Element Distribution and Its Induced Peritectic Reaction during Solidification of Ti-Al-Nb Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
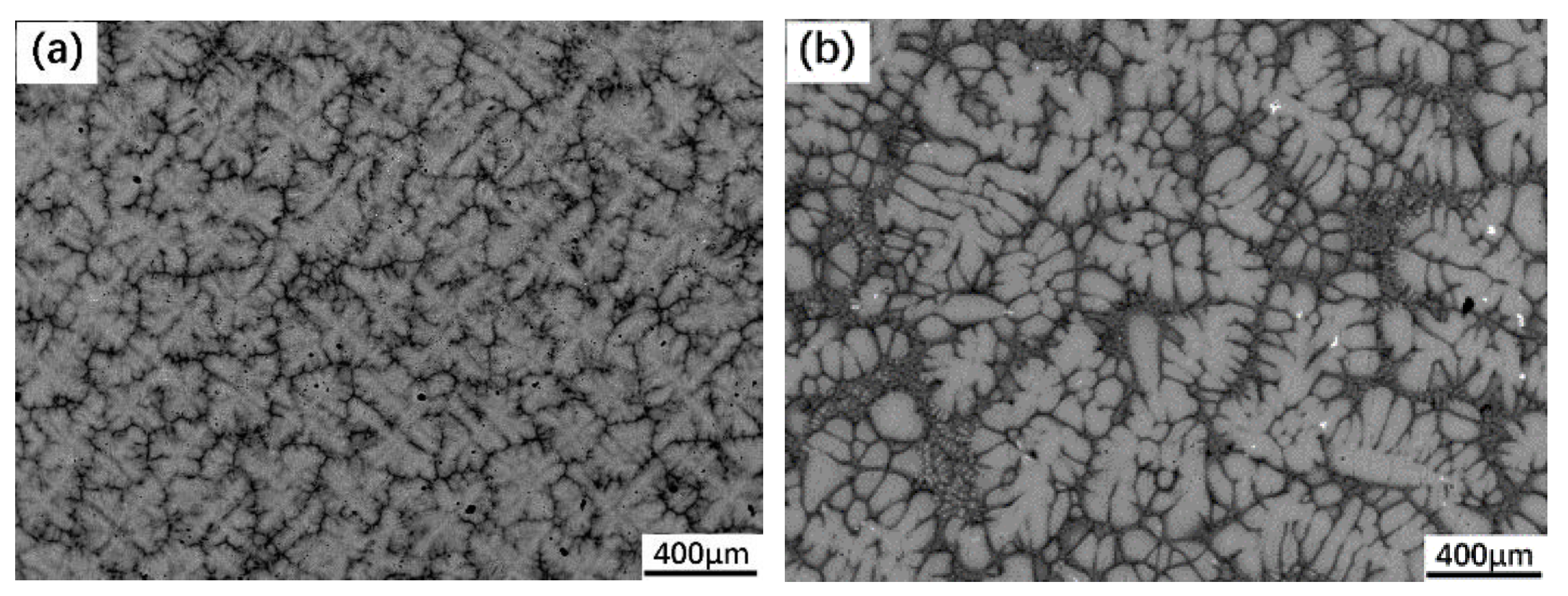
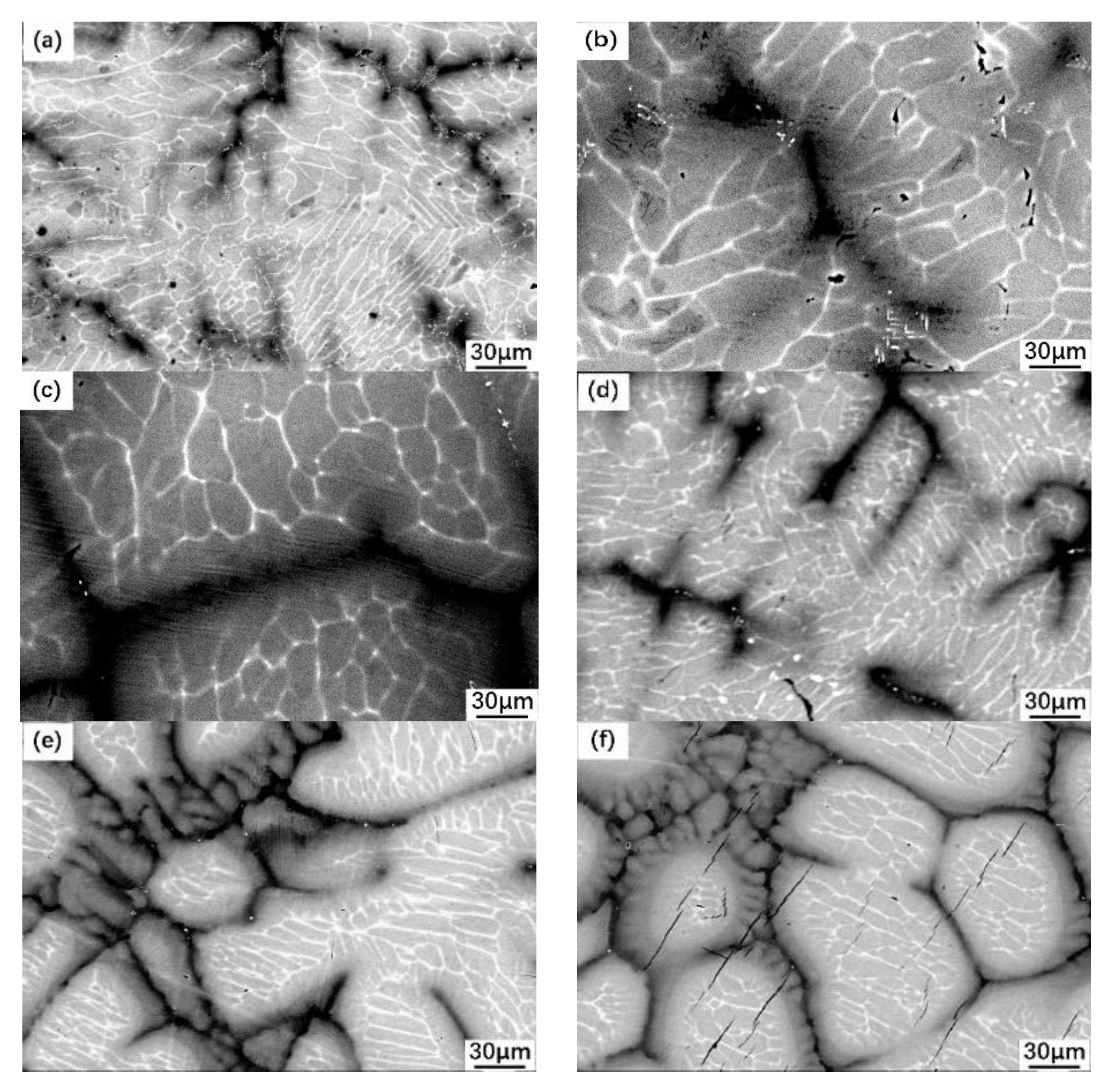
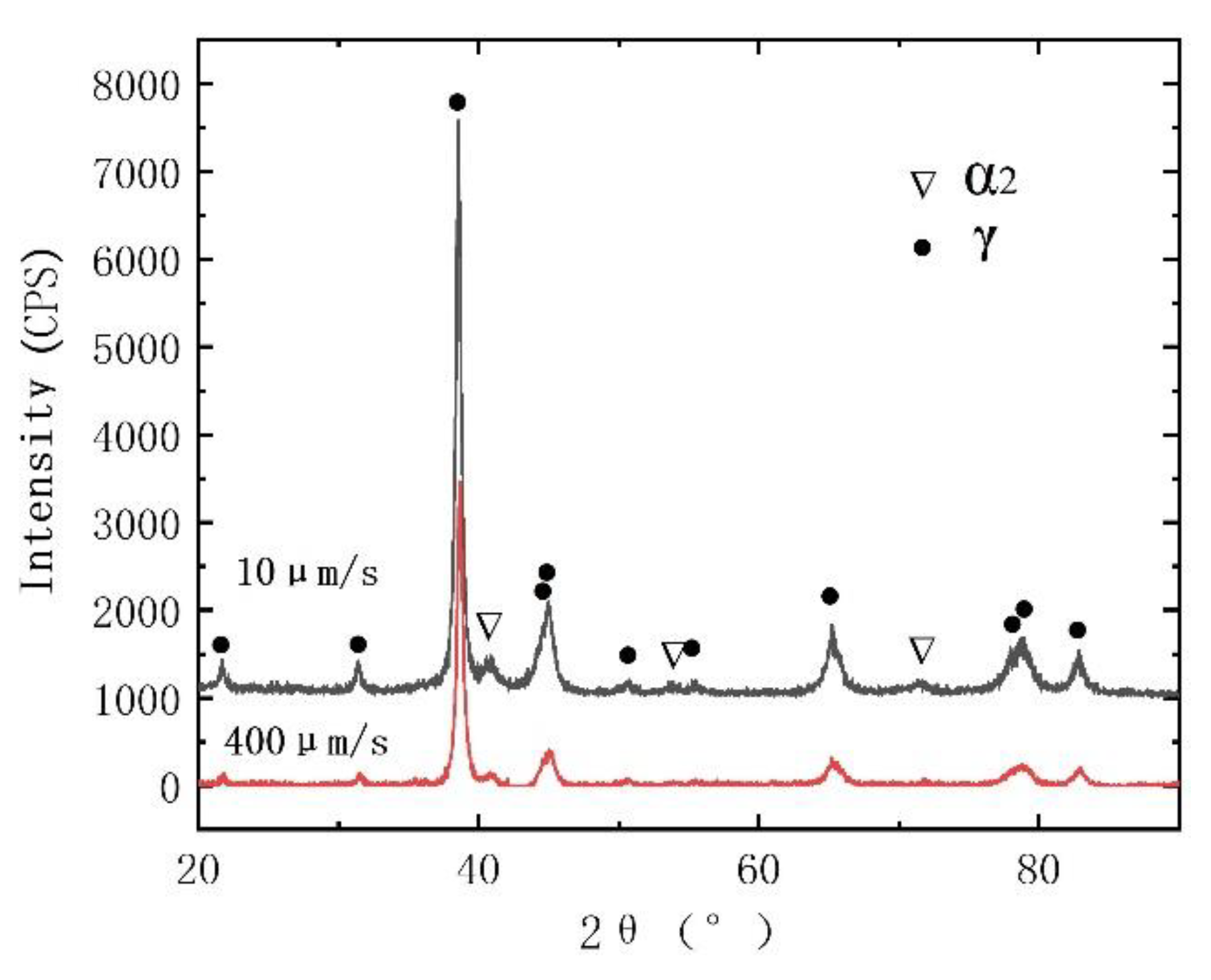
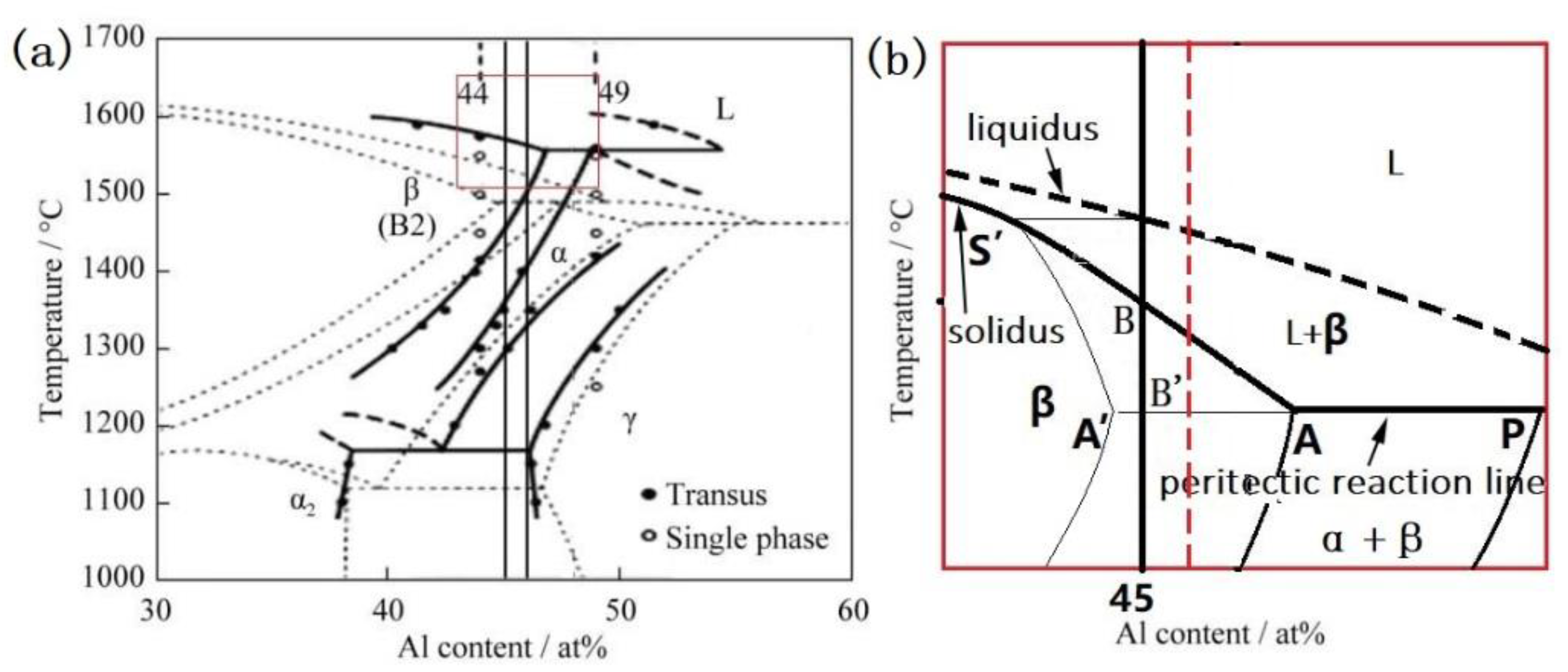
3.1. Peritectic a Phase in Solidificaiton Microstructures
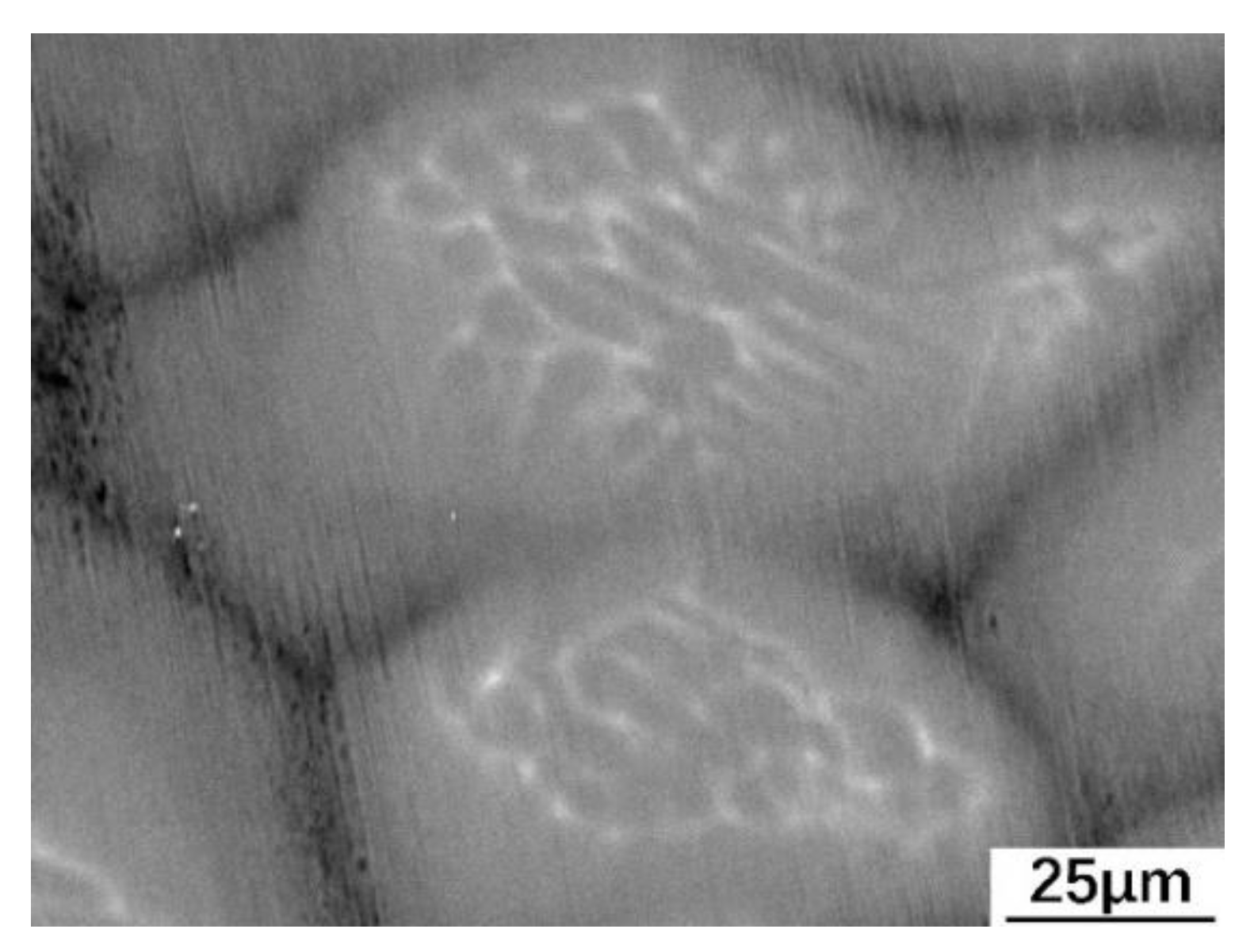
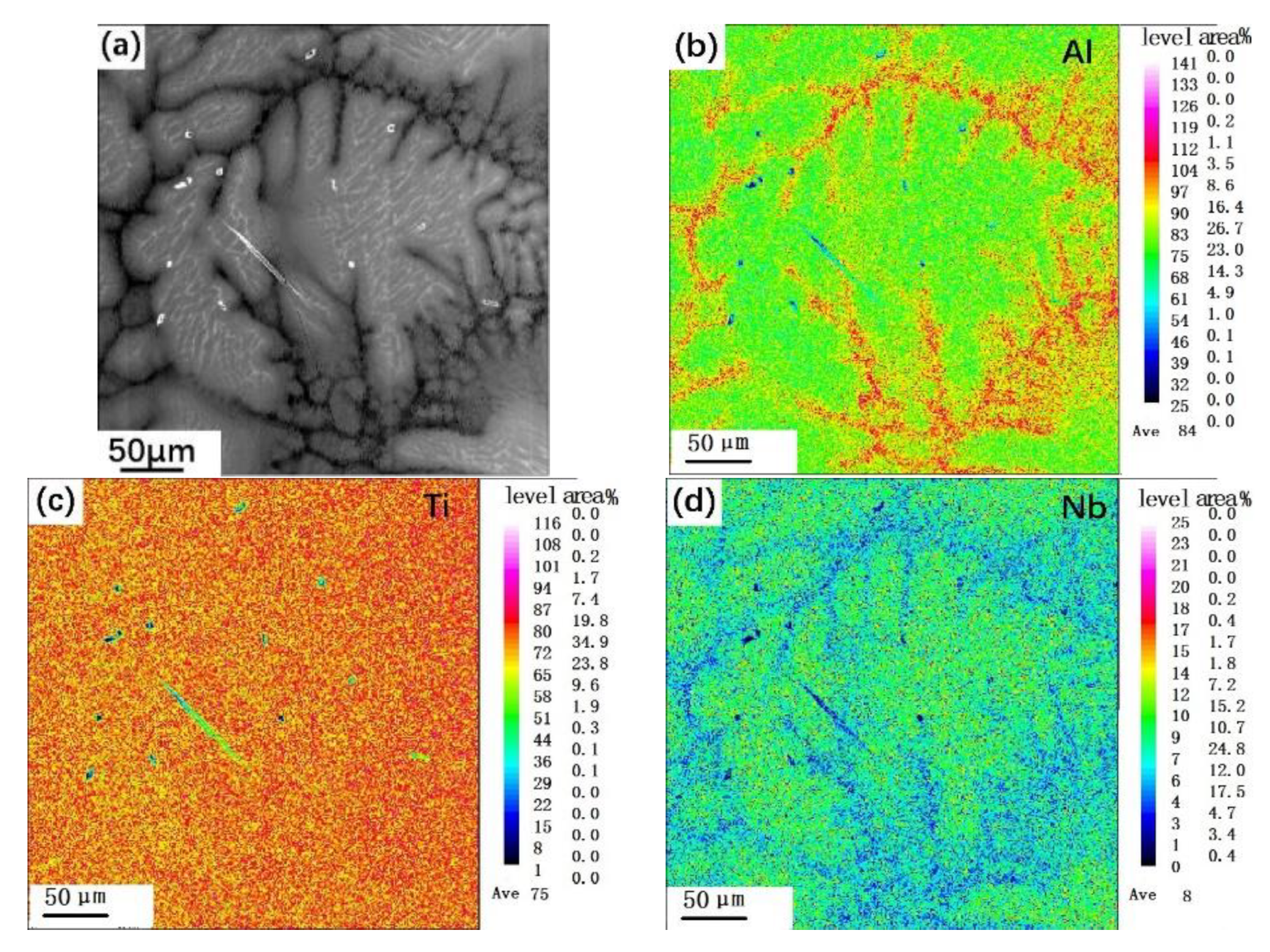
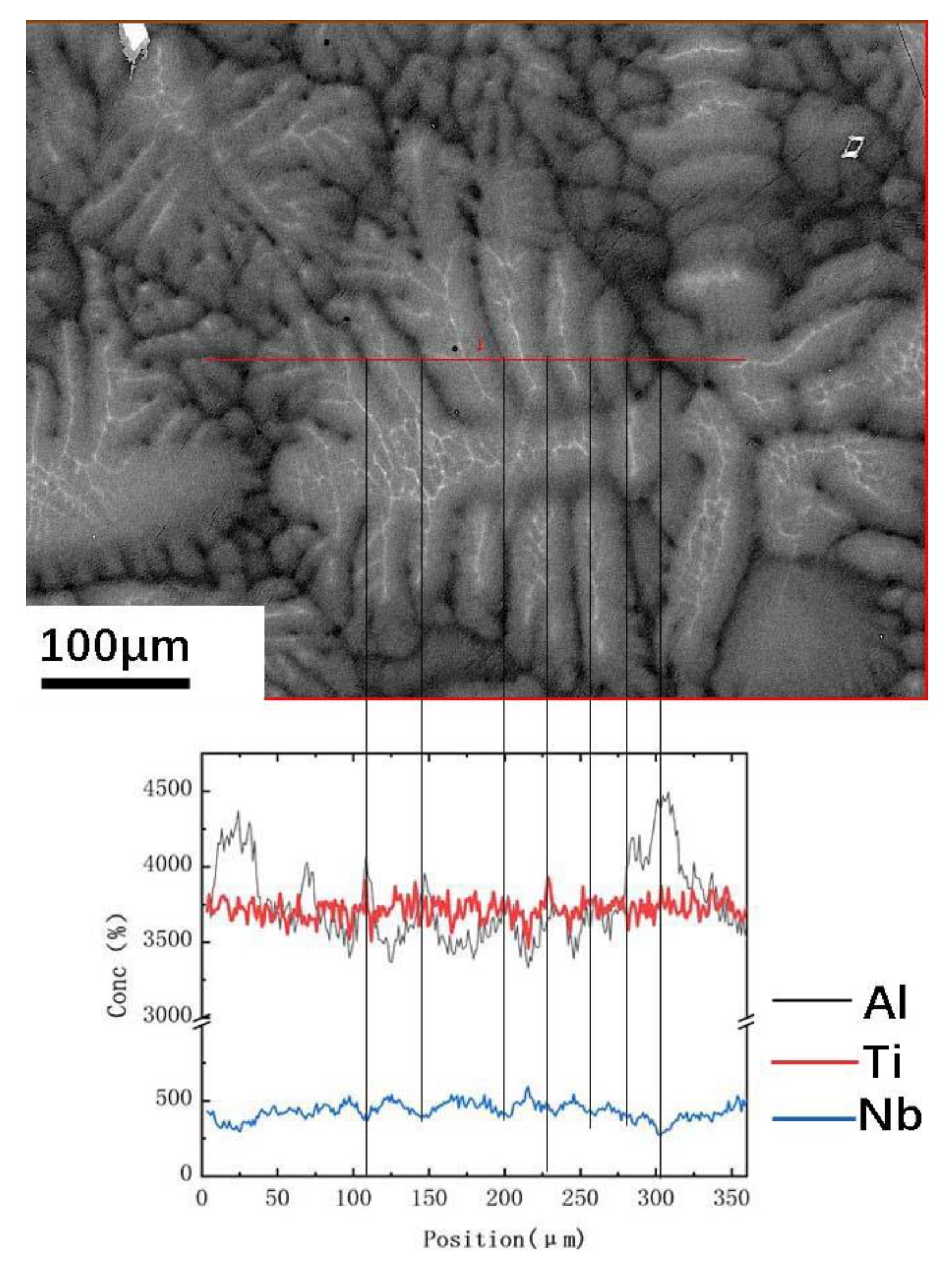
3.2. Distribution of Elements in Peritectic a Phase
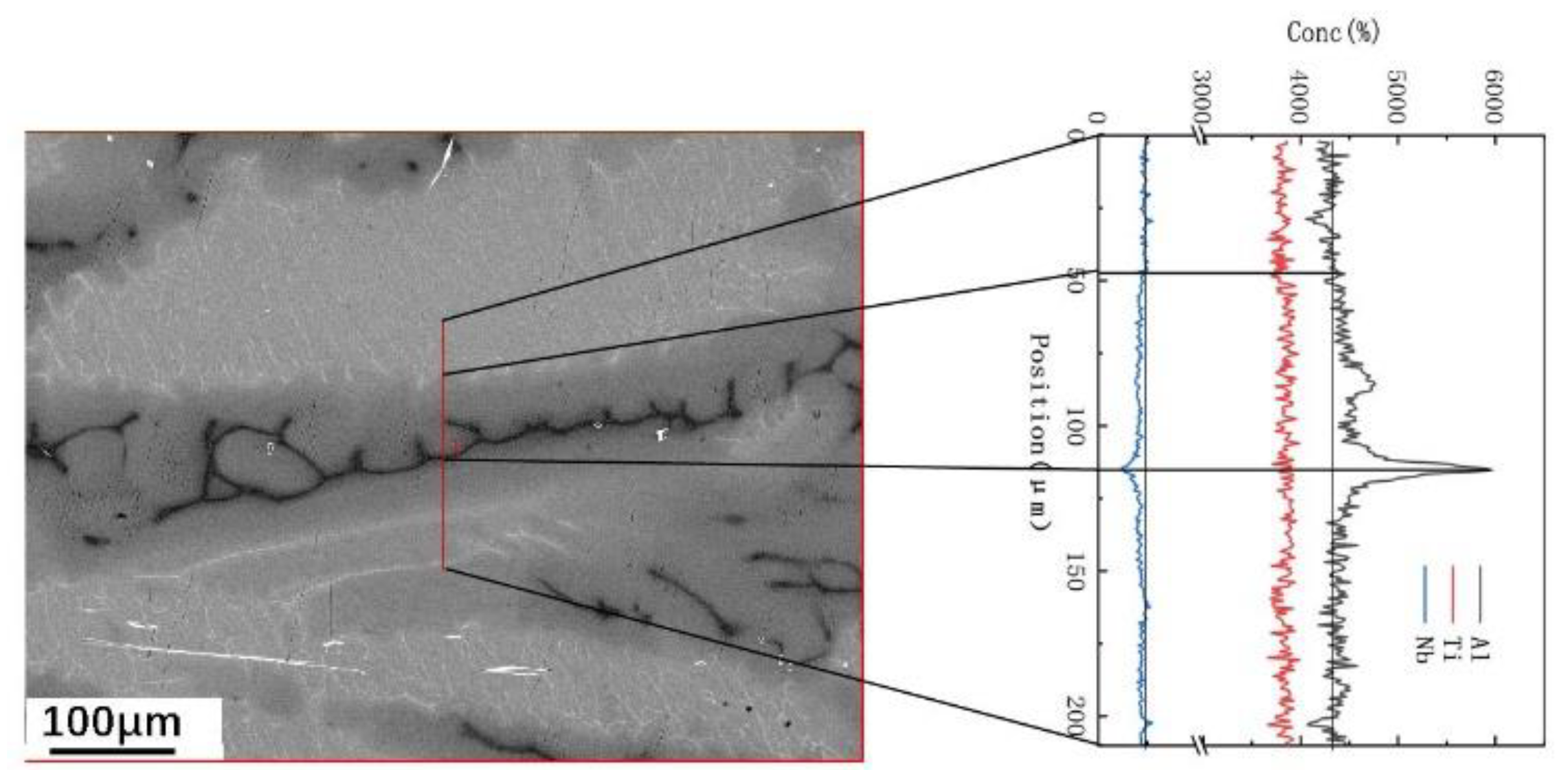
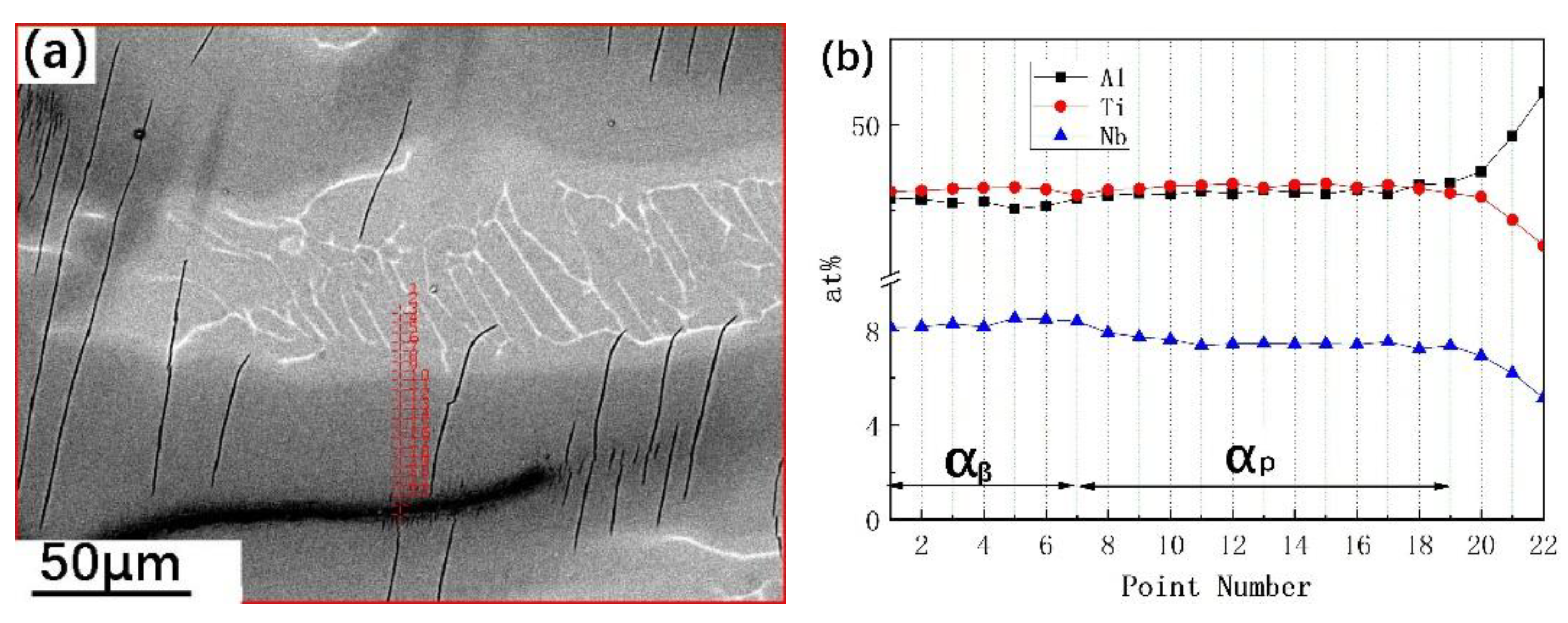
3.3. Effect of Element Distribution on the Peritectic Reaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Appel, F.; Paul, J.D.H.; Oehring, M. Gamma