Surface Micromorphology and Structure of Stainless and Maraging Steel Obtained via Selective Laser Melting: A Mössbauer Spectroscopy Study
Abstract
1. Introduction
2. Materials and Methods
2.1. SLM Technology
2.2. Mössbauer Spectroscopy
2.3. Scanning Electron Microscopy and Digital Optical Microscopy
2.4. X-ray Diffraction
2.5. CL20ES and CL50WS Steel Powders
2.6. Test Samples
3. Results and Discussion
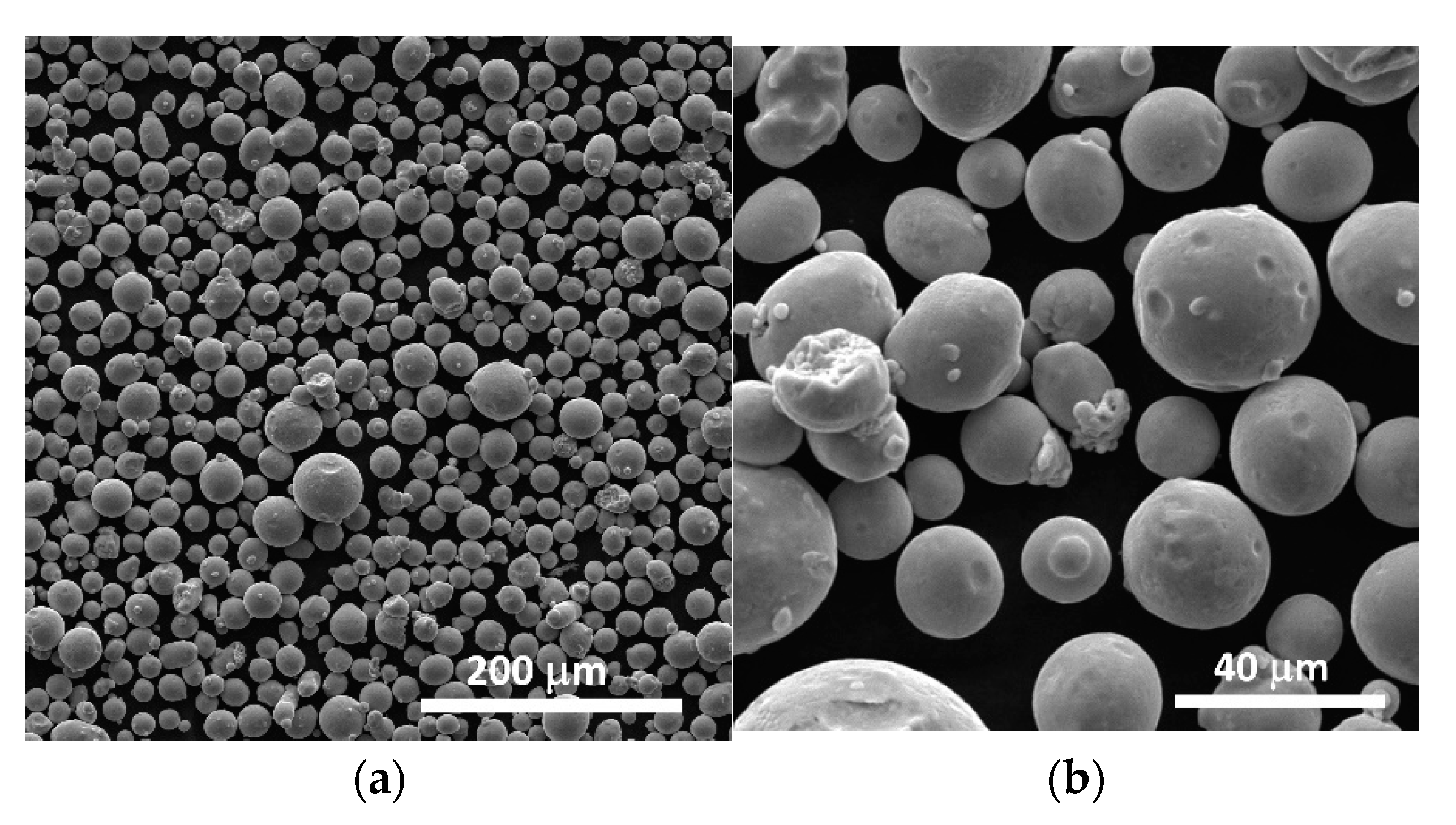
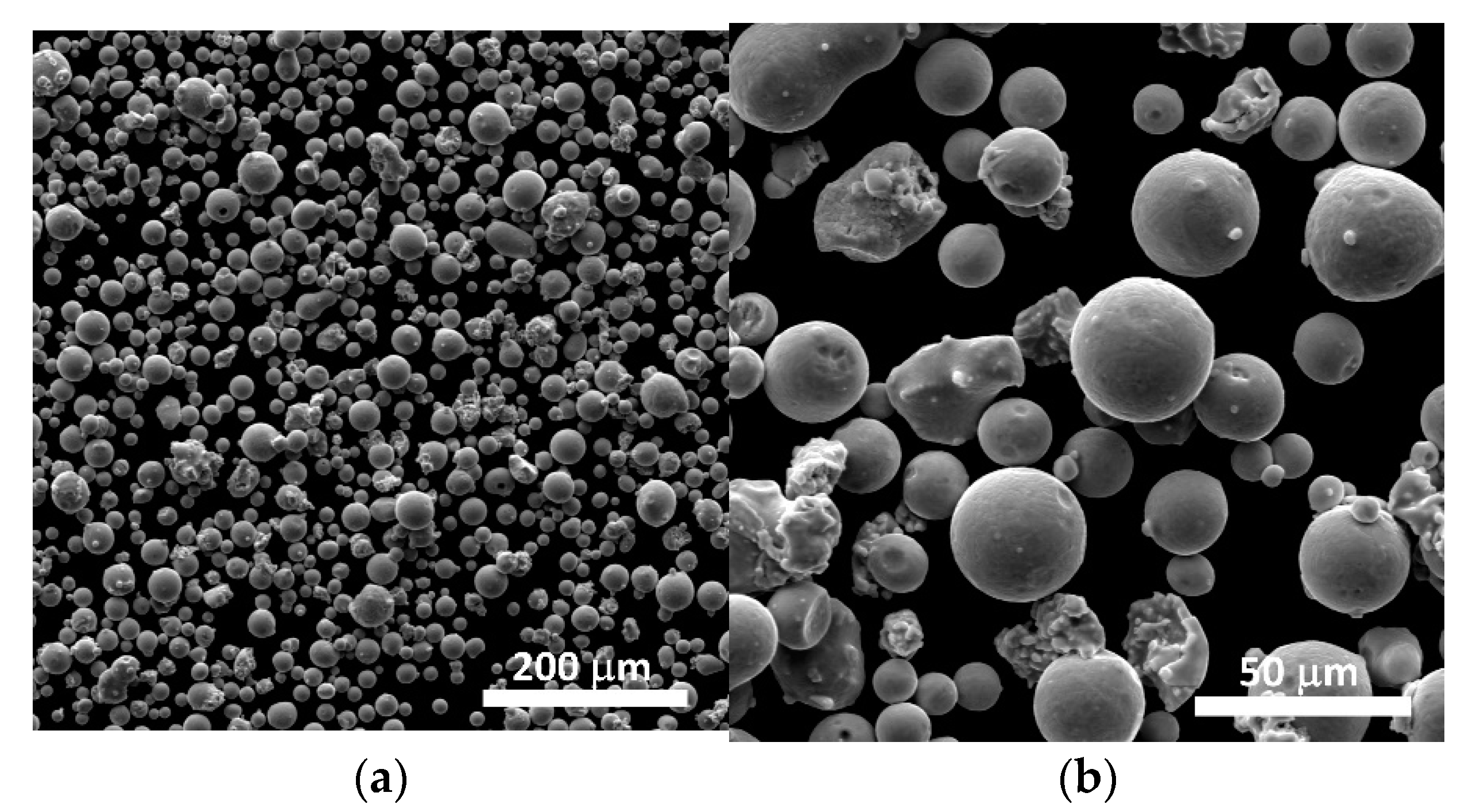
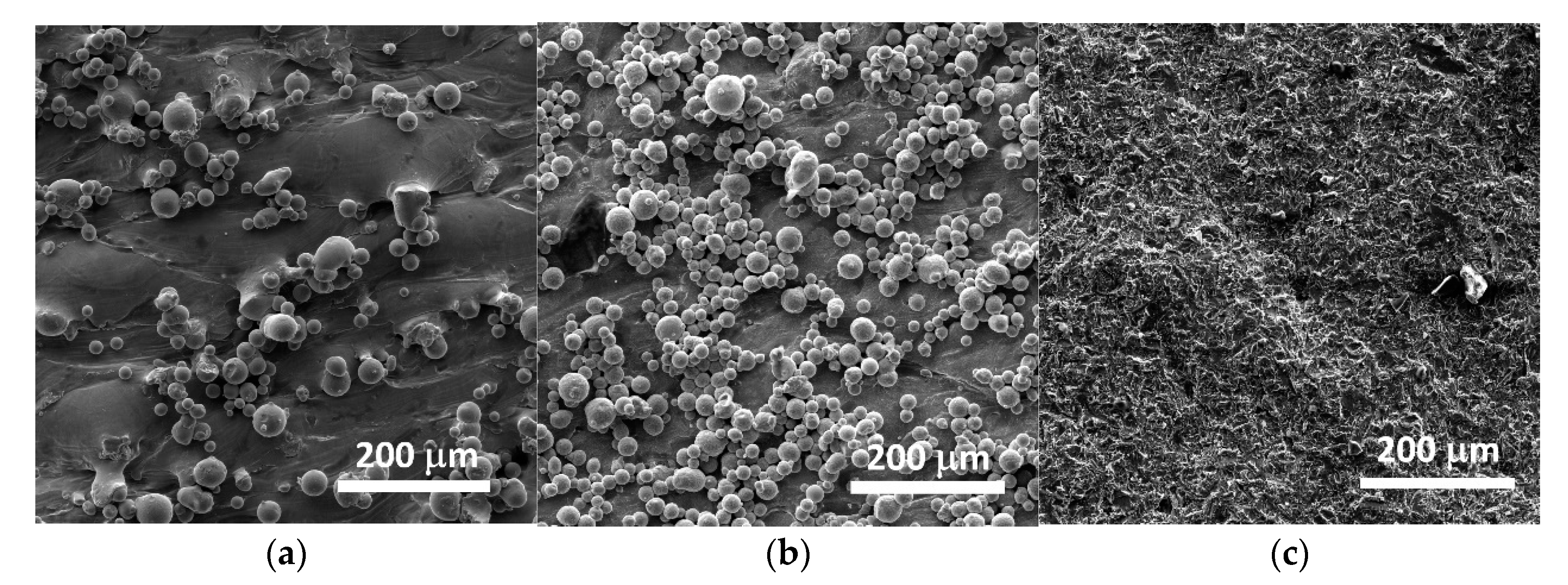
3.1. Scanning Electron Microscopy
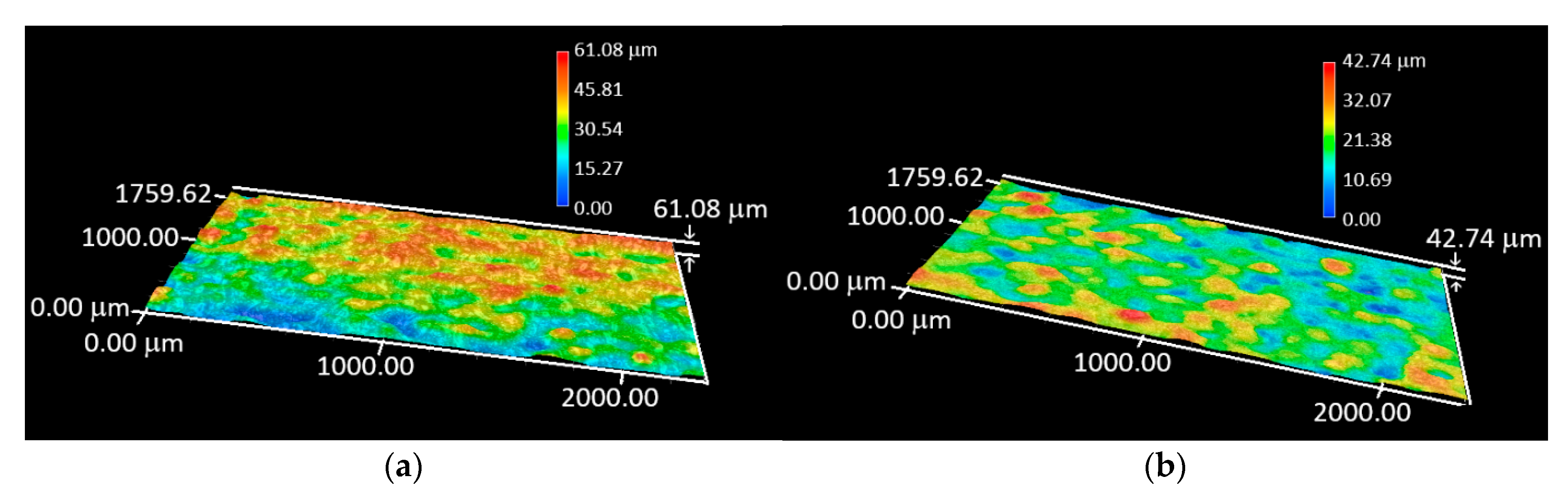
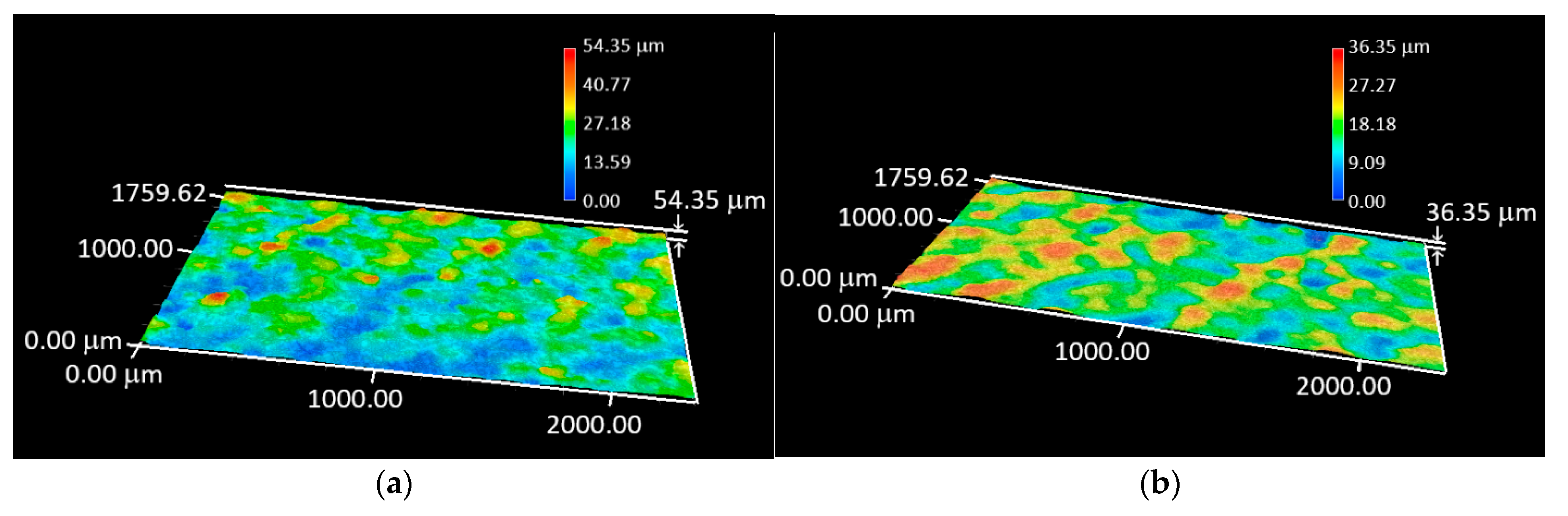
3.2. Digital Optical Microscopy
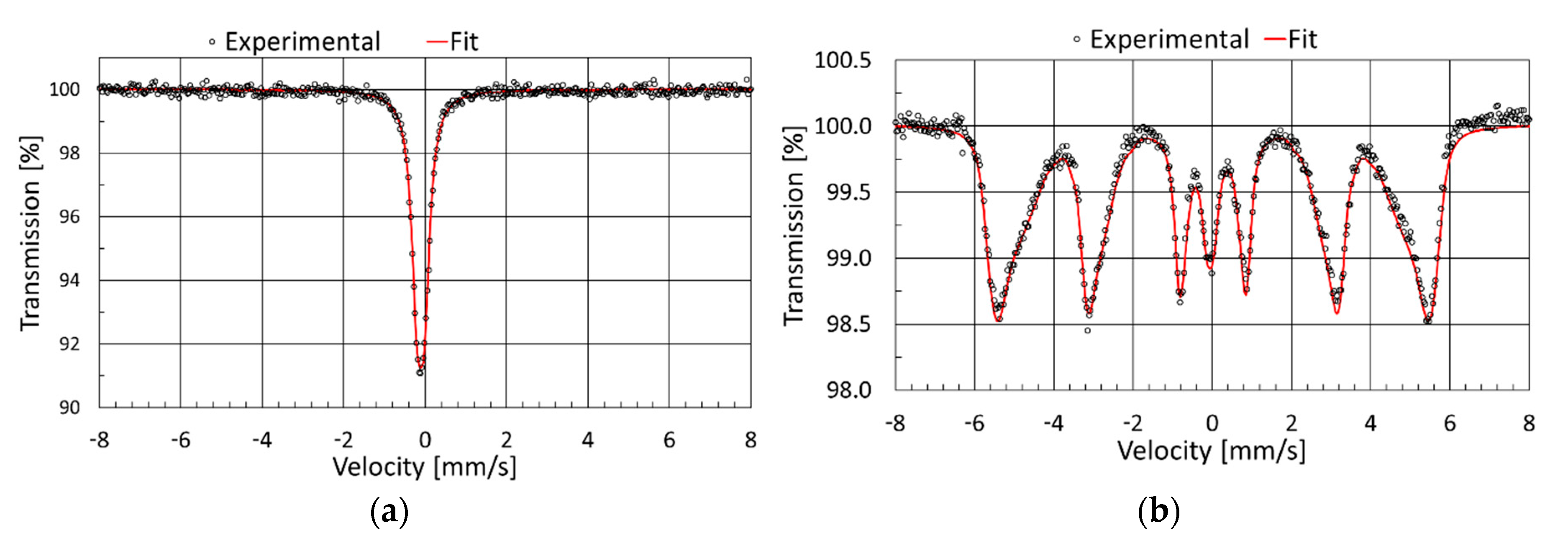
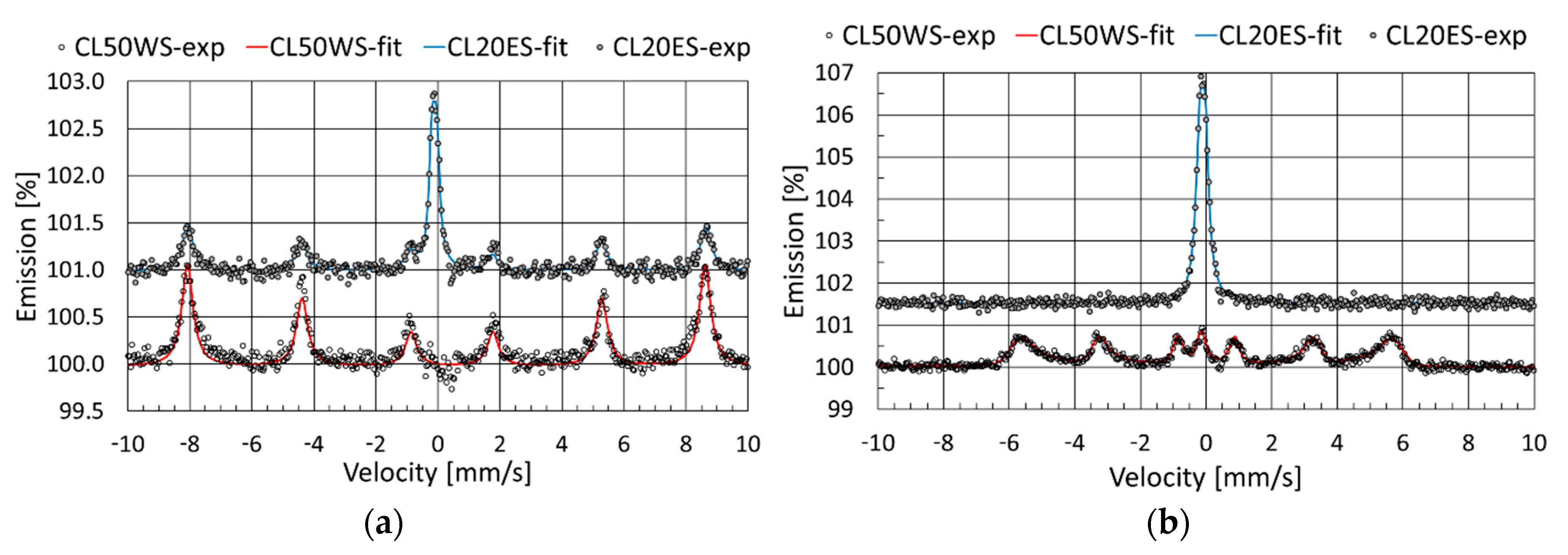
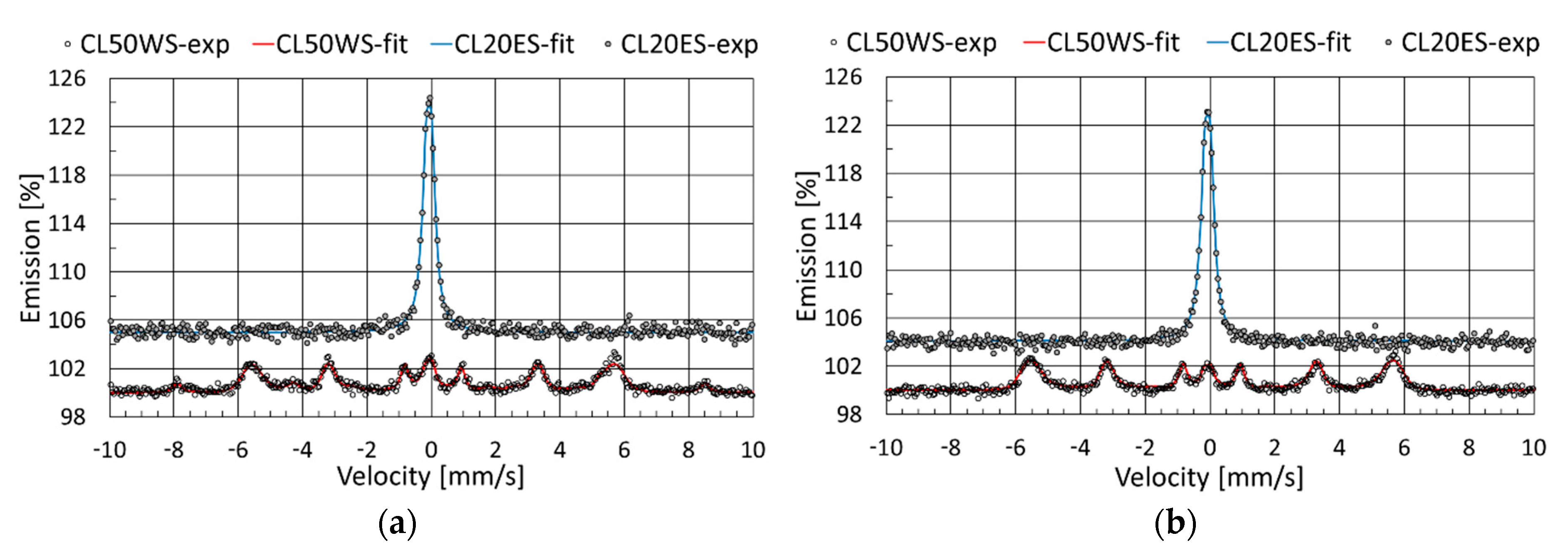
3.3. Mössbauer Spectroscopy
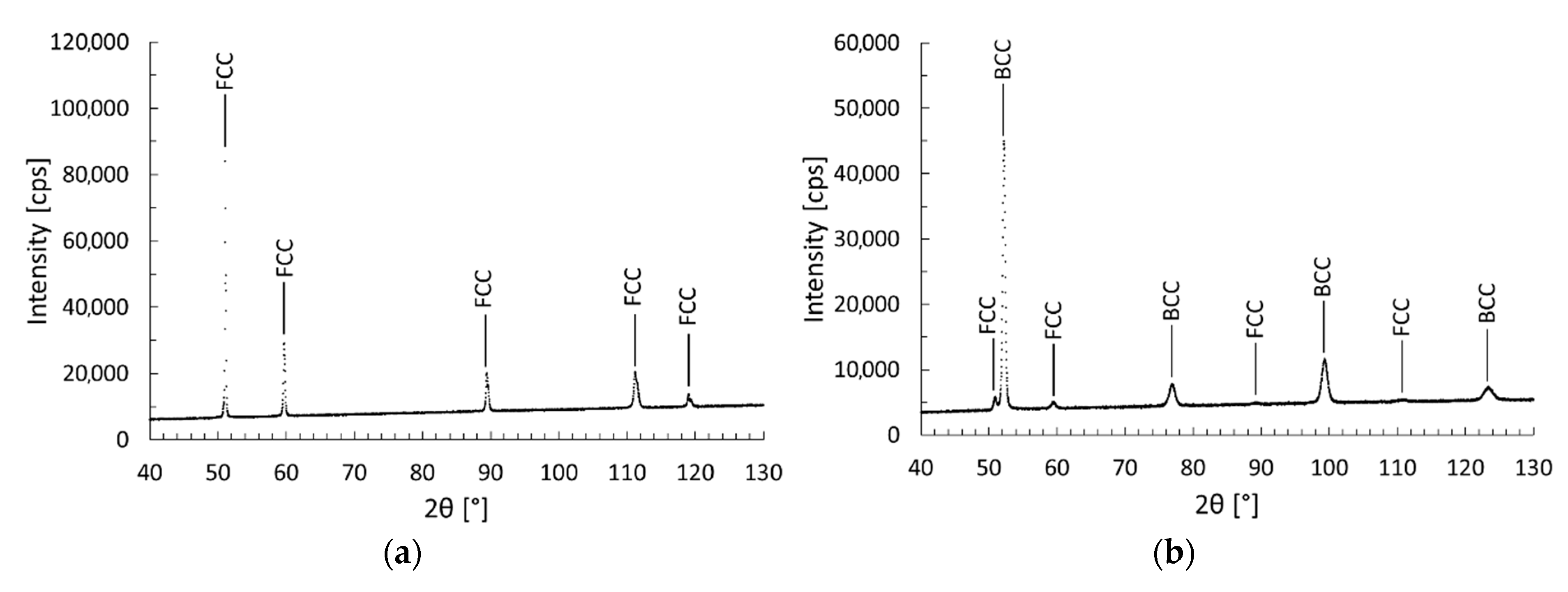
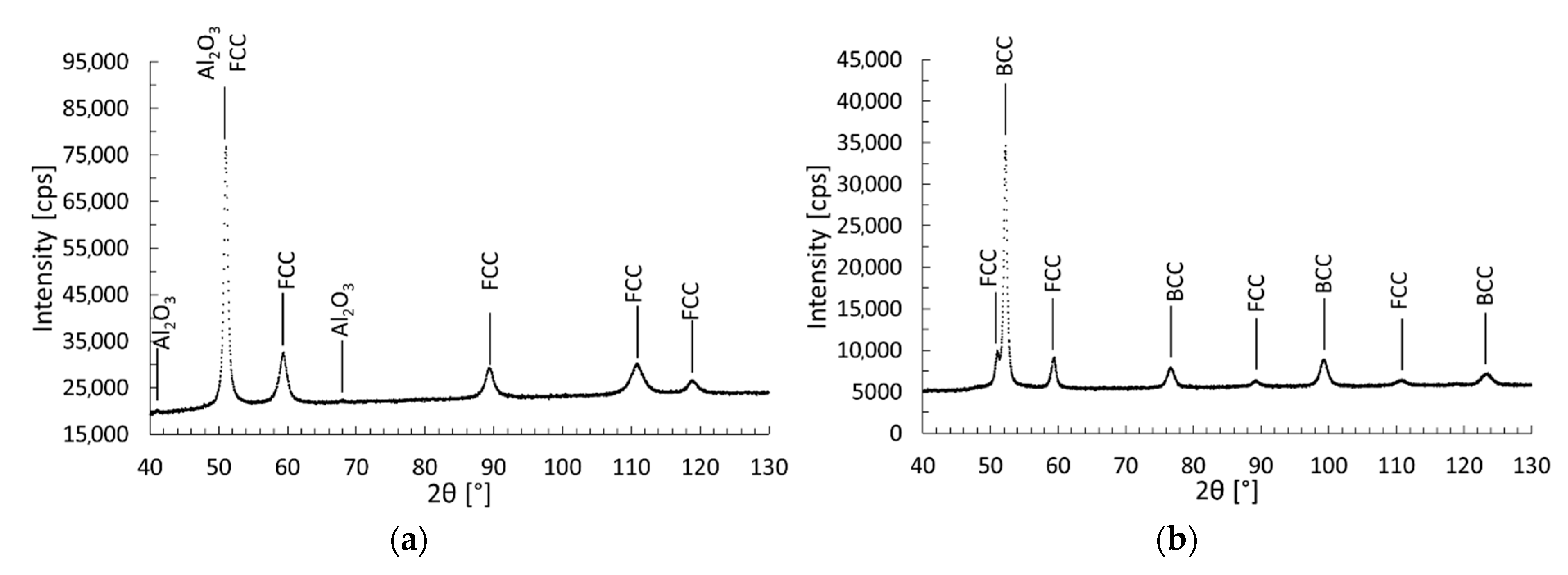
3.4. X-ray Diffraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afkhami, S.; Dabiri, M.; Alavi, S.H.; Björk, T.; Salminen, A. Fatigue characteristics of steels manufactured by selective laser melting. Int. J. Fatigue 2019, 122, 72–83. [Google Scholar] [CrossRef]
- Kruth, J.; Froyen, L.; Van Vaerenbergh, J.; Mercelis, P.; Rombouts, M.; Lauwers, B. Selective laser melting of iron-based powder. J. Mater. Process. Technol. 2004, 149, 616–622. [Google Scholar] [CrossRef]
- Frazier, W.E. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Patel, C.M.; Patel, S.B.; Shah, M.K. Experimental Investigation of Mechanical Properties and Surface Roughness of CL50WS Material Parts Made by Selective Laser Sintering Process. Int. J. Sci. Res. Dev. 2015, 3, 306–310. [Google Scholar]
