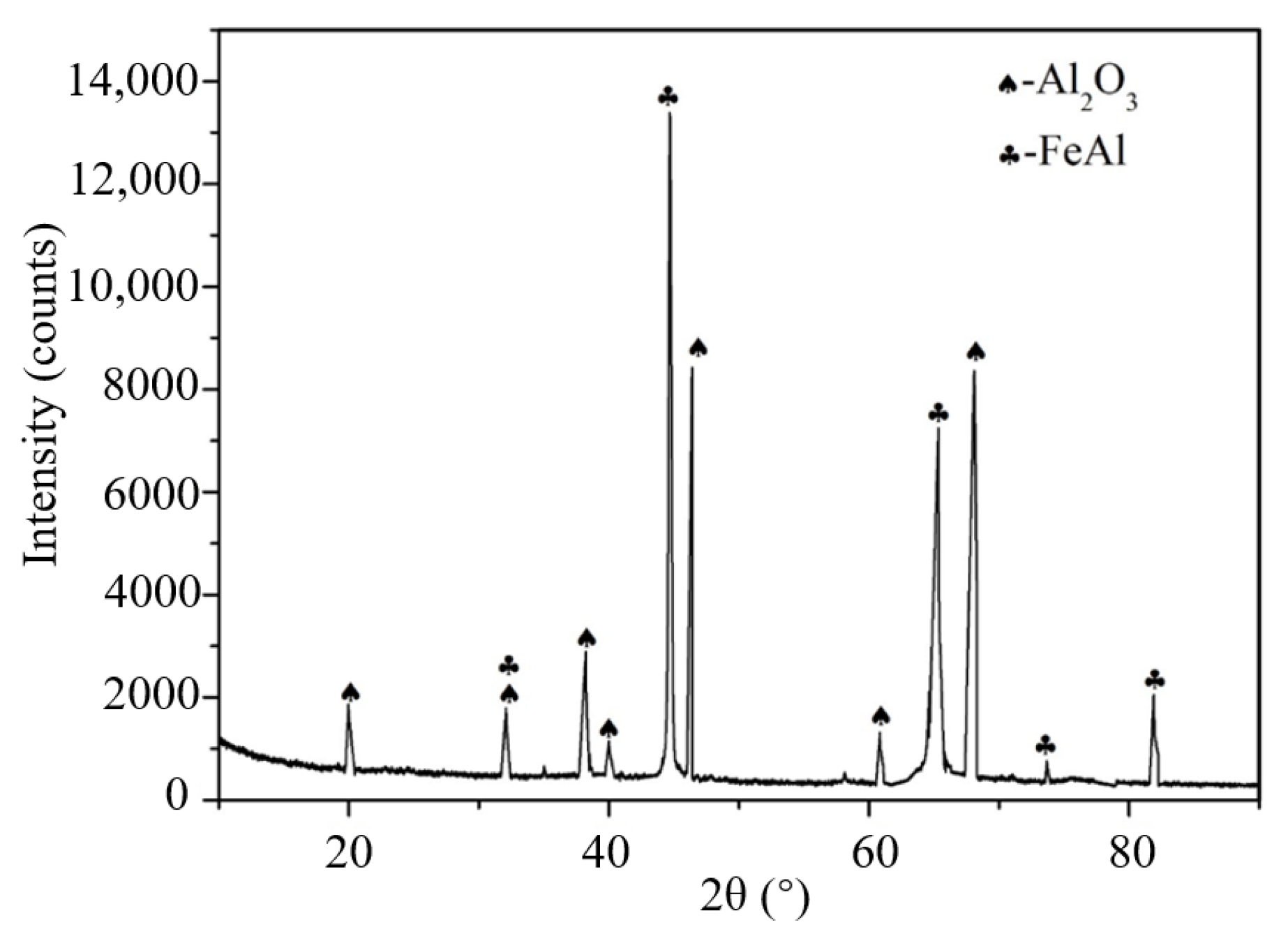
3.5. Phase Structure of Inclusions
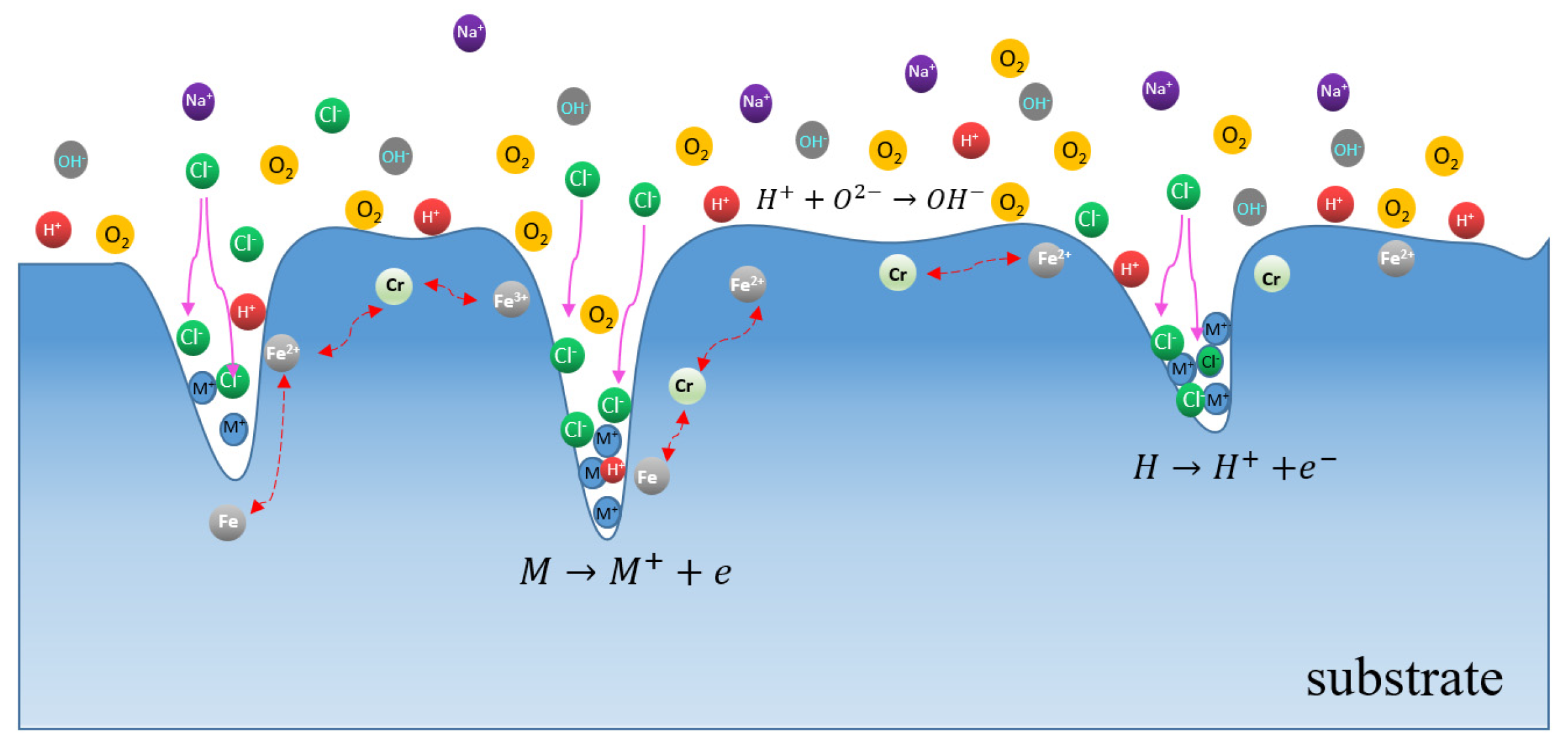
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cai, W.; Zhao, G.; Zhao, D.; Wei, A. Corrosion resistance and semiconductor properties of passive films formed on super 13Cr stainless steel. J. Univ. Sci. Technol. Beijing 2011, 33, 1226–1230. [Google Scholar]
- Hampel, M.; Schenderlein, M.; Schary, C.; Dimper, M.; Ozcan, O. Efficient detection of localized corrosion processes on stainless steel by means of scanning electrochemical microscopy (SECM) using a multi-electrode approach. Electrochem. Commun. 2019, 101, 52–55. [Google Scholar] [CrossRef]
- Yule, L.C.; Bentley, C.L.; West, G.; Shollock, B.A.; Unwin, P.R. Scanning electrochemical cell microscopy: A versatile method for highly localised corrosion related measurements on metal surfaces. Electrochim. Acta 2019, 298, 80–88. [Google Scholar] [CrossRef]
- Zhou, H.R.; Ma, J.; Li, X.G.; Dong, C.F.; Lu, Q.K.; Feng, H. Corrosion study of pure aluminum in 0.6 mol/L NaCl solution by SECM. Hangkong Cailiao Xuebao 2009, 29, 8–12. [Google Scholar]
