The Role of Retained Austenite in Tempered Martensite Embrittlement of 4340 and 300-M Steels Investigated through Rapid Tempering
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Tensile Properties
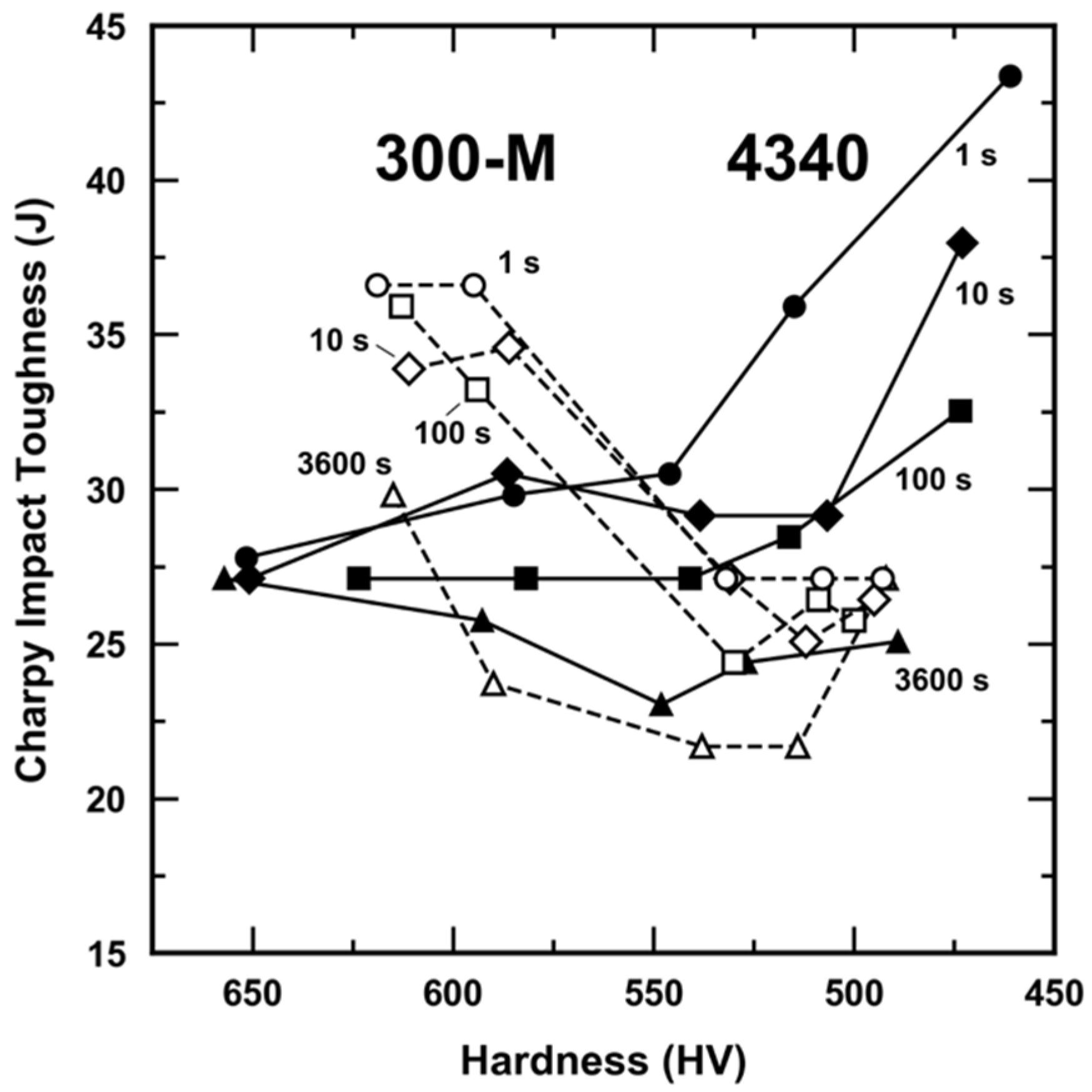
3.2. Room Temperature Impact Toughness
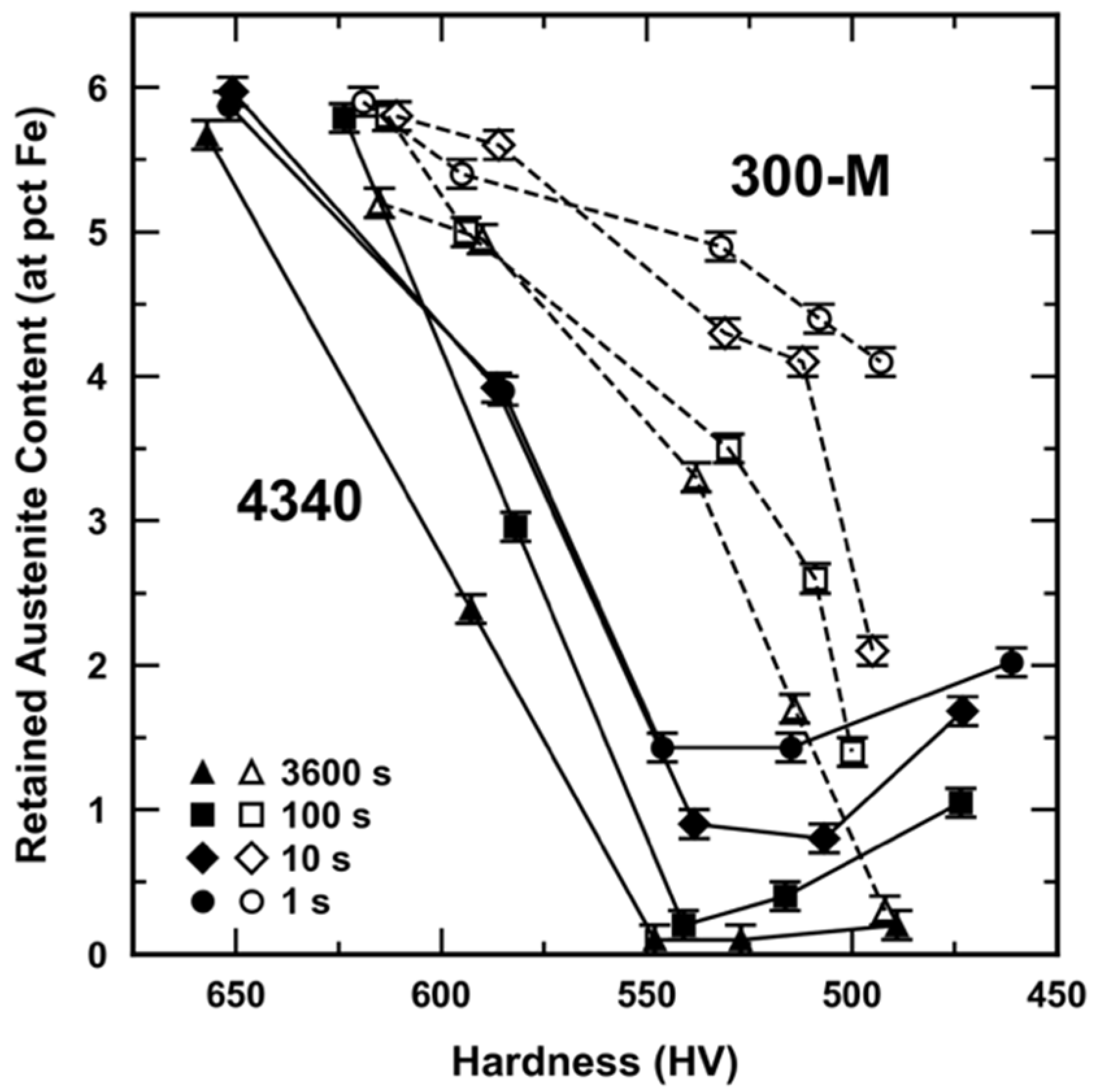
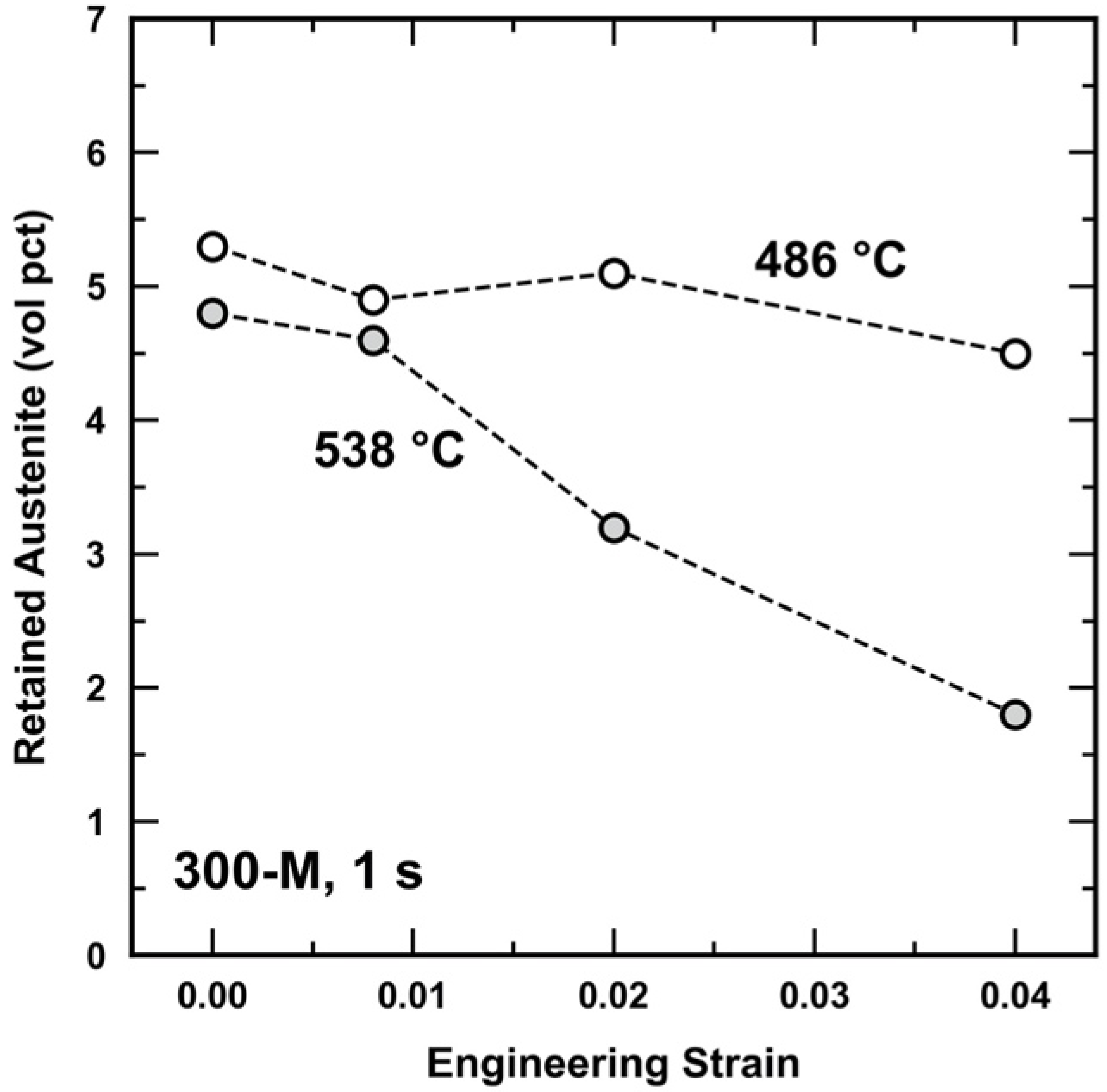
3.3. Retained Austenite
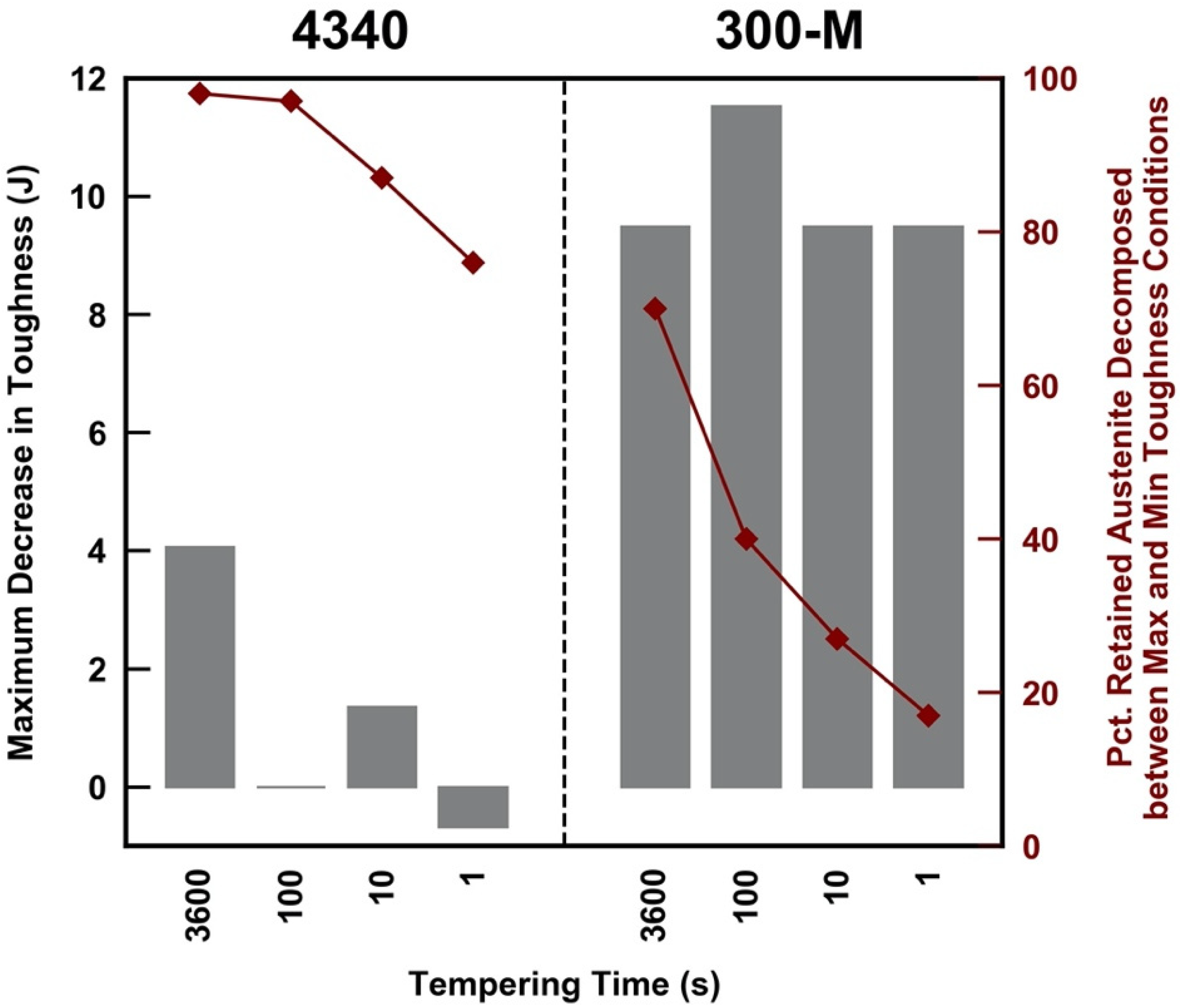
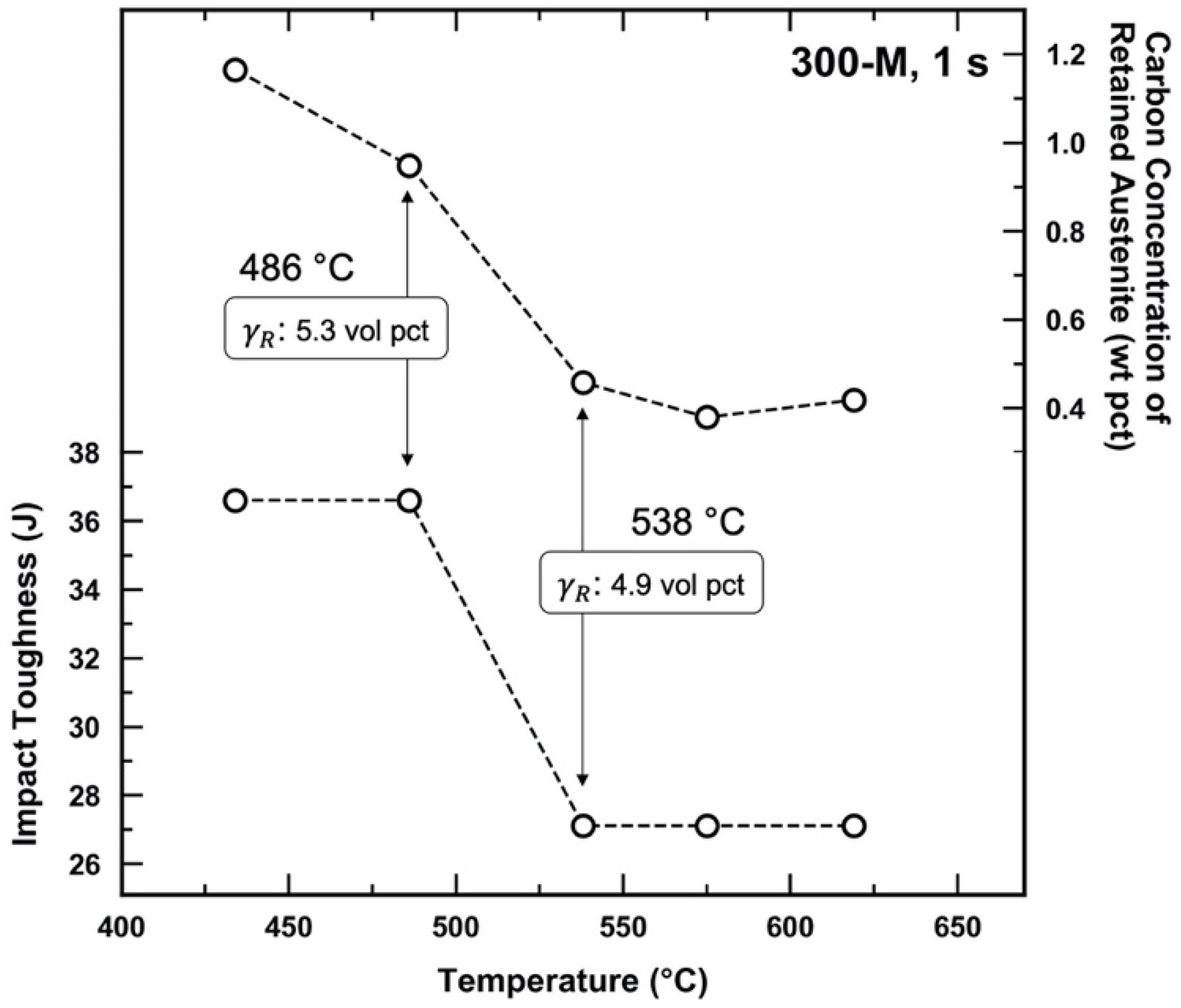
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krauss, G. Steels: Processing, Structure, and Performance, 1st ed.; ASM International: Materials Park, OH, USA, 2015. [Google Scholar]
- Judge, V.K.; Speer, J.G.; Clarke, K.D.; Findley, K.O.; Clarke, A.J. Rapid Thermal Processing to Enhance Steel Toughness. Sci. Rep. 2018, 8, 445. [Google Scholar] [CrossRef] [Green Version]
- Euser, V.K.; Williamson, D.L.; Clarke, K.D.; Findley, K.O.; Speer, J.G.; Clarke, A.J. Effects of Short-Time Tempering on Impact Toughness, Strength, and Phase Evolution of 4340 Steel Within the Tempered Martensite Embrittlement Regime. Metall. Mater. Trans. A 2019, 50, 3654–3662. [Google Scholar] [CrossRef]
