3.1. Structural Properties for C11b-VSi2 with Different Atomic Vacancies
Here, VSi
2 is a tetragonal (C11
b) structure. To investigate the relationship between different vacancies and properties, we constructed a crystal structure containing nine V atoms and ten Si atoms as shown in
Figure 1 and discussed C11
b-VSi
2 with different vacancies, such as V-va1, V-va2, Si-va1 and Si-va2 [
31]. By discussing the forming energies of perfect C11
b-VSi
2 and different atomic vacancies C11
b-VSi
2, the thermodynamic stability of C11
b-VSi
2 containing vacancies is studied. The vacancy formation energy (
Ef), which is the energy necessary to generate a vacancy and is dependent on the relationship between the atom and its nearby atoms whenever the element is removed, is used to determine the thermodynamic stability of vacancies [
32]. Only when the formation energy is negative, it is known that a solid material is thermodynamically stable. In particular, the lower the value of the vacancy formation energy, the better the thermodynamic stability [
33]. When atomic vacancies are present, C11
b-VSi
2 with different atomic vacancies have different levels of thermodynamic stability. Therefore, to investigate the structural stability of C11
b-VSi
2, we estimated the atomic vacancy formation energy as follows:
The total energy of C11b-VSi2 with an M (M = V and Si) vacancy and a C11b-VSi2 perfect crystal without a vacancy are and , respectively. μM is the chemical potential of the M atom that has been eliminated.
The vacancy formation energies and lattice parameters of perfect C11
b-VSi
2 and C11
b-VSi
2 containing different atomic vacancies are listed in
Table 1, as well as the other theoretical results [
10]. Because the vacancy formation energies in C11
b-VSi
2 are less than zero, these vacancies are thermodynamically stable in the ground state [
34]. Moreover, with the introduction of vacancies, the vacancy formation energy of C11
b-VSi
2 containing vacancies is slightly larger compared with that of the perfect C11
b-VSi
2. It shows that the introduction of vacancies increases the thermodynamic instability of C11
b-VSi
2. The calculated vacancy formation energies of V vacancies are smaller than those of Si vacancies, as shown in
Table 1, indicating that the V vacancies are more thermodynamically stable than the Si-vacancies. As a result, C11
b-VSi
2 is more inclined to form V vacancies.
Moreover, the lattice parameters of perfect C11
b-VSi
2 and C11
b-VSi
2 containing different vacancies are discussed in depth, which have also been discussed in NbSi
2 [
35]. In general, the lattice parameters of C11
b-VSi
2 containing vacancies are slightly smaller than that of perfect C11
b-VSi
2 along the a-axis and the b-axis, but slightly larger than that of perfect C11
b-VSi
2 along the c-axis. The differences in the lattice parameters determine the elastic properties. For V vacancies, the lattice constants of V-va1 are approximately equal to those of V-va2. Additionally, C11
b-VSi
2 with V vacancies have larger values of lattice parameters a and b than Si vacancies; however, the c values are smaller than those of Si vacancies. For Si vacancies, the lattice constant values for Si-va2 vacancies are larger than those for Si-va1 in the a and b directions, and the c values are approximately equal. The position and atomic type of the removed atoms lead to differences in the variation of the lattice constants.
The computed phonon dispersion of perfect C11
b-VSi
2 and C11
b-VSi
2 with vacancies in the Brillouin zone along the high symmetric direction is shown in
Figure 2. The phonon spectrum is an important indicator to judge whether the system is stable [
36]. If the phonon frequencies are all above the zero point, it means that the material does not have imaginary frequencies, indicating that the material is stable [
37]. From
Figure 2, the phonon frequencies of both perfect C11
b-VSi
2 and C11
b-VSi
2 in the presence of vacancies have no imaginary frequencies, indicating that they are dynamically stable. This yields results that are consistent with those obtained for the vacancy formation energy.
3.2. Elastic Properties
Before analyzing the elastic properties of C11
b-VSi
2 with various vacancies, it is necessary to consider the mechanical stability of the material [
38]. The elastic constants (
Cij) and the elastic flexibility matrix (
Sij) are calculated for perfect C11
b-VSi
2 and C11
b-VSi
2 containing different vacancies in
Table 2 and
Table 3, respectively. The generalized mechanical stability criterion for the tetragonal system is
C11 > 0,
C33 > 0,
C44 > 0,
C66 > 0,
C11 >
C12,
C11 +
C33 − 2
C13 > 0, 2
C11 +
C33 + 2
C12 + 4
C13 > 0 [
39]. Clearly, the perfect C11
b-VSi
2 and C11
b-VSi
2 containing different vacancies satisfy the stability criterion for all elastic constants. Therefore, they have mechanical stability.
