Effect of Sulfur Content on the Inclusion and Mechanical Properties in Ce-Mg Treated Resulfurized SCr420H Steel
Abstract
:1. Introduction
2. Methods
2.1. Material Preparation
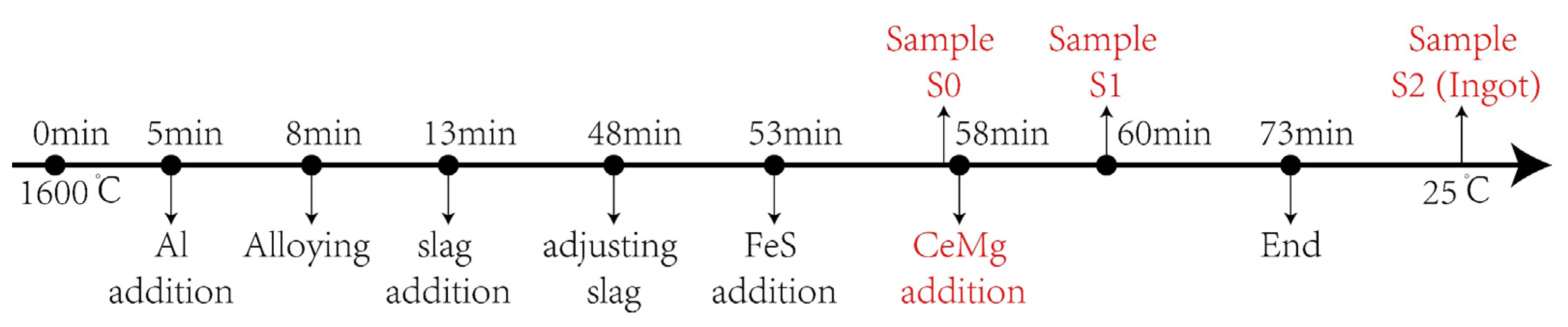
2.2. Experiment Procedure
2.3. Analysis
3. Results and Discussion
3.1. Steel Composition
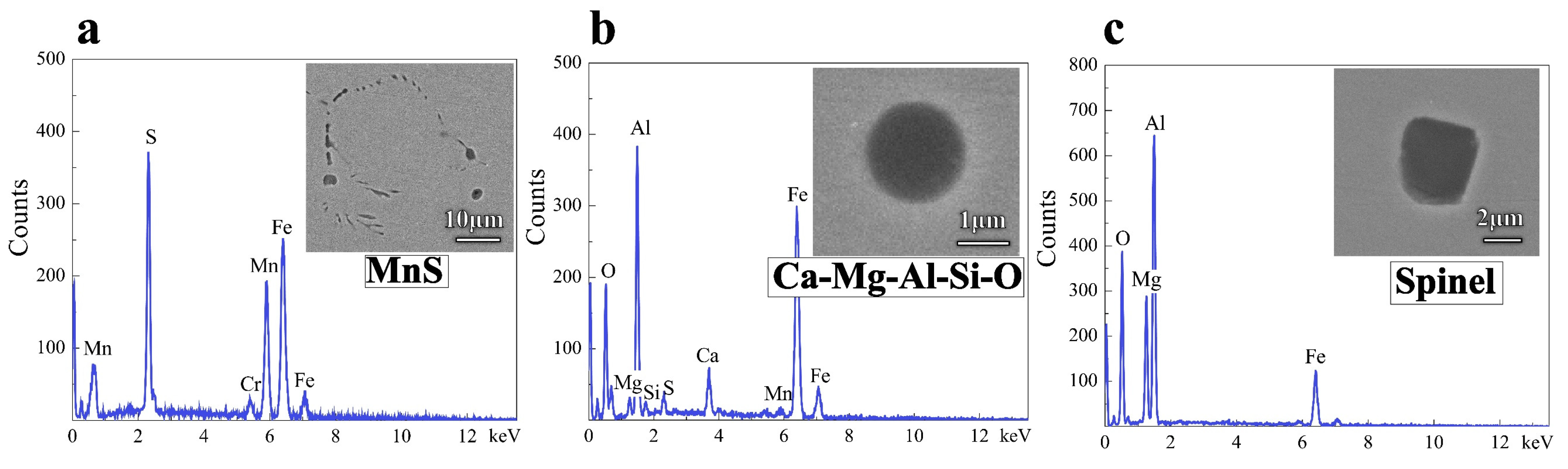
3.2. Evolution of Inclusions in Liquid Steel