- Euser, V.K.; Clarke, A.J.; Speer, J.G. Rapid Tempering: Opportunities and Challenges. J. Mater. Eng. Perform. 2020, 29, 4155–4161. [Google Scholar] [CrossRef]
- Clarke, A.J. Perspectives on Quenching and Tempering 4340 Steel. Metall. Mater. Trans. A 2020, 22, 4984–5005. [Google Scholar] [CrossRef]
- Euser, V.K.; Williamson, D.L.; Clarke, A.J.; Speer, J.G. Limiting Retained Austenite Decomposition in Quenched and Tempered Steels: Influences of Rapid Tempering and Silicon. ISIJ Int. 2020, 60, 2990–3000. [Google Scholar] [CrossRef]
- Speich, G.R.; Leslie, W.C. Tempering of Steel. Metall. Trans. 1972, 13, 1043–1054. [Google Scholar] [CrossRef]
- Grange, R.A.; Baughman, R.W. Hardness of Tempered Martensite in Carbon and Low Alloy Steels. Trans. ASM 1956, 48, 165–197. [Google Scholar] [CrossRef]
- Saeglitz, M.; Krauss, G. Deformation, Fracture, and Mechanical Properties of Low-Temperature-Tempered Martensite in SAE 43xx Steels. Metall. Mater. Trans. A 1997, 28, 377–387. [Google Scholar] [CrossRef]
- Materkowski, J.P.; Krauss, G. Tempered Martensite Embrittlement in SAE 4340 Steel. Metall. Trans. A 1979, 10, 1643–1651. [Google Scholar] [CrossRef]
- Horn, R.M.; Ritchie, R.O. Mechanisms of Tempered Martensite Embrittlement in Low Alloy Steels. Metall. Trans. A 1978, 9, 1039–1053. [Google Scholar] [CrossRef]
- Thomas, G. Retained Austenite and Tempered Martensite Embrittlement. Metall. Trans. A 1978, 9A, 439–450. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, C.H. Tempered Martensite Embrittlement in Fe-Mo-C and Fe-W-C Steel. Metall. Trans. A 1983, 14, 1389–1394. [Google Scholar] [CrossRef]
- Sarikaya, M.; Jhingan, A.K.; Thomas, G. Retained Austenite and Tempered Martensite Embrittlement in Medium Carbon Steels. Metall. Mater. Trans. A 1983, 14, 1121–1133. [Google Scholar] [CrossRef] [Green Version]
- King, J.E.; Smith, R.F.; Knott, J.F. Toughness Variations during the Tempering of a Plain Carbon Martensitic Steel. In Proceedings of the 4th International Conference on Fracture, Waterloo, ON, Canada, 19–24 June 1977; pp. 279–286. [Google Scholar]
- Bhadeshia, H.K.D.H.; Edmonds, D.V. Tempered Martensite Embrittlement: Role of Retained Austenite and Cementite. Met. Sci. 1979, 13, 325–334. [Google Scholar]
- Peters, J.A.; Bee, J.V.; Kolk, B.; Garrett, G.G. On the Mechanisms of Tempered Martensite Embrittlement. Acta Metall. 1989, 37, 675–686. [Google Scholar] [CrossRef]
