Comparