2.2. Methodology
First, the mining district of Linares was studied in order to identify those waste dumps belonging to different mine workings that could present viable percentages of interesting metallic elements such as copper. The selected waste dumps were visited to take samples of representative materials, taking into account the most abundant formations therein and collecting this type of material. Subsequently, the material from the different samples was treated for subsequent analysis with micro-X-ray fluorescence tests, identifying the existence of copper in the dumps. Based on the results obtained, the dump with the highest potential percentage of copper was selected for extraction by hydrometallurgical techniques.
The selected waste dump was analyzed in more detail by taking samples from different points of the waste dump in order to quantify in more detail the chemical elements present, the percentage of copper present, as well as the variability of the results. For this purpose, samples were taken and, subsequently, analyzed chemically. Once the chemical composition of the selected waste dump was evaluated, the material was milled and leached in acidic media. These acidic media consisted of sulfuric acid solutions of different molarities (0.25 mol, 0.50 mol, 1.00 mol, and 2.00 mol), measuring the pH, electrical conductivity, and copper concentration at different times. With these data, it was possible to obtain the appropriate leaching solution and the time required for the recovery of an economic percentage of copper.
The leaching residue was subjected to a leachate test in order to determine its suitability for the conformation of bituminous mixtures. This process was carried out in order to assess that the leachate from the aggregate did not cause significant environmental contamination. Subsequently, the leaching residue was subjected to different physical and mechanical tests to determine its suitability as an aggregate for bituminous mixtures.
Finally, and having determined the suitability of the leaching residue for conformation bituminous mixtures, different discontinuous grading and bitumen emulsion bituminous mixtures were conformed. These bituminous mixtures were conformed with increasing percentages of binder and were subsequently subjected to physical and mechanical tests in order to establish the optimum percentage of bitumen emulsion that developed the best properties.
The following sections describe the tests carried out in greater detail in the different blocks into which this research is divided.
2.2.3. Leaching in Acidic Media and Atmospheric Pressure
Once the chemical composition of the selected waste dump sample was analyzed, we proceeded to study the leaching of copper with different sulfuric acid solutions at different leaching times and atmospheric pressure.
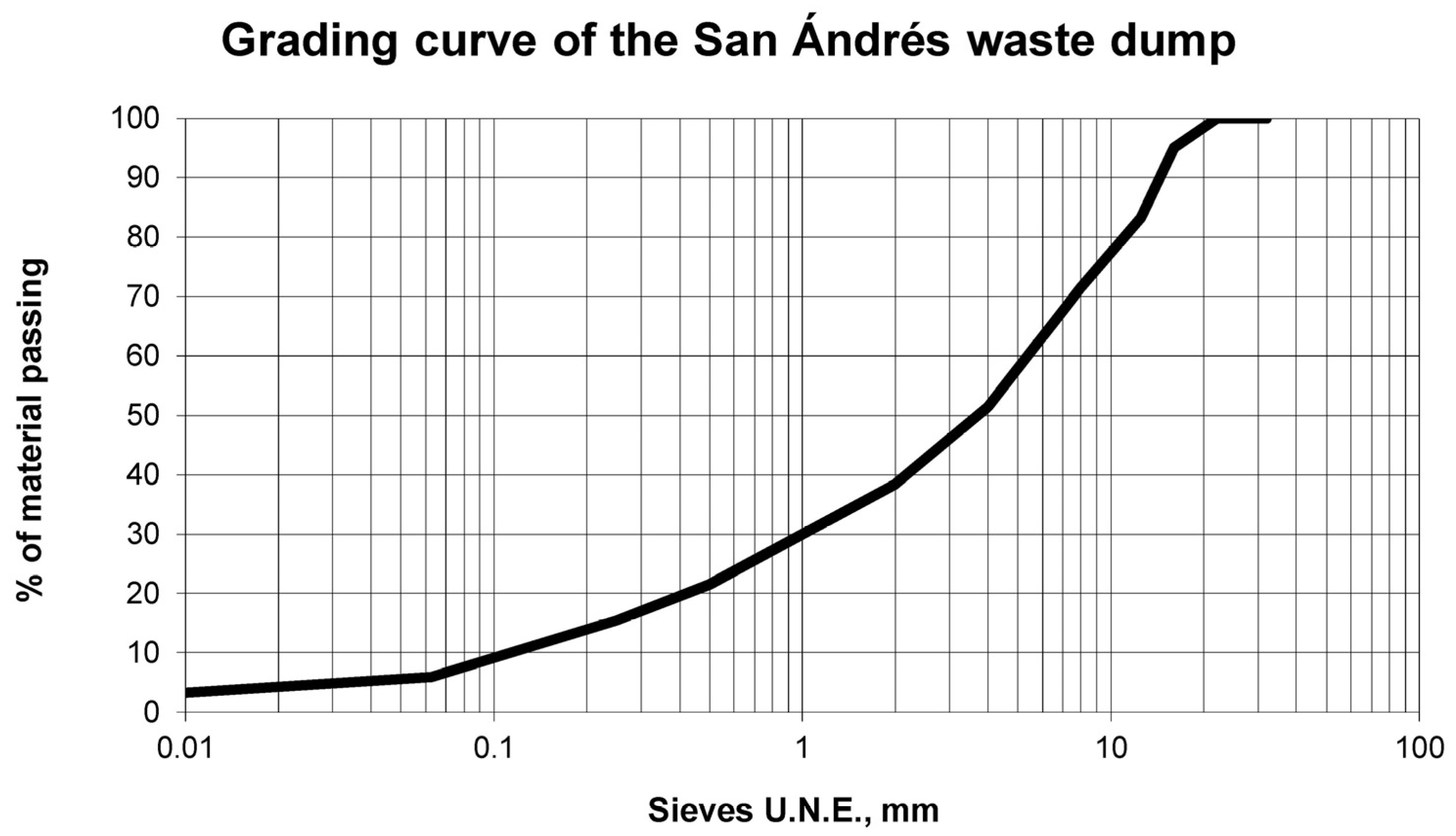
First, the sample of the selected waste dump was milled, and different representative sub-samples were obtained according to the UNE-EN 932-2 standard. The grading curve of the crushed waste dump sample was determined using the UNE-EN 933-1 grading curve analysis test. The milling was carried out with a double purpose: on the one hand, to reduce the sample size so that it was easier to handle in the industrial process; on the other hand, and due to the fact that the granite of the waste dump was pre-cracked and in these cracks there were copper hydrothermal chemical compounds, the milling produced the fracture of the rock through these cracks obtaining, consequently, the exposure of the interesting chemical compounds to the acid dissolution.