- Jägle, E.A.; Sheng, Z.; Kürnsteiner, P.; Ocylok, S.; Weisheit, A.; Raabe, D. Comparison of Maraging Steel Micro- and Nanostructure Produced Conventionally and by Laser Additive Manufacturing. Materials 2016, 10, 8. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Rowshan, R.; Abu Al-Rub, R.K. Topology-mechanical property relationship of 3D printed strut, skeletal, and sheet based periodic metallic cellular materials. Addit. Manuf. 2018, 19, 167–183. [Google Scholar] [CrossRef]
- Gainov, R.; Faidel, D.; Behr, W.; Natour, G.; Pauly, F.; Willms, H.; Vagizov, F. Investigation of LPBF A800H steel parts using Computed Tomography and Mössbauer spectroscopy. Addit. Manuf. 2020, 32, 101035. [Google Scholar] [CrossRef]
- Zirhli, O.; Akdogan, N.G.; Odeh, Y.N.; Misirlioglu, I.B.; Devlin, E.; Akdogan, O. Fabrication and Characterization of Fe 16 N 2 Micro-Flake Powders and Their Extrusion-Based 3D Printing into Permanent Magnet Form. Adv. Eng. Mater. 2020, 22, 2000311. [Google Scholar] [CrossRef]
- Mashlan, M.; Linderhof, F.; Davidova, M.; Kubickova, H.; Zemtsova, E. Changes of phase composition of maraging steel 1.2709 during selective laser melting. Hyperfine Interact. 2019, 241, 11. [Google Scholar] [CrossRef]
- Tyczyński, P.; Siemiątkowski, Z.; Bąk, P.; Warzocha, K.; Rucki, M.; Szumiata, T. Performance of Maraging Steel Sleeves Produced by SLM with Subsequent Age Hardening. Materials 2020, 13, 3408. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Ujihira, Y.; Vertes, A. Applications of conversion electron Mössbauer spectrometry (CEMS). J. Radioanal. Nucl. Chem. 1996, 202, 103–199. [Google Scholar] [CrossRef]
