Surface Properties and Biocompatibility of Anodized Titanium with a Potential Pretreatment for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Analysis of Surface Property
2.3. Wettability Testing
2.4. Biocompatibility Evaluation
2.5. Statistical Analysis
3. Results
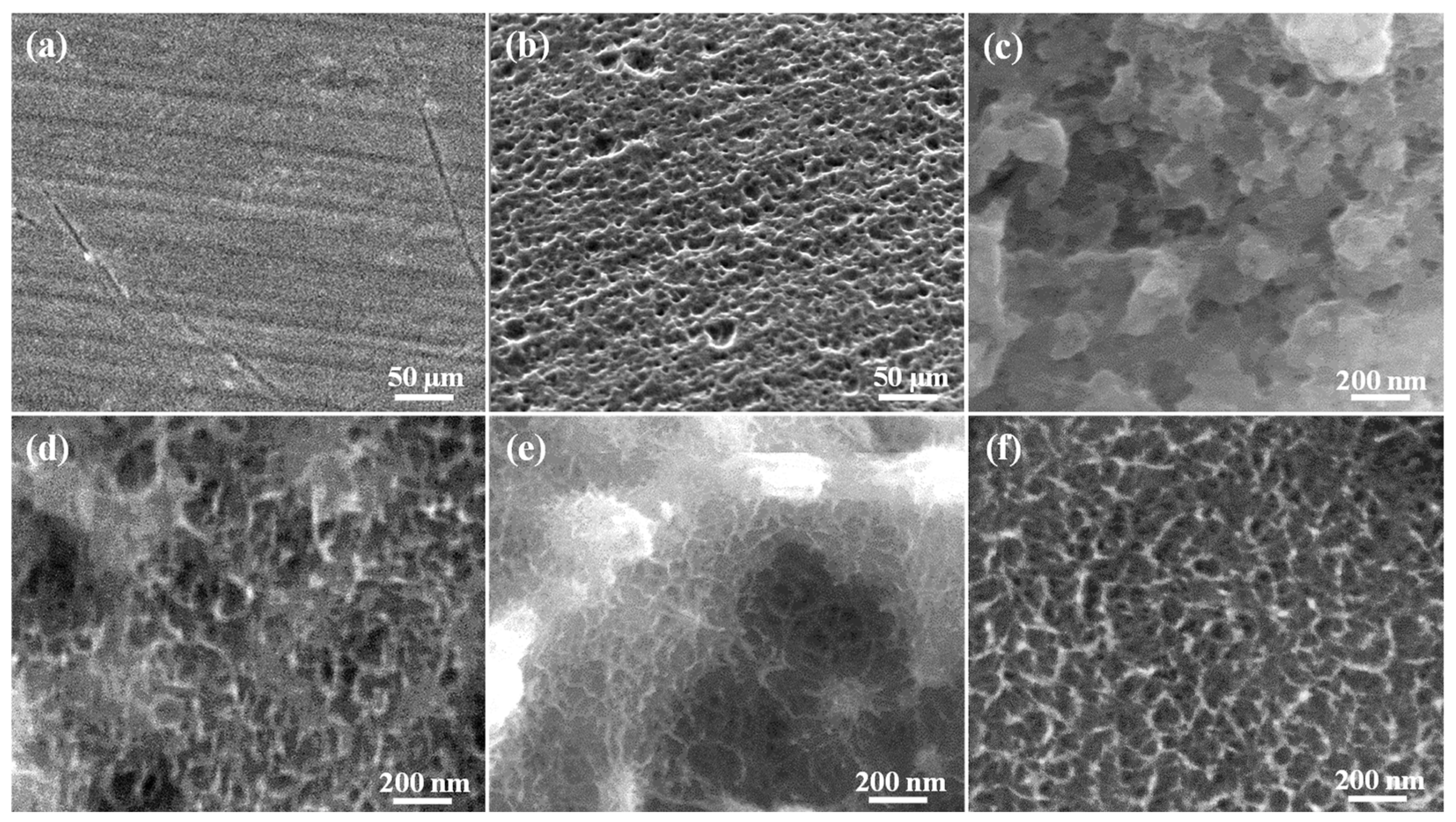
3.1. Morphology of the Investigated Samples
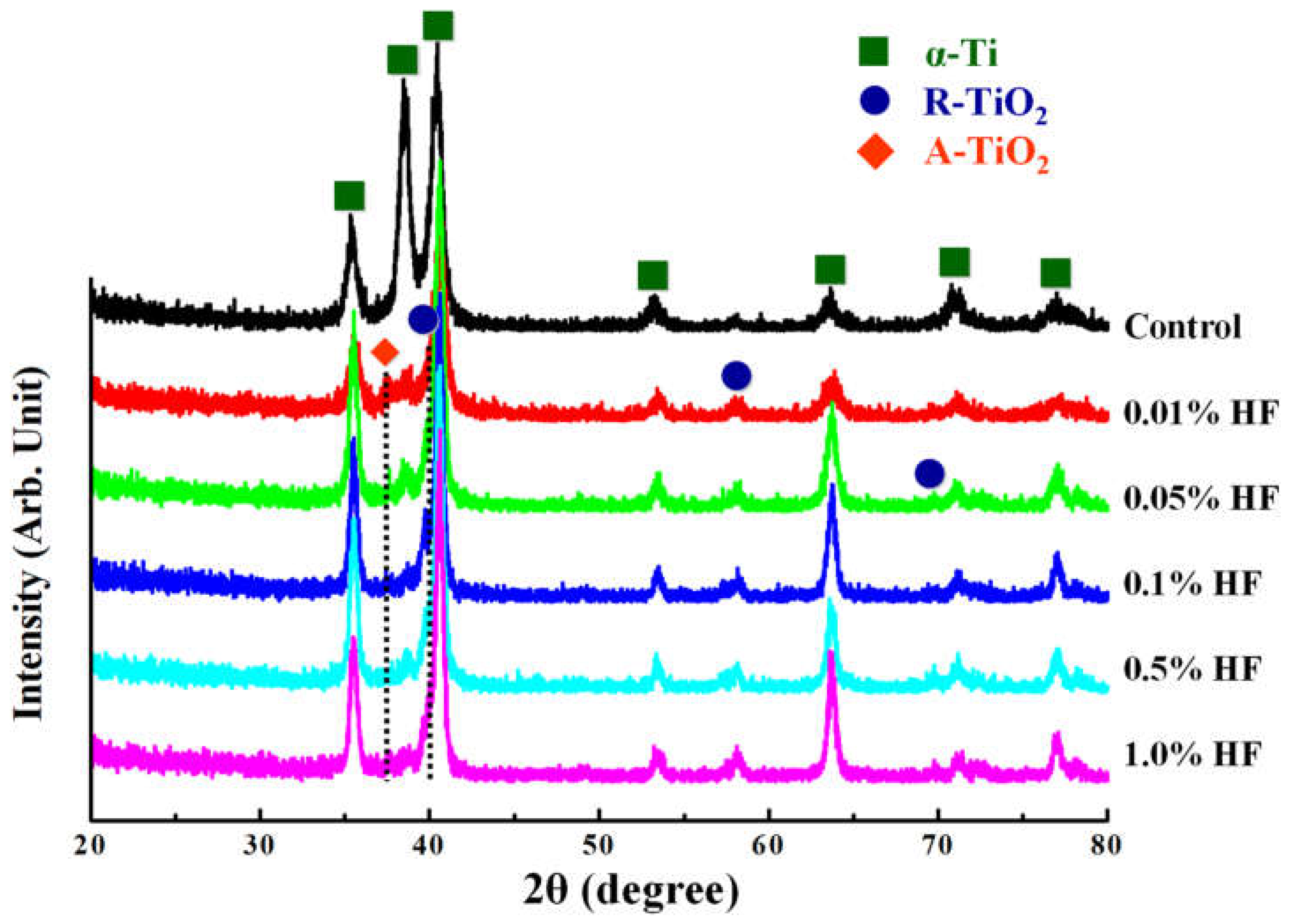
3.2. Microstructure of the Investigated Samples
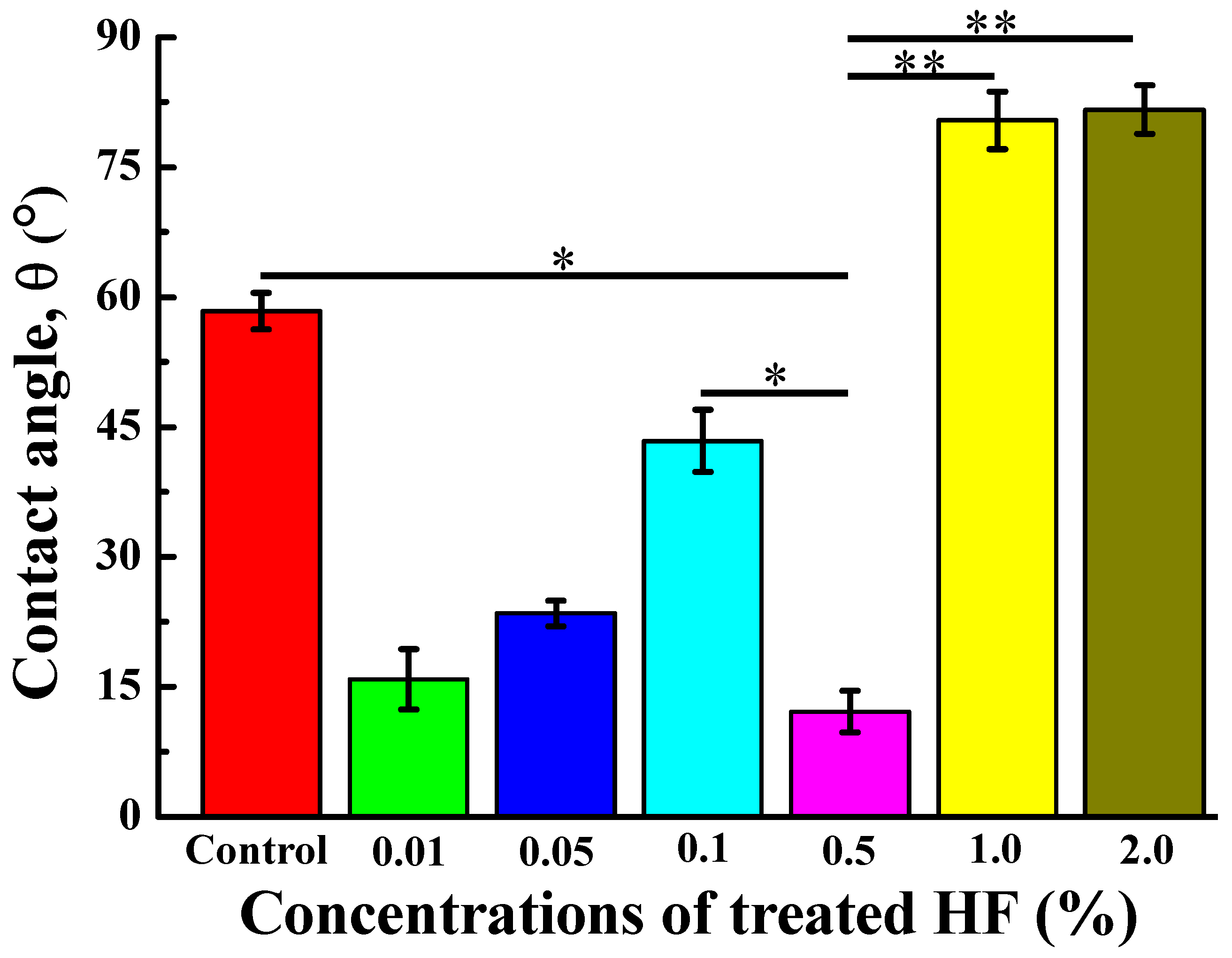
3.3. Wettability of the Investigated Samples
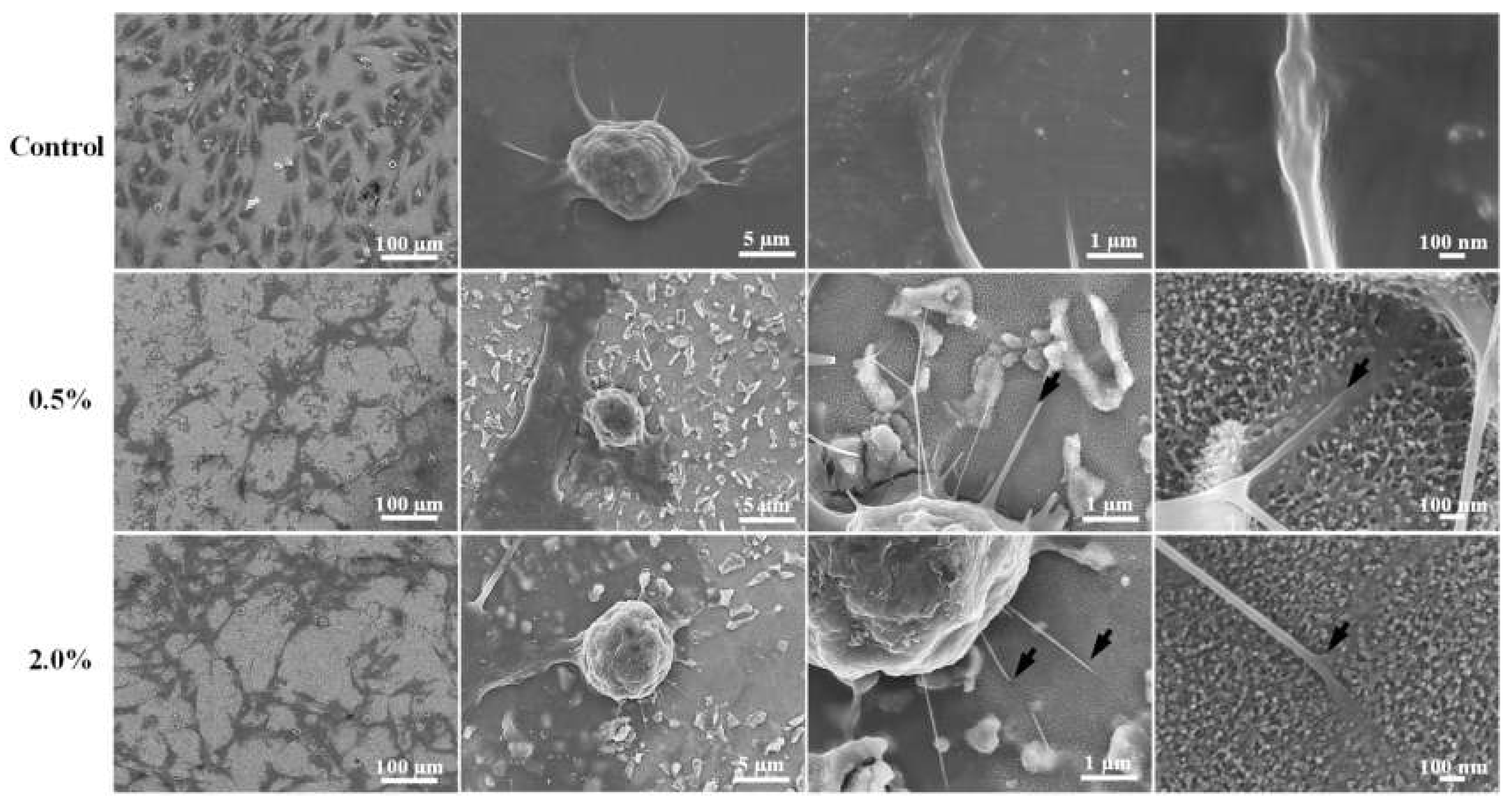
3.4. Cell Response and Adhesion Behavior of the Investigated Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.; Lee, B.A.; Piao, X.H.; Chung, H.J.; Kim, Y.J. Surface characteristics and bioactivity of an anodized titanium surface. J. Periodontal Implant Sci. 2013, 43, 198–205. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Jurczyk, K.; Koper, J.K.; Paszel-Jaworska, A.; Romaniuk, A.; Lipińska, N.; Żurawski, J.; Urbaniak, P.; Jakubowicz, J.; Jurczyk, M.U. In vitro biocompatibility of anodized titanium with deposited silver nanodendrites. J. Mater. Sci. 2016, 51, 5259–5270. [Google Scholar] [CrossRef]
- Barjaktarević, D.R.; Cvijović-Alagić, I.L.; Dimić, I.D.; Đokić, V.R.; Rakin, M.P. Anodization of Ti-based materials for biomedical applications: A review. Metall. Mater. Eng. 2016, 22, 129–144. [Google Scholar] [CrossRef]
