Energetic Materials Based on W/PTFE/Al: Thermal and Shock-Wave Initiation of Exothermic Reactions
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Wang, H.; Ge, C. Characterization of the Dynamic Response and Constitutive Behavior of PTFE/Al/W Reactive Materials. Propellants Explos. Pyrotech. 2020, 45, 788–797. [Google Scholar] [CrossRef]
- Ge, C.; Maimaitituersun, W.; Dong, Y.; Tian, C. A Study on the Mechanical Properties and Impact-Induced Initiation Characteristics of Brittle PTFE/Al/W Reactive Materials. Materials 2017, 10, 452. [Google Scholar] [CrossRef] [Green Version]
- Feng, B.; Fang, X.; Wang, H.-X.; Dong, W.; Li, Y.-C. The Effect of Crystallinity on Compressive Properties of Al-PTFE. Polymers 2016, 8, 356. [Google Scholar] [CrossRef] [Green Version]
- Feng, B.; Li, Y.C.; Wu, S.Z. A Crack-induced Initiation Mechanism of Al-PTFE under Quasi-static Compression and the Investigation of Influencing Factors. J. Mater. Des. 2016, 108, 411–417. [Google Scholar] [CrossRef]
- Feng, B.; Qiu, C.L.; Zhang, T.H.; Hu, Y.F.; Li, H.G.; Xu, B.C. Sensitivity of Al-PTFE upon low-speed impact. Propellants Explos. Pyrotech. 2019, 44, 630–636. [Google Scholar] [CrossRef]
- Xiao, J.G.; Zhang, X.P.; Guo, Z.X.; Wang, H.F. Enhanced damage effects of multi-layered concrete target produced by reactive materials liner. Propellants Explos. Pyrotech. 2018, 43, 955–961. [Google Scholar] [CrossRef]
- Zheng, Y.-F.; Su, C.-H.; Guo, H.-G.; Yu, Q.-B.; Wang, H.-F. Chain damage effects of multi-spaced plates by reactive jet impact. J. Def. Technol. 2021, 17, 393–404. [Google Scholar] [CrossRef]
- Hastings, D.L.; Dreizin, E.L. Reactive Structural Materials: Preparation and Characterization. Adv. Eng. Mater. 2017, 20, 1700631. [Google Scholar] [CrossRef]
