Antimicrobial Activity and Degradation of Superhydrophobic Magnesium Substrates in Bacterial Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Analysis of Bactericidal Properties of the Superhydrophobic Magnesium Substrates
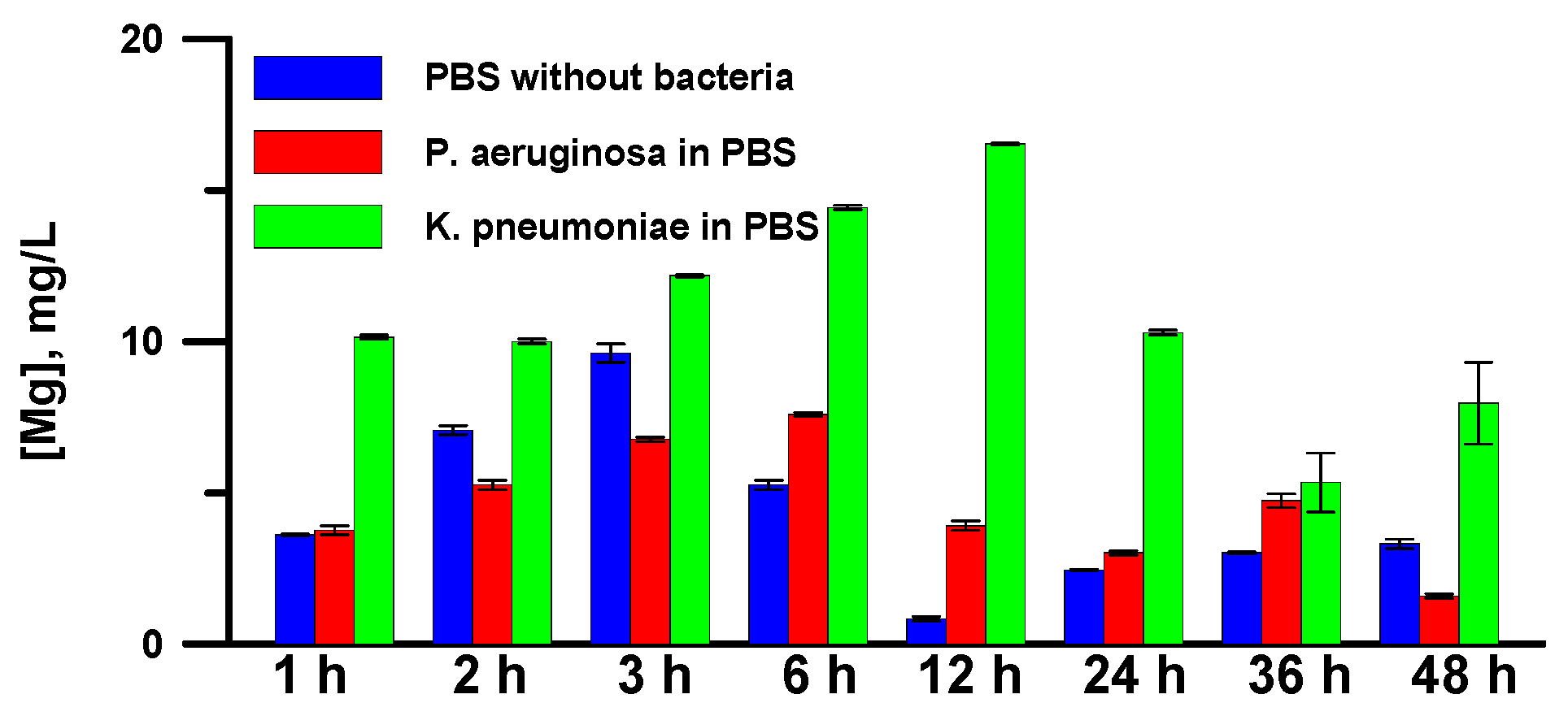
2.3. Determination of the Concentration of Mg2+ in a Dispersion Medium
2.4. Characterization of Surface Morphology, Chemical and Phase Composition
2.5. Characterization of Surface Wettability and pH of the Dispersion Medium
2.6. Characterization of Electrochemical Properties of Surfaces after Contact with Bacterial Suspensions
3. Results and Discussion
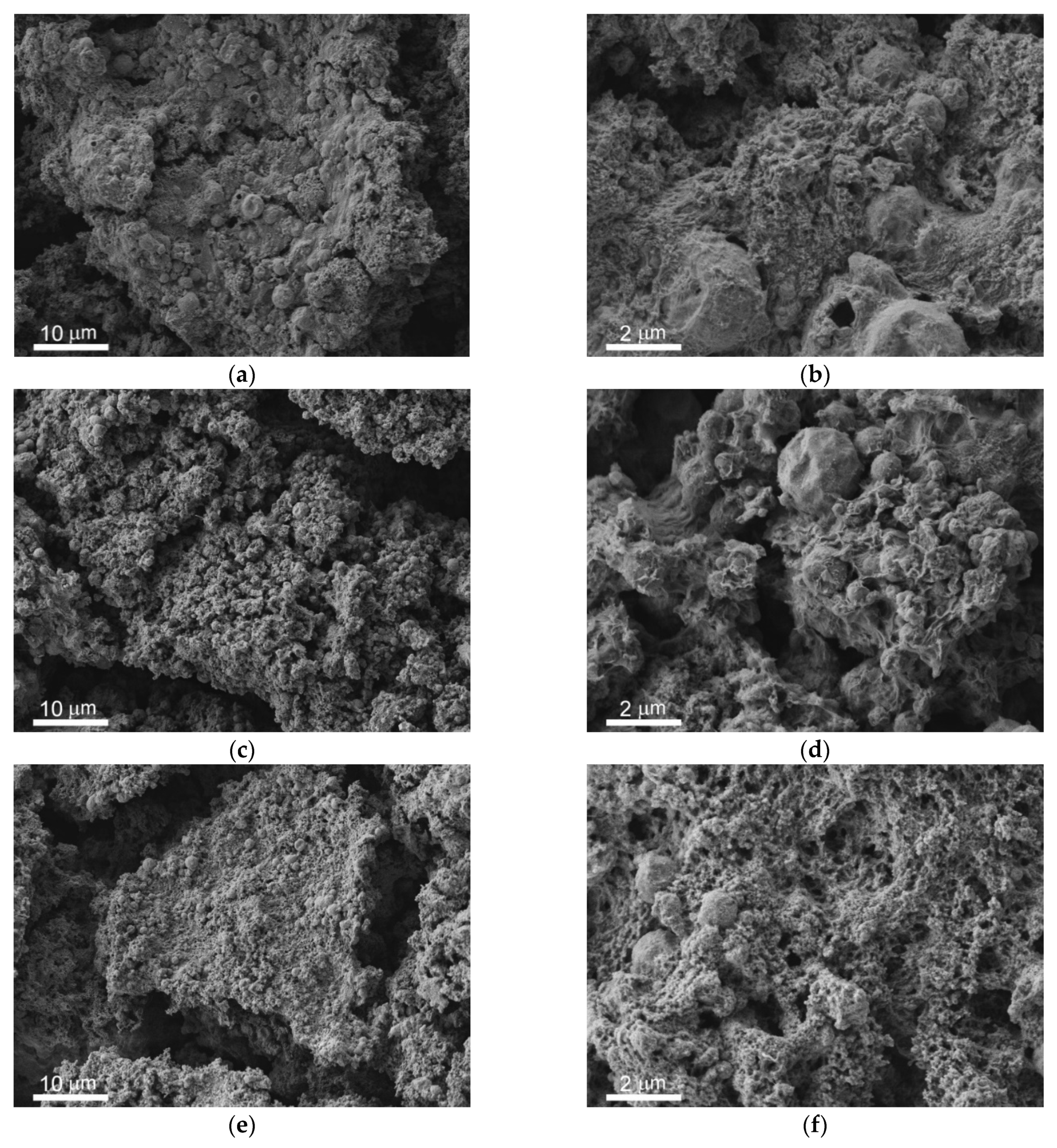
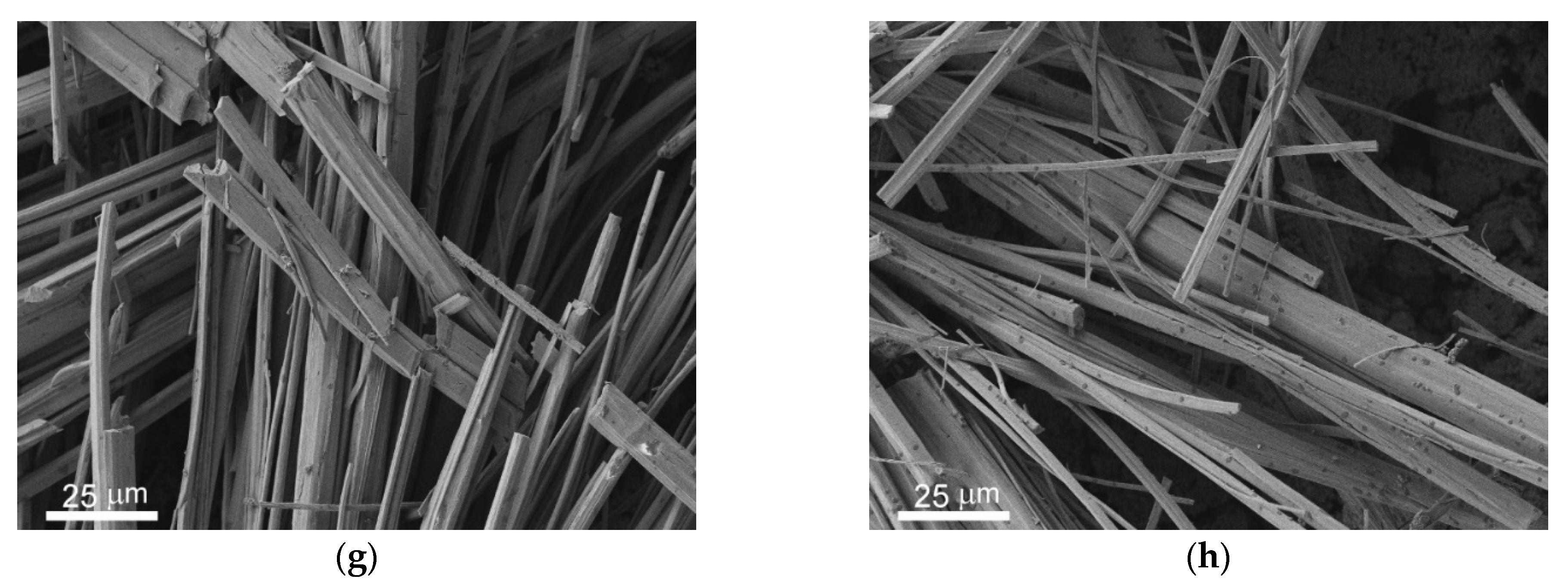
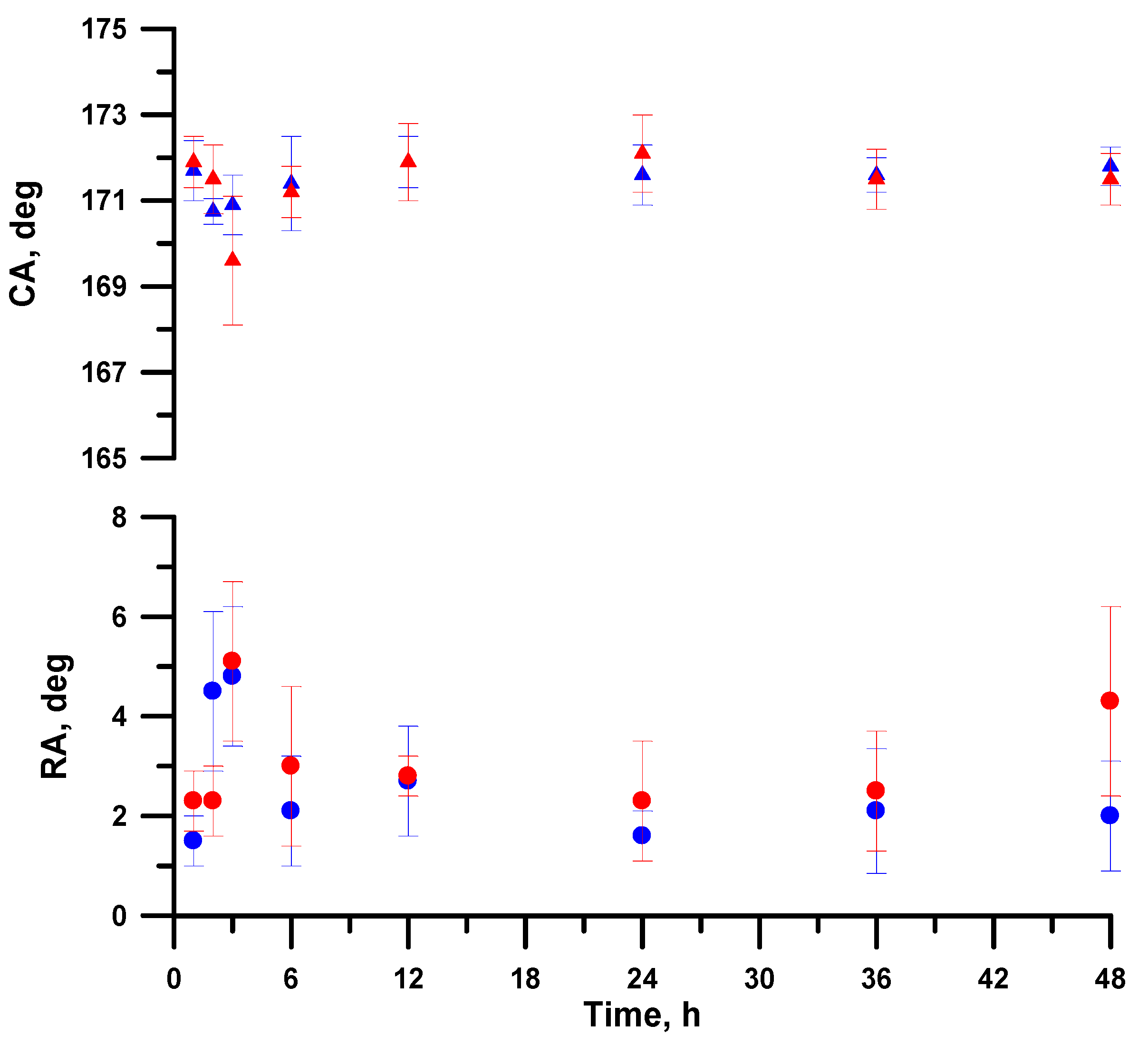
3.1. Morphology and Wettability of Superhydrophobic Coating
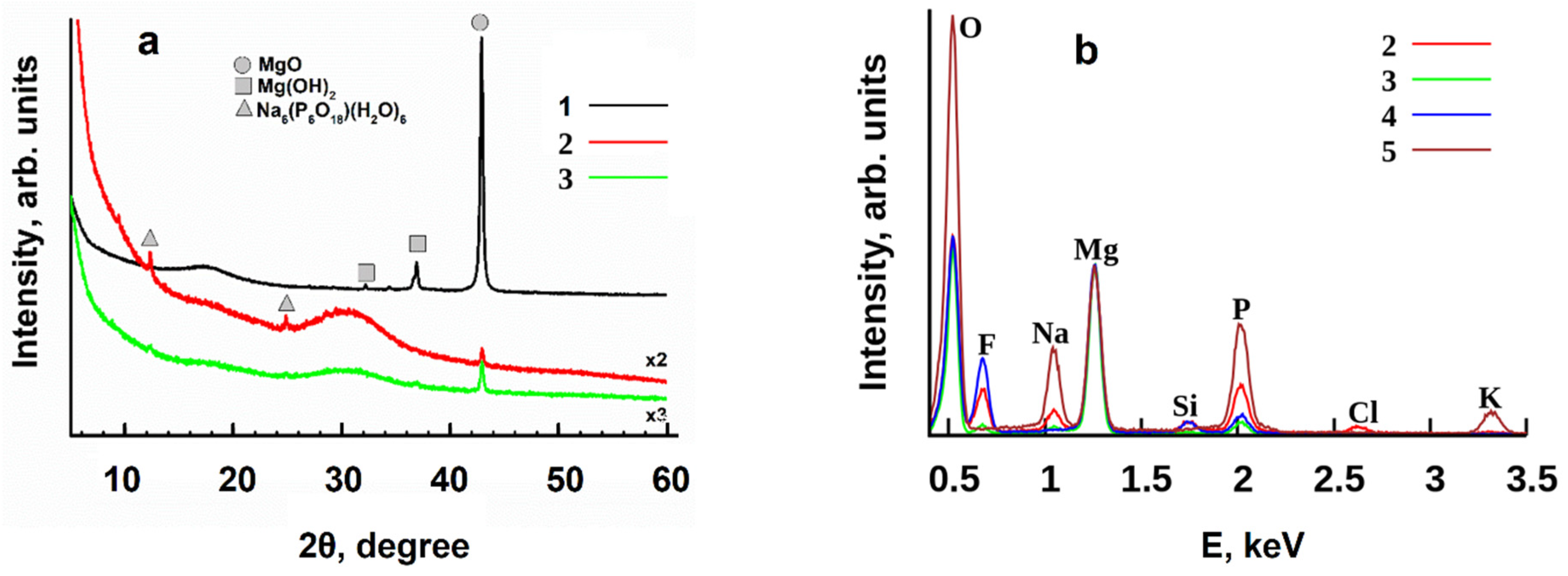
3.2. Variation in Surface Composition of a Superhydrophobic Coating
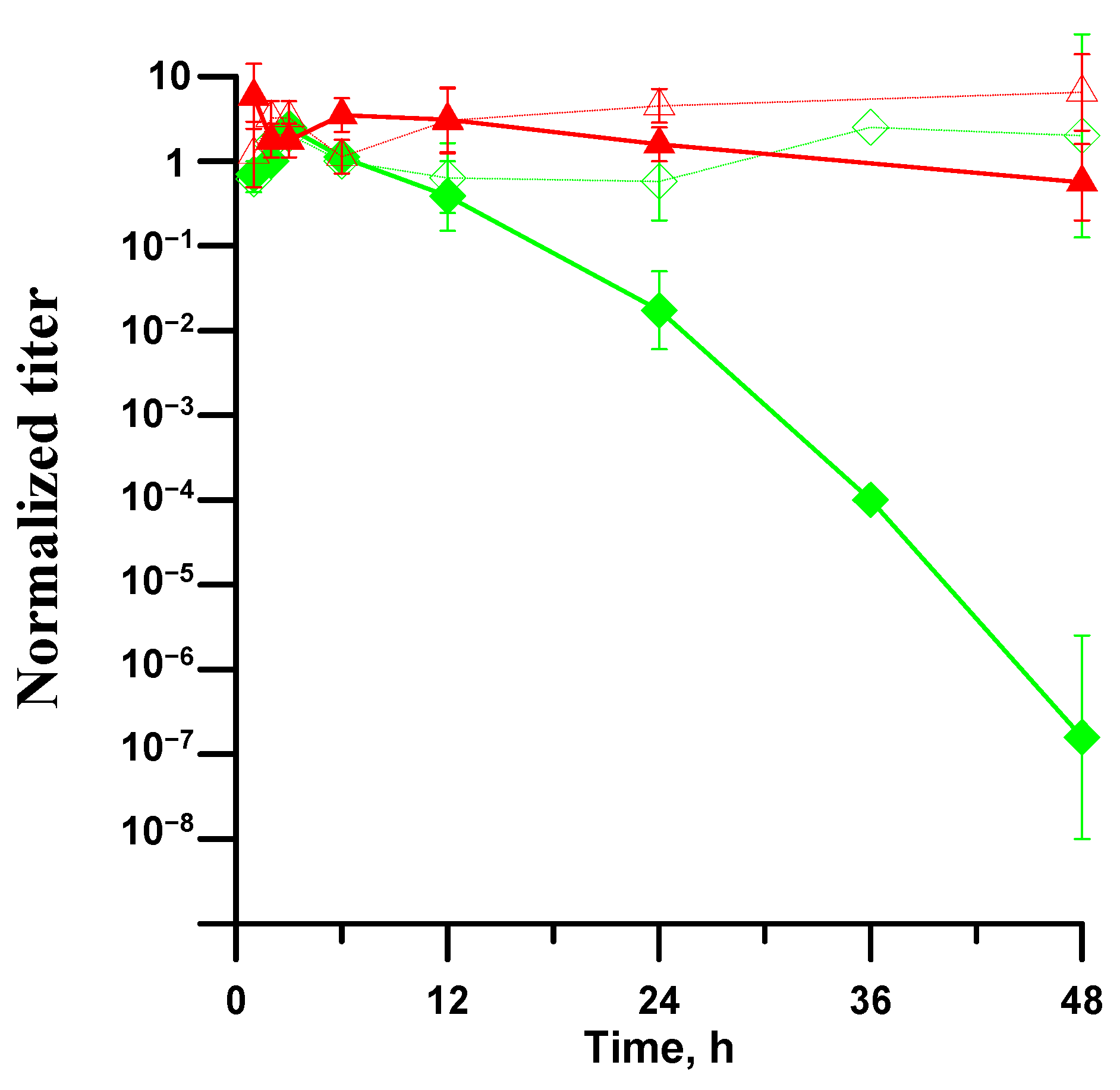
3.3. Antibacterial Activity of Superhydrophobic Coatings in Bacterial Dispersions
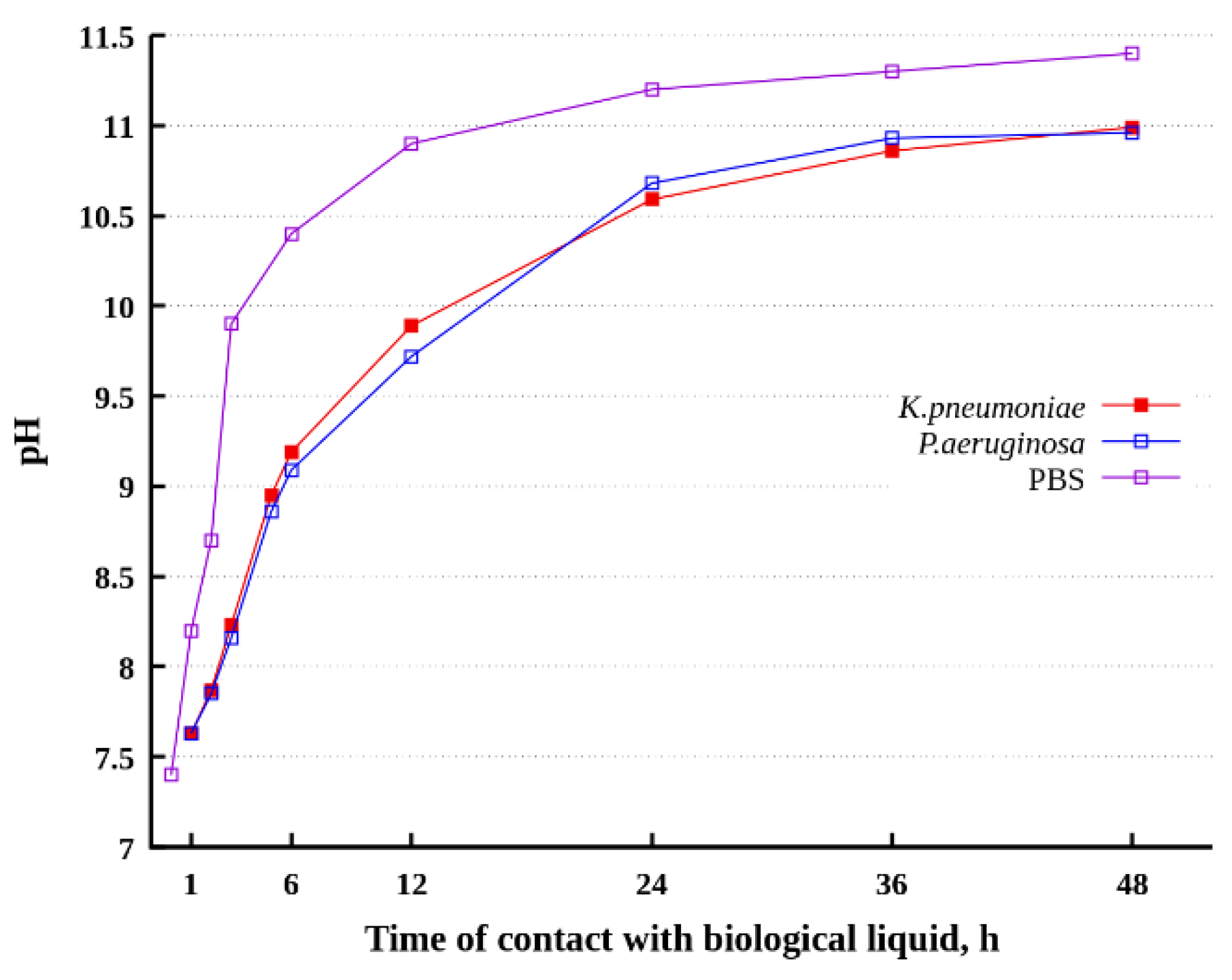
3.4. Variation of pH in Dispersion Medium during Its Contact with a Superhydrophobic Sample
3.5. Anticorrosion Behavior of Superhydrophobic Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maier, P.; Hort, N. Magnesium alloys for biomedical applications. Metals 2020, 10, 1328. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Fernández-Calderón, M.C.; Pacha-Olivenza, M.A.; Pérez-Giraldo, C.; Gallardo-Moreno, A.M.; González-Martín, M.L. The role of magnesium in biomaterials related infections. Colloids Surf. B Biointerfaces 2020, 191, 110996. [Google Scholar] [CrossRef]
- Rahim, M.I.; Eifler, R.; Rais, B.; Mueller, P.P. Alkalization is responsible for antibacterial effects of corroding magnesium. J. Biomed. Mater. Res. Part A 2015, 103, 3526–3532. [Google Scholar] [CrossRef]
- Feng, H.; Wang, G.; Jin, W.; Zhang, X.; Huang, Y.; Gao, A.; Wu, H.; Wu, G.; Chu, P.K. Systematic study of inherent antibacterial properties of magnesium-based biomaterials. ACS Appl. Mater. Interfaces 2016, 8, 9662–9673. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Oh, J.K.; Cho, D.H.; Akbulut, M.; Teizer, W. Bacterial inactivation characteristics of magnesium–calcium–zinc alloys for bone implants. MRS Commun. 2020, 10, 609–612. [Google Scholar] [CrossRef]
- Rosalbino, F.; De Negri, S.; Scavino, G.; Saccone, A. Microstructure and in vitro degradation performance of Mg–Zn–Mn alloys for biomedical application. J. Biomed. Mater. Res. Part A 2013, 101, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Li, S.; Liu, L.; Bin, S.; Yang, Y.; Peng, S.; Shuai, C. Dual alloying improves the corrosion resistance of biodegradable Mg alloys prepared by selective laser melting. J. Magnes. Alloys 2021, 9, 305–316. [Google Scholar] [CrossRef]
