Formation and Evolution of DS-Type Inclusions in 15-5PH Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedures
2.2. Analysis of Steel and Inclusion Composition
3. Results and Discussion
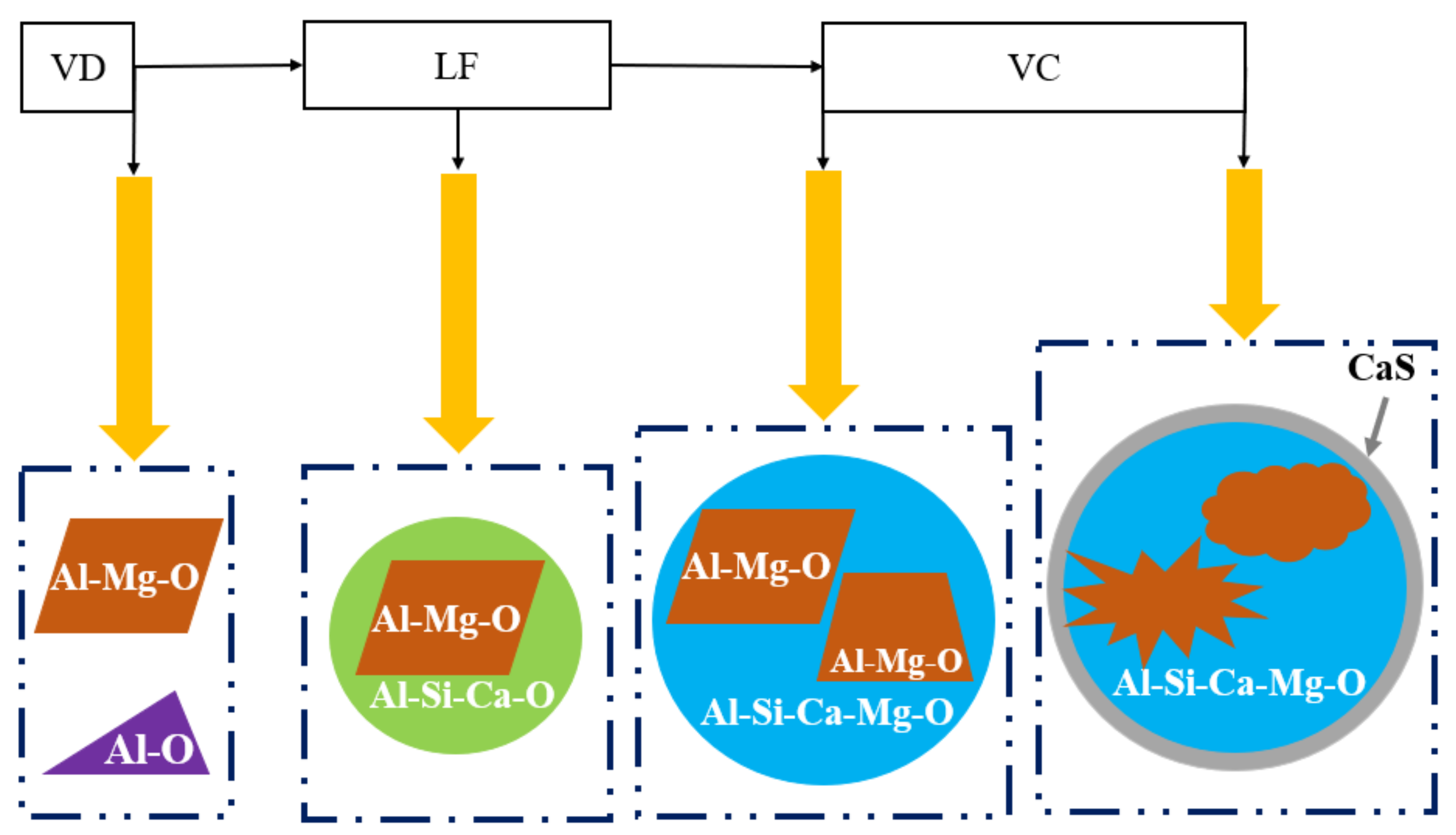
3.1. Inclusion Characteristics
3.2. Thermodynamic Calculation of Inclusion Formation
3.3. DS-Type Inclusion Formation Mechanisms and Controlling Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vengosh, A.; Jackson, R.B.; Warner, N.; Thomas, H.; Andrew, K. A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ. Sci. Technol. 2014, 48, 8334–8348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhou, H.; Hua, J. Study on Influence of Material on Self-Reinforcing Performance of Pump Head of Ultra-High Pressure Fracturing Pump. Mech. Des. Manuf. 2010, 7, 67–68. [Google Scholar]
- Zhang, S. Study on Crack Propagation and Fatigue Life of Fracturing Pump Head; Yangtze University: Jingzhou, China, 2018. [Google Scholar]