Once the sample was crushed, the leaching test was carried out in acidic media and with different solutions. The samples selected for leaching had a mass of 300 ± 0.1 g, after drying to remove humidity. The sulfuric acid solutions, 3000 ± 10 mL volume per process, were 0.25 mol, 0.50 mol, 1.00 mol, and 2.00 mol. The use of these low molarity solutions is motivated by different reasons: on the one hand, the use of low molarity solutions of sulfuric acid develops a leaching residue that is easier to treat afterwards; on the other hand, a lower concentration of sulfuric acid makes it possible to increase the working life of the equipment; and finally, higher molarities of sulfuric acid would create a higher percentage of sludge that is difficult to treat. Furthermore, it should be noted that the use of the aforementioned solid/liquid ratio is to avoid the need to carry out subsequent concentration processes at a higher economic cost, as would be the case if higher temperatures than ambient were used.
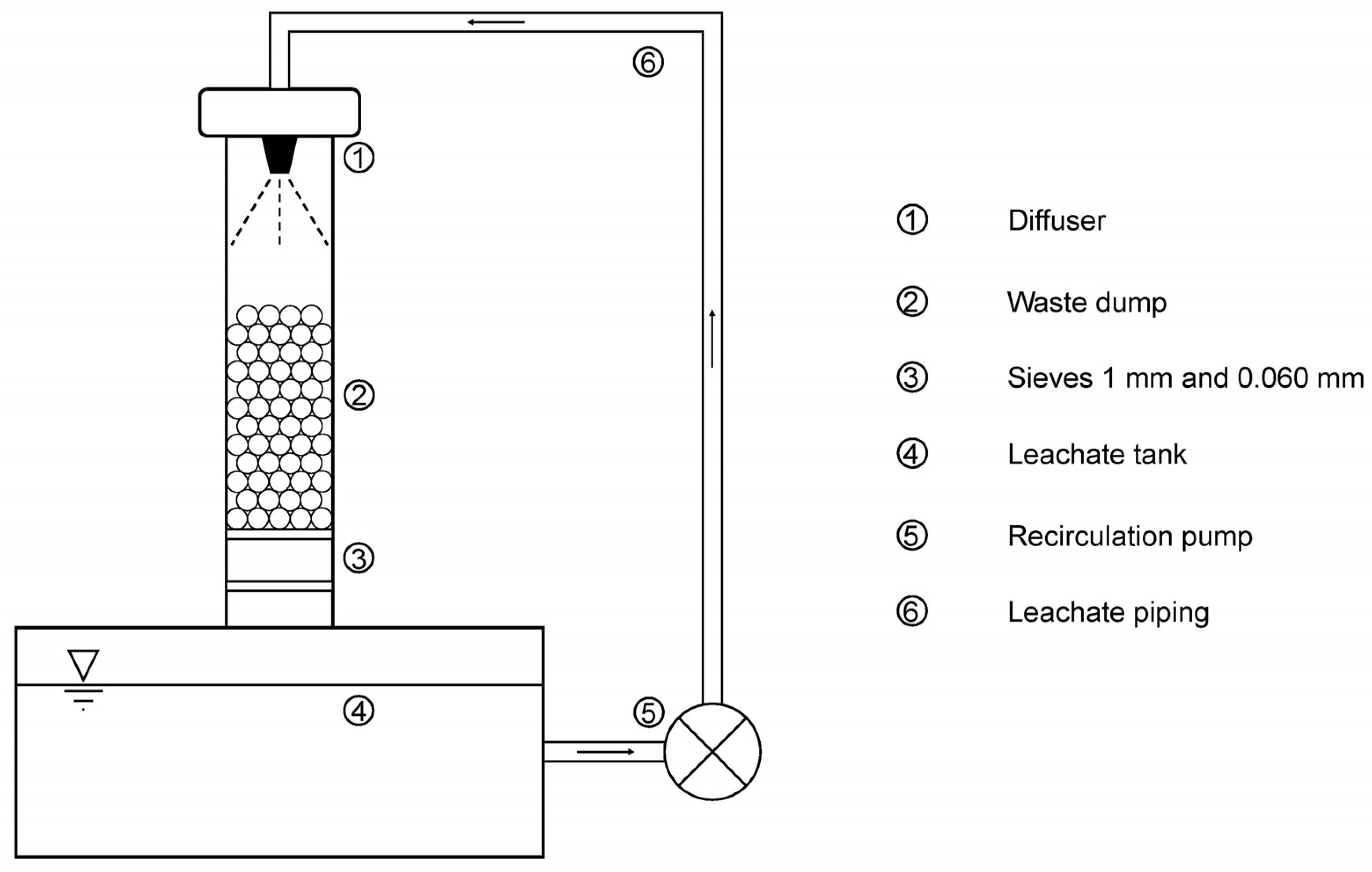
The leaching process was carried out in equipment specially designed for the research. This equipment consisted mainly of sieves that retained the sample and an upper pulverizer. The sprayer sprayed the sample remaining on the sieves, depositing the fluid by gravity in a leaching tank. The fluid from the leach tank was continuously pumped into the pulverizer for a continuous recirculation process. This process was maintained constant during the 35 days of testing without interruption, controlling the temperature at 25 ± 1 °C. It should be noted that the operation of the equipment is very similar to that of the industrial process, in which large quantities of material are irrigated with acid solutions that are continuously recirculated. The image of the equipment can be seen in
Figure 2.