The linear compression resistance along the
a-axis,
b-axis, and
c-axis is represented by
C11,
C22, and
C33, respectively [
40].
Table 2 shows that the
C11 values for perfect C11
b-VSi
2 and C11
b-VSi
2 containing atomic vacancies are generally lower than the
C33 values, indicating that the deformation resistance along the
a-axis is lower than the deformation resistance along the
c-axis. The
C11 values of C11
b-VSi
2 with different vacancies are larger than those of perfect C11
b-VSi
2, yet the
C33 values are lower than those of perfect C11
b-VSi
2. It shows that the vacancies created by the removal of atoms increase the resistance to deformation along the
a-axis but weaken resistance to deformation along the
c-axis. Additionally, since the
C11 value of C11
b-VSi
2 containing Si vacancies is larger than that of C11
b-VSi
2 containing V vacancies, the deformation resistance produced by the removal of Si atoms is larger than that produced by the removal of V atoms in the direction along the
a-axis. In addition to this, it is well known that
C44 and
C66 are correlated to shear modulus, with larger values of
C44 and
C66 corresponding to larger shear modulus.
Table 2 shows that the
C44 and
C66 values of C11
b-VSi
2 with different vacancies are larger than those of perfect C11
b-VSi
2, indicating that the atom vacancies significantly enhance the shear deformation resistance of C11
b-VSi
2. Moreover, the V-va2 vacancies has the largest
C33,
C44 and
C66 values compared to other vacancy types, which implies that C11
b-VSi
2 with V-va2 vacancies have a greater resistance to deformation.
The mechanical properties of C11
b-VSi
2 containing these vacancies are investigated in order to establish the link between vacancies and mechanical performance. The elastic moduli (including bulk modulus
B, Poisson’s ratio
v, Young’s modulus
E, and shear modulus
G) of perfect C11
b-VSi
2 and C11
b-VSi
2 with different atomic vacancies for the C11
b structure obtained by the Voigt–Reuss–Hill method are presented in
Table 4, where
BV (
GV) and
BR (
GR) are
B (
G) in the Voigt and Reuss approximations, respectively, and the expressions are as follows [
41,
42,
43,
44,
45]:
The ratio of bulk modulus to shear modulus (
B/
G) validates the solid material’s brittleness/ductility [
46]. If the
B/
G ratio is higher than 1.75, the solid material will exhibit ductility. Otherwise, the material will exhibit brittleness. The higher the
B/
G value, the more ductile the solid material is [
47]. Based on the
B/
G ratios of C11
b-VSi
2 with varying vacancies as well as perfect C11
b-VSi
2 shown in
Table 4, the
B/
G of perfect C11
b-VSi
2 is 1.642, which is less than 1.75, showing that C11
b-VSi
2 is brittle. Meanwhile, with the introduction of vacancies, the calculated
B/
G values of C11
b-VSi
2 containing these vacancies generally decrease, except the
B/
G value of V
-va1 (1.668). Notably, the
B/
G values of C11
b-VSi
2 with V vacancies are all larger than those of C11
b-VSi
2 with Si vacancies, and thus, C11
b-VSi
2 containing V vacancies have less brittleness.
Bulk modulus is a macroscopic property of a material that reflects its resistance to external compression, i.e., incompressibility, as is widely known. As seen in
Table 4, the bulk modulus of C11
b-VSi
2 containing different vacancies is larger than that of perfect C11
b-VSi
2, indicating that the removal of atoms instead makes the material more incompressible. The force that resists form change under shear stress is known as the shear modulus (
G). Shear stress is more closely related to hardness than bulk modulus, and shear modulus is a more appropriate predictor of hardness [
48]. In
Table 4, C11
b-VSi
2 with the introduction of V-va1 vacancy has the smallest shear modulus and is smaller than perfect C11
b-VSi
2. Therefore, its ductility is slightly better than that of perfect C11
b-VSi
2. Additionally, all the remaining types of vacancies increase the shear modulus, compared to perfect C11
b-VSi
2. Thus, the hardness values of C11
b-VSi
2 with V-va2, Si-va1, and Si-va2 increase, and this variation of hardness with vacancy has been found in chromium silicide [
49]. In addition, the stiffness of a solid can be described using Young’s modulus. The stiffness of a solid is proportional to its Young’s modulus. As can be seen from
Table 4, the introduction of atomic vacancies increases the Young’s modulus of C11
b-VSi
2, with the largest being for Si-va1 vacancies and only those containing V-va1 vacancies having lower Young’s modulus. Accordingly, removing atoms increases the elastic stiffness of VSi
2. It is congruent with the results of the elastic constants. From the point of view of interatomic interactions, elastic modulus is closely related to the interaction between atoms. The increased elastic modulus of C11
b-VSi
2 containing different vacancies, except for the V-va1 vacancy, can be tentatively judged that the removal of atoms enhances the atomic interaction of C11
b-VSi
2.