- Tomita, Y.; Okawa, T. Effect of Microstructure on Mechanical Properties of Isothermally Bainite-Transformed 300M Steel. Mater. Sci. Eng. A 1993, 172, 145–151. [Google Scholar] [CrossRef]
- Allten, A.G.; Payson, P. The Effect of Silicon on the Tempering of Martensite. Trans. Am. Soc. Met. 1953, 45, 498. [Google Scholar]
- Owen, W.S. The Effect of Silicon on the Kinetics of Tempering. Trans. Am. Soc. Met. 1954, 46, 812–829. [Google Scholar]
- ASM International. Heat Treater’s Guide: Practices and Procedures for Irons and Steels, 2nd ed.; Chandler, H., Ed.; ASM International: Materials Park, OH, USA, 1996. [Google Scholar]
- Euser, V.K. The Effect of Rapid Tempering on Microstructural Evolution and Toughness within the Tempered Martensite Embrittlement Regime of 4340 and 300-M. Ph.D. Thesis, Colorado School of Mines, Golden, CO, USA, 2020. [Google Scholar]
- ASTM International. Standard Methods for Notched Bar Impact Testing of Metallic Materials E23; ASTM International: West Conshohocken, PA, USA, 1982; Volume 552. [Google Scholar]
- Pierce, D.T.; Coughlin, D.R.; Williamson, D.L.; Clarke, K.D.; Clarke, A.J.; Speer, J.G.; De Moor, E. Characterization of Transition Carbides in Quench and Partitioned Steel Microstructures by Mossbauer Spectroscopy and Complementary Techniques. Acta Mater. 