Titanium Aluminide Alloys: Science and Technology; Wiley-VCH Verlag GmbH and Co. KGaA: Weinheim, Germany, 2011; pp. 1–3. [Google Scholar]
- Pflumm, R.; Friedle, S.; Schütze, M. Oxidation protection of γ-TiAl-based alloys-A review. Intermetallics 2015, 56, 1–14. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, S.L. Advances in gammalloy materials-processes-application technology: Successes, dilemmas, and future. JOM 2018, 70, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Bewlay, B.P.; Weimer, M.; Kelly, T.; Suzuki, A.; Subramanian, P.R. The science, technology, and implementation of TiAl alloys in commercial aircraft engines. Mater. Res. Soc. Symp. Proc. 2013, 1516, 49–57. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Bondar, A.A.; Hecht, U.; Rex, S.; Velikanova, T.Y. The Al-B-Nb-Ti system: III. Thermodynamic re-evaluation of the constituent binary system Al-Ti. J. Alloys. Compd. 2008, 456, 64–77. [Google Scholar] [CrossRef]
- Lin, J.P.; Zhao, L.L.; Li, G.Y.; Zhang, L.Q.; Song, X.P.; Ye, F.; Chen, G.L. Effect of Nb on oxidation behavior of high Nb containing TiAl alloys. Intermetallics 2011, 19, 131–136. [Google Scholar] [CrossRef]
- Lin, J.P.; Xu, X.J.; Wang, Y.L.; He, S.F.; Zhang, Y.; Song, X.P.; Chen, G.L. High temperature deformation behaviors of a high Nb containing TiAl alloy. Intermetallics 2007, 15, 668–674. [Google Scholar] [CrossRef]