- Mueller, W.D.; Nascimento, M.L.; De Mele, M.F.L. Critical discussion of the results from different corrosion studies of Mg and Mg alloys for biomaterial applications. Acta Biomater. 2010, 6, 1749–1755. [Google Scholar] [CrossRef]
- Leones, A.; Lieblich, M.; Benavente, R.; Gonzalez, J.L.; Peponi, L. Potential applications of magnesium-based polymeric nanocomposites obtained by electrospinning technique. Nanomaterials 2020, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Cui, L.Y.; Zeng, R.C.; Li, S.Q.; Chen, X.B.; Zheng, Y.F.; Kannan, M.B. Advances in functionalized polymer coatings on biodegradable magnesium alloys—A review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Liu, Z.; Wang, Q. Advances in antibacterial functionalized coatings on Mg and its alloys for medical use—A review. Coatings 2020, 10, 828. [Google Scholar] [CrossRef]
- Ren, L.; Lin, X.; Tan, L.; Yang, K. Effect of surface coating on antibacterial behavior of magnesium based metals. Mater. Lett. 2011, 65, 3509–3511. [Google Scholar] [CrossRef]
- Gray-Munro, J.; Campbell, J. Mimicking the hierarchical surface topography and superhydrophobicity of the lotus leaf on magnesium alloy AZ31. Mater. Lett. 2017, 189, 271–274. [Google Scholar] [CrossRef]
- Ishizaki, T.; Kumagai, S.; Tsunakawa, M.; Furukawa, T.; Nakamura, K. Ultrafast fabrication of superhydrophobic surfaces on engineering light metals by single-step immersion process. Mater. Lett. 2017, 193, 42–45. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Pashinin, A.S.; Gnedenkov, S.V.; Egorkin, V.S.; Sinebryukhov, S.L. Mg alloy treatment for superhydrophobic anticorrosion coatings formation. Surf. Innov. 2013, 1, 162–172. [Google Scholar] [CrossRef]
- Yeganeh, M.; Mohammadi, N. Superhydrophobic surface of Mg alloys: A review. J. Magnes. Alloys 2018, 6, 59–70. [Google Scholar] [CrossRef]
- Emelyanenko, K.A.; Domantovsky, A.G.; Chulkova, E.V.; Emelyanenko, A.M.; Boinovich, L.B. Thermally induced gradient of properties on a superhydrophobic magnesium alloy surface. Metals 2021, 11, 41. [Google Scholar] [CrossRef]
- Chobaomsup, M.; Metzner, M.; Boonyongmaneerat, Y. Superhydrophobic surface modification for corrosion protection of metals and alloys. J. Coat. Technol. Res. 2020, 17, 583–595. [Google Scholar] [CrossRef]
- Yao, W.; Wu, L.; Huang, G.; Jiang, B.; Atrens, A.; Pan, F. Superhydrophobic coatings for corrosion protection of magnesium alloys. J. Mater. Sci. Technol. 2020, 52, 100–118. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, J.; Li, B.; Li, S.; Tian, N.; Jing, L.; Zhang, J. A self-healing superamphiphobic coating for efficient corrosion protection of magnesium alloy. J. Colloid Interface Sci. 2020, 575, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.B.; Emelyanenko, A.M. The behaviour of fluoro- and hydrocarbon surfactants used for fabrication of superhydrophobic coatings at solid/water interface. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 167–175. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Pytskii, I.S.; Kaminsky, V.V.; Chulkova, E.V.; Domantovsky, A.G.; Emelyanenko, K.A.; Sobolev, V.D.; Aleshkin, A.V.; Boinovich, L.B. Superhydrophobic copper in biological liquids: Antibacterial activity and microbiologically induced or inhibited corrosion. Colloids Surf. B Biointerfaces 2020, 185, 110622. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.R.; Butina, K.; Herting, G.; Rajarao, G.K.; Richter-Dahlfors, A.; Blomberg, E.; Wallinder, I.O.; Leygraf, C. The interplay between atmospheric corrosion and antimicrobial efficiency of Cu and Cu5Zn5Al1Sn during simulated high-touch conditions. Corros. Sci. 2021, 185, 109433. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M. Analysis of wetting as an efficient method for studying the characteristics of coatings and surfaces and the processes that occur on them: A review. Inorg. Mater. 2011, 47, 1667–1675. [Google Scholar] [CrossRef]
- Heimann, R.B. Magnesium alloys for biomedical application: Advanced corrosion control through surface coating. Surf. Coat. Technol. 2021, 405, 126521. [Google Scholar] [CrossRef]
- Little, B.; Wagner, P.; Mansfeld, F. Microbiologically influenced corrosion of metals and alloys. Int. Mater. Rev. 1991, 36, 253–272. [Google Scholar] [CrossRef]