2015, 90, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Pierce, D.T.; Coughlin, D.R.; Williamson, D.L.; Kähkönen, J.; Clarke, A.J.; Clarke, K.D.; Speer, J.G.; De Moor, E. Quantitative Investigation into the Influence of Temperature on Carbide and Austenite Evolution during Partitioning of a Quenched and Partitioned Steel. Scr. Mater. 2016, 121, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Böttger, A.; de Keijser, T.H.; Mittemeijer, E.J. Lattice Parameters of Iron-Carbon and Iron-Nitrogen Martensites and Austenites. Scr. Metall. Mater. 1990, 24, 509–514. [Google Scholar] [CrossRef]
- Uwakweh, O.N.C.; Bauer, J.P.; Génin, J.M.R. Mössbauer Study of the Distribution of Carbon Interstitials in Iron Alloys and the Isochronal Kinetics of the Aging of Martensite: The Clustering-Ordering Synergy. Metall. Trans. A 1990, 21, 589–602. [Google Scholar] [CrossRef]
- Williamson, D.L.; Nakazawa, K.; Krauss, G. A Study on the Early Stages of Tempering in an Fe-1.2C Pct Alloy. Metall. Trans. A 1979, 10A, 1351–1363. [Google Scholar] [CrossRef]
- ASTM International. Standard Practice for X-Ray Determination of Retained Austenite in Steel with Near Random Crystallographic Orientation; ASTM International: West Conshohocken, PA, USA, 2013; pp. 1–7. [Google Scholar]
- Jatczak, C.F. Retained Austenite and Its Measurement by X-ray Diffraction. SAE Tech. Pap. Ser. 1980, 108, 800426. [Google Scholar]
- Hidalgo, J.; Findley, K.O.; Santofimia, M.J. Thermal and Mechanical Stability of Retained Austenite Surrounded by Martensite with Different Degrees of Tempering. Mater. Sci. Eng. A 2017, 690, 337–347. [Google Scholar] [CrossRef]
- Xiong, X.C.; Chen, B.; Huang, M.X.; Wang, J.F.; Wang, L. The Effect of Morphology on the Stability of Retained Austenite in a Quenched and Partitioned Steel. Scr. Mater. 2013, 68, 321–324. [Google Scholar] [CrossRef]
- Clarke, A.J.; Miller, M.K.; Field, R.D.; Coughlin, D.R.; Gibbs, P.J.; Clarke, K.D.; Alexander, D.J.; Powers, K.A.; Papin, P.A.; Krauss, G. Atomic and Nanoscale Chemical and Structural Changes in Quenched and Tempered 4340 Steel. Acta Mater. 2014, 77, 17–27. [Google Scholar] [CrossRef]
- Joshi, A.; Stein, D.F. Impurity Segregation to Grain Boundaries. J. Test. Eval. 1973, 1, 202–208. [Google Scholar]


| wt pct | C 1 | Mn | Si | Ni | Cr | Mo | Ti |
|---|---|---|---|---|---|---|---|
| 4340 | 0.41 | 0.71 | 0.25 | 1.76 | 0.75 | 0.26 | - |
| 300-M | 0.42 | 0.72 | 1.58 | 1.93 | 0.80 | 0.40 | 0.003 |