- Molina, J.; Fernández, J.; Inés, J.C.; Del Río, A.I.; Bonastre, J.; Cases, F. Electrochemical characterization of reduced graphene oxide-coated polyester fabrics. Electrochim. Acta 2013, 93, 44–52. [Google Scholar] [CrossRef]
- Yu, J.G.; Luo, J.L.; Norton, P.R. Investigation of hydrogen induced pitting active sites. Electrochim. Acta 2002, 47, 4019–4025. [Google Scholar] [CrossRef]
- Lin, C.J.; Xie, Z.X.; Tian, Z.W. Electrochemical scanning tunneling microscope studies of initial pitting corrosion 277 of stainless steel. Corros. Sci. Prot. Technol. 1997, 4, 3–8. [Google Scholar]
- Sun, P.; Zhang, Z.; Gao, Z.; Shao, Y. Probing fast facilitated ion transfer across an externally polarized liquid–liquid interface by scanning electrochemical microscopy. Angew. Chem. 2002, 114, 3595–3598. [Google Scholar] [CrossRef]
- Meng, F.; Wang, J.; Han, E.H.; Ke, W. Effects of scratching on corrosion and stress corrosion cracking of Alloy 690TT at 58 °C and 330 °C. Corros. Sci. 2009, 51, 2761–2769. [Google Scholar] [CrossRef]
- Reclaru, L.; Lerf, R.; Eschler, P.Y.; Blatter, A.; Meyer, J.M. Pitting, crevice and galvanic corrosion of REX stainless-steel/CoCr orthopedic implant material. Biomaterials 2002, 23, 3479–3485. [Google Scholar] [CrossRef]