- Singh, A.; Singh, B.P.; Wani, M.R.; Kumar, D.; Singh, J.K.; Singh, V. Effect of anodization on corrosion behaviour and biocompatibility of Cp-titanium is simulated body fluid. Bull. Mater. Sci. 2013, 36, 8. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.X.; Takao, Y.; Wang, W.X.; Matsubara, T.; Ren, L.M. Surface characteristics and in vitro biocompatibility of titanium anodized in a phosphoric acid solution at different voltages. Biomed. Mater. 2009, 4, 065003. [Google Scholar] [CrossRef]
- Martinez, E.F.; Ishikawa, G.J.; de Lemos, A.B.; Barbosa Bezerra, F.J.; Sperandio, M.; Napimoga, M.H. Evaluation of a Titanium Surface Treated with Hydroxyapatite Nanocrystals on Osteoblastic Cell Behavior: An In Vitro Study. Int. J. Oral Maxillofac. Implant. 2018, 33, 597–602. [Google Scholar] [CrossRef]
- SergiyKyrylenko, S.; Warchoł, F.; Oleshko, O.; Husak, Y.; Kazek-Kęsik, A.; Korniienko, V.; Deineka, V.; Sowa, M.; Maciej, A.; Michalska, J.; et al. Effects of the sources of calcium and phosphorus on the structural and functional properties of ceramic coatings on titanium dental implants produced by plasma electrolytic oxidation. Mater. Sci. Eng. C 2020, 119, 111607. [Google Scholar]
- Myakinin, A.; Turlybekuly, A.; Pogrebnjak, A.; Mirek, A.; Bechelany, M.; Liubchak, I.; Oleshko, O.; Husak, Y.; Korniienko, V.; Leśniak-Ziółkowska, K.; et al. In vitro evaluation of electrochemically bioactivated Ti6Al4V 3D porous scaffolds. Mater. Sci. Eng. C 2021, 121, 111870. [Google Scholar] [CrossRef]
- Yanovska, A.; Husak, Y.; Mishchenko, O.; Gudakov, A.; Oleshko, O.; Yusupova, A.; Vielikov, M.; Radwan-Pragłowska, J.; Piątkowski, M.; Janus, Ł.; et al. Cell viability and collagen deposition on hydroxyapatite coatings formed on pretreated substrates. Mater. Chem. Phys. 2021, 258, 123978. [Google Scholar] [CrossRef]