3.3. Discussion on Evolution Mechanism of Inclusions in Liquid Steel
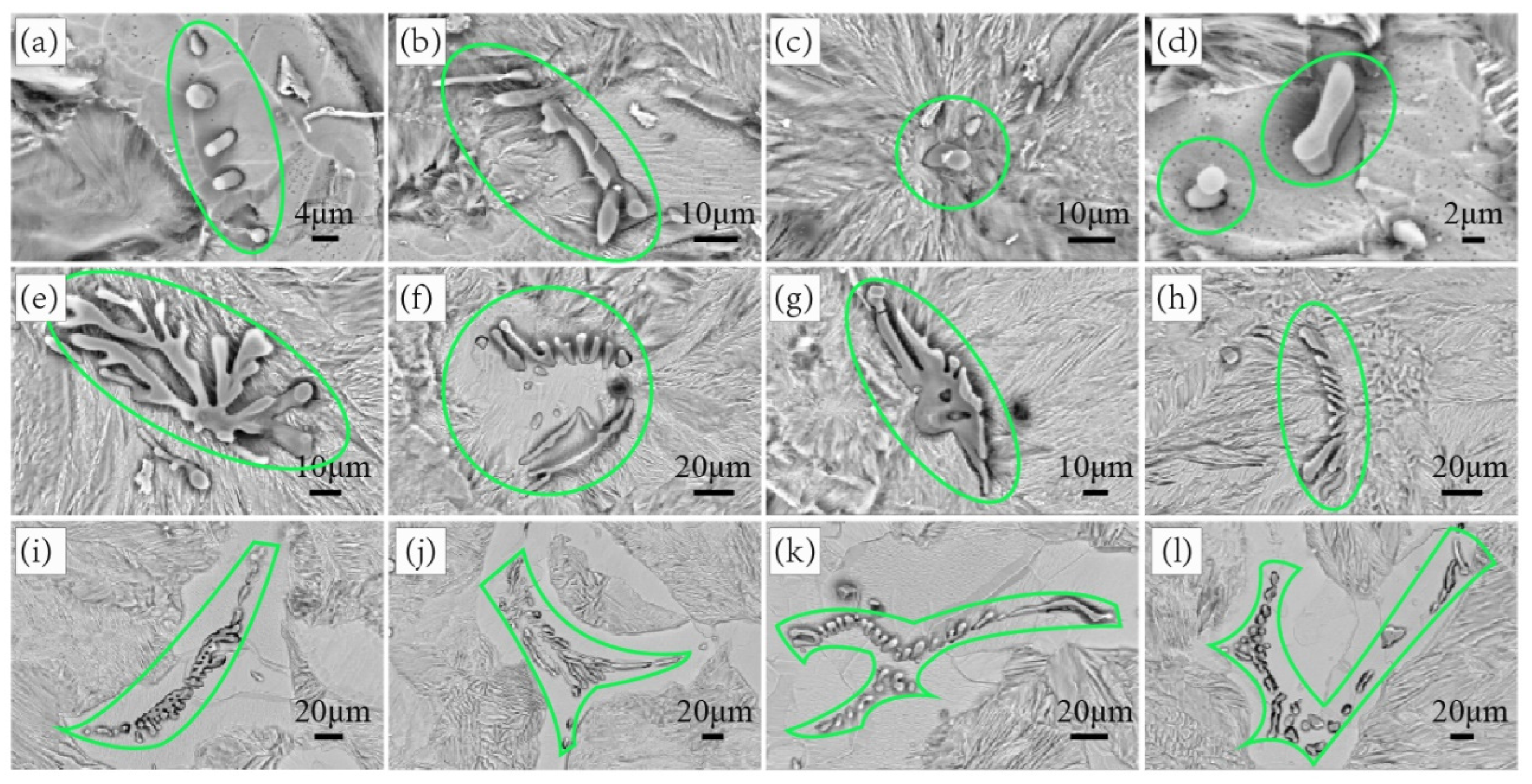
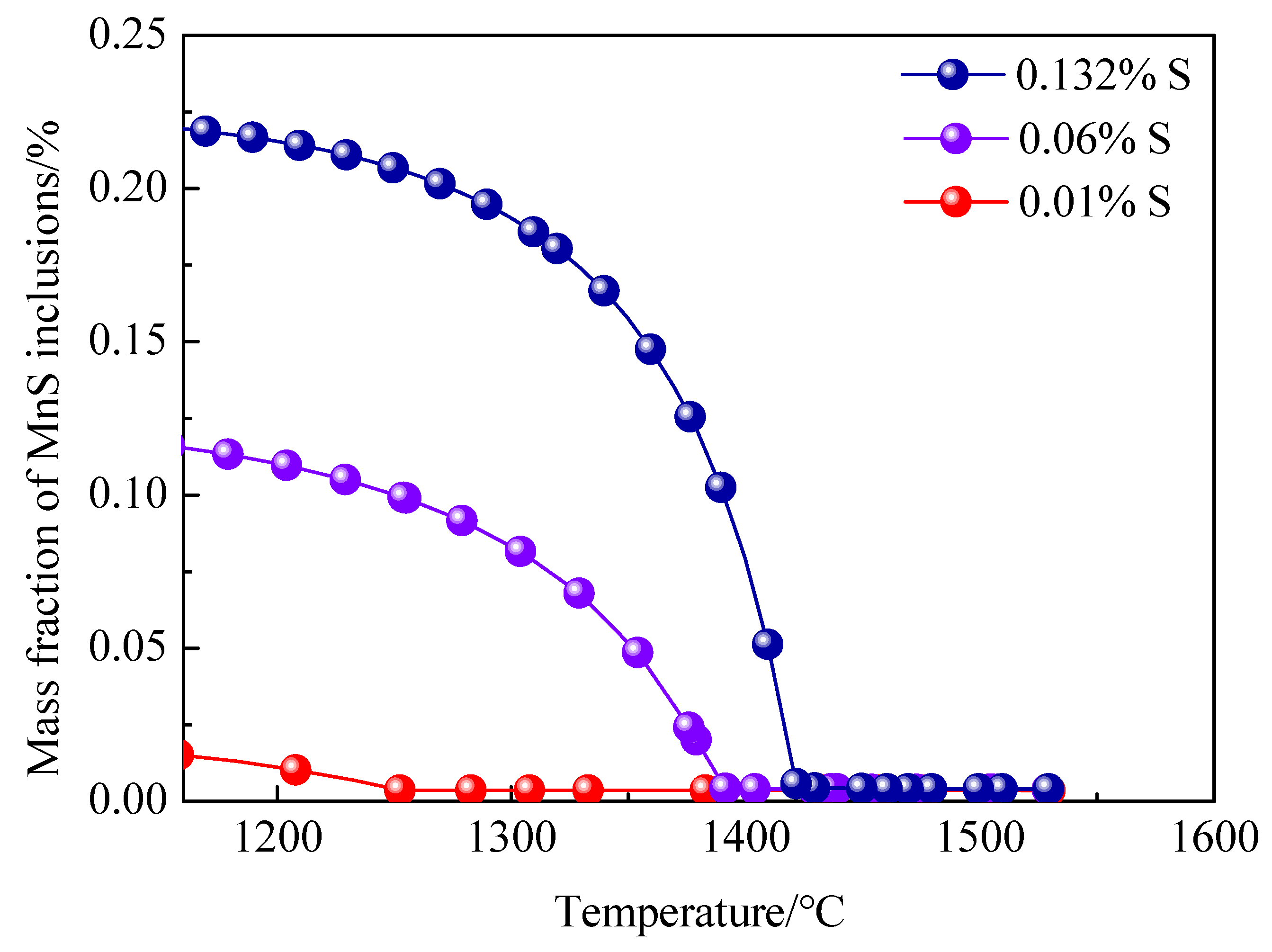
3.4. Precipitation of MnS Inclusion during Solidification
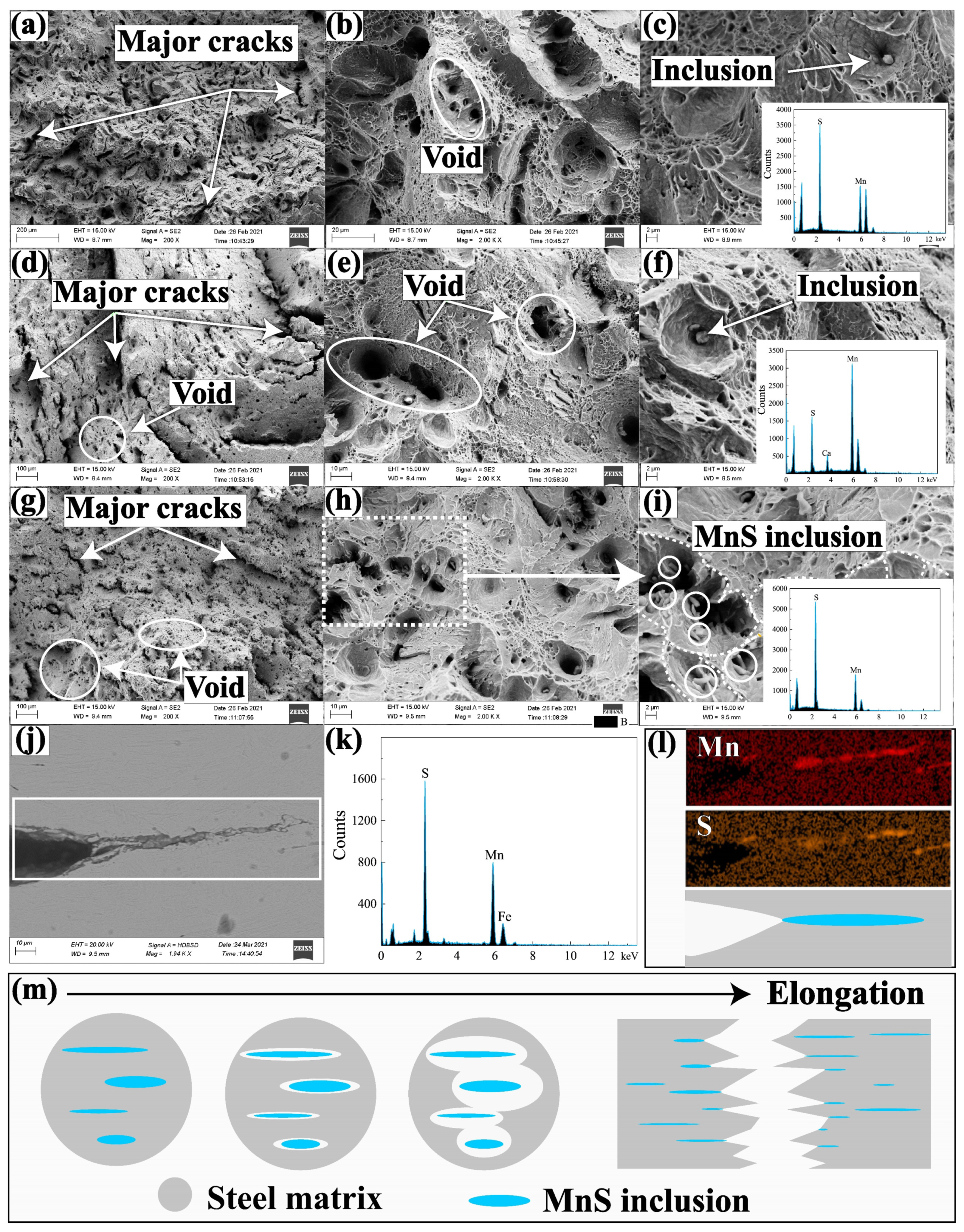
3.5. Effects of MnS Inclusions on Mechanical Properties
4. Conclusions
- (1)
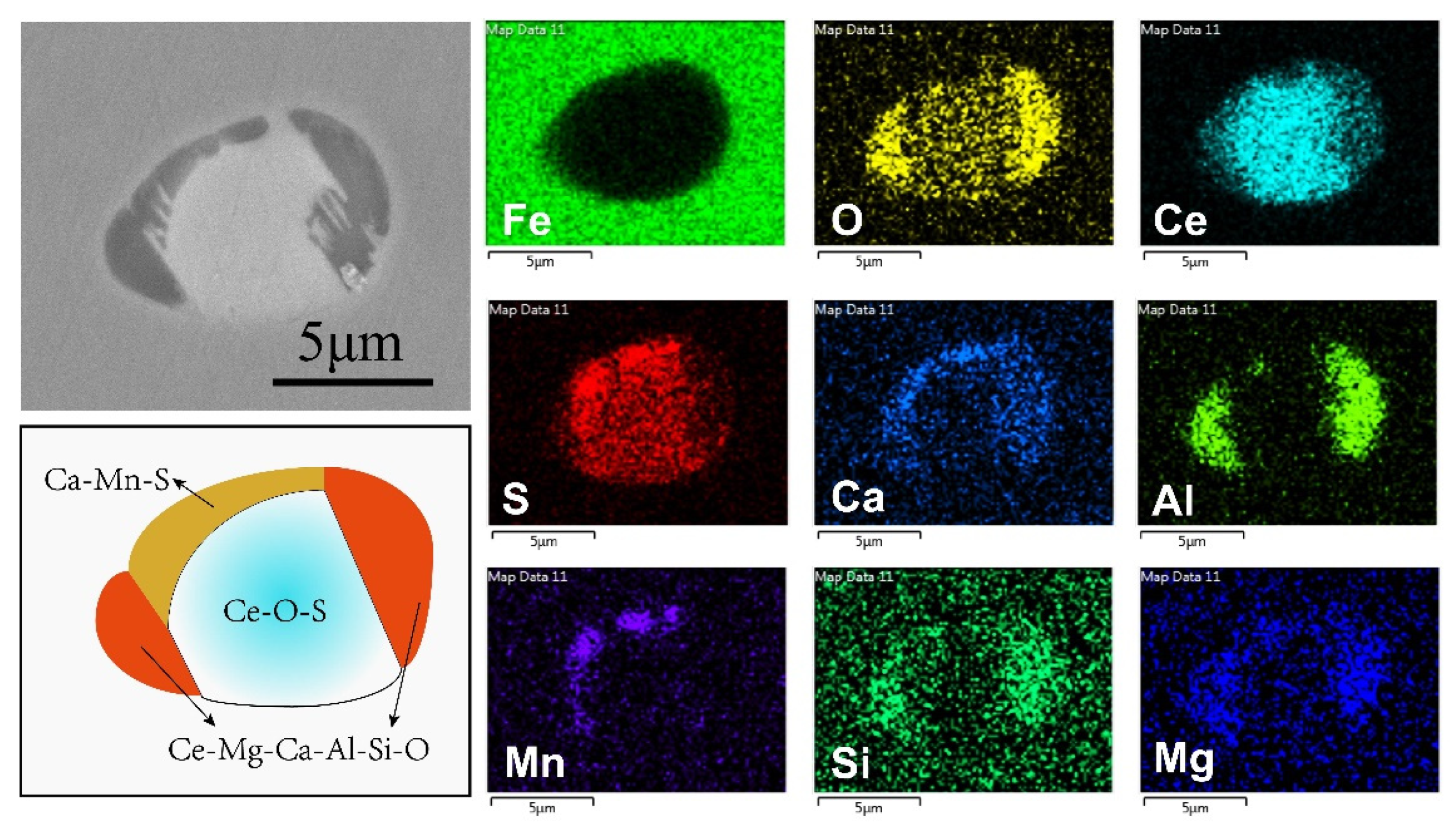