- Daniels, A.; Baker, E.; DeFisher, S.; Pham, J.; Ng, K. Bam Bam: Large scale unitary demolition warheads. In Proceedings of the 23rd International Symposium on Ballistics (ISB’23), Tarragona, Spain, 16–20 April 2007. [Google Scholar]
- Committee on Advanced Energetic Materials and Manufacturing Technologies, National Research Council. Advanced Energetic Materials; National Academies Press: Washington, DC, USA, 2004; pp. 1–23. [Google Scholar]
- He, W.; Liu, P.J.; He, G.Q.; Gozin, M.; Yan, Q.L. Highly reactive metastable intermixed composites (MICs): Preparation and characterization. Adv. Mater. 2018, 30, 1706293. [Google Scholar] [CrossRef] [PubMed]
- Hunt, E.M.; Pantoya, M.L. Impact sensitivity of intermetallic nanocomposites: A study on compositional and bulk density. Intermetallics 2010, 18, 1612–1616. [Google Scholar] [CrossRef]
- Tu, J.; Qiao, L.; Shan, Y.; Xin, C.; Liu, J. Study on the Impact-Induced Energy Release Characteristics of Zr68.5Cu12Ni12Al7.5 Amorphous Alloy. Materials 2021, 14, 1447. [Google Scholar] [CrossRef]
- Glavier, L.; Taton, G.; Ducere, J.; Baijot, V.; Pinon, S.; Calais, T.; Esteve, A.; Rouhani, M.D.; Rossi, C. Nanoenergetics as pressure generator for nontoxic impact primers: Comparison of Al/Bi2O3, Al/CuO, Al/MoO3 nanothermites and Al/PTFE. Combust. Flame 2015, 162, 1813–1820. [Google Scholar] [CrossRef]
- Bacciochini, A.; Radulescu, M.I.; Yandouzi, M.; Maines, G.; Lee, J.J.; Jodoin, B. Reactive structural materials consolidated by cold spray: Al-CuO thermite. Surf. Coat. Technol. 2013, 226, 60–67. [Google Scholar] [CrossRef]
- Wang, H.; Jian, G.; Egan, G.C.; Zachariah, M.R. Assembly and reactive properties of Al/CuO based nanothermite microparticles. Combust. Flame 2014, 161, 2203–2208. [Google Scholar] [CrossRef]