- Principi, G. The Mössbauer Effect: A Romantic Scientific Page. Metals 2020, 10, 992. [Google Scholar] [CrossRef]
- Bandyopadhyay, D. Study of materials using Mössbauer spectroscopy. Int. Mater. Rev. 2006, 51, 171–208. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Sagaradze, V.V.; Kozlov, K.A.; Ustyugov, Y.M. Atomic Order and Submicrostructure in Iron Alloys at Megaplastic Deformation. Metals 2018, 8, 995. [Google Scholar] [CrossRef]
- Kholmetskii, A.L.; Uglov, V.V.; Khodasevich, V.V.; Anischik, V.M.; Ponaryadov, V.V.; Mashlan, M. Application of the Mössbauer Effect in Some Tribological Problems. Mössbauer Spectrosc. Mater. Sci. 1999, 66, 215–226. [Google Scholar] [CrossRef]
- Krauss, G. Tempering of Lath Martensite in Low and Medium Carbon Steels: Assessment and Challenges. Steel Res. Int. 2017, 88, 1700038. [Google Scholar] [CrossRef]
- Kalman, E.; Lakatos, M.; Kármán, F.; Nagy, F.; Klencsár, Z.; Vértes, A. Mössbauer Spectroscopy for Characterization of Corrosion Products and Electrochemically Formed Layers. Corros. Rev. 2005, 23, 1–106. [Google Scholar] [CrossRef]
- Graham, M.J.; Hussey, R.J. Analytical techniques in high temperature corrosion. Oxid. Met. 1995, 44, 339–374. [Google Scholar] [CrossRef]
- Schaaf, P. Laser nitriding of metals. Prog. Mater. Sci. 2002, 47, 1–161. [Google Scholar] [CrossRef]
- Carbucicchio, M.; Palonibarini, G. Surface structures produced in 1C-1.5Cr and 0.38C-Ni-Cr-Mo steels by high-power CO2 laser processing. J. Mater. Sci. 1986, 21, 75–82. [Google Scholar] [CrossRef]
- Tan, J.H.; Wong, W.L.E.; Dalgarno, K.W. An overview of powder granulometry on feedstock and part performance in the selective laser melting process. Addit. Manuf. 2017, 18, 228–255. [Google Scholar] [CrossRef]