- Gao, S.; Xu, X.; Shen, Z.; Ye, T.; Xu, S.; Lin, J. Microstructure and properties of forged plasma arc melted pilot ingot of Ti-45Al-8.5Nb-(W, B, Y) alloy. Mater. Sci. Eng. A 2016, 677, 89–96. [Google Scholar] [CrossRef]
- Chen, G.L.; Xu, X.J.; Teng, Z.K.; Wang, Y.L.; Lin, J.P. Microsegregation in high Nb containing TiAl alloy ingots beyond laboratory scale. Intermetallics 2007, 15, 625–631. [Google Scholar] [CrossRef]
- Song, L.; Zhang, L.Q.; Xu, X.J.; Sun, J.; Lin, J.P. Omega phase in as-cast high-Nb-containing TiAl alloy. Scr. Mater. 2013, 68, 929–932. [Google Scholar] [CrossRef]
- Xu, X.; Song, L.; Jin, X.; Han, D.; Wang, X.; Lin, J. Microstructure and microsegregation of directionally solidified Ti-45Al-8Nb alloy with different solidification rates. Rare Met. 2016, 35, 70–76. [Google Scholar] [CrossRef]
- Xu, X.; Hu, R.; Lin, J.; Guo, J. Evolution of Microstructure and Microsegregation of Ti-45Al-8Nb Alloy during Directional Solidification. Adv. Mater. Sci. Eng. 2018, 2018, 1713835. [Google Scholar] [CrossRef] [Green Version]
- Witusiewicz, V.T.; Bondar, A.A.; Hecht, U.; Velikanova, T.Y. The Al–B–Nb–Ti system: IV. Experimental study and thermodynamic re-evaluation of the binary Al-Nb and ternary Al-Nb-Ti systems. J. Alloys Compd. 2009, 472, 133–161. [Google Scholar] [CrossRef]
- He, T.; Hu, R.; Yang, J.; Fu, H. Phase selection and solidification path transition of Ti–48Al–xNb alloys with different cooling rates. Rare Met. 2017, 1–8. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Design, processing, microstructure, properties, and applications of advanced intermetallic TiAl alloys. Adv. Eng. Mater. 2013, 15, 191–215. [Google Scholar] [CrossRef]