- Wu, J.-X.; Fang, X.; Gao, Z.-R.; Wang, H.-X.; Huang, J.-Y.; Wu, S.-Z.; Li, Y.-C. Investigation on Mechanical Properties and Reaction Characteristics of Al-PTFE Composites with Different Al Particle Size. Adv. Mater. Sci. Eng. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mock, W., Jr.; Holt, W.H. Impact initiation of rods of pressed Polytetrafluoroethylene (PTFE) and Aluminum powders, shock compression condens matter. AIP Conf. Proc. 2006, 845, 1097–1100. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Q.M.; Zhang, L.S. Reaction behavior of Polytetrafluoroethylene/al granular composites subjected to planar shock wave, propellants explosives pyrotechnics. Propellants Explos. Pyrotech. 2017, 42, 300–307. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Jiang, C.; Niu, H. Experimental Study on Impact-Induced Reaction Characteristics of PTFE/Ti Composites Enhanced by W Particles. Materials 2017, 10, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Tang, E.; Zhu, W.; Han, Y.; Gao, Q. Modified model of Al/PTFE projectile impact reaction energy release considering energy loss. Exp. Therm. Fluid Sci. 2020, 116, 110132. [Google Scholar] [CrossRef]
- Yu, Z.S.; Fang, X.; Gao, Z.R.; Wang, H.X.; Huang, J.Y.; Yao, M.; Li, Y.C. Mechanical and reaction properties of Al/TiH2/PTFE under quasi-static compression. J. Adv. Eng. Mater. 2018, 20, 1800019. [Google Scholar] [CrossRef]
- Ding, L.; Zhou, J.; Tang, W.; Ran, X.; Hu, Y. Impact Energy Release Characteristics of PTFE/Al/CuO Reactive Materials Measured by a New Energy Release Testing Device. Polymers 2019, 11, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, B.; Fang, X.; Li, Y.C.; Wang, H.X.; Mao, Y.M.; Wu, S.Z. An Initiation Phenomenon of Al–PTFE under Quasi-Static Compression. Chem. Phys. Lett. 2015, 637, 38–41. [Google Scholar] [CrossRef]