- Wang, Z.; Chen, L.; Zhang, X. Fatigue Life Analysis of Drilling Pump Valve Box. J. Chongqing Univ. Sci. Technol. 2014, 16, 77–79. [Google Scholar]
- Park, J.H.; Kang, Y. Inclusions in Stainless Steels—A Review. Steel Res. Int. 2017, 88, 1700130. [Google Scholar] [CrossRef]
- Li, J.; Cheng, G.; Ruan, Q.; Pan, J.; Chen, X. Evolution Mechanism of Oxide Inclusions in Titanium-Stabilized AISI 443 Stainless Steel. Met. Mater. Trans. B 2018, 49, 2357–2369. [Google Scholar] [CrossRef]
- Park, J.H. Formation Mechanism of Spinel-Type Inclusions in High-Alloyed Stainless Steel Melts. Met. Mater. Trans. B 2007, 38, 657–663. [Google Scholar] [CrossRef]
- Park, J.H.; Todoroki, H. Control of MgO·Al2O3 Spinel Inclusions in Stainless Steels. ISIJ Int. 2010, 50, 1333–1346. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Wang, Q.; Zhang, L.; Li, J.; Peaslee, K. Formation and Modification of MgO·Al2O3-Based Inclusions in Alloy Steels. Met. Mater. Trans. B 2012, 43, 731–750. [Google Scholar] [CrossRef]
- Pan, C.; Hu, X.; Lin, P.; Chou, K. Evolution of Inclusions after Cerium and Titanium Addition in Aluminum Deoxidized Fe-17Cr-9Ni Austenitic Stainless Steel. ISIJ Int. 2020, 60, 1878–1885. [Google Scholar] [CrossRef]
- Li, J.; Cheng, G.; Ruan, Q.; Pan, J.; Chen, X. Evolution behaviour of nonmetallic inclusions in Ti-bearing 11Cr stainless steel with calcium treatment. Ironmak. Steelmak. 2020, 47, 31–39. [Google Scholar] [CrossRef]
- Li, J.; Cheng, G.; Ruan, Q.; Li, J.; Pan, J.; Chen, X. Evolution Mechanism of Inclusions in Al-killed, Ti-bearing 11Cr Stainless Steel with Ca Treatment. ISIJ Int. 2018, 58, 1042–1051. [Google Scholar] [CrossRef] [Green Version]
- He, Q.X.; Sun, S.Q. Introduction to Steel-Determination of Content of Nonmetallic Inclusions-Micrographic Method Using Standards Diagrams GB/T 10561–2005. Ptca (Part: Aphystest) 2007, 43, 103–107. [Google Scholar]
- Wu, H.; Li, Q.; Wei, C.; Wang, Z. Study on the behaviour of DS-Class inclusions in advanced bearing steel. Met. Res. Technol. 2019, 116, 223. [Google Scholar] [CrossRef] [Green Version]