Poisson’s ratio is a well-known method for determining a solid’s stability under shear deformation [
50]. Solids having a Poisson’s ratio of −1 to 0.5 are relatively stable under shear deformation in general. Furthermore, a larger Poisson’s ratio indicates that the solid is more malleable. As shown in
Table 4, the Poisson’s ratios of perfect C11
b-VSi
2 and C11
b-VSi
2 containing different vacancies is in the range of 0.229~0.25, which is between −1 and 0.5, indicating that they are stable solids. Among them, VSi
2 containing V-va1 vacancies has the largest Poisson’s ratio, indicating that it has better plasticity than the other compounds. It also corroborates that the Vickers hardness of VSi
2 containing V-va1 vacancies is the smallest among them. Poisson’s ratio also can be used to estimate the solid’s brittleness and ductility. If the solid has a
ν > 0.33, the material is ductile; otherwise, it is fragile. From the calculations in
Table 4,
ν < 0.33 for perfect C11
b-VSi
2 and C11
b-VSi
2 with different vacancies; thus, they are brittle. This result agrees well with that of
B/
G.
3.3. Elastic Anisotropy
The elastic anisotropy is an important index reflecting the mechanical anisotropy of materials and plays a very large role in the generation of microcracks. To better describe the elastic anisotropy, in this paper, we use elastic anisotropy indices such as the universal anisotropy index (
AU), compression and shear anisotropy percentages (
Acomp and
Ashear), and shear anisotropy factors (
A1,
A2, and
A3) to investigate the elastic anisotropy [
51]. The calculation equations are as follows:
Furthermore, due to the fact that the VSi
2 we study in this paper is a tetragonal crystal structure, considering its shear anisotropy factor:
A1,
A2, and
A3 represent the degree of shear anisotropy corresponding to the (100), (010) and (001) planes, respectively. For these elastic anisotropy indices, if
A1 =
A2 =
A3 = 1 and
AU =
Acomp =
Ashear = 0 [
52], the solid shows elastic isotropy; otherwise, it is anisotropic. At the same time, when the solid has larger values of
AU,
Acomp and
Ashear, it has higher elastic anisotropy.
The calculated elastic anisotropic indices are listed in
Table 5. From
Table 5, both perfect C11
b-VSi
2 and C11
b-VSi
2 with different vacancies are anisotropic, since their elastic anisotropy indices deviate from 0. The greater the deviation, the greater the anisotropy [
53]. It is obvious that the changes corresponding to
Acomp and
Ashear are different.
Ashear increases with the introduction of vacancies. However,
Acomp is decreasing. This may be due to the difference in the variation of bulk and shear moduli. Therefore, using
Acomp and
Ashear alone to evaluate the material elastic anisotropy has limitations. On the contrary,
AU is more accurate to evaluate the elastic anisotropy by considering both bulk modulus and shear modulus. As can be seen from
Table 5, the
AU values of C11
b-VSi
2 containing different vacancies are larger than those of VSi
2, and in contrast, the
AU values of C11
b-VSi
22 containing V vacancies increase sharply. It indicates that the introduction of vacancies improves the anisotropy of the material, especially the V vacancies. Among them, the elastic anisotropy of vacancy V-va1 is the largest. In addition, the shear anisotropy indices
A1,
A2 and
A3 are not equal to 1, indicating that they are all anisotropic. The introduction of the vacancy increases
A1,
A2 and
A3, meaning that the vacancy increases the shear anisotropy of C11
b-VSi
2 in the (100), (010) and (001) plane all. For
A3, the increase is the largest. It shows that the vacancy enhances the shear anisotropy of C11
b-VSi
2 in the (001) plane. the increase in V-va1 is particularly significant.