- Lan, W.-C.; Wang, C.-H.; Huang, B.-H.; Cho, Y.-C.; Saito, T.; Huang, C.-C.; Huang, M.-S. Fabrication of a Promising Hierarchical Porous Surface on Titanium for Promoting Biocompatibility. Appl. Sci. 2020, 10, 1363. [Google Scholar] [CrossRef]
- Baxter, L.C.; Frauchiger, V.; Textor, M.; ap Gwynn, I.; Richards, R.G. Fibroblast and osteoblast adhesion and morphology on calcium phosphate surfaces. Eur. Cell. Mater. 2002, 4, 18. [Google Scholar] [CrossRef]
- Carrado, A.; Perrin-Schmitt, F.; Le, Q.V.; Giraudel, M.; Fischer, C.; Koenig, G.; Jacomine, L.; Behr, L.; Chalom, A.; Fiette, L.; et al. Nanoporous hydroxyapatite/sodium titanate bilayer on titanium implants for improved osteointegration. Dent. Mater. 2017, 33, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Shayganpour, A.; Rebaudi, A.; Cortella, P.; Diaspro, A.; Salerno, M. Electrochemical coating of dental implants with anodic porous titania for enhanced osteointegration. Beilstein J. Nanotechnol. 2015, 6, 2183–2192. [Google Scholar] [CrossRef]
- Aboushelib, M.N.; Salem, N.A.; Taleb, A.L.; El Moniem, N.M. Influence of surface nano-roughness on osseointegration of zirconia implants in rabbit femur heads using selective infiltration etching technique. J. Oral Implantol. 2013, 39, 583–590. [Google Scholar] [CrossRef]
- El-Gammal, M.N.; El-Gammal, N.Y.; Fadhil, O.N.; Maria, O.M. Biological reactions to different dental implant surface treatments. Int. J. Contemp. Dent. Med. Rev. 2016, 2015, 9. [Google Scholar] [CrossRef]
- Tomsia, A.P.; Launey, M.E.; Lee, J.S.; Mankani, M.H.; Wegst, U.G.K.; Saiz, E. Nanotechnology approaches for better dental implant. Int. J. Oral Maxillofac. Implant. 2011, 26, 25. [Google Scholar]