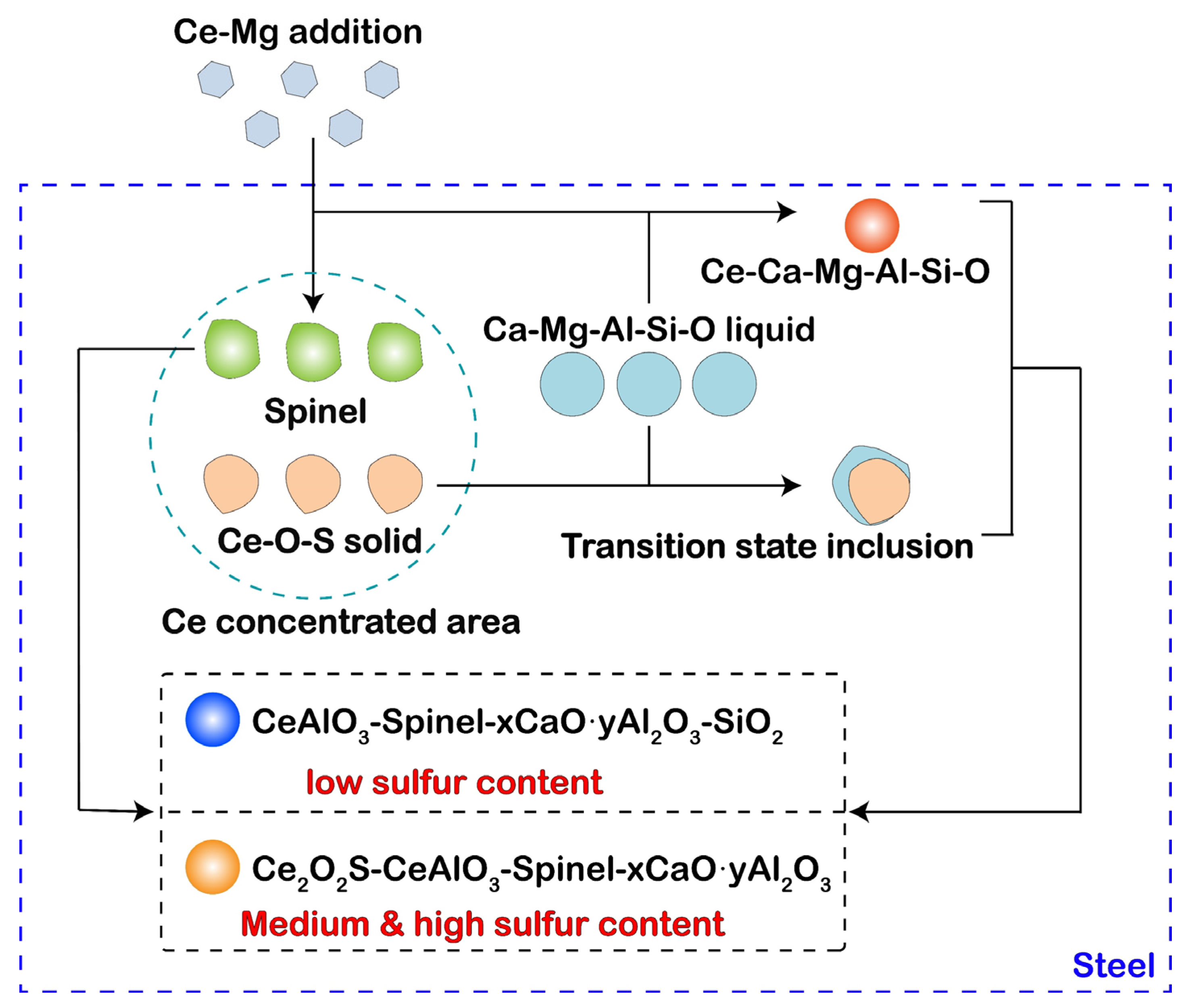
- After the Ce-Mg addition, the cerium concentration area formed in liquid steel. This may result in the formation of transition state inclusions. In current experiment, solid Ce2O2S formed in the cerium concentration area and collides with Ca-Mg-Al-Si-O liquid inclusion, and the liquid phase adhered to the outside of the solid phase, and formed a composite inclusion with a double-layer structure. As a type of transition state inclusion, it only exists in a short time after Ce-Mg addition. As the homogenization of liquid steel, cerium concentration area disappeared, and the difference of sulfur content in steel can result in this type of transition state inclusions transformed into different inclusions.