- Fayazfar, H.; Salarian, M.; Rogalsky, A.; Sarker, D.; Russo, P.; Paserin, V.; Toyserkani, E. A critical review of powder-based additive manufacturing of ferrous alloys: Process parameters, microstructure and mechanical properties. Mater. Des. 2018, 144, 98–128. [Google Scholar] [CrossRef]
- Sander, G.; Tan, J.; Balan, P.; Gharbi, O.; Feenstra, D.; Singer, L.; Thomas, S.; Kelly, R.; Scully, J.; Birbilis, N. Corrosion of Additively Manufactured Alloys: A Review. Corrosion 2018, 74, 1318–1350. [Google Scholar] [CrossRef]
- Zai, L.; Zhang, C.; Wang, Y.; Guo, W.; Wellmann, D.; Tong, X.; Tian, Y. Laser Powder Bed Fusion of Precipitation-Hardened Martensitic Stainless Steels: A Review. Metals 2020, 10, 255. [Google Scholar] [CrossRef]
- Farias, M.; Souza, R.; Sinatora, A.; Tanaka, D. The influence of applied load, sliding velocity and martensitic transformation on the unlubricated sliding wear of austenitic stainless steels. Wear 2007, 263, 773–781. [Google Scholar] [CrossRef]
- Waanders, F.; Vorster, S.; Engelbrecht, A. Mössbauer and SEM characterisation of the scale on type 304 stainless steel. Scr. Mater. 2000, 42, 997–1000. [Google Scholar] [CrossRef]
- Dubiel, S.M.; Cieślak, J.; Żukrowski, J. Distribution of Cr atoms in the surface zone of Fe-rich Fe–Cr alloys quenched into various media: Mössbauer spectroscopic study. Appl. Surf. Sci. 2015, 359, 526–532. [Google Scholar] [CrossRef]
- Idczak, R.; Idczak, K.; Konieczny, R. Oxidation and surface segregation of chromium in Fe–Cr alloys studied by Mössbauer and X-ray photoelectron spectroscopy. J. Nucl. Mater. 2014, 452, 141–146. [Google Scholar] [CrossRef]