- Dehua, L.; Qinghua, C. Cause Analysis of Large Particle Inclusions (Ds) in GCr15 Steel. Steelmaking 2007, 2, 21–24. [Google Scholar]
- Chen, R.; Chen, Z.; Xu, Y. In-situ observation of behaviors of inclusions in bearing steel. J. Iron Steel Res. 2015, 27, 48–53. [Google Scholar]
- Jiang, M.; Wang, X.-H.; Pak, J.-J.; Yuan, P. In Situ Observation on Behaviors of CaO-MgO-Al2O3-SiO2 Complex Inclusions at Solid–Liquid Interface of Low-Oxygen Special Steel. Met. Mater. Trans. B 2014, 45, 1656–1665. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, J.H. Effect of CaO/Al2O3 Ratio of Ladle Slag on Formation Behavior of Inclusions in Mn and V Alloyed Steel. ISIJ Int. 2018, 58, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wang, L.; Zhai, J.; Li, J.; Chou, K. Calcium Treatment for FeSi-killed Fe-13 Pct Cr Stainless Steel with Various Top Slag Compositions. Met. Mater. Trans. B 2018, 49, 325–333. [Google Scholar] [CrossRef]
- Escobar, J.D.; Faria, G.; Maia, E.; Oliveira, J.; Boll, T.; Seils, S.; Mei, P.; Ramirez, A. Fundamentals of isothermal austenite reversion in a Ti-stabilized 12cr-6 Ni-2 Mo super martensitic stainless steel: Thermodynamics versus experimental assessments. Acta Mater. 2019, 174, 246–259. [Google Scholar] [CrossRef]
- Martin, A.C.; Oliveira, J.P.; Fink, C. Elemental Effects on Weld Cracking Susceptibility in AlxCoCrCuyFeNi High-Entropy Alloy. Met. Mater. Trans. A 2020, 51, 778–787. [Google Scholar] [CrossRef]
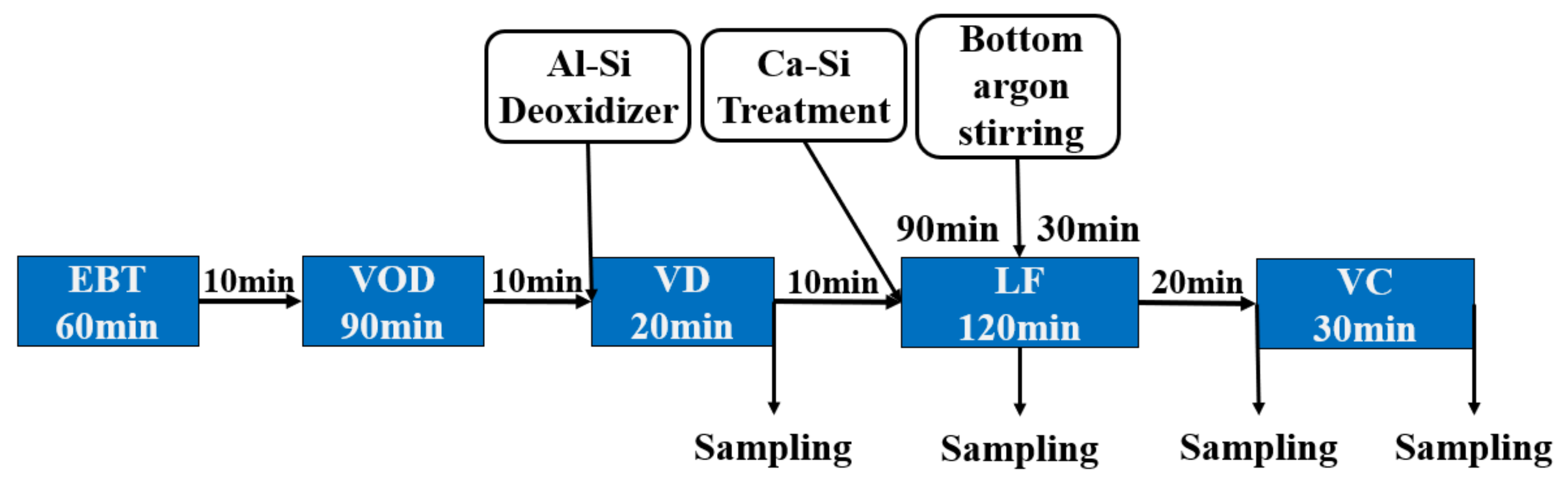

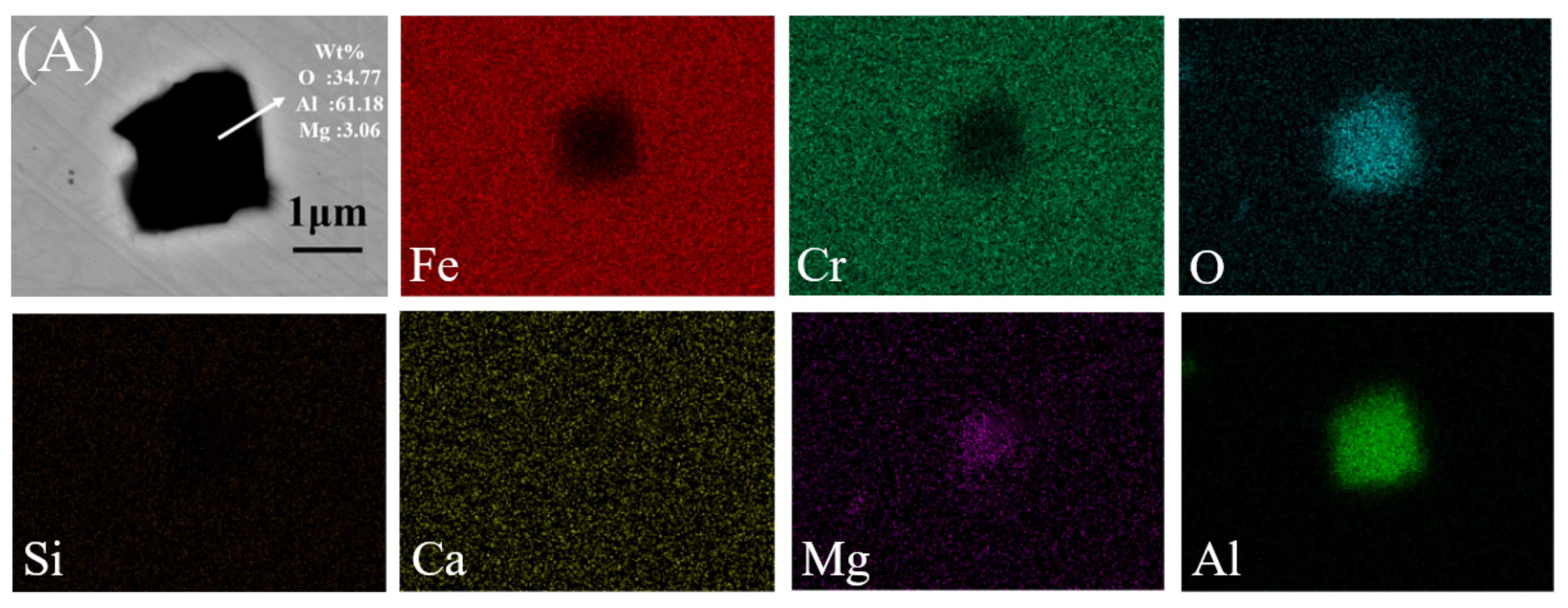


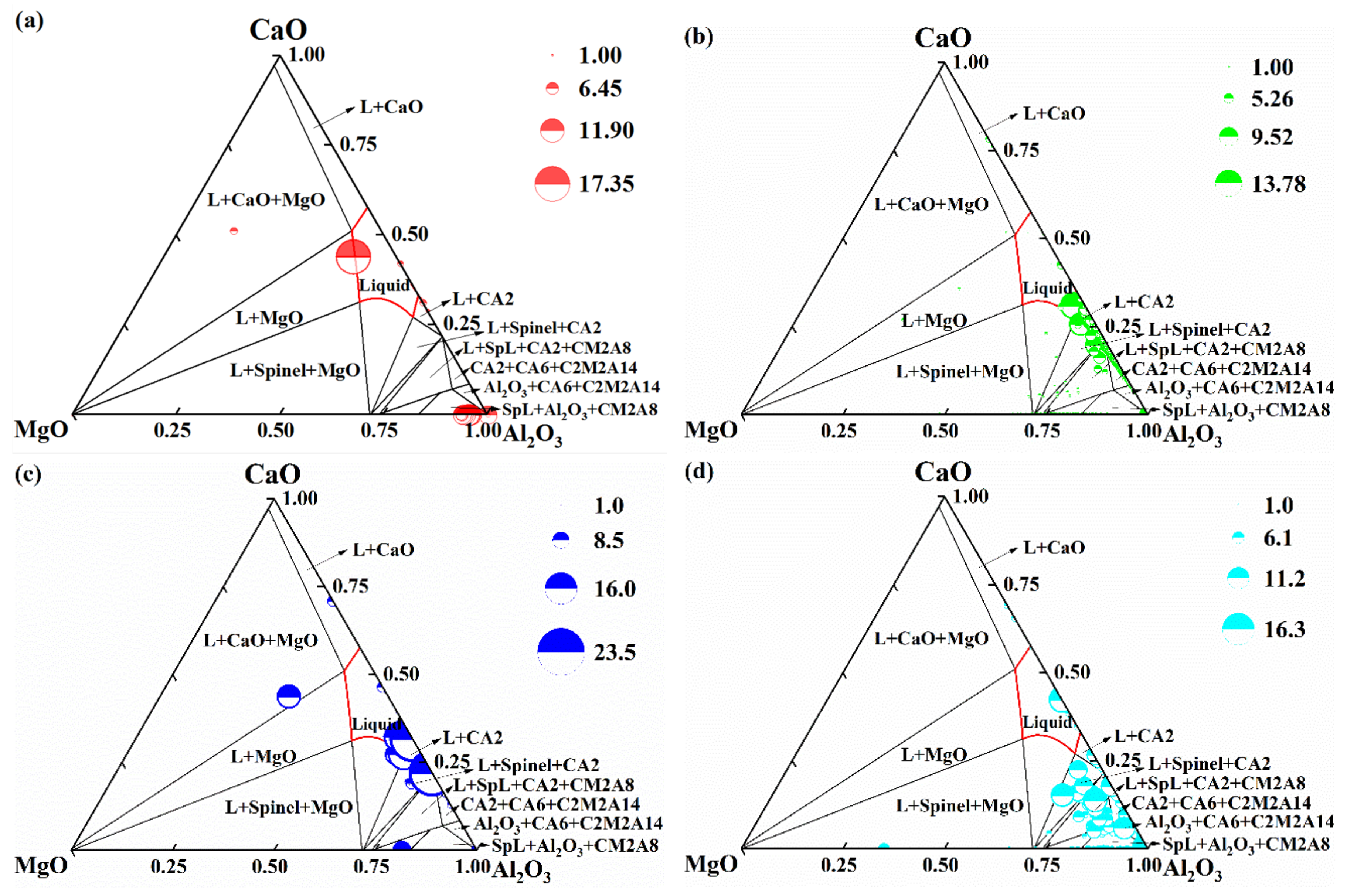

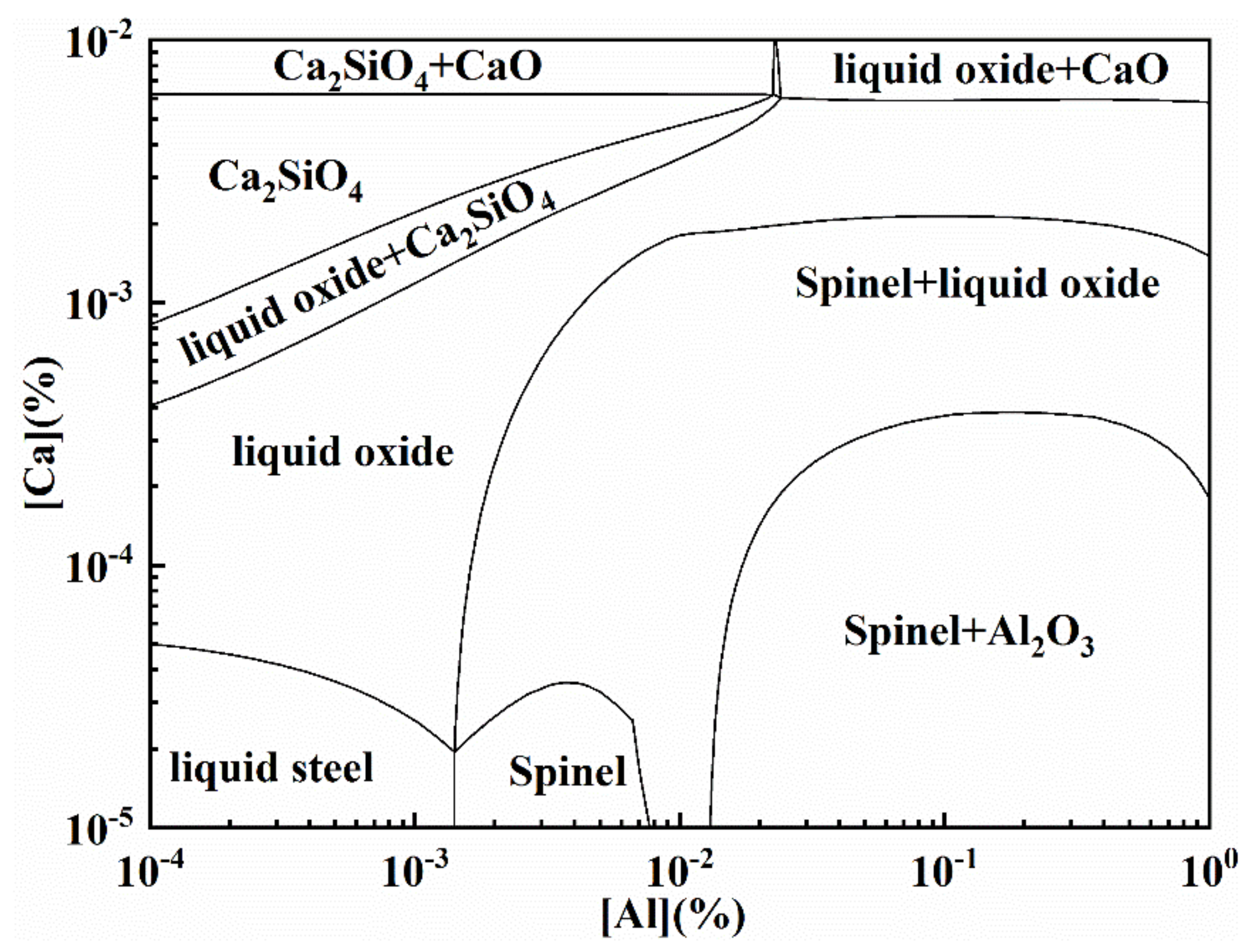

| Sample No | C | Si | Mn | S | Cr | Ni | Cu | Als | Ca ppm | Mg ppm | O ppm | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VD-end | 0.036 | 0.3 | 0.69 | 0.006 | 14.5 | 5.1 | 2.9 | 0.0056 | <5(4) | <5(2) | 122 | bal |
| LF-end | 0.03 | 0.56 | 0.61 | 0.007 | 14.5 | 5.1 | 2.9 | 0.01 | 19 | 5 | 54 | bal |