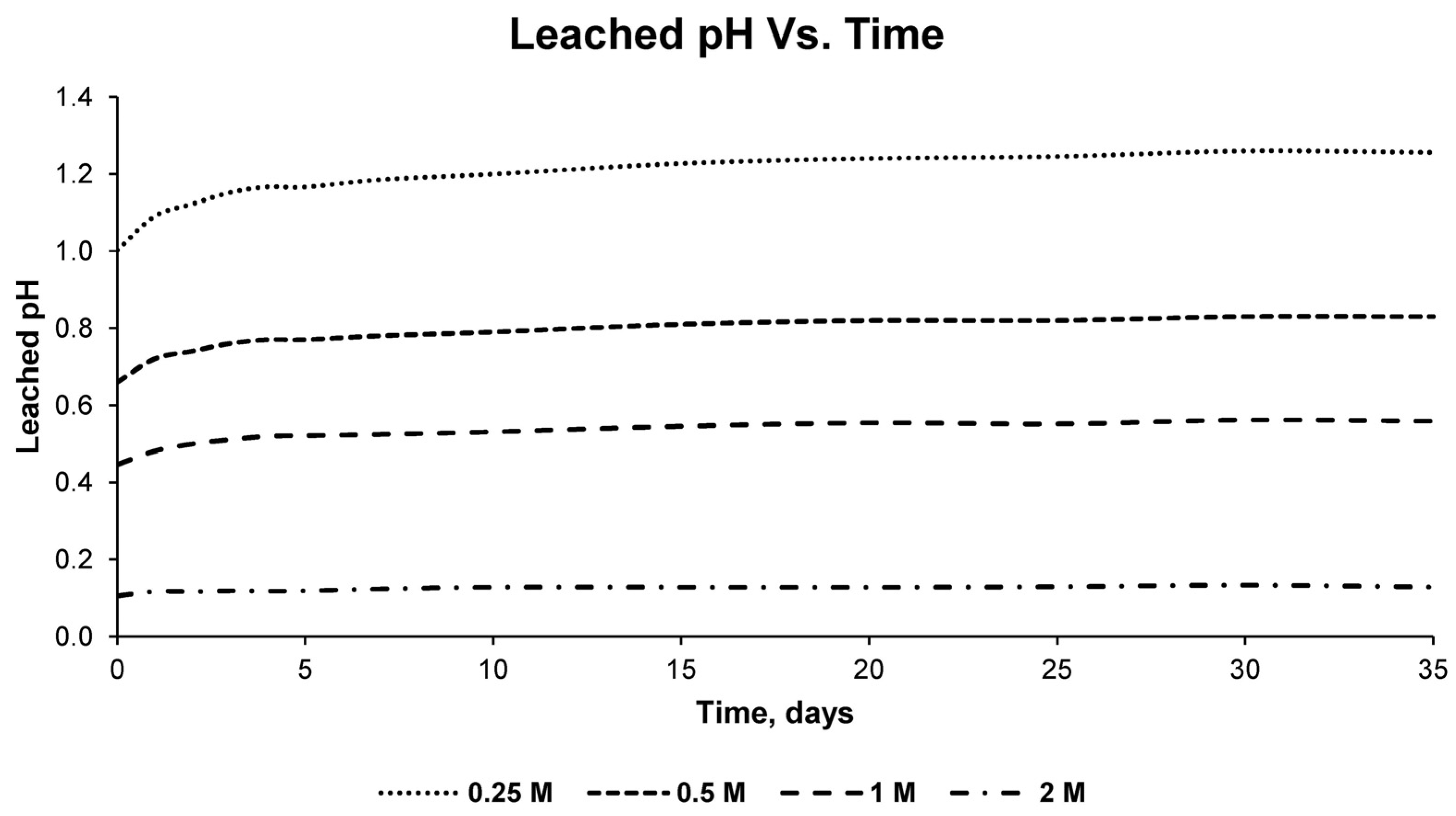
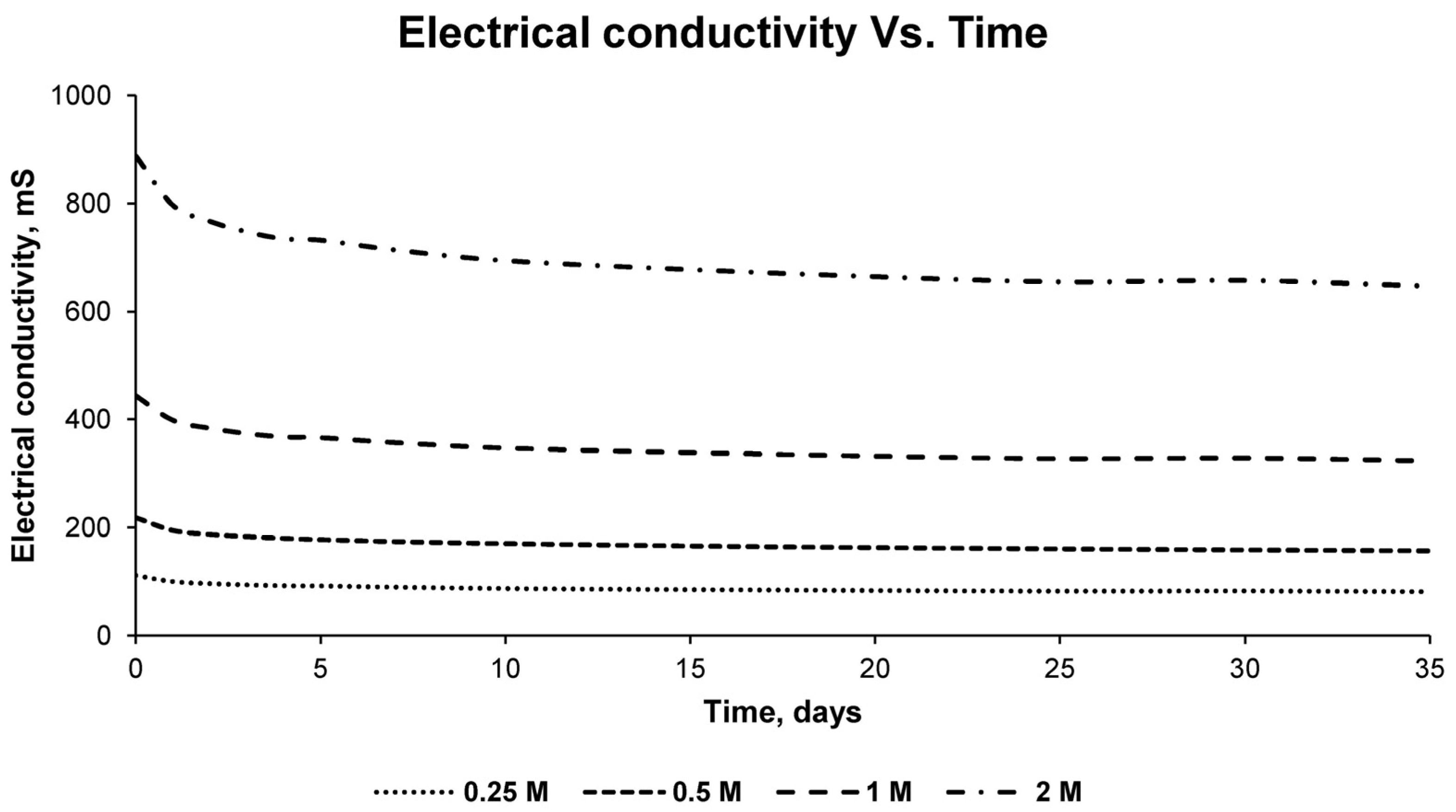
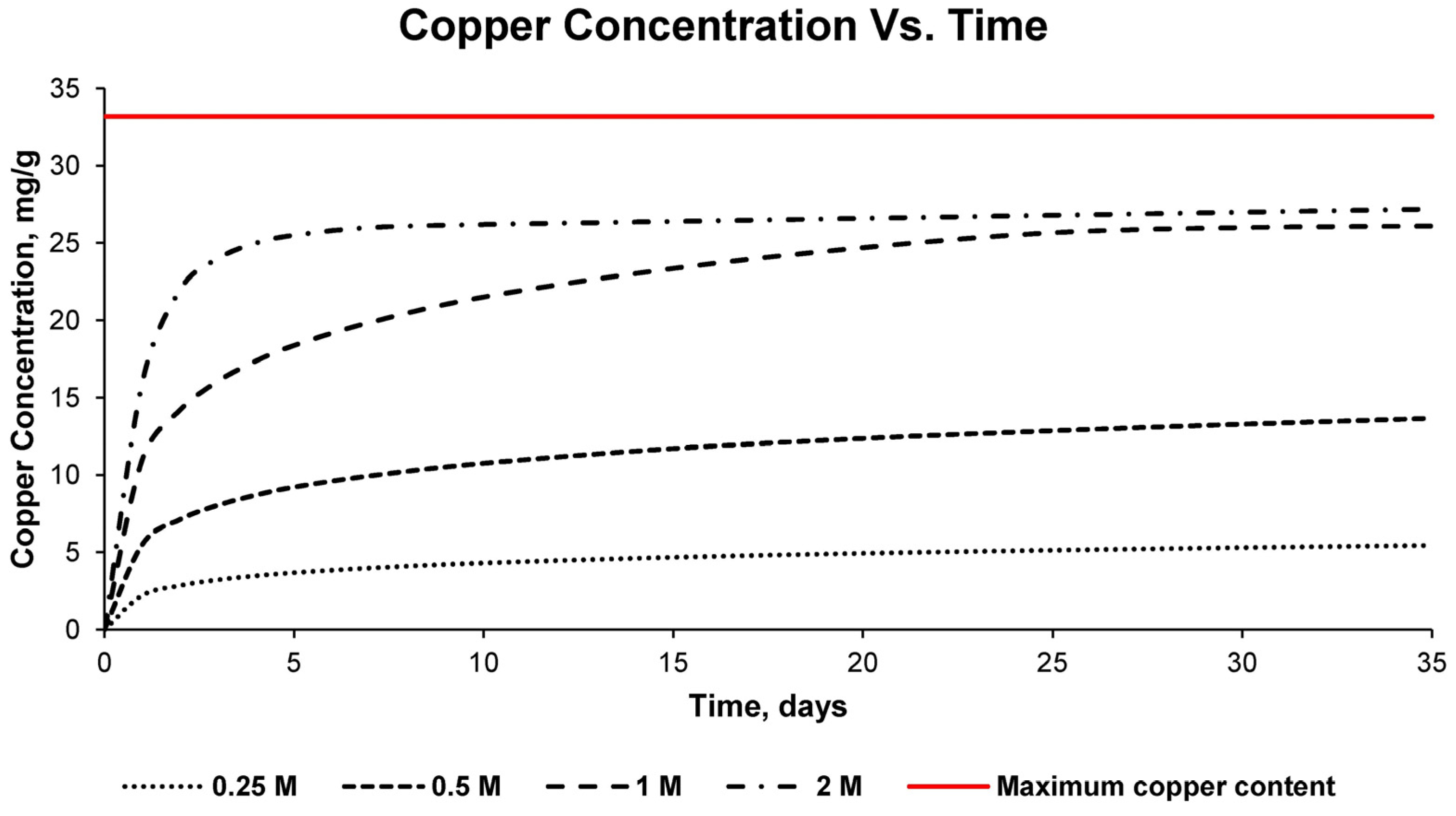
This process was carried out for the four detailed sulfuric acid solutions, measuring the pH and electrical conductivity of the solution every 24 h. In addition, samples were taken every 24 h for subsequent analysis by inductively coupled plasma mass spectrometry (7900, Agilent, Santa Clara, CA, USA). For the chemical analysis with the detailed equipment, standardized standards of the chemical element copper were used, repeating each measurement three times to obtain statistically reliable results. In this way, the percentage of copper leached in the solution used and at different times could be verified, as well as the percentage of recovery that occurred with respect to the total copper in the sample.
With the results of the variation of the pH of the solution, the variation of the electrical conductivity, as well as the concentration of copper in the different solutions for different times, the optimum solution was determined, as well as the time necessary for the leaching of copper at an economically viable recovery percentage.
2.2.5. Bituminous Mixtures Conforming and Testing
Once the chemical, physical, and mechanical suitability of the leaching residue had been analyzed, the bituminous mix used was formulated and named a discontinuous grading mixture, hereinafter referred to as AF12. This type of bituminous mix was developed with the fundamental aim of obtaining a sustainable, low-cost mix with a high use of waste. It should be noted that in this work, the best practice guide provided by the Technical Association for Bitumen Emulsions (hereinafter ATEB) will be used for the formulation of bituminous mixes. This guide was written by experts in the field and sets out the necessary steps for assessing the suitability of a mixture of this type.