- Amulevičius, A.; Mažeika, K.; Sipavičius, Č. Oxidation of Stainless Steel by Laser Cutting. Acta Phys. Pol. A 2009, 115, 880–885. [Google Scholar] [CrossRef]
- Sartowska, B.; Piekoszewski, J.; Walis, L.; Starosta, W.; Barlak, M.; Nowicki, L.; Ratajczak, R. Application of nuclear tech-niques for characterization of materials surfaces: Own investigations examples. Nukleonika 2012, 57, 521–528. [Google Scholar]
- Agudelo, A.; Gancedo, J.; Marco, J.; Creus, M.; Gallego-Lluesma, E.; Desimoni, J.; Mercader, R. Characterization and corrosion studies of laser-melted carbon steel surfaces. Appl. Surf. Sci. 1999, 148, 171–182. [Google Scholar] [CrossRef]
- Nomura, K. Basics and Application of Scattering Mössbauer Spectrometry to Analysis of Solid Surface. Radioisotopes 2014, 63, 405–427. [Google Scholar] [CrossRef][Green Version]
- Kalogirou, O.; Stergioudis, G.; Haidar, O.; Tsipas, D. Identification of corrosion products resulting from accelerated oxidation process. Corros. Eng. Sci. Technol. 2009, 44, 469–473. [Google Scholar] [CrossRef]
- Ghayoor, M.; Lee, K.; He, Y.; Chang, C.-H.; Paul, B.K.; Pasebani, S. Selective laser melting of 304L stainless steel: Role of volumetric energy density on the microstructure, texture and mechanical properties. Addit. Manuf. 2020, 32, 101011. [Google Scholar] [CrossRef]
- Tascioglu, E.; Karabulut, Y.; Kaynak, Y. Influence of heat treatment temperature on the microstructural, mechanical, and wear behavior of 316L stainless steel fabricated by laser powder bed additive manufacturing. Int. J. Adv. Manuf. Technol. 2020, 107, 1947–1956. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, Y.; Gu, R.; Zhang, X.; Quach, W.-M.; Yan, M. A Comprehensive Study of Steel Powders (316L, H13, P20 and 18Ni300) for Their Selective Laser Melting Additive Manufacturing. Metals 2019, 9, 86. [Google Scholar] [CrossRef]