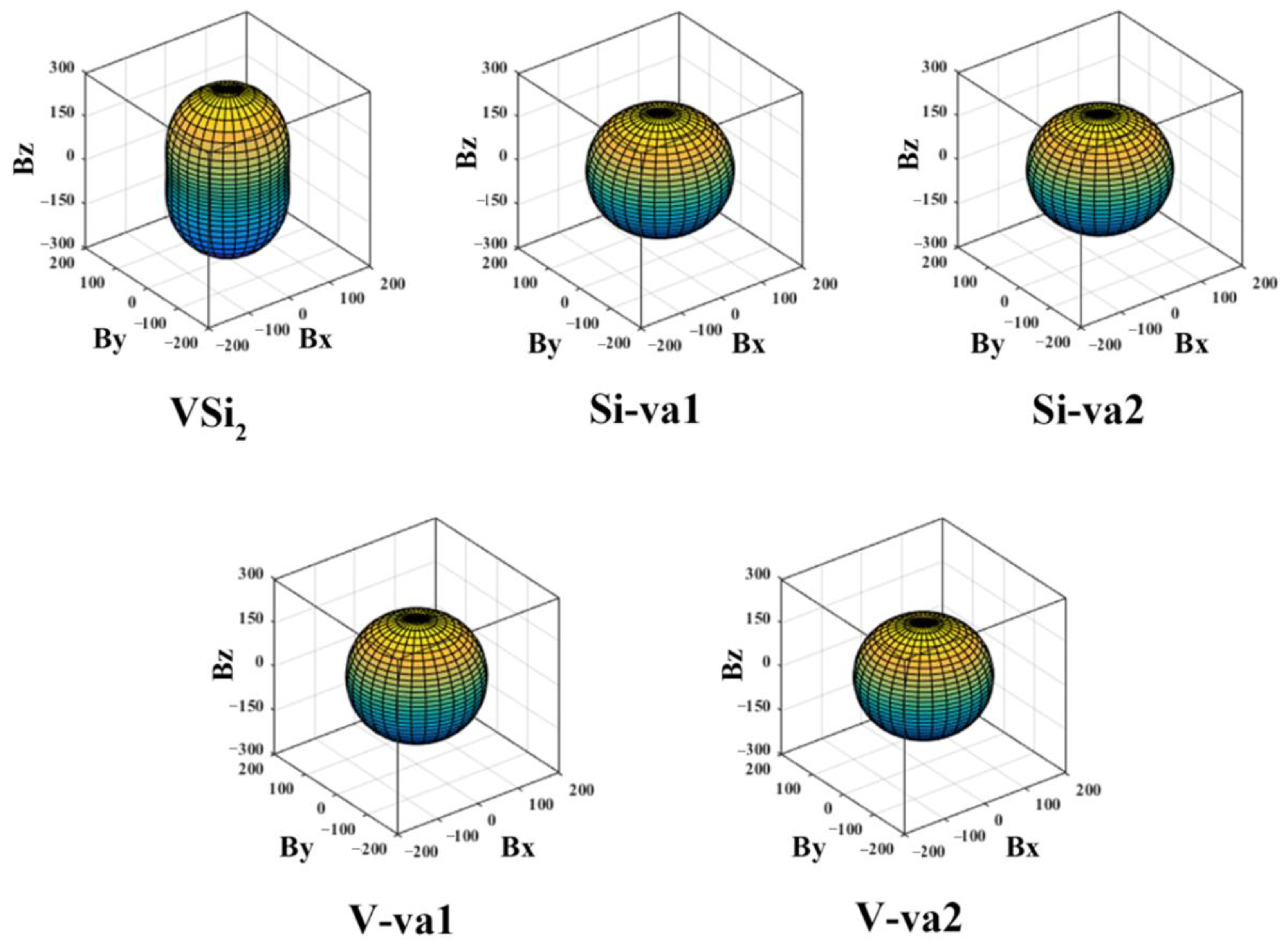
The elastic anisotropy of the crystal can also be visualized by the three-dimension (3D) surface construction diagram of the elastic modulus. When the crystal is isotropic, the 3D surface construction diagram is perfectly spherical. On the contrary, it is anisotropic. In this work, we focus on the elastic anisotropy of the bulk modulus
B and Young’s modulus
E through the 3D surface construction diagram. For the tetragonal structure, the inverse of the bulk modulus
B and Young’s modulus
E are calculated as follows [
54]:
Here,
Sij is the elastic compliance constant listed in
Table 3, and
l1,
l2, and
l3 are the direction cosines.