- (2)
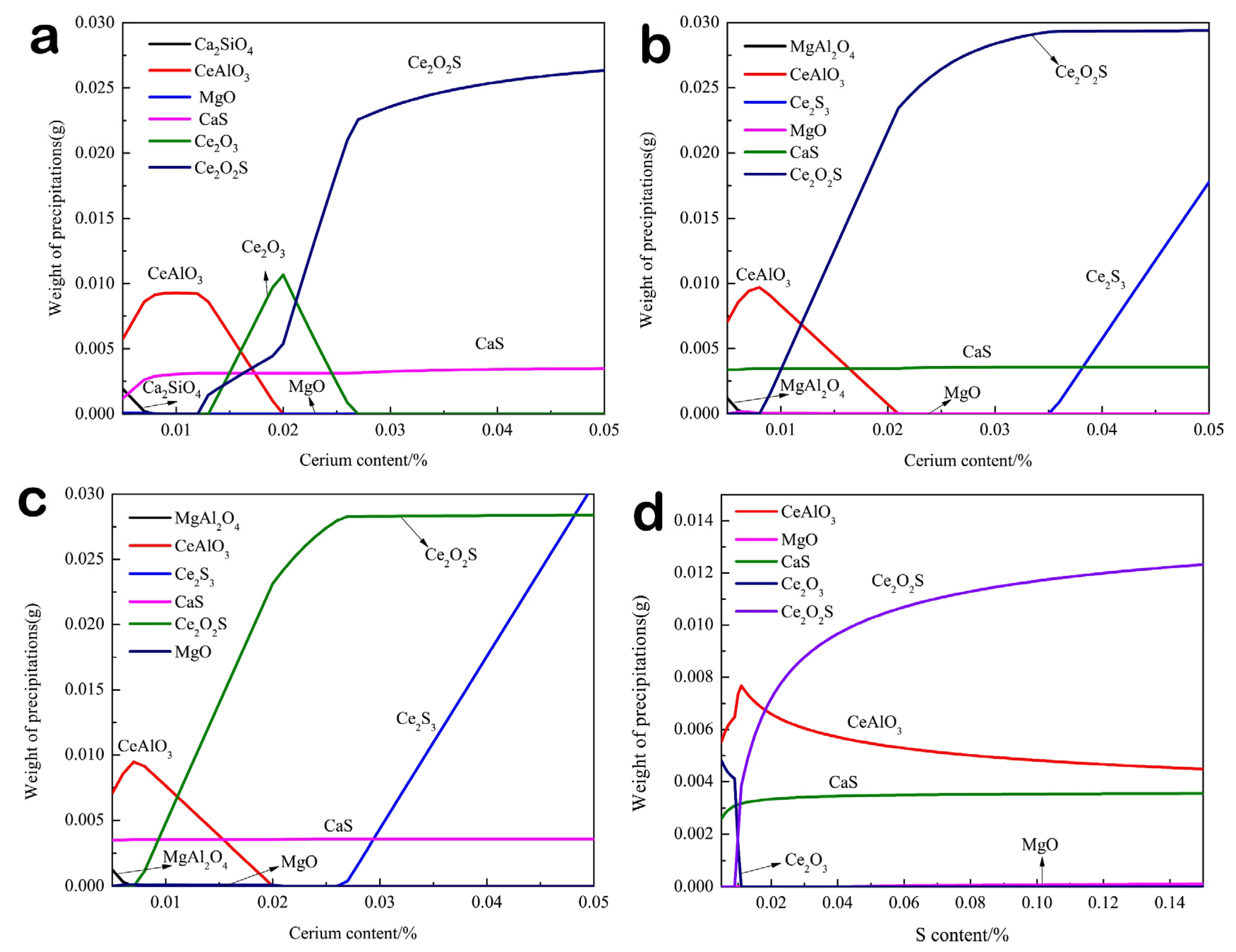
- The thermodynamic calculation results indicated that with the increase of cerium content, the evolution sequence of Ce-containing inclusions in 0.01% sulfur content steel is CeAlO3→Ce2O3→Ce2O2S, and the evolution sequence of Ce-containing inclusions in 0.06% and 0.132% sulfur content steel is CeAlO3→Ce2O2S→Ce2S3.