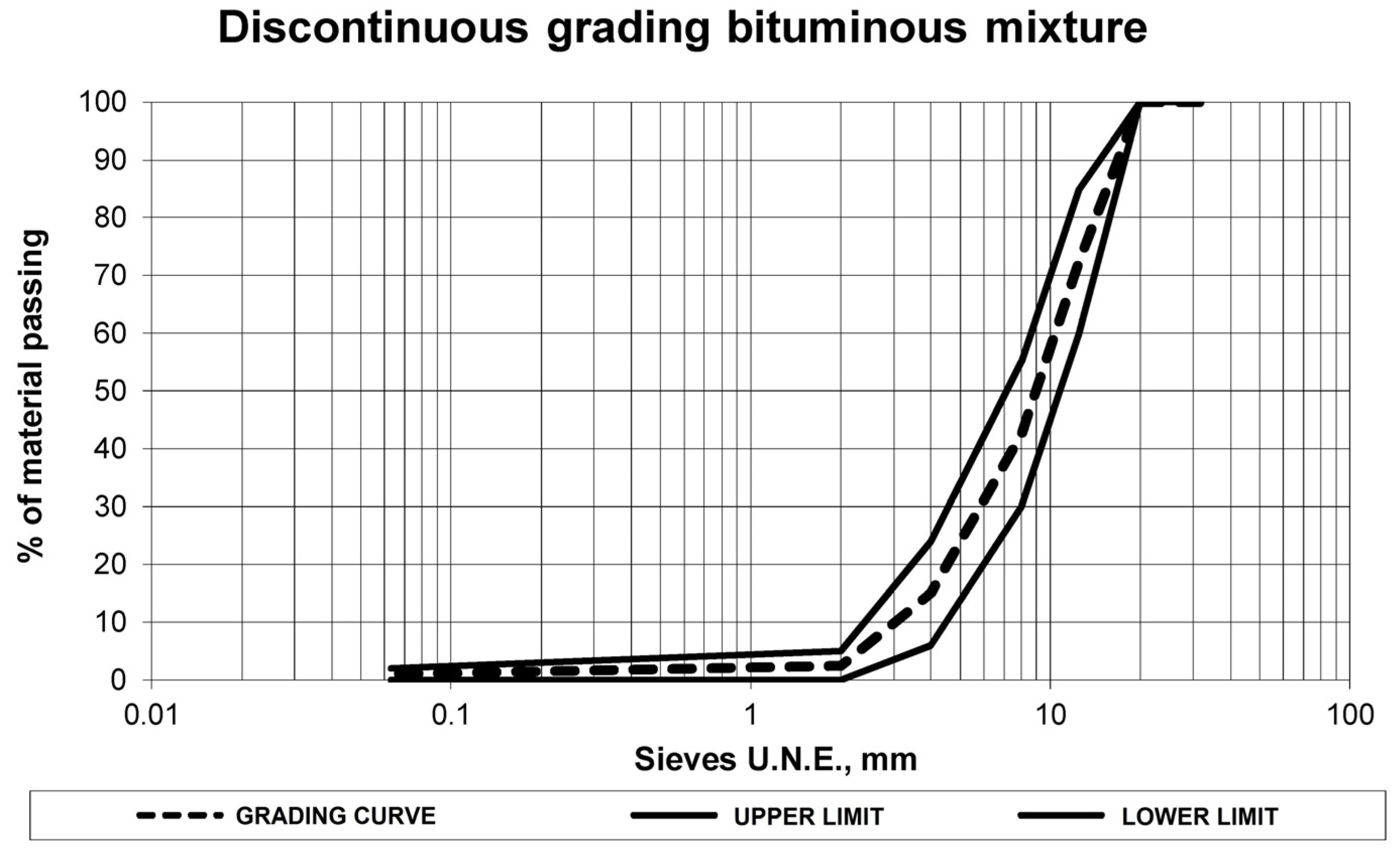
The grading curve of the leaching residue was that established by the envelope grading curve detailed in the aforementioned standard. This grading curve, shown in
Figure 3, was intermediate between the upper and lower limit of the grading envelope in order not to induce more variables.
Once the grading curve had been defined, the analysis of the compatibility of the aggregate with the emulsion to be used was carried out. For this purpose, the coating test was carried out in accordance with the NLT-196/84 standard. This test evaluates the quality of the aggregate’s coating by the emulsion and, consequently, the quality of the final mix to be developed.
First, the minimum percentage of emulsion, residual bitumen, that the bituminous mix could contain was calculated. To do this, the percentage of emulsion to be added to the bituminous mix was calculated according to the mathematical formula described by ATEB in its good practice guide. It should be noted that the bitumen emulsion is mainly composed of bitumen and water, and that after the mixture is spread, the water evaporates by natural processes. Therefore, the calculation of the percentage of residual bitumen from the percentage of bitumen emulsion is immediate if the technical data sheet of the bitumen emulsion is known. In this case the bitumen emulsion used had a residual bitumen percentage of 60%. The formula provided by ATEB for this type of bituminous mixture is based on the specific surface area of a conventional aggregate and is detailed below.
where:
BR = Proportion of residual bitumen on the dry mass of the aggregates.
K = Coefficient of enrichment, the value of which is 1 in the wearing course and 0.9 in the lower course.
A = Proportion of aggregates retained by the sieve UNE 20 mm.
B = Proportion of aggregates passing through the sieve UNE 20 mm and retained by the sieve UNE 8 mm.
C = Proportion of aggregates passing through the sieve UNE 8 mm and retained by the sieve UNE 4 mm.
D = Proportion of aggregates passing through the sieve UNE 4 mm and retained by the sieve UNE 2 mm.
E = Proportion of aggregates passing through the sieve UNE 2 mm and retained by the sieve UNE 0.063 mm.
F = Proportion of aggregates passing through the sieve UNE 0.063 mm.
Consequently, and according to the grading defined above, the ATEB formula determined a residual bitumen content of the aggregate of 3.5%, corresponding to a percentage of bitumen emulsion of the aggregate of 5.8%. Once the optimum point was calculated empirically using the formula provided by ATEB, different families of test samples were made with increasing percentages of bitumen from 0.25% onwards. As mentioned above, when conforming, it must be taken into account that the water in the emulsion will be evaporated later, so the reference values are the bitumen that will remain in the bituminous mix after evaporation.
The test samples of each family of specimen with increasing percentages of bitumen were conformed and compacted according to UNE-EN 12697-30. Subsequently, all the test samples were subjected to a curing process to eliminate the water from the emulsion. For this purpose, the Marshall type test samples manufactured were leveled with the mold so that they could be placed on sieves with an opening of less than 2 mm. The subsequent curing process consisted of 2 days at a temperature of 75 ± 2 °C and 5 more days at a temperature of 90 ± 2 °C (the curing processes are detailed in ATEB). During the curing process, binder drainage must be continuously observed; for this purpose, a filter paper was placed at the bottom of the test samples during the curing process. The test sample family that caused binder drainage was considered as null, and consequently, the percentage of bitumen or emulsion in this family was the maximum permissible. Therefore, the bituminous mixes viable for use were those incorporating a percentage of emulsion from the minimum set by ATEB to the maximum produced by binder drainage.