| VC-onset | 0.05 | 0.4 | 0.66 | 0.008 | 14.4 | 5.1 | 2.9 | 0.0062 | 11 | 5.4 | 40 | bal |
| Casting | 0.05 | 0.4 | 0.71 | 0.006 | 14.5 | 5.1 | 2.9 | 0.0054 | 7.6 | <5(4) | 29 | bal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, Z.; Zhang, W.; Zhang, Y.; Shi, R.; Cheng, G. Formation and Evolution of DS-Type Inclusions in 15-5PH Stainless Steel. Metals 2021, 11, 1129. https://doi.org/10.3390/met11071129
Zhan Z, Zhang W, Zhang Y, Shi R, Cheng G. Formation and Evolution of DS-Type Inclusions in 15-5PH Stainless Steel. Metals. 2021; 11(7):1129. https://doi.org/10.3390/met11071129
Chicago/Turabian StyleZhan, Zhonghua, Weifeng Zhang, Yanling Zhang, Ruxing Shi, and Guoguang Cheng. 2021. "Formation and Evolution of DS-Type Inclusions in 15-5PH Stainless Steel" Metals 11, no. 7: 1129. https://doi.org/10.3390/met11071129
APA StyleZhan, Z., Zhang, W., Zhang, Y., Shi, R., & Cheng, G. (2021). Formation and Evolution of DS-Type Inclusions in 15-5PH Stainless Steel. Metals, 11(7), 1129. https://doi.org/10.3390/met11071129