- (3)
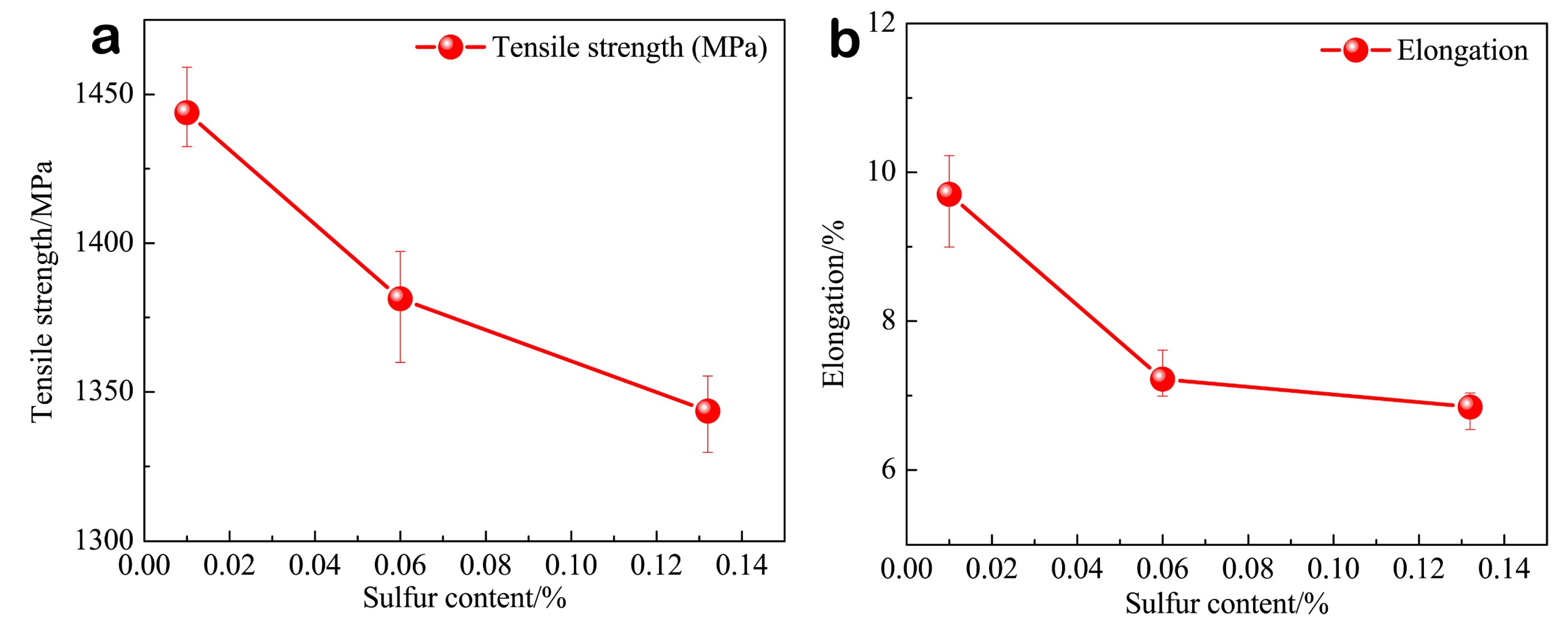
- With the sulfur content increasing from 0.01% to 0.132%, MnS becomes the prominent inclusion in steel, and inclusion spacing decreases from 21.20 μm to 10.19 μm, the tensile strength decreases from 1443.8 MPa to 1343.57 MPa, and the elongation decreases from 9.7% to 6.8%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, J.B.; Zhang, D.; Yang, Q.K.; An, J.M.; Huang, Z.Z.; Fu, J.X. Exploration of morphology evolution of the inclusions in Mg-treated 16MnCrS5 steel. Ironmak. Steelmak. 2019, 46, 564–573. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.P.; Xie, J.B.; Ai, K.N.; Zeng, Z.Q.; Shen, P.; Fu, J.X. Inclusions modification and improvement of machinability in a non-quenched and tempered steel with Mg treatment. Metall. Res. Technol. 2020, 117, 208. [Google Scholar] [CrossRef]
- Ånmark, N.; Karasev, A.; Jönsson, P.G. The effect of different non-metallic inclusions on the machinability of steels. Materials 2015, 8, 751–783. [Google Scholar] [CrossRef]
- Sui, H.; Wang, L.J.; Wang, Q.; Wang, H.M.; Che, D.H.; Li, J.M. The formation and growth of sulfides in free-cutting stainless steel. Steel Res. Int. 2018, 89, 1800179. [Google Scholar] [CrossRef]
- Ånmark, N.; Karasev, A.; Jönsson, P.G. The influence of microstructure and non-metallic inclusions on the machinability of clean steels. Steel Res. Int. 2017, 88, 1600111. [Google Scholar] [CrossRef] [Green Version]
- An, X.X.; Tian, Y.; Wang, H.J.; Shen, Y.F.; Wang, Z.D. Suppression of austenite grain coarsening by using Nb-Ti microalloying in high temperature carburizing of a gear steel. Adv. Eng. Mater. 2019, 21, 1900132. [Google Scholar] [CrossRef]
- An, X.X.; Tian, Y.; Wang, H.J.; Wang, Z.D. Effect of preheat treatment on microstructure and properties of a gear steel for high-temperature carburizing. Steel Res. Int. 2020, 91, 2000180. [Google Scholar] [CrossRef]
- Wen, B.; Song, B.; Pan, N.; Hu, Q.Y.; Mao, J.H. Effect of austenitizing temperature on microstructure in 16Mn steel treated by cerium. Int. J. Miner. Metall. Mater. 2011, 18, 652–658. [Google Scholar] [CrossRef]
- Ji, Y.P.; Zhang, M.X.; Ren, H.P. Roles of lanthanum and cerium in grain refinement of steels during solidification. Metals 2018, 8, 884. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.P.; Li, Y.M.; Zhang, M.X.; Qu, W.; Zhao, T.X.; Ren, H.P. Grain refinement mechanism of the δ-ferrite in steels through cerium addition. Metall. Mater. Trans. A 2020, 51, 1707–1718. [Google Scholar] [CrossRef]
- Jiang, X.; Song, S.H. Enhanced hot ductility of a Cr-Mo low alloy steel by rare earth cerium. Mater. Sci. Eng. A 2014, 613, 171–177. [Google Scholar] [CrossRef]
- Gao, J.Z.; Fu, P.X.; Liu, H.W.; Li, D.Z. Effects of rare earth on the microstructure and impact toughness of H13 steel. Metals 2015, 5, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.H.; Wang, C.; Gong, W.; Wang, H.D. Evolution of inclusions and change of as-cast microstructure with Mg addition in high carbon and high chromium die steel. Ironmak. Steelmak. 2015, 42, 669–674. [Google Scholar] [CrossRef]
- Cui, X.K.; Song, B.; Yang, Z.B.; Liu, Z.; Li, L.F.; Wang, L. Effect of Mg on the evolution of inclusions and formation of acicular ferrite in La-Ti-treated steels. Steel Res. Int. 2020, 91, 1900563. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Xu, G.; Li, Y.; Li, H.B.; Lv, J.B.; Wang, Q. Effect of ultra-high magnesium on SKS51 liquid steel cleanliness and microstructure. ISIJ Int. 2019, 59, 1234–1241. [Google Scholar] [CrossRef] [Green Version]
- Bao, D.H.; Cheng, G.G.; Huang, Y.; Qiao, T.; Dai, W.X. Refinement of Solidification Structure of H13 Steel by Rare Earth Sulfide. Steel Res. Int. 2021, 93, 2100304. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, J.H. Formation mechanism of oxide-sulfide complex inclusions in high-sulfur containing steel melts. Metall. Mater. Trans. A 2017, 49, 311–324. [Google Scholar] [CrossRef]