| wt pct | Nb | V | Al | S | P | Cu | Sn |
| 4340 | 0.005 | 0.047 | 0.008 | 0.001 | 0.009 | 0.14 | - |
| 300-M | - | 0.08 | 0.038 | 0.001 | 0.006 | 0.12 | 0.009 |
| Alloy | Time (s) | Temperature (°C) | ||||
|---|---|---|---|---|---|---|
| 4340 | 3600 | 200 | 250 | 300 | 350 | 400 |
| 100 | 241 | 295 | 350 | 404 | 458 | |
| 10 | 271 | 329 | 386 | 444 | 501 | |
| 1 | 305 | 366 | 427 | 489 | 550 | |
| Range of Average Hardness (HV) | 624–657 | 582–593 | 538–548 | 507–527 | 461–489 | |
| 300-M | 3600 | 350 | 400 | 450 | 500 | 550 |
| 100 | 404 | 434 | 517 | 544 | 567 | |
| 10 | 441 | 462 | 519 | 555 | 612 | |
| 1 | 434 | 486 | 538 | 575 | 619 | |
| Range of Average Hardness (HV) | 611–619 | 586–595 | 526–538 | 508–514 | 492–500 | |
| Condition | Alloy | Preservation of | Mechanical Stability of | TME Severity | Embrittling Agent(s) |
|---|---|---|---|---|---|
| Conventional | 4340 | None | n/a | Medium | Interlath Cementite |
| 300-M | Medium-High | Low: Transforms to fresh martensite during deformation | High | Interlath Cementite + Fresh Martensite | |
| Rapid | 4340 | Medium-Low | High | Low | Interlath Cementite |