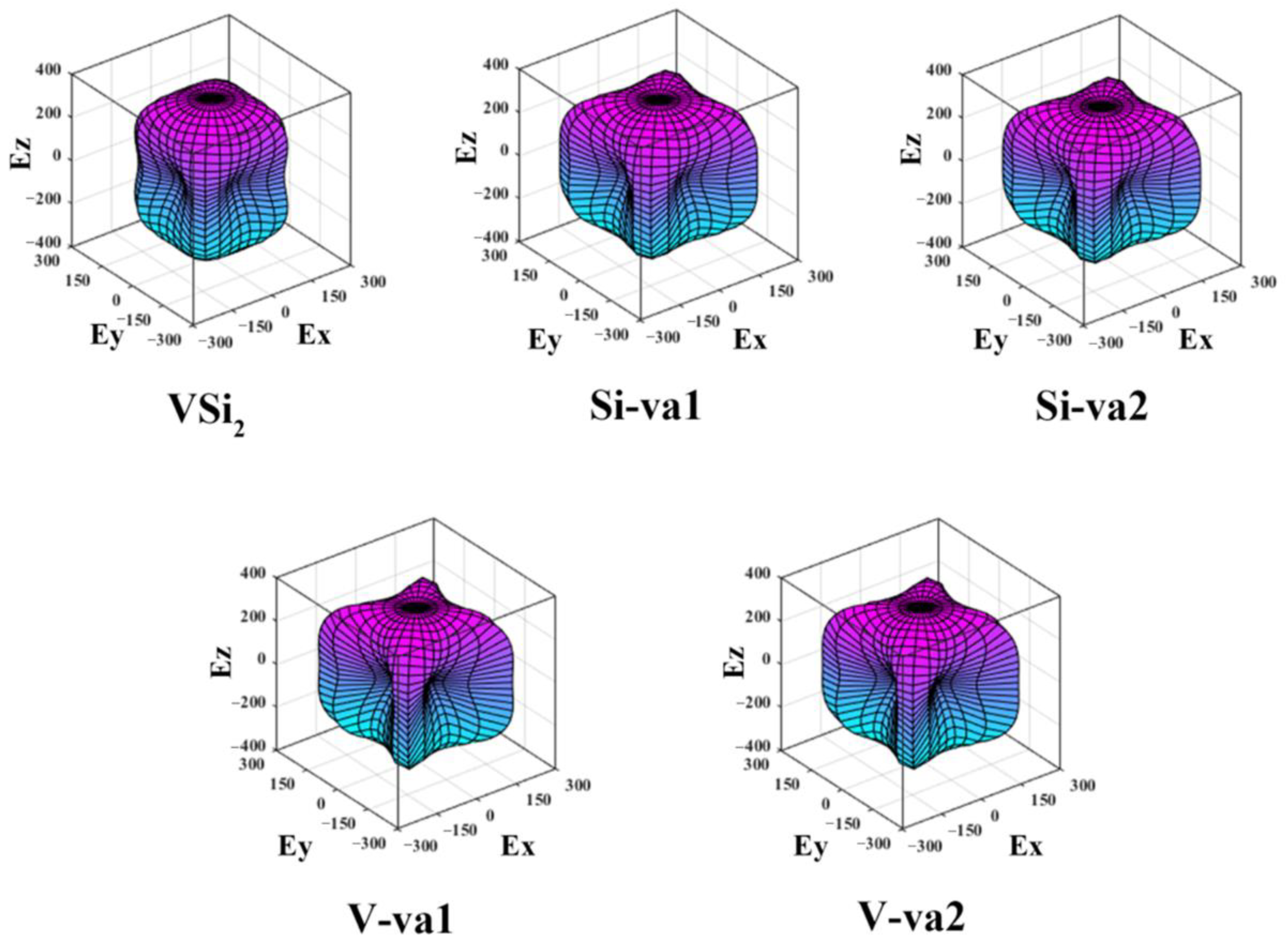
Figure 3 and
Figure 4 show the 3D surface construction diagrams of the bulk modulus and Young’s modulus, respectively. When the deviation of the 3D surface construction diagram from the spherical shape is greater, the anisotropy of the solid is greater [
55]. As can be seen from
Figure 3, there is no significant difference between the 3D diagrams of bulk modulus of C11
b-VSi
2 containing vacancies, then it is necessary to consider the 3D diagram of Young’s modulus. It is obvious from
Figure 4 that the 3D diagrams of Young’s modulus of C11
b-VSi
2 containing different vacancies and perfect C11
b-VSi
2 are non-spherical. Compared with the 3D diagrams of bulk modulus, the 3D diagrams of Young’s modulus are more irregular in shape, indicating that they are all anisotropic, and the anisotropic feature is greater than that of the bulk modulus. Moreover, with the introduction of vacancies, the deviation of 3D diagrams of Young’s modulus from the spherical shape is greater, indicating that the different atomic vacancies enhance the anisotropy of Young’s modulus. This result is in good agreement with the results corresponding to
AU in
Table 5.