- Raftenberg, M.N.; Mock, W., Jr.; Kirby, G.C. Modeling the impact deformation of rods of a pressed PTFE/Al composite mixture. Int. J. Impact Eng. 2008, 35, 1735–1744. [Google Scholar] [CrossRef]
- Adamenko, N.A.; Kazurov, A.V.; Agafonova, G.V.; Savin, D.V. Structure Formation in Nickel-Polytetrafluorethylene Compo-site Materials upon Explosive Pressing of Powders. Inorg. Mater. Appl. Res. 2020, 11, 982–990. [Google Scholar] [CrossRef]
- Tolkachev, V.F.; Ivanova, O.V.; Zelepugin, S.A. Initiation and development of exothermic reactions during solid-phase synthesis under explosive loading. Therm. Sci. 2019, 23, 505–511. [Google Scholar] [CrossRef]
- Zelepugin, S.A.; Ivanova, O.V.; Yunoshev, A.S.; Zelepugin, A.S. Problems of Solid-Phase Synthesis in Cylindrical Ampoules under Explosive Loading. IOP Conf. Ser. Mater. Sci. Eng. 2016, 127, 012057. [Google Scholar] [CrossRef]
- Ge, C.; Yu, Q.; Zhang, H.; Qu, Z.; Wang, H.; Zheng, Y. On dynamic response and fracture-induced initiation characteristics of aluminum particle filled PTFE reactive material using hat-shaped specimens. Mater. Des. 2020, 188, 108472. [Google Scholar] [CrossRef]
- Zhou, Q.; Hu, Q.W.; Wang, B.; Zhou, B.B.; Chen, P.W.; Liu, R. Fabrication and characterization of the Ni–Al energetic structural material with high energy density and mechanical properties. J. Alloys Compd. 2020, 832, 154894. [Google Scholar] [CrossRef]