- Duncan, A.B.F.; Gordy, W.; Jones, R.N.; Matsen, F.A.; Sandorfy, C.; West, W. Technique of Organic Chemistry. Volume IX: Chemical applications of spectroscopy; West, W., Ed.; Interscience Publishers: New York, NY, USA, 1956. [Google Scholar]
- Lima, A.C.; Mano, J.F. Micro/nano-structured superhydrophobic surfaces in the biomedical field: Part I: Basic concepts and biomimetic approaches. Nanomedicine 2015, 10, 103–119. [Google Scholar] [CrossRef] [Green Version]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef] [Green Version]
- Boinovich, L.B.; Modin, E.B.; Aleshkin, A.V.; Emelyanenko, K.A.; Zulkarneev, E.R.; Kiseleva, I.A.; Vasiliev, A.L.; Emelyanenko, A.M. Antibacterial effect of textured surfaces induced by extreme wettability and bacteriophage seeding. ACS Appl. Nanomater. 2018, 1, 1348–1359. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef]
- Muller, D.W.; Losslein, S.; Terriac, E.; Moeller, R.; Kautenburger, R.; Mucklich, F. Increasing antibacterial efficiency of Cu surfaces by targeted surface functionalization via ultrashort pulsed direct laser interference patterning. Adv. Mater. Interfaces 2021, 8, 2001656. [Google Scholar] [CrossRef]
- Lin, Z.; Sun, X.; Yang, H. The role of antibacterial metallic elements in simultaneously improving the corrosion resistance and antibacterial activity of magnesium alloys. Mater. Des. 2021, 198, 109350. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2021, 5 (Suppl. S1), i3–i14. [Google Scholar] [CrossRef] [Green Version]