- Pechoušek, J.; Mashlan, M. Mössbauer spectrometer as a virtual instrument in the PXI/Compact PCI modular system. Czechoslov. J. Phys. 2005, 55, 853–863. [Google Scholar] [CrossRef]
- Kholmetskii, A.; Misevich, O.; Mashlan, M.; Chudakov, V.; Anashkevich, A.; Gurachevskii, V. Air scintillation detector for conversion electrons Mössbauer spectroscopy (CEMS). Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1997, 129, 110–116. [Google Scholar] [CrossRef]
- Klencsár, Z.; Kuzmann, E.; Vértes, A. User-friendly software for Mössbauer spectrum analysis. J. Radioanal. Nucl. Chem. 1996, 210, 105–118. [Google Scholar] [CrossRef]
- Cook, D.C. Strain induced martensite formation in stainless steel. Met. Mater. Trans. A 1987, 18, 201–210. [Google Scholar] [CrossRef]
- Ovchinnikov, V.V. Mössbauer Analysis of the Atomic and Magnetic Structure of Alloys; Cambridge International Science Publishing: Cambridge, UK, 2006; p. 248. [Google Scholar]
- Kuzmann, E.; Nagy, S.; Vertes, A. Critical review of analytical applications of Mössbauer spectroscopy illustrated by mineralogical and geological examples (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 801–858. [Google Scholar] [CrossRef]




| Steel Powder | Element Concentration, wt % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | C | Si | Mn | P | S | Cr | Mo | Ni | Ti | Co | |
| CL20ES | Balance | ≤0.03 | 0–1.0 | 0–2.0 | ≤0.045 | ≤0.03 | 16.5–18.5 | 2.0–2.5 | 10.0–13.0 | - | - |