- Zhang, W.; Cao, H.; Zhang, X.; Li, G.; Chang, Q.; Zhao, J.; Qiao, Y.; Ding, X.; Yang, G.; Liu, X.; et al. A strontium-incorporated nanoporous titanium implant surface for rapid osseointegration. Nanoscale 2016, 8, 5291–5301. [Google Scholar] [CrossRef]
- Ou, K.-L.; Lin, C.-T.; Chen, S.-L.; Huang, C.-F.; Cheng, H.-C.; Yeh, Y.-M.; Lin, K.-H. Effect of Multi-nano-titania Film on Proliferation and Differentiation of Mouse Fibroblast Cell on Titanium. J. Electrochem. Soc. 2008, 155. [Google Scholar] [CrossRef]
- Cheng, H.-C.; Lee, S.-Y.; Chen, C.-C.; Shyng, Y.-C.; Ou, K.-L. Influence of Hydrogen Charging on the Formation of Nanostructural Titania by Anodizing with Cathodic Pretreatment. J. Electrochem. Soc. 2007, 154. [Google Scholar] [CrossRef]
- Shih, Y.-H.; Lin, C.-T.; Liu, C.-M.; Chen, C.-C.; Chen, C.-S.; Ou, K.-L. Effect of nano-titanium hydride on formation of multi-nanoporous TiO 2 film on Ti. Appl. Surf. Sci. 2007, 253, 3678–3682. [Google Scholar] [CrossRef]
- Ross, A.P.; Webster, T.J. Anodizing color coded anodized Ti6Al4V medical devices for increasing bone cell functions. Int. J. Nanomedicine 2013, 8, 109–117. [Google Scholar] [CrossRef][Green Version]
- Marenzi, G.; Spagnuolo, G.; Sammartino, J.C.; Gasparro, R.; Rebaudi, A.; Salerno, M. Micro-Scale Surface Patterning of Titanium Dental Implants by Anodization in the Presence of Modifying Salts. Materials 2019, 12, 1753. [Google Scholar] [CrossRef]