- Niu, H.Z.; Chen, X.J.; Chen, Y.F.; Zhao, S.; Liu, G.H.; Zhang, D.L. Microstructural stability, phase transformation and mechanical properties of a fully-lamellar microstructure of a Mo-modified high-Nb γ-TiAl alloy. Mater. Sci. Eng. A 2020, 748, 139313. [Google Scholar] [CrossRef]
- Imayev, V.; Oleneva, T.; Imayev, R.; Christ, H.-J.; Fecht, H.-J. Microstructure and mechanical properties of low and heavy alloyed γ-TiAl + α2-Ti3Al based alloys subjected to different treatments. Intermetallics 2012, 26, 91–97. [Google Scholar] [CrossRef]
- Liu, Y.L.; Kang, S.B. Solidification and segregation of Al-Mg alloys and influence of alloy and influence of alloy composition and cooling rate. Mat. Sci. Tech. 1997, 13, 331–336. [Google Scholar] [CrossRef]
- Ses, E.; Billdal, H. Non-equilibrium solidification of hyperperitetic Al-Zr alloys. Acta Metal. 1977, 25, 1031–1037. [Google Scholar]
- Johnson, D.R.; Inui, H.; Muto, S.; Omiya, Y.; Yamanaka, T. Microstructural development during directional solidification of α-seeded TiAl alloys. Acta Metal. 2006, 54, 1077–1085. [Google Scholar] [CrossRef]
- Ishida, H.; Natsume, Y.; Ohsasa, K. Characterization of dendrite morphology for evaluating interdendritic fluidity based on phase-field simulation. ISIJ Int. 2009, 49, 37–43. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Lin, J.; Xiang, C.; Liang, Y. Element Distribution and Its Induced Peritectic Reaction during Solidification of Ti-Al-Nb Alloys. Metals 2021, 11, 1386. https://doi.org/10.3390/met11091386
Xu X, Lin J, Xiang C, Liang Y. Element Distribution and Its Induced Peritectic Reaction during Solidification of Ti-Al-Nb Alloys. Metals. 2021; 11(9):1386. https://doi.org/10.3390/met11091386
Chicago/Turabian StyleXu, Xiangjun, Junpin Lin, Chao Xiang, and Yongfeng Liang. 2021. "Element Distribution and Its Induced Peritectic Reaction during Solidification of Ti-Al-Nb Alloys" Metals 11, no. 9: 1386. https://doi.org/10.3390/met11091386
APA StyleXu, X., Lin, J., Xiang, C., & Liang, Y. (2021). Element Distribution and Its Induced Peritectic Reaction during Solidification of Ti-Al-Nb Alloys. Metals, 11(9), 1386. https://doi.org/10.3390/met11091386