| CL50WS | Balance | ≤0.03 | ≤0.1 | ≤0.15 | ≤0.01 | ≤0.01 | ≤0.25 | 4.5–5.2 | 17.0–19.0 | 0.80–1.20 | 8.50–10.0 |
| Powder | Phase | IS (mm/s) | QS (mm/s) | FWHM (mm/s) | B (T) | A (%) |
|---|---|---|---|---|---|---|
| CL20ES | austenitic | −0.11 ± 0.11 | 0.18 ± 0.01 | 0.32 ± 0.01 | – | 100 |
| CL50WS | austenitic | −0.06 ± 0.01 | 0.16 ± 0.02 | 0.34 ± 0.02 | – | 8 ± 1 |
| ferritic | 0.02 ± 0.01 | – | 0.27 ± 0.01 | 31.7 1 | 92 ± 1 |
| Sample | Powder | Phase | IS (mm/s) | QS (mm/s) | FWHM (mm/s) | B (T) | A (%) |
|---|---|---|---|---|---|---|---|
| Sample1 | CL20ES | austenitic | −0.12 ± 0.01 | 0.15 ± 0.01 | 0.25 ± 0.02 | – | 100 |
| Sample4 | CL50WS | austenitic | −0.12 ± 0.04 | 0.25 ± 0.08 | 0.29 ± 0.02 | – | 8 ± 1 |
| ferritic | 0.02 ± 0.01 | – | 0.19 ± 0.02 | 31.4 1 | 92 ± 1 | ||
| Sample2 | CL20ES | austenitic | −0.12 ± 0.01 | 0.15 ± 0.01 | 0.24 ± 0.02 | – | 42 ± 1 |
| α-Fe2O3 | 0.36 ± 0.01 | −0.15 ± 0.02 | 0.41 ± 0.02 | 51.9 ± 0.5 | 58 ± 1 | ||
| Sample5 | CL50WS | α-Fe2O3 | 0.36 ± 0.01 | −0.18 ± 0.02 | 0.44 ± 0.02 | 51.9 ± 0.5 | 100 |
| Sample3 | CL20ES | austenitic | −0.11 ± 0.01 | 0.16 ± 0.01 | 0.27 ± 0.01 | – | 100 |
| Sample6 | CL50WS | austenitic | −0.15 ± 0.02 | 0.11 ± 0.04 | 0.29 ± 0.03 | – | 9 ± 1 |
| ferritic | 0.01 ± 0.01 | – | 0.23 ± 0.03 | 31.4 1 | 91 ± 1 |
| Sample | Powder | Phase | IS (mm/s) | QS (mm/s) | FWHM (mm/s) | B (T) | A (%) |
|---|---|---|---|---|---|---|---|
| Sample1 | CL20ES | austenitic | −0.12 ± 0.02 | 0.17 ± 0.01 | 0.31 ± 0.01 | – | 100 |
| Sample4 | CL50WS | austenitic | −0.08 ± 0.01 | 0.21 ± 0.08 | 0.27 ± 0.03 | – | 9 ± 1 |
| ferritic | 0.05 ± 0.01 | – | 0.21 ± 0.03 | 29.5 1 | 91 ± 1 | ||
| Sample2 | CL20ES | austenitic | −0.08 ± 0.01 | 0.17 ± 0.01 | 0.30 ± 0.01 | – | 100 |