- Bezait, Z.; Tatar, D.; Ertugrul, M. Effect of oxygen addition on the formation of anatase TiO2 nano-coatings obtained by spray pyrolysis technique. J. Appl. Res. Technol. 2018, 16, 9. [Google Scholar]
- Chiang, H.-J.; Chou, H.-H.; Ou, K.-L.; Sugiatno, E.; Ruslin, M.; Waris, R.A.; Huang, C.-F.; Liu, C.-M.; Peng, P.-W. Evaluation of Surface Characteristics and Hemocompatibility on the Oxygen Plasma-Modified Biomedical Titanium. Metals 2018, 8, 513. [Google Scholar] [CrossRef]
- Kota, A.K.; Kwon, G.; Tuteja, A. The design and applications of superomniphobic surfaces. NPG Asia Mater. 2014, 6, e109. [Google Scholar] [CrossRef]
- Barbosa, T.P.; Naves, M.M.; Menezes, H.H.M.; Pinto, P.H.C.; De Mello, J.D.B.; Costa, H.L. Topography and surface energy of dental implants: A methodological approach. J. Braz. Soc. Mech. Sci. Eng. 2017, 39, 1895–1907. [Google Scholar] [CrossRef]
- Liu, X.M.; Wu, S.L.; Chu, P.K.; Chung, C.Y.; Chu, C.L.; Chan, Y.L.; Lam, K.O.; Yeung, K.W.; Lu, W.W.; Cheung, K.M.; et al. Nano-scale surface morphology, wettability and osteoblast adhesion on nitrogen plasma-implanted NiTi shape memory alloy. J. Nanosci. Nanotechnol. 2009, 9, 3449–3454. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 5. [Google Scholar] [CrossRef]
- Wu, F.; Xu, R.; Yu, X.; Yang, J.; Liu, Y.; Ouyang, J.; Zhang, C.; Deng, F. Enhanced Biocompatibility and Antibacterial Activity of Selective Laser Melting Titanium with Zinc-Doped Micro-Nano Topography. J. Nanomater. 2019, 2019, 5432040. [Google Scholar] [CrossRef]
- Syam, S.; Wu, C.-J.; Lan, W.-C.; Ou, K.-L.; Huang, B.-H.; Lin, Y.-Y.; Saito, T.; Tsai, H.-Y.; Chuo, Y.-C.; Yen, M.-L.; et al. The Potential of a Surface-Modified Titanium Implant with Tetrapeptide for Osseointegration Enhancement. Appl. Sci. 2021, 11, 2616. [Google Scholar] [CrossRef]