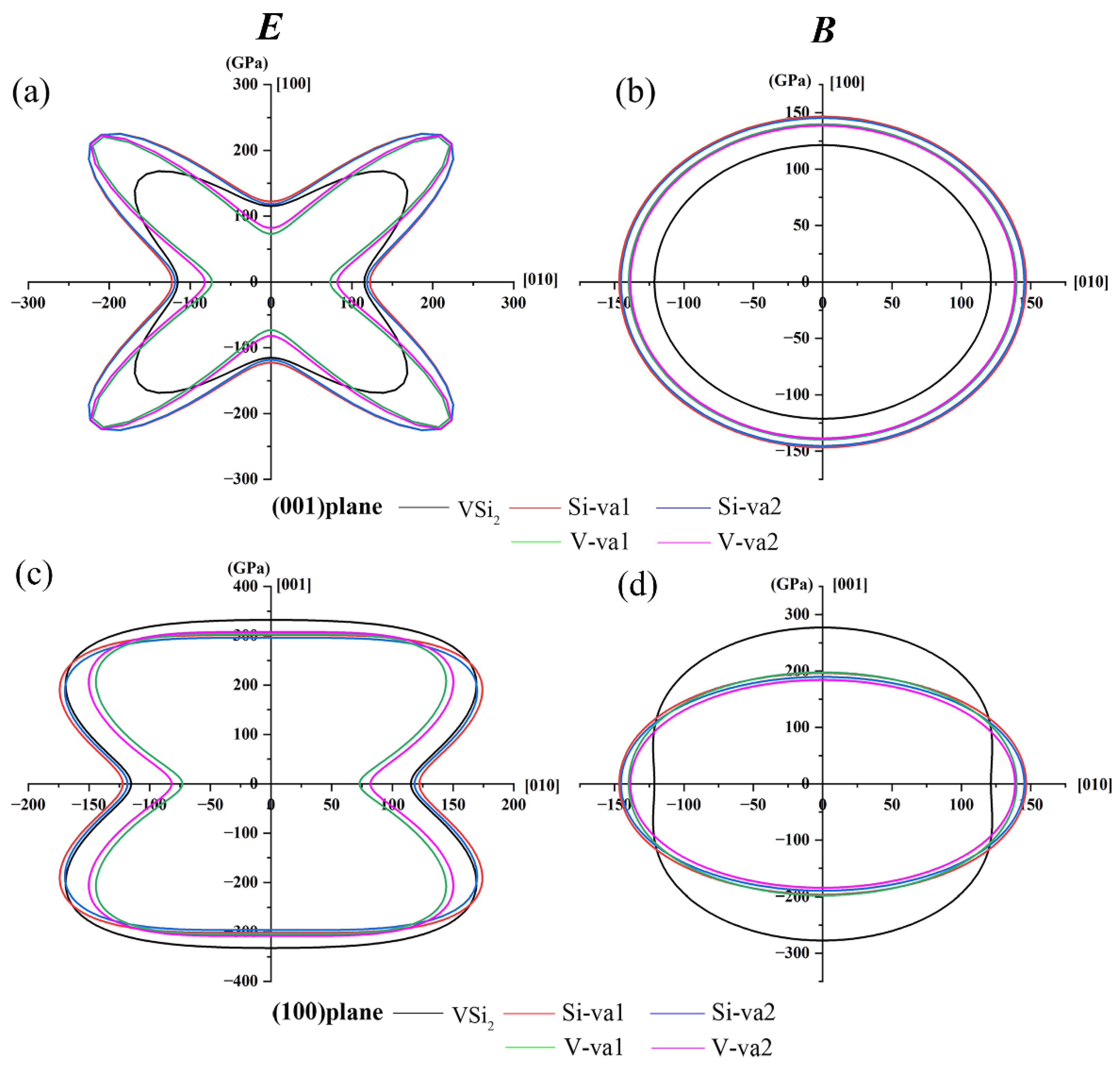
However, the 3D construction diagrams in
Figure 4 do not clearly show the subtle differences in the elastic modulus anisotropy of C11
b-VSi
2 containing different vacancies. Therefore, to see more details of the elastic anisotropy, the two-dimension (2D) projections of bulk modulus and Young’s modulus in the (001) and (100) planes, which have been employed successfully [
56], for both perfect C11
b-VSi
2 and C11
b-VSi
2 containing different atomic vacancies are shown in
Figure 5. From
Figure 5, in general, the introduction of vacancies increases the anisotropy of the elastic modulus. For the Young’s modulus,
Figure 5a shows that the shape of C11
b-VSi
2 containing different atomic vacancies in the (001) plane is more deviated from circular than that of perfect C11
b-VSi
2. It means that the vacancies make Young’s modulus more anisotropic. As shown in
Figure 5c, the 2D projection of C11
b-VSi
2 containing Si atomic vacancies in the (100) plane is approximately or slightly anisotropic to that of perfect C11
b-VSi
2. In contrast, C11
b-VSi
2 containing V-atom vacancies is more obviously irregular in shape and more anisotropic. This result is in agreement with the one corresponding to
AU. For the bulk modulus, in the (001) plane, the shapes of the graphs are all circular and the crystals show no significant anisotropy. However, in the (100) plane, the shape of C11
b-VSi
2 containing different vacancies deviates significantly from a circle and shows a higher anisotropy.