| Sample5 | CL50WS | austenitic | −0.13 ± 0.03 | 0.17 ± 0.03 | 0.26 ± 0.05 | – | 9 ± 1 |
| ferritic | 0.03 ± 0.01 | - | 0.22 ± 0.03 | 31.2 1 | 80 ± 1 | ||
| α-Fe2O3 | 0.37 ± 0.02 | −0.20 ± 0.05 | 0.34 ± 0.07 | 50.8 ± 0.3 | 11 ± 1 | ||
| Sample3 | CL20ES | austenitic | −0.08 ± 0.01 | 0.18 ± 0.01 | 0.30 ± 0.01 | – | 100 |
| Sample6 | CL50WS | austenitic | −0.08 ± 0.01 | 0.18 ± 0.01 | 0.27 | – | 9 |
| ferritic | 0.05 ± 0.01 | – | 0.23 ± 0.02 | 32.0 1 | 91 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linderhof, F.; Mashlan, M.; Doláková, H.; Ingr, T.; Ivanova, T. Surface Micromorphology and Structure of Stainless and Maraging Steel Obtained via Selective Laser Melting: A Mössbauer Spectroscopy Study. Metals 2021, 11, 1028. https://doi.org/10.3390/met11071028
Linderhof F, Mashlan M, Doláková H, Ingr T, Ivanova T. Surface Micromorphology and Structure of Stainless and Maraging Steel Obtained via Selective Laser Melting: A Mössbauer Spectroscopy Study. Metals. 2021; 11(7):1028. https://doi.org/10.3390/met11071028
Chicago/Turabian StyleLinderhof, Fredericus, Miroslav Mashlan, Hana Doláková, Tomáš Ingr, and Tatiana Ivanova. 2021. "Surface Micromorphology and Structure of Stainless and Maraging Steel Obtained via Selective Laser Melting: A Mössbauer Spectroscopy Study" Metals 11, no. 7: 1028. https://doi.org/10.3390/met11071028
APA StyleLinderhof, F., Mashlan, M., Doláková, H., Ingr, T., & Ivanova, T. (2021). Surface Micromorphology and Structure of Stainless and Maraging Steel Obtained via Selective Laser Melting: A Mössbauer Spectroscopy Study. Metals, 11(7), 1028. https://doi.org/10.3390/met11071028