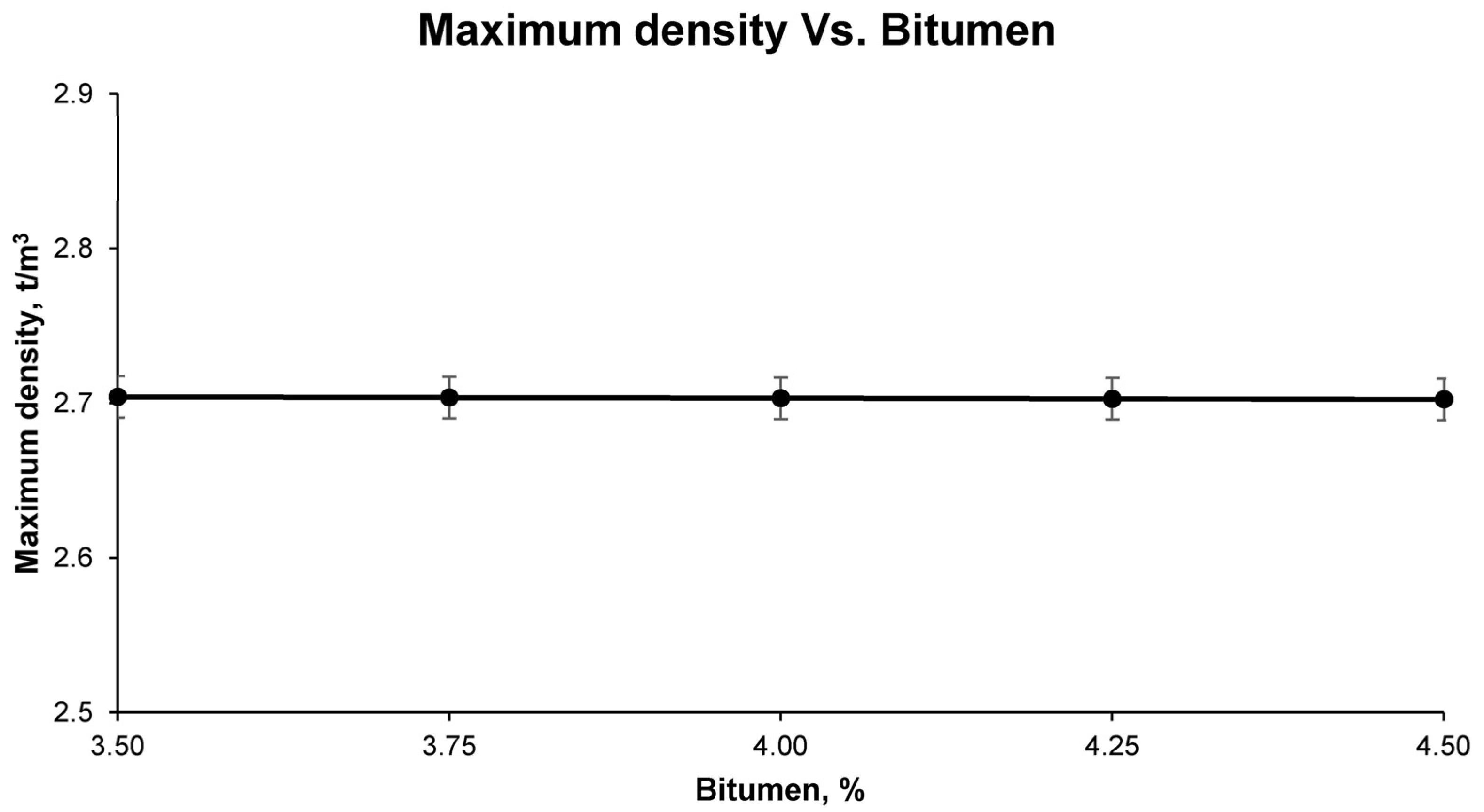
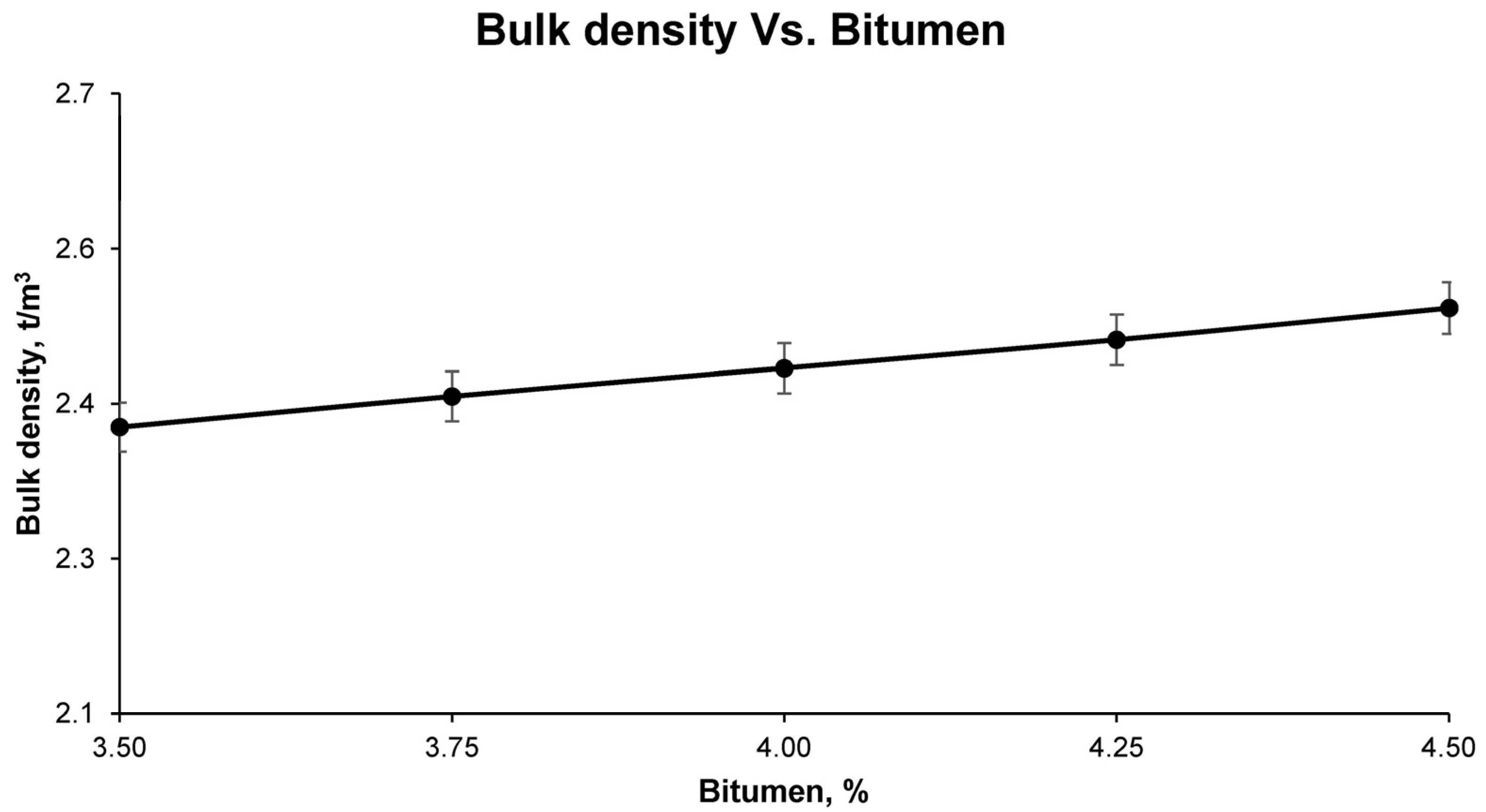
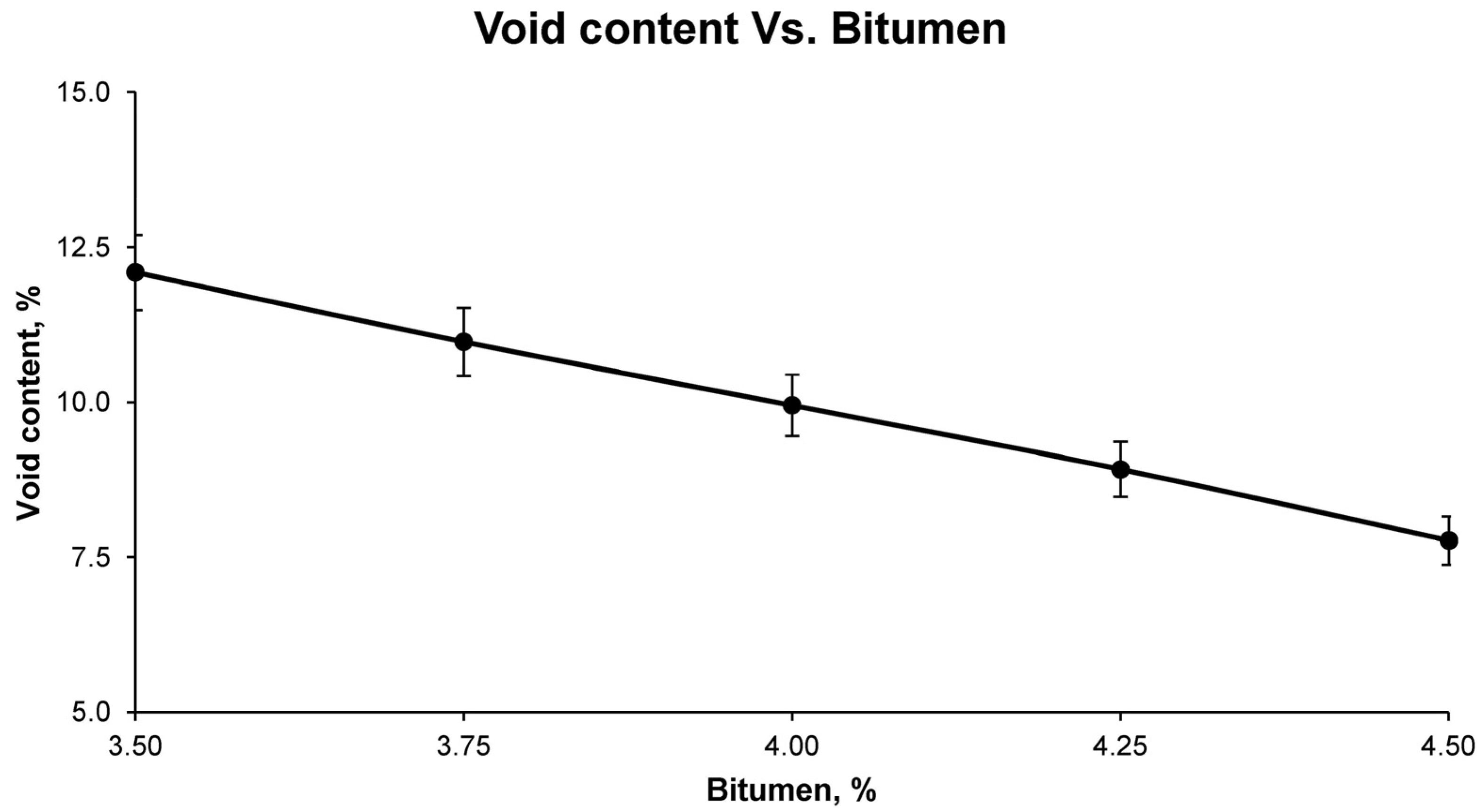
After curing, the test samples were stripped and subjected to a series of physical tests to determine the general physical properties. The first test samples were tested for maximum density, according to UNE-EN 12697-5; bulk density, according to UNE-EN 12697-6; and void characteristics in the mix, according to UNE-EN 12697-8.
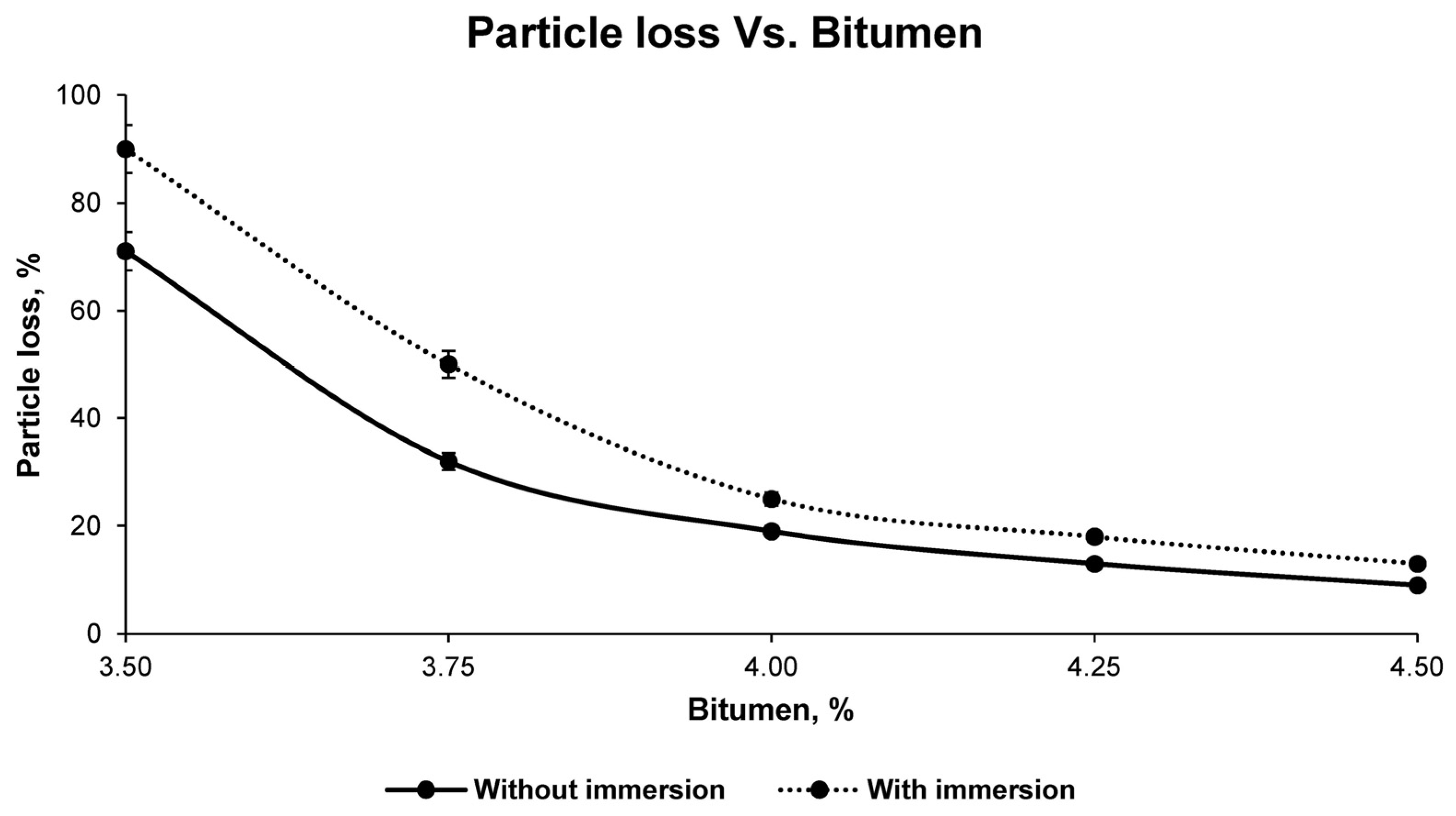
Subsequently, and in order to determine the resistance of the bituminous mixtures to fracture and aggregate pull-out, the particle loss test was carried out in accordance with the UNE-EN 12697-17 standard for all families of mixtures. In turn, in order to evaluate the influence of water on the bituminous mix and, consequently, to determine the compatibility between the aggregate and the emulsion, the same type of test was carried out with immersion in water at 45 ± 1 °C for 24 h, in accordance with standard NLT-362/92.