- Olney, K.L.; Benson, D.J.; Nesterenko, V.F. Modeling shear instability and fracture in dynamically deformed Al/W granular composites. AIP Conf. Proc. 2012, 1426, 729–732. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wang, H.; Fang, X.; Li, Y.; Mao, Y.; Yang, L.; Yin, Q.; Wu, S.; Yao, M.; Song, J. Investigation on the Thermal Behavior, Mechanical Properties and Reaction Characteristics of Al-PTFE Composites Enhanced by Ni Particle. Materials 2018, 11, 1741. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, J.; Li, S.; Zhang, X. Investigation on reaction energy, mechanical behavior and impact insensitivity of W–PTFE–Al composites with different W percentage. Mater. Des. 2016, 92, 397–404. [Google Scholar] [CrossRef]
- Geng, B.; Wang, H.; Yu, Q.; Zheng, Y.; Ge, C. Bulk Density Homogenization and Impact Initiation Characteristics of Porous PTFE/Al/W Reactive Materials. Materials 2020, 13, 2271. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Li, Y.C.; Feng, B.; Huang, J.Y.; Zhang, S.; Fang, X. Compressive Properties of PTFE/Al/Ni Composite Under Uniaxial Loading. J. Mater. Eng. Perform. 2017, 26, 2331–2336. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Wang, L.; Li, S.; Zhang, S.; Lan, J.; Wang, Y. Effects of Al and W Particle Size on Combustion Characteristics and Dynamic Response of W-PTFE-Al Composites. Rare Metal Mater. Eng. 2018, 47, 1723–1728. [Google Scholar] [CrossRef]
- Jiang, C.; Cai, S.; Mao, L.; Wang, Z. Effect of Porosity on Dynamic Mechanical Properties and Impact Response Characteristics of High Aluminum Content PTFE/Al Energetic Materials. Materials 2020, 13, 140. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Liu, J.; Yang, M.; Wang, L.; Lan, J.; Li, S.; He, C.; Xue, X. Effects of multi-component co-addition on reaction characteristics and impact damage properties of reactive material. Mater. Des. 2018, 153, 1–8. [Google Scholar] [CrossRef]