Table 6 shows the elastic moduli in the [100], [010] and [001] directions obtained according to
Figure 5. It is obvious from
Table 6 that the values of Young’s modulus and bulk modulus in the [100] and [010] directions are smaller than those in the [001] direction because the value of
C11 is smaller than that of
C33, making the crystal easier to compress along the
a-axis [
57].
3.4. Electronic Properties
As mentioned above, the reason for the change in the elastic modulus of C11
b-VSi
2 containing atomic vacancies is related to the inter-charge interactions, where the type and position of the atoms determine the inter-charge interactions [
58]. The removal of V and Si atoms in C11
b-VSi
2 due to inter-charge interactions changes the electron equilibrium concentration between adjacent atoms and alters the chemical bonding in VSi
2. There are two different types of chemical bonds in perfect C11
b-VSi
2, the V-Si bond and the Si-Si covalent bond. The bond lengths of its Si-Si covalent and V-Si bonds were calculated to be 2.529 Å and 2.579 Å, respectively, which is consistent with earlier theoretical predictions. The bond lengths of Si-Si covalent and V-Si bonds for perfect C11
b-VSi
2 and C11
b-VSi
2 with different vacancies are shown in
Table 7. As shown in
Table 7, the insertion of Si vacancies boosts the charge interaction between the V atom and the Si atom while weakening the charge interaction between the Si atom and the other Si atom. As a result, the single cell cohesion energy is weakened due to electron collapse, leading to lattice contraction. This is the major reason why Si vacancies have a greater elastic modulus than perfect C11
b-VSi
2.
For the V-atom vacancy, the presence of the V atom vacancy shortens the bond length of both the V-Si bond and the Si-Si covalent bond. In other words, the removal of the V atom enhances the interatomic charge interactions. We suggest that the loss of the V-Si bond on the shear surface causes the enhancement of the elastic modulus.
The electron density difference can be sued to further investigate electron transfer in chemical bonding [
59].
Figure 6 shows the electron density difference for perfect C11
b-VSi
2 and C11
b-VSi
2 with V and Si atomic vacancies. In each graph, the electron density difference ranges from −0.1e/Å
3 to 0.1e/Å
3. The blue color implies the maximum localization of electrons in this region, and the red color indicates the maximum delocalization of electrons in this region. In
Figure 6a, intact V-Si bonds and Si-Si covalent bonds in perfect C11
b-VSi
2 are observed. The removal of atoms clearly disrupts the localized hybridization between adjacent atoms compared to the figure with atomic vacancies. By comparison, we find that the electron delocalization of V vacancies and Si vacancies is weaker than that of perfect C11
b-VSi
2. In other words, the localized hybridization between V and Si atoms is improved by the removed atoms, which has been confirmed in TaSi
2 that the removals of Ta and Si can enhance the electron hybridization between Ta and Si [
60]. As can be seen in
Figure 6, for C11
b-VSi
2 containing vacancies, the inter-atomic charge interactions are stronger than the corresponding charge interactions for perfect C11
b-VSi
2. This is the reason that the elastic modulus of C11
b-VSi
2 containing vacancies increases.
In
Figure 6b,c, for Si vacancies, the removal of Si atoms enhances the local hybridization between V and Si atoms, but the Si-Si atom interactions are weakened. This is consistent with the results obtained from the discussion of bond lengths above. Thus, the enhanced elastic modulus comes from the electronic bonding properties. From
Figure 6d,e, the removal of V atoms when V vacancies are present also results in enhanced Si-Si inter-atomic interactions.