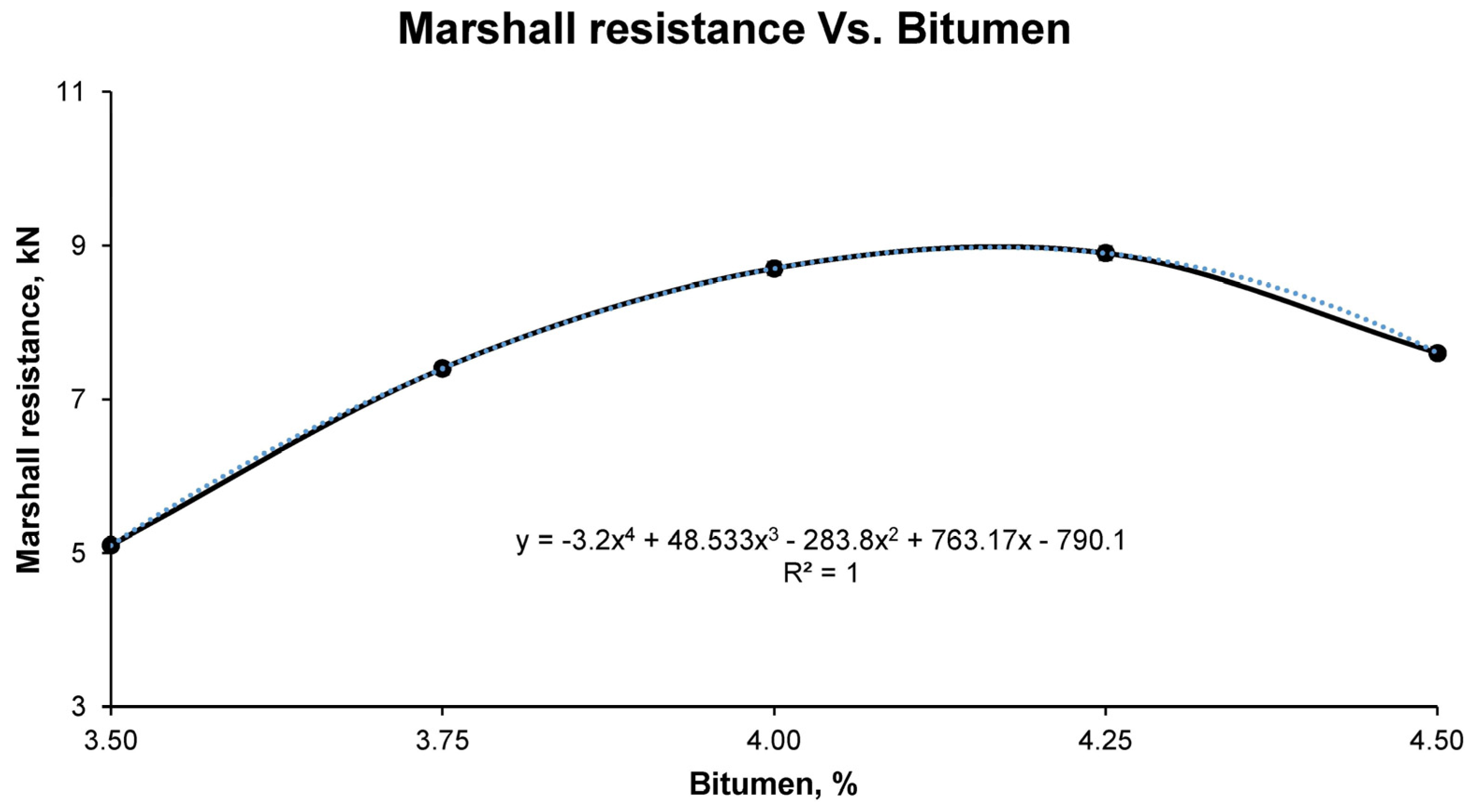
In this way, the quality of the mastic conformed with the filler and bitumen in the emulsion could be determined. However, due to the incorporation of high percentages of bitumen, the problem of plastic deformation in the infrastructure can occur. To avoid this and to quantify the resistance of the mix at high temperatures, the Marshall test was carried out in accordance with the UNE-EN 12697-34 standard.
Finally, with the results of the aforementioned tests for the different groups of samples, it was possible to observe, graphically and mathematically, the variation of the physical and mechanical properties of the bituminous mixes with increasing bitumen percentage. In this way, the study of the graphical and mathematical models provided an optimum percentage of bitumen emulsion that developed the best physical and mechanical characteristics. This combination is the one that was called optimum, with which all the above-mentioned tests were carried out again to corroborate the mathematically determined properties. These tests for determining the physical properties of the final bituminous mix are: maximum density UNE-EN 12697-5, bulk density UNE-EN 12697-6, and void characteristics UNE-EN 12697-8; and for the mechanical properties: loss of particles without immersion and after immersion UNE-EN 12697-17 and the Marshall test UNE-EN 12697-34.