- Emelyanenko, A.M.; Emelyanenko, K.A.; Boinovich, L.B. Deep undercooling of aqueous droplets on a superhydrophobic surface: The specific role of cation hydration. J. Phys. Chem. Lett. 2020, 11, 3058–3062. [Google Scholar] [CrossRef]
- Little, B.; Ray, R. A perspective on corrosion inhibition by biofilms. Corrosion 2002, 58, 424–428. [Google Scholar] [CrossRef]
- Zarasvand, K.A.; Rai, V.R. Microorganisms: Induction and inhibition of corrosion in metals. Int. Biodeterior. Biodegrad. 2014, 87, 66–74. [Google Scholar] [CrossRef]
- Ishizaki, T.; Shigematsu, I.; Saito, N. Surface & coatings technology anticorrosive magnesium phosphate coating on AZ31 magnesium alloy. Surf. Coat. Technol. 2009, 203, 2288–2291. [Google Scholar] [CrossRef]
- Van Phuong, N.; Gupta, M.; Moon, S. Enhanced corrosion performance of magnesium phosphate conversion coating on AZ31 magnesium alloy. Trans. Nonferrous Met. Soc. China 2017, 27, 1087–1095. [Google Scholar] [CrossRef]
- El-Taib Heakal, F.; Shehata, O.S.; Tantawy, N.S. Degradation behaviour of AZ80E magnesium alloy exposed to phosphate buffer saline medium. Corros. Sci. 2014, 86, 285–294. [Google Scholar] [CrossRef]
- Cabeza, S.; Zubiaur, P.P.; Garcés, G.; Andrade, C.; Adeva, P. Corrosion behaviour of Mg98.5Nd1Zn0.5 (at. %) alloy in phosphate buffered saline solution. Metals 2020, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Li, D.; Zheng, T.L. Research on water based coating for magnesium alloy. Adv. Mater. Res. 2006, 15–17, 485–490. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emelyanenko, A.M.; Kaminsky, V.V.; Pytskii, I.S.; Emelyanenko, K.A.; Domantovsky, A.G.; Chulkova, E.V.; Shiryaev, A.A.; Aleshkin, A.V.; Boinovich, L.B. Antimicrobial Activity and Degradation of Superhydrophobic Magnesium Substrates in Bacterial Media. Metals 2021, 11, 1100. https://doi.org/10.3390/met11071100
Emelyanenko AM, Kaminsky VV, Pytskii IS, Emelyanenko KA, Domantovsky AG, Chulkova EV, Shiryaev AA, Aleshkin AV, Boinovich LB. Antimicrobial Activity and Degradation of Superhydrophobic Magnesium Substrates in Bacterial Media. Metals. 2021; 11(7):1100. https://doi.org/10.3390/met11071100
Chicago/Turabian StyleEmelyanenko, Alexandre M., Valery V. Kaminsky, Ivan S. Pytskii, Kirill A. Emelyanenko, Alexander G. Domantovsky, Elizaveta V. Chulkova, Andrei A. Shiryaev, Andrei V. Aleshkin, and Ludmila B. Boinovich. 2021. "Antimicrobial Activity and Degradation of Superhydrophobic Magnesium Substrates in Bacterial Media" Metals 11, no. 7: 1100. https://doi.org/10.3390/met11071100
APA StyleEmelyanenko, A. M., Kaminsky, V. V., Pytskii, I. S., Emelyanenko, K. A., Domantovsky, A. G., Chulkova, E. V., Shiryaev, A. A., Aleshkin, A. V., & Boinovich, L. B. (2021). Antimicrobial Activity and Degradation of Superhydrophobic Magnesium Substrates in Bacterial Media. Metals, 11(7), 1100. https://doi.org/10.3390/met11071100