- Yu, Z.; Fang, X.; Li, Y.; Wu, J.; Wu, S.; Zhang, J.; Ren, J.; Zhong, M.; Chen, L.; Yao, M. Investigation on the Reaction Energy, Dynamic Mechanical Behaviors, and Impact-Induced Reaction Characteristics of PTFE/Al with Different TiH2 Percentages. Materials 2018, 11, 2008. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jiang, C. Influence of Molding Parameters on Quasi-Static Mechanical Properties of Al-Rich Al/PTFE/TiH2 Active Materials. Materials 2021, 14, 2750. [Google Scholar] [CrossRef]
- Wu, J.-X.; Liu, Q.; Feng, B.; Yin, Q.; Li, Y.-C.; Wu, S.-Z.; Yu, Z.-S.; Huang, J.-Y.; Ren, X.-X. Improving the energy release characteristics of PTFE/Al by doping magnesium hydride. J. Def. Technol. 2021, in press. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, X.; Li, Y.; Yu, Z.; Huang, J.; Song, J.; Gao, Z.; Wu, S.; Wu, J.; Kui, J. Mechanical Properties and Reaction Characteristics of Al-ZrH2-PTFE Composites under Quasi-Static Compression. Metals 2019, 9, 421. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Ding, L.; Tang, W.; Ran, X. Experimental Study of Mechanical Properties and Impact-Induced Reaction Characteristics of PTFE/Al/CuO Reactive Materials. Materials 2020, 13, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Fang, X.; Wu, S.; Yang, L.; Yu, Z.; Li, Y. Mechanical Response and Shear-Induced Initiation Properties of PTFE/Al/MoO3 Reactive Composites. Materials 2018, 11, 1200. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Huang, J.; Fang, X.; Li, Y.; Yu, Z.; Gao, Z.; Wu, S.; Yang, L.; Wu, J.; Kui, J. Thermal Decomposition and Thermal Reaction Process of PTFE/Al/MnO2 Fluorinated Thermite. Materials 2018, 11, 2451. [Google Scholar] [CrossRef] [Green Version]