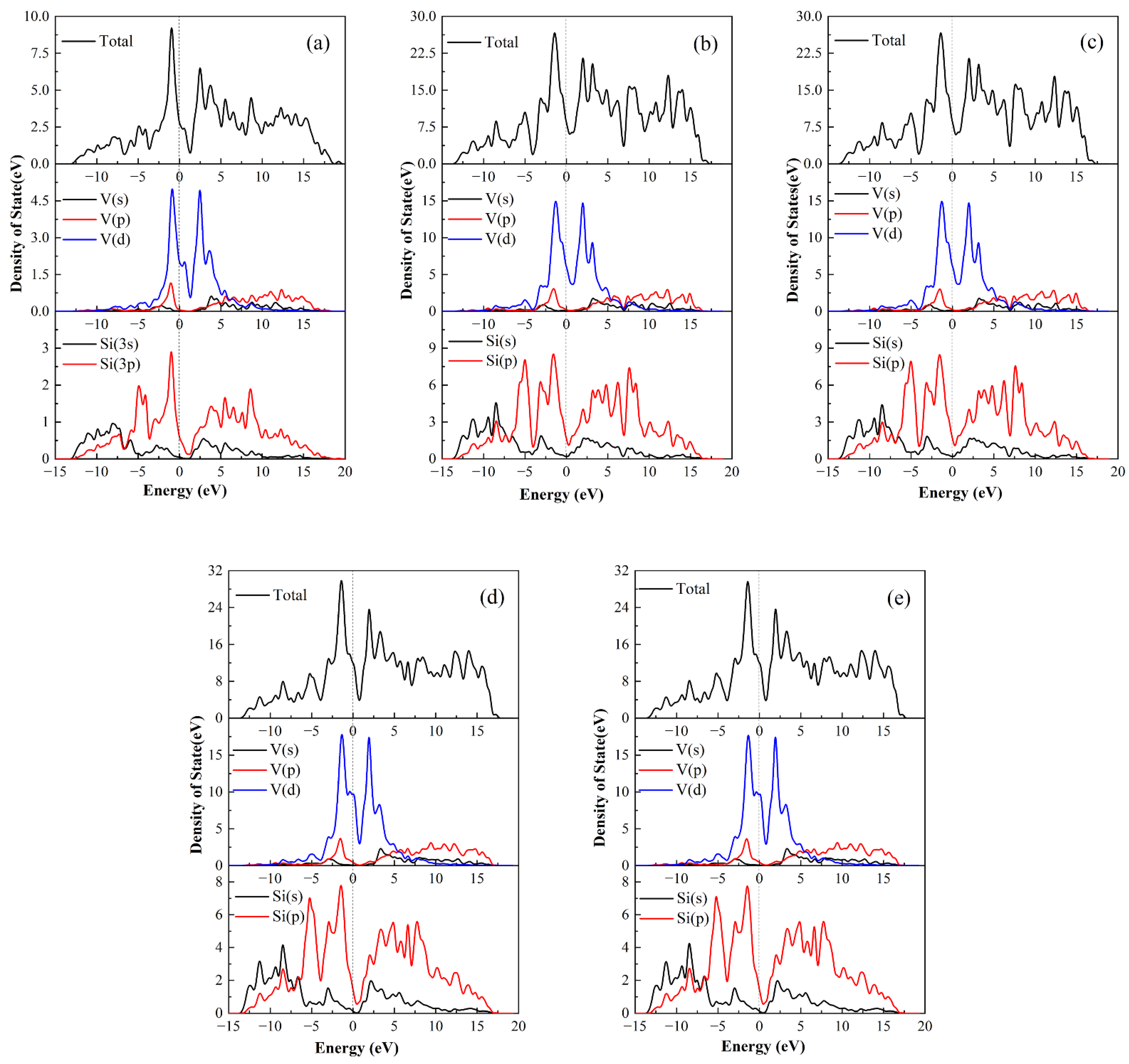
We calculated the total and partial densities of states (DOS and PDOS) to discuss the bonding properties of perfect C11
b-VSi
2 and C11
b-VSi
2 containing different atomic vacancies in
Figure 7. DOS denotes the number of electron states per unit energy interval when the electron energy levels are quasi-continuously distributed [
61,
62]. The dashed line with zero energy represents the Fermi energy level [
63]. The valley at the Fermi energy level is called the pseudogap, which indicates the presence of a stable phase. From the comparison in
Figure 7, the pseudogap of C11
b-VSi
2 with V vacancies is the smallest, proving that V vacancies are more stable than Si vacancies. This result is in line with the vacancy formation energy conclusion. The TDOS profile of C11
b-VSi
2 around the Fermi energy level is predominantly from the V-3d state and the Si-3p state, showing substantial hybridization between V and Si atoms, as seen in
Figure 7.
Figure 7a shows that the Si-3p state is divided into two parts by the Si-3s state, indicating the formation of hybridization between Si and Si atoms in VSi
2. However, the PDOS profile resulting from the removal of V and Si atoms is slightly different from that of the perfect C11
b-VSi
2. The energy of the V vacancies is determined by a tiny change around the Fermi energy level. As can be seen in
Figure 7b,c, for the V vacancy, the PDOS produces some small peaks near the Fermi energy level. The charge energy of V and Si atoms increases compared to that of perfect C11
b-VSi
2. The introduction of Si vacancies has a similar feature. The DOS profile of C11
b-VSi
2 with Si vacancies is similar to that of C11
b-VSi
2 with V vacancies, while more electrons are transferred from the lower energy region to the Fermi energy level compared with the density of states of V vacancies. It leads to stronger local hybridization between the Si-3s and Si-3p states, forming Si-Si covalent bonds. The loss of Si atoms, in particular, causes a charge transfer from the Si-3s state to the Si-3p state.