| 300-M | High | Low: Transforms to fresh martensite during deformation | High | Interlath Cementite + Fresh Martensite |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Euser, V.K.; Williamson, D.L.; Findley, K.O.; Clarke, A.J.; Speer, J.G. The Role of Retained Austenite in Tempered Martensite Embrittlement of 4340 and 300-M Steels Investigated through Rapid Tempering. Metals 2021, 11, 1349. https://doi.org/10.3390/met11091349
Euser VK, Williamson DL, Findley KO, Clarke AJ, Speer JG. The Role of Retained Austenite in Tempered Martensite Embrittlement of 4340 and 300-M Steels Investigated through Rapid Tempering. Metals. 2021; 11(9):1349. https://doi.org/10.3390/met11091349
Chicago/Turabian StyleEuser, Virginia K., Don L. Williamson, Kip O. Findley, Amy J. Clarke, and John G. Speer. 2021. "The Role of Retained Austenite in Tempered Martensite Embrittlement of 4340 and 300-M Steels Investigated through Rapid Tempering" Metals 11, no. 9: 1349. https://doi.org/10.3390/met11091349
APA StyleEuser, V. K., Williamson, D. L., Findley, K. O., Clarke, A. J., & Speer, J. G. (2021). The Role of Retained Austenite in Tempered Martensite Embrittlement of 4340 and 300-M Steels Investigated through Rapid Tempering. Metals, 11(9), 1349. https://doi.org/10.3390/met11091349