- Shiryaev, A.A. Thermodynamics of SHS: Modern approach. Int. J. Self-Propag. High-Temp. Synth. 1995, 4, 351–362. [Google Scholar]
- Alymov, M.I.; Vadchenko, S.G.; Saikov, I.V.; Kovalev, I.D. Shock-wave treatment of tungsten/fluoropolymer powder compositions. Inorg. Mater. Appl. Res. 2017, 8, 340–343. [Google Scholar] [CrossRef]
- Seropyan, S.; Saikov, I.; Andreev, D.; Saikova, G.; Alymov, M. Reactive Ni–Al-Based Materials: Strength and Combustion Behavior. Metals 2021, 11, 949. [Google Scholar] [CrossRef]
- Vadchenko, S.G.; Alymov, M.I.; Saikov, I.V. Ignition of Some Powder Mixtures of Metals with Teflon. Inorg. Mater. Appl. Res. 2018, 9, 517–522. [Google Scholar] [CrossRef]
- Gaurav, M.; Ramakrishna, P.A. Effect of mechanical activation of high specific surface area with PTFE on composite solid propellants. Combust. Flame 2016, 166, 203–215. [Google Scholar] [CrossRef]


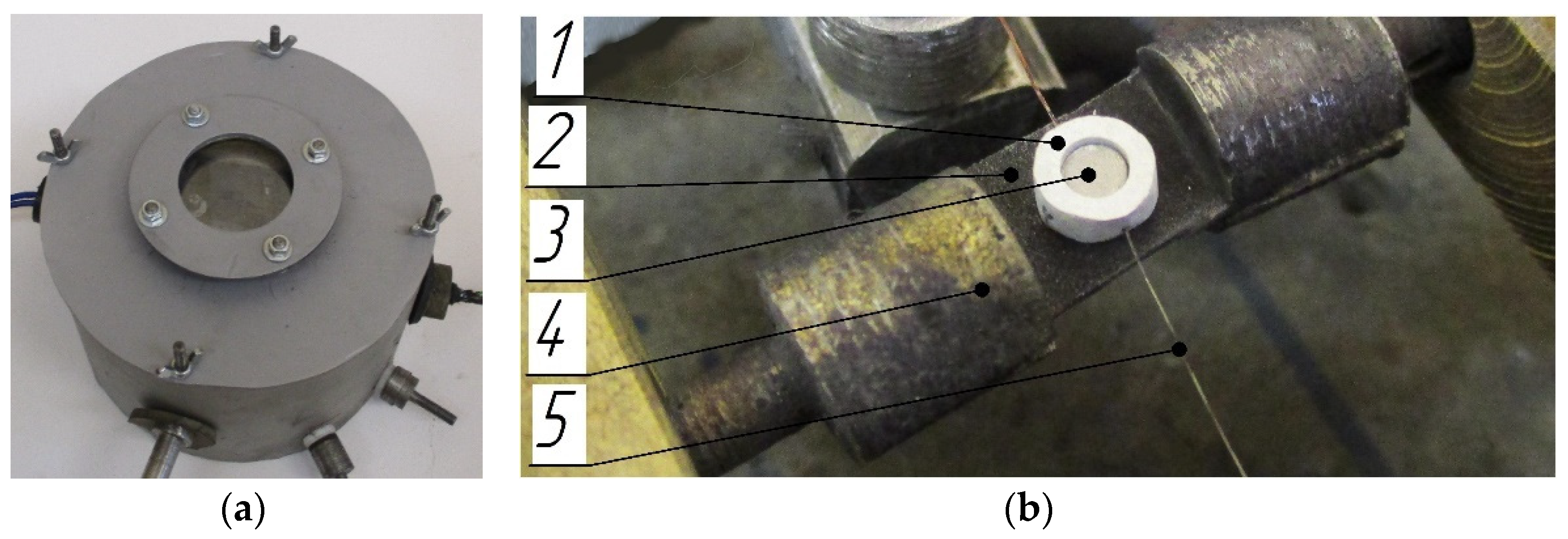

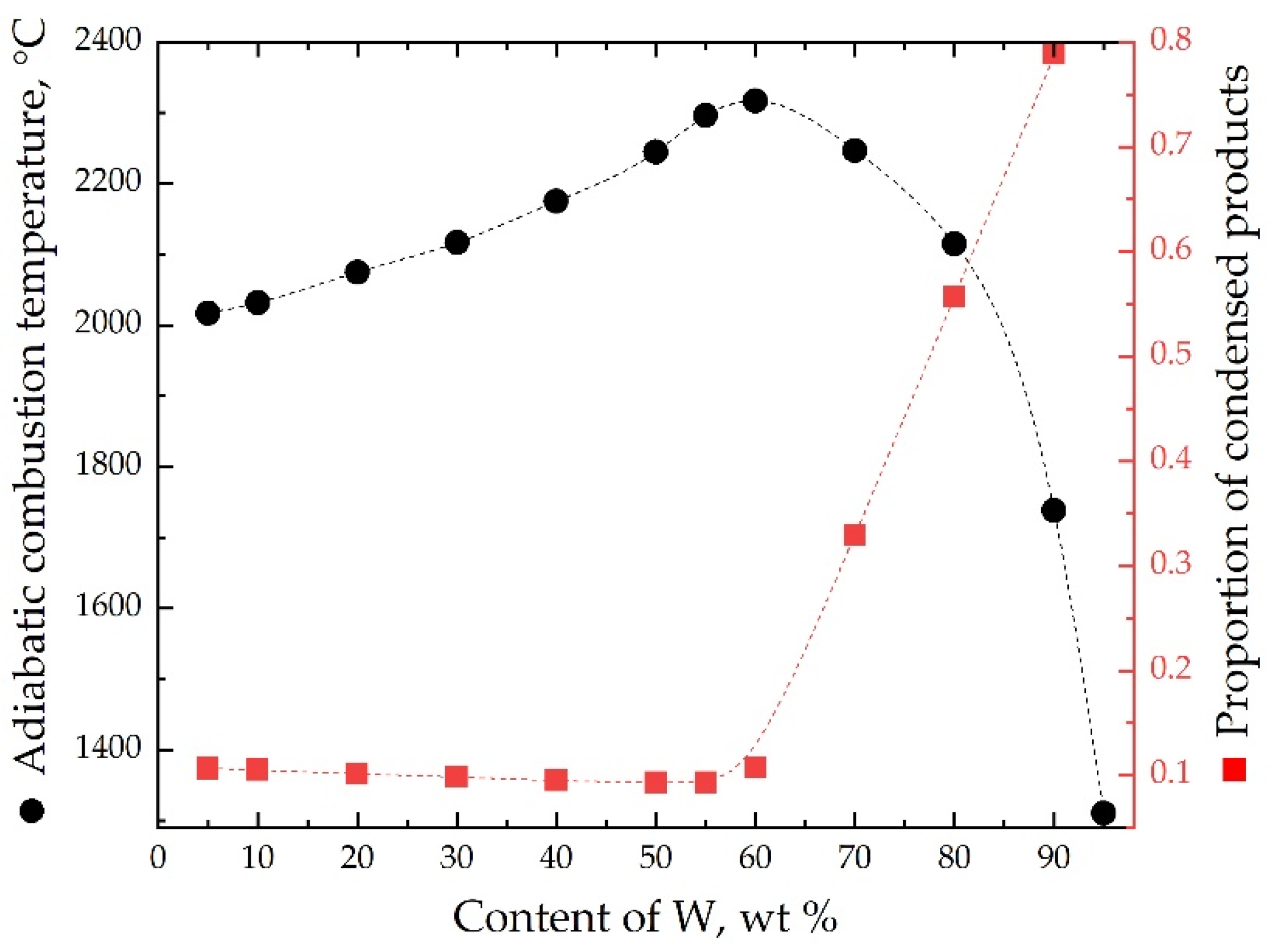
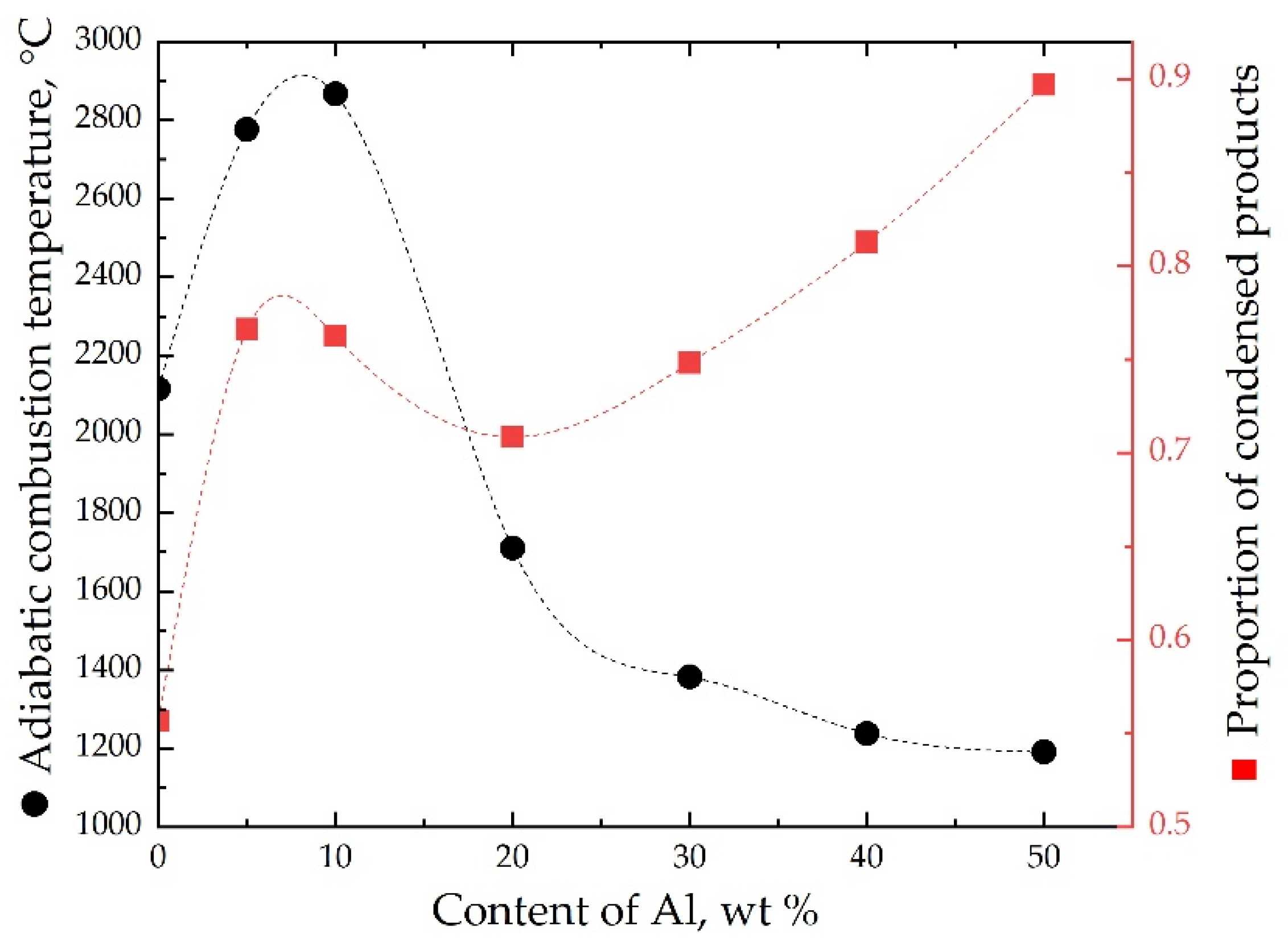

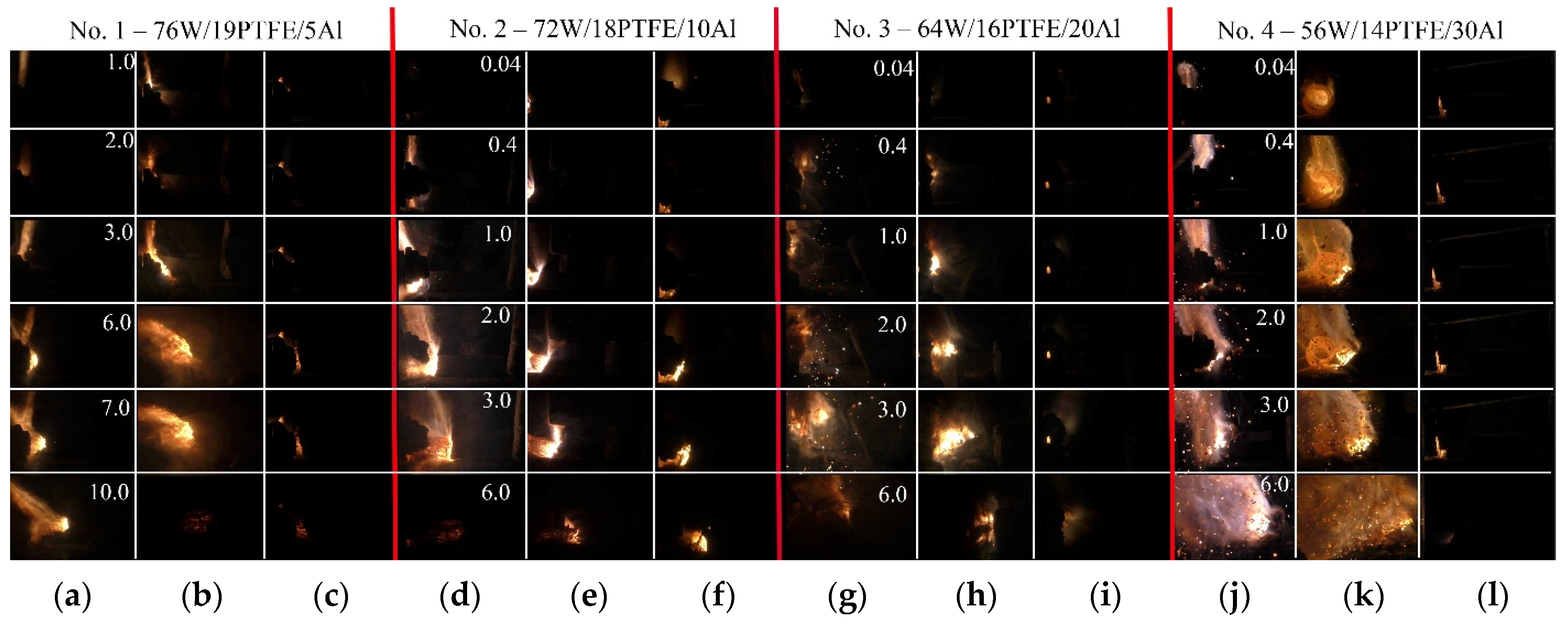
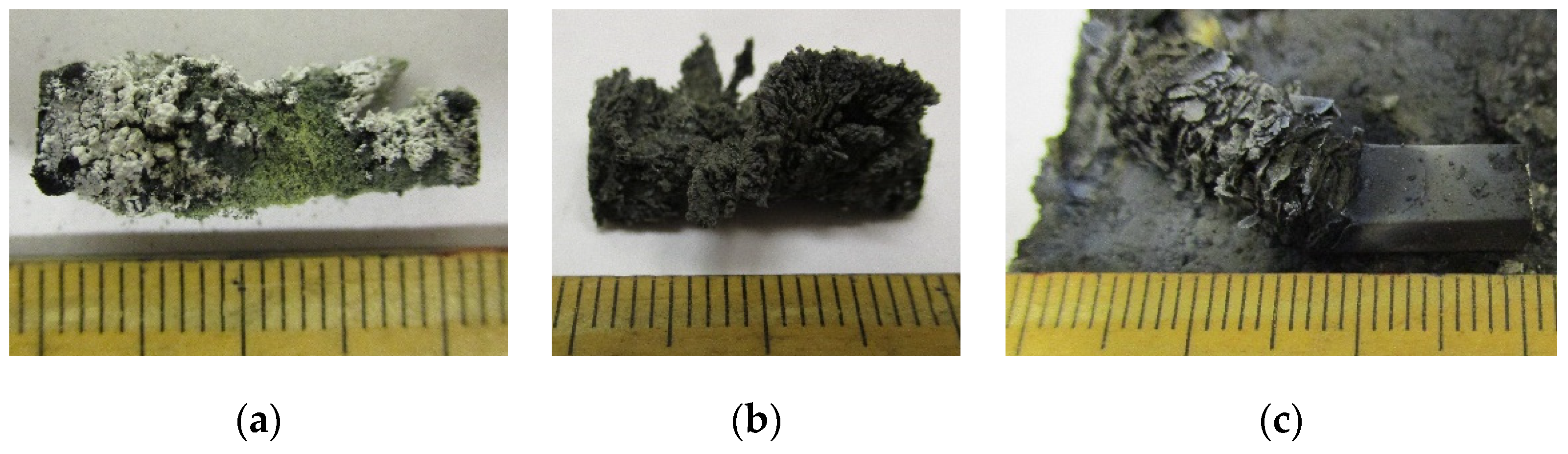
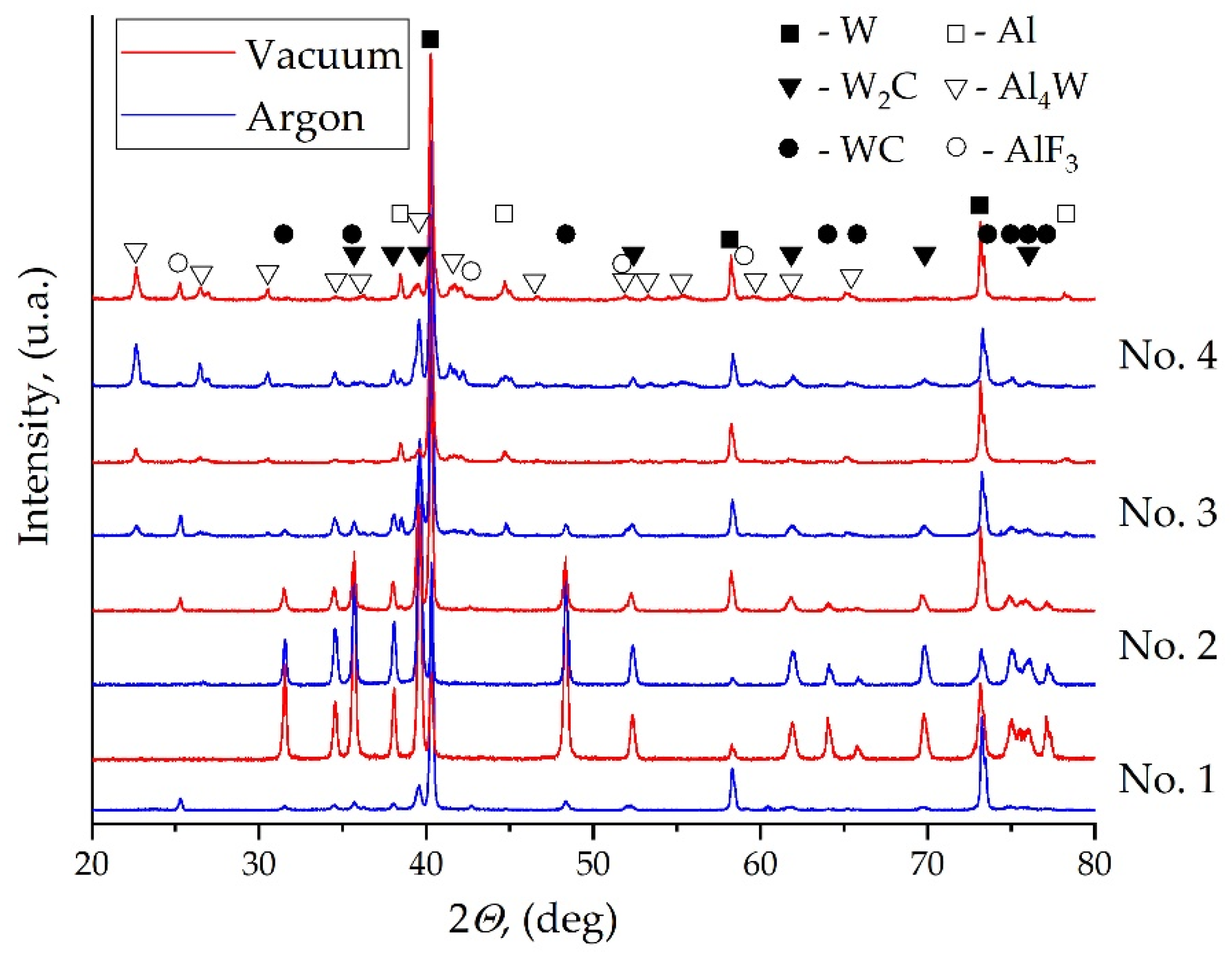


| No. | Mass Ratios (wt%) | TMD (g/cm3) | Actual Density (g/cm3) | Relative Density | ||
|---|---|---|---|---|---|---|
| W | PTFE | Al | ||||
| 0 | 80 | 20 | 0 | 7.55 | 7.17 | 0.95 |
| 1 | 76 | 19 | 5 | 6.93 | 6.58 | |
| 2 | 72 | 18 | 10 | 6.4 | 6.08 | |
| 3 | 64 | 16 | 20 | 5.55 | 5.28 | |
| 4 | 56 | 14 | 30 | 4.91 | 4.66 | |
| No. | Composition (wt%) | Tad (°C) | Gas Product (wt%) | Condensed Product (wt%) |
|---|---|---|---|---|
| 0 | 80W/20PTFE | 2115 | 20 (WF6), 14 (WF5), 10 (WF5) | 53 (W2C) |
| 1 | 76W/19PTFE/5Al | 2776 | 16 (AlF3), 3 (WF4) | 75 (W2C) |
| 2 | 72W/18PTFE/10Al | 2866 | 11 (AlF3), 7 (AlF), 5 (AlF2), | 74 (W2C) |