- Li, X.; Long, X.; Wang, L.; Tong, S.; Wang, X.; Zhang, Y.; Li, Y. Inclusion characteristics in 95CrMo steels with different calcium and sulfur contents. Materials 2020, 13, 619. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Zhu, C.Y.; Wang, W.L.; Li, X. In situ observation of the MnS precipitation behavior in high-sulfur microalloyed steel under different cooling rates. Metall. Mater. Trans. B 2020, 51, 2522–2531. [Google Scholar] [CrossRef]
- Maciejewski, J. The effects of sulfide inclusions on mechanical properties and failures of steel components. J. Failure Anal. Prev. 2015, 15, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Cheng, G.G.; Li, S.J.; Dai, W.X. Effect of cerium on the behavior of inclusions in H13 steel. Steel Res. Int. 2018, 89, 1800371. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, L.F. Effect of cerium content on inclusions in an ultra-low-carbon aluminum-killed steel. Metall. Mater. Trans. B 2020, 51, 589–600. [Google Scholar] [CrossRef]
- Hamidzadeh, M.A.; Meratian, M.; Saatchi, A. Effect of cerium and lanthanum on the microstructure and mechanical properties of AISI D2 tool steel. Mater. Sci. Eng. A 2013, 571, 193–198. [Google Scholar] [CrossRef]
- Lin, C.K.; Pan, Y.C.; Su, Y.H.F.; Lin, G.R.; Hwang, W.S.; Kuo, J.C. Effects of Mg-Al-O-Mn-S inclusion on the nucleation of acicular ferrite in magnesium-containing low-carbon steel. Mater. Charact. 2018, 141, 318–327. [Google Scholar] [CrossRef]
- Du, G.; Li, J.; Wang, Z.B.; Shi, C.B. Effect of magnesium addition on behavior of collision and agglomeration between solid inclusion particles on H13 steel meltes. Steel Res. Int. 2017, 88, 1600185. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Shi, C.B.; Li, J. Eolution of Al2O3 inclusions by magnesium treatment in H13 hot work die steel. Ironmak. Steelmak. 2016, 44, 128–133. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, G.G.; Xie, Y. Modification Mechanism of Cerium on the Inclusions in Drill Steel. Acta Metall. Sin. 2018, 54, 1253–1261. [Google Scholar]
- Roos, E.; Karasev, A.; Jönsson, P.G. Effect of Si and Ce contents on the nozzle clogging in a REM alloyed stainless steel. Steel Res. Int. 2015, 86, 1279–1288. [Google Scholar] [CrossRef]
- Shen, P.; Fu, J. Morphology study on inclusion modification using Mg-Ca treatment in resulfurized special steel. Materials 2019, 12, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X. Metallurgical Quality and Composition Optimization of H13 Hot Work Die Steel. Ph.D. Thesis, Northeastern University, Shenyang, China, 2020. [Google Scholar]
- Luyckx, L.; Bell, J.R.; Mclean, A.; Korchynsky, M. Sulfide Shape Control in High Strength Low Alloy Steels. Metall. Trans. 1970, 1, 3341–3350. [Google Scholar]
- Dahl, W.; Gammal, T.E.; Lorenz, L.L. Einfluß sehr niedriger Schwefelgehalte auf die mechanischen Eigenschaften des Stahles St 52-3. Arch. Eisenhüttenwesen 1973, 44, 843–846. [Google Scholar] [CrossRef]
- Banks, T.M.; Gladman, T. Sulphide shape control. Met. Technol. 1979, 6, 81–94. [Google Scholar] [CrossRef]
- Handerhan, K.J.; Garrison, W.M.; Moody, N.R. A Comparison of the Fracture Behavior of Two Heats of the Secondary Hardening Steel AF1410. Metall. Trans. A 1989, 20, 105–123. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Z.H.; Geng, X.; Chen, M.J.; Cui, S. Effect of rare earth-magnesium alloy on inclusion evolution in industrial production of die steel. Steel Res. Int. 2019, 90, 1900103. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Z.H.; Geng, X.; Chen, M.J.; Peng, L.Z. Evolution mechanism of inclusions in H13 steel with rare earth magnesium alloy addition. ISIJ Int. 2019, 59, 1552–1561. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.Z.; Gao, G.; Zheng, F.Z.; Shi, X.F. Effect of rare earth and magnesium complex treatment on inclusions in GCr15 bearing steel. J. Chem. Eng. 2019, 41, 763–771. [Google Scholar]
- Maloney, J.L.; Garrison, W.M. The effect of sulfide type on the fracture behavior of HY180 steel. Acta Mater. 2005, 53, 533–551. [Google Scholar] [CrossRef]