- Olorundaisi, E.; Jamiru, T.; Adegbola, A.T. Mitigating the effect of corrosion and wear in the application of high strength low alloy steels (HSLA) in the petrochemical transportation industry—A review. Mater. Res. Express 2020, 6, 1265k9. [Google Scholar] [CrossRef]
- Amphlett, J.L.; Denuault, G. Scanning electrochemical microscopy (SECM): An investigation of the effects of tip geometry on amperometric tip response. J. Phys. Chem. B 1998, 102, 9946–9951. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, L.; Wang, C.; Zhu, Y. Study of the effects of hydrogen on the pitting processes of X70 carbon steel with SECM. Electrochem. Commun. 2010, 12, 1804–1807. [Google Scholar] [CrossRef]
- Dong, C.F.; Luo, H.; Xiao, K.; Li, X.G.; Cheng, Y.F. In situ characterization of pitting corrosion of stainless steel by a scanning electrochemical microscopy. J. Mater. Eng. Perform. 2012, 21, 406–410. [Google Scholar] [CrossRef]
- Wang, F.; Shan, Q.; Zhang, F.; Lu, F.; Li, J.; Yu, T.; Qu, C. Pitting corrosion behavior of metal materials and research methods. IOP Conf. Ser. Earth Environ. Sci. 2021, 651, 032039. [Google Scholar] [CrossRef]
- Venugopal, A.; Raja, V.S. AC impedance study on the activation mechanism of aluminium by indium and zinc in 3.5% NaCl medium. Corros. Sci. 1997, 39, 2053–2065. [Google Scholar] [CrossRef]
- Aligizaki, K.K. Analytical Methods in Corrosion Science and Engineering. Corrosion 2008, 64, 86. [Google Scholar]
- Lister, T.E.; Pinhero, P.J. The effect of localized electric fields on the detection of dissolved sulfur species from Type 304 stainless steel using scanning electrochemical microscopy. Electrochim. Acta 2003, 48, 2371–2378. [Google Scholar] [CrossRef]
- Cruz, J.; Martınez, R.; Genesca, J.; Garcıa-Ochoa, E. Experimental and theoretical study of 1-(2-ethylamino)-2-methylimidazoline as an inhibitor of carbon steel corrosion in acid media. J. Electroanal. Chem. 2004, 566, 111–121. [Google Scholar] [CrossRef]
- Katemann, B.B.; Inchauspe, C.G.; Castro, P.A.; Schulte, A.; Calvo, E.J.; Schuhmann, W. Precursor sites for localised corrosion on lacquered tinplates visualised by means of alternating current scanning electrochemical microscopy. Electrochim. Acta 2003, 48, 1115–1121. [Google Scholar] [CrossRef]