| 3 | 64W/16PTFE/20Al | 1710 | 29 (AlF) | 66 (W2C), 4 (Al4C3) |
| 4 | 56W/14PTFE/30Al | 1382 | 24 (AlF), | 57 (W2C), 6 (Al4C3), 11 (Al) |
| Composition | No. 1 | No. 2 | No. 3 | No. 4 |
|---|---|---|---|---|
| Medium | Combustion Rates (mm/s) | |||
| Air | 1.6 | 0.8–1.2 | 3–4 | 2.5–3 |
| Ar | 1.8 | 2.5 | 4–5 | 2.5–3 |
| Vacuum | 0.2 | 0.3 | 0.6 | 0.2 |
| Composition | No. 1 | No. 2 | No. 3 | No. 4 |
|---|---|---|---|---|
| Medium | Combustion Temperature (°C) | |||
| Air | 1900 | 1800 | 1600 | 1500 |
| Ar | 2000 | 2000 | 1500 | 1400 |
| Vacuum | 1200 | 1000 | 1000 | 1000 |
| Temperature of Ignition (°C) | ||||
| Air/Ar/Vacuum | 830 | 800 | 800 | 920 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saikov, I.; Seropyan, S.; Malakhov, A.; Saikova, G.; Denisov, I.; Petrov, E. Energetic Materials Based on W/PTFE/Al: Thermal and Shock-Wave Initiation of Exothermic Reactions. Metals 2021, 11, 1355. https://doi.org/10.3390/met11091355
Saikov I, Seropyan S, Malakhov A, Saikova G, Denisov I, Petrov E. Energetic Materials Based on W/PTFE/Al: Thermal and Shock-Wave Initiation of Exothermic Reactions. Metals. 2021; 11(9):1355. https://doi.org/10.3390/met11091355
Chicago/Turabian StyleSaikov, Ivan, Stepan Seropyan, Andrey Malakhov, Gulnaz Saikova, Igor Denisov, and Evgenii Petrov. 2021. "Energetic Materials Based on W/PTFE/Al: Thermal and Shock-Wave Initiation of Exothermic Reactions" Metals 11, no. 9: 1355. https://doi.org/10.3390/met11091355
APA StyleSaikov, I., Seropyan, S., Malakhov, A., Saikova, G., Denisov, I., & Petrov, E. (2021). Energetic Materials Based on W/PTFE/Al: Thermal and Shock-Wave Initiation of Exothermic Reactions. Metals, 11(9), 1355. https://doi.org/10.3390/met11091355