- Li, K.Q.; Jia, S.S.; Ma, M.; Shen, H.Z.; Xu, L.; Liu, G.P.; Huang, S.Y.; Zhang, D.S. Effects of fluoride on proliferation and mineralization in periodontal ligament cells in vitro. Braz. J. Med. Biol. Res. 2016, 49. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Y.; Zeng, B.; Zhang, H. Effects of sodium fluoride treatment in vitro on cell proliferation, BMP-2 and BMP-3 expression in human osteosarcoma MG-63 cells. Biol. Trace Elem. Res. 2014, 162, 18–25. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, J.Y.; Jeong, Y.J.; Park, J.H.; Yang, K.H.; Choi, N.K.; Kim, S.H.; Kim, W.J. Involvement of both mitochondrial- and death receptor-dependent apoptotic pathways regulated by Bcl-2 family in sodium fluoride-induced apoptosis of the human gingival fibroblasts. Toxicology 2008, 243, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.H.; Haugen, H.J.; Rinna, A.; Ellingsen, J.E.; Reseland, J.E. Hydrofluoric acid treatment of titanium surfaces the proliferation of human gingival fibroblasts. J. Tissue Eng. 2019, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Ks, S.K.; Grandhi, V.V.; Gupta, V. The Effects of Titanium Implant Surface Topography on Osseointegration: Literature Review. JMIR Biomed. Eng. 2019, 4. [Google Scholar] [CrossRef]
- Guo, J.; Padilla, R.J.; Ambrose, W.; De Kok, I.J.; Cooper, L.F. The effect of hydrofluoric acid treatment of TiO2 grit blasted titanium implants on adherent osteoblast gene expression in vitro and in vivo. Biomaterials 2007, 28, 5418–5425. [Google Scholar] [CrossRef]