- Lister, T.E.; Pinhero, P.J. Scanning electrochemical microscopy study of corrosion dynamics on type 304 stainless steel. Electrochem. Solid-State Lett. 2002, 5, B33. [Google Scholar] [CrossRef]
- Chen, G.; Liss, K.D.; Chen, C.; He, Y.; Qu, X.; Cao, P. Porous FeAl alloys via powder sintering: Phase transformation, microstructure and aqueous corrosion behavior. J. Mater. Sci. Technol. 2021, 86, 64–69. [Google Scholar] [CrossRef]
- Nagaoka, A.; Nose, K.; Nokami, K.; Kajimura, H. The role of micro pits in the initiation process of crevice corrosion of SUS304 stainless steel in an aqueous chloride solution. Mater. Trans. 2022, 63, 335–342. [Google Scholar] [CrossRef]
- Paik, C.H.; White, H.S.; Alkire, R.C. Scanning electrochemical microscopy detection of dissolved sulfur species from inclusions in stainless steel. J. Electrochem. Soc. 2000, 147, 4120. [Google Scholar] [CrossRef]
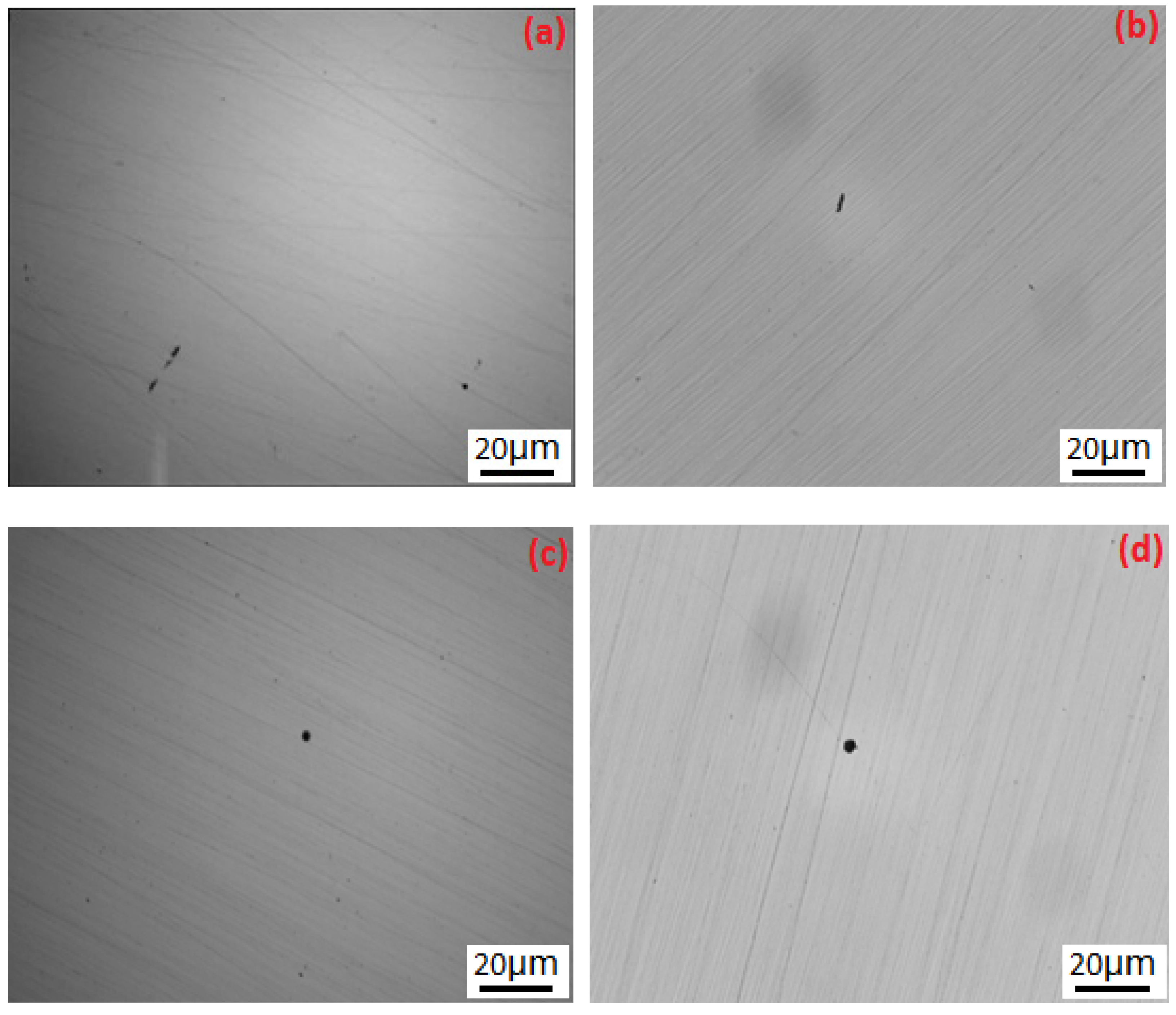
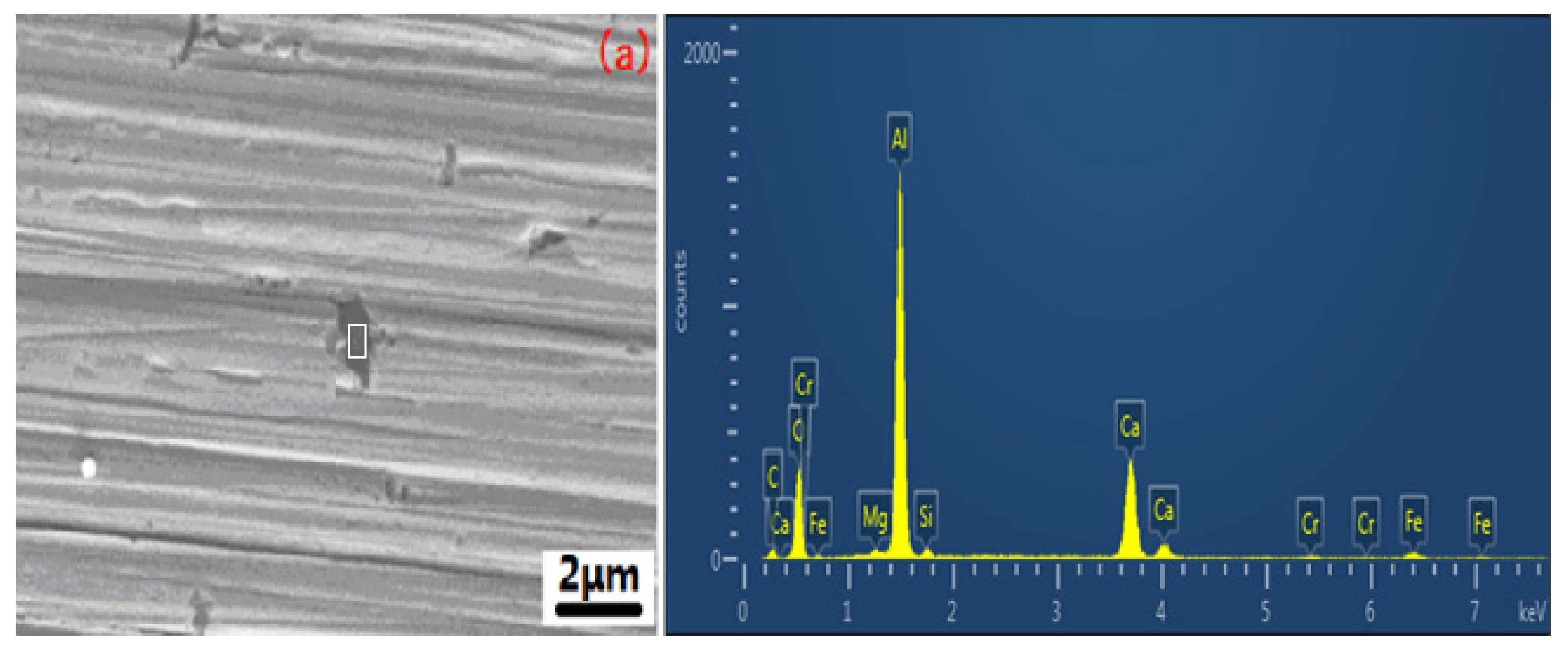

| Element | C | O | Al | Cr | Fe | Ni | Si | Ca | Mg |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.59 | 34.24 | 45.53 | 2.69 | 11.95 | − | 1.01 | 14.27 | 0.44 |