- Ma, Y.; Pan, T.; Jiang, B.; Cui, Y.H.; Su, H.; Peng, Y. Study of the effect of sulfur contents on frature toughness on railway wheel steels for high speed train. Acta Metall. Sin. 2011, 47, 978–983. [Google Scholar]
- Yang, C.Y.; Luan, Y.K.; Li, D.Z.; Li, Y.Y. Very hgih cycle fatigue properties of bearing steel with different aluminum and sulfur content. Int. J. Fatigue 2018, 116, 396–408. [Google Scholar] [CrossRef]
- Kang, M.H.; Lee, J.S.; Koo, Y.M.; Kim, S.J.; Heo, N.H. Correlation between MnS precipitation, sulfur segregation kinetics, and hot ductility in C-Mn steel. Metall. Mater. Trans. A 2014, 45, 5295–5299. [Google Scholar] [CrossRef] [Green Version]
- Oikawa, K.; Ohtani, H.; Ishida, K.; Nishizawa, T. The control of the morphology of MnS inclusions in steel during solidification. ISIJ Int. 1995, 4, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Wijk, O.; Brabie, V. The purity of ferrosilicon and its influence on inclusion cleanliness of steel. ISIJ Int. 1996, 36, 132–135. [Google Scholar] [CrossRef]
- Mizuno, K.; Todoroki, H.; Noda, M.; Tohge, T. Effects of Al and Ca in ferrosilicon alloys for deoxidation on inclusion composition in type 304 stainless steel. Iron Steelmak. 2001, 28, 93–101. [Google Scholar]
- Deng, Z.Y.; Zhu, M.Y. Evolution mechanism of non-metallic inclusions in Al-killed alloyed steel during secondary refining process. ISIJ Int. 2013, 53, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.Y.; Huang, F.X.; Wang, X.H. The effect of refining slag and refractory on inclusion transformation in extra low oxygen steels. Metall. Mater. Trans. B 2016, 47, 999–1009. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.H.; Chen, B.; Wang, W.J. Laboratory study on evolution mechanisms of non-metallic inclusions in high strength alloyed steel refined by high basicity slag. ISIJ Int. 2010, 50, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Gulliver, G.H. The quantitative effect of rapid cooling upon the constitution of binary alloys. J. Inst. Met. 1913, 9, 120–157. [Google Scholar]
- Yamamoto, K.I.; Yamamura, H.; Suwa, Y. Behavior of non-metallic inclusions in steel during hot deformation and the effects of deformed inclusions on local ductility. ISIJ Int. 2011, 12, 1987–1994. [Google Scholar] [CrossRef] [Green Version]





| Steel | C | Si | Mn | Cr | Ni | Cu | Mo | Als | Ca | Mg | Ce | T.O | S | Category |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0.191 | 0.320 | 0.865 | 1.210 | 0.16 | 0.17 | 0.03 | 0.020 | 0.0019 | 0.0010 | 0.014 | 0.0026 | 0.01 | Low sulfur |
| B | 0.203 | 0.298 | 0.872 | 1.210 | 0.15 | 0.17 | 0.03 | 0.017 | 0.0022 | 0.0009 | 0.013 | 0.0029 | 0.06 | Medium sulfur |
| C | 0.214 | 0.313 | 0.869 | 1.225 | 0.15 | 0.17 | 0.03 | 0.016 | 0.0020 | 0.0008 | 0.013 | 0.0030 | 0.132 | High sulfur |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Jiang, Z.; Li, Y.; Chen, C.; Ma, S.; Ji, Y.; Wang, J.; Liu, H. Effect of Sulfur Content on the Inclusion and Mechanical Properties in Ce-Mg Treated Resulfurized SCr420H Steel. Metals 2022, 12, 136. https://doi.org/10.3390/met12010136
Sun M, Jiang Z, Li Y, Chen C, Ma S, Ji Y, Wang J, Liu H. Effect of Sulfur Content on the Inclusion and Mechanical Properties in Ce-Mg Treated Resulfurized SCr420H Steel. Metals. 2022; 12(1):136. https://doi.org/10.3390/met12010136
Chicago/Turabian StyleSun, Meng, Zhouhua Jiang, Yang Li, Changyong Chen, Shuai Ma, Yongshuai Ji, Ju Wang, and Hang Liu. 2022. "Effect of Sulfur Content on the Inclusion and Mechanical Properties in Ce-Mg Treated Resulfurized SCr420H Steel" Metals 12, no. 1: 136. https://doi.org/10.3390/met12010136
APA StyleSun, M., Jiang, Z., Li, Y., Chen, C., Ma, S., Ji, Y., Wang, J., & Liu, H. (2022). Effect of Sulfur Content on the Inclusion and Mechanical Properties in Ce-Mg Treated Resulfurized SCr420H Steel. Metals, 12(1), 136. https://doi.org/10.3390/met12010136