- Hattar, S.; Berdal, A.; Asselin, A.; Loty, S.; Greenspan, D.C.; Sautier, J.M. Behaviour of moderately differentiated osteoblast-like cells cultured in contact with bioactive glasses. Eur. Cell. Mater. 2002, 4, 61–69. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.-H.; Lu, Y.-J.; Lan, W.-C.; Ruslin, M.; Lin, H.-Y.; Ou, K.-L.; Saito, T.; Tsai, H.-Y.; Lee, C.-H.; Cho, Y.-C.; et al. Surface Properties and Biocompatibility of Anodized Titanium with a Potential Pretreatment for Biomedical Applications. Metals 2021, 11, 1090. https://doi.org/10.3390/met11071090
Huang B-H, Lu Y-J, Lan W-C, Ruslin M, Lin H-Y, Ou K-L, Saito T, Tsai H-Y, Lee C-H, Cho Y-C, et al. Surface Properties and Biocompatibility of Anodized Titanium with a Potential Pretreatment for Biomedical Applications. Metals. 2021; 11(7):1090. https://doi.org/10.3390/met11071090
Chicago/Turabian StyleHuang, Bai-Hung, Yi-Jung Lu, Wen-Chien Lan, Muhammad Ruslin, Hung-Yang Lin, Keng-Liang Ou, Takashi Saito, Hsin-Yu Tsai, Chen-Han Lee, Yung-Chieh Cho, and et al. 2021. "Surface Properties and Biocompatibility of Anodized Titanium with a Potential Pretreatment for Biomedical Applications" Metals 11, no. 7: 1090. https://doi.org/10.3390/met11071090
APA StyleHuang, B.-H., Lu, Y.-J., Lan, W.-C., Ruslin, M., Lin, H.-Y., Ou, K.-L., Saito, T., Tsai, H.-Y., Lee, C.-H., Cho, Y.-C., Yang, T.-S., Liu, C.-M., & Hou, P.-J. (2021). Surface Properties and Biocompatibility of Anodized Titanium with a Potential Pretreatment for Biomedical Applications. Metals, 11(7), 1090. https://doi.org/10.3390/met11071090