| 2 | 5.61 | 23.50 | 30.18 | 6.52 | 32.51 | − | 0.92 | 16.08 | 1.34 |
| 3 | 14.94 | 35.02 | 30.96 | 0.75 | 3.05 | − | − | − | − |
| 4 | 9.80 | 36.74 | 32.86 | 0.66 | 2.50 | 1.68 | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Yang, H.; Li, X.; Zhang, Z.; Lin, Y.; Deng, K. Effect of Chloride and Iodide on the Corrosion Behavior of 13Cr Stainless Steel. Metals 2022, 12, 1833. https://doi.org/10.3390/met12111833
Liu W, Yang H, Li X, Zhang Z, Lin Y, Deng K. Effect of Chloride and Iodide on the Corrosion Behavior of 13Cr Stainless Steel. Metals. 2022; 12(11):1833. https://doi.org/10.3390/met12111833
Chicago/Turabian StyleLiu, Wanying, Hong Yang, Xiaopeng Li, Zhi Zhang, Yuanhua Lin, and Kuanhai Deng. 2022. "Effect of Chloride and Iodide on the Corrosion Behavior of 13Cr Stainless Steel" Metals 12, no. 11: 1833. https://doi.org/10.3390/met12111833
APA StyleLiu, W., Yang, H., Li, X., Zhang, Z., Lin, Y., & Deng, K. (2022). Effect of Chloride and Iodide on the Corrosion Behavior of 13Cr Stainless Steel. Metals, 12(11), 1833. https://doi.org/10.3390/met12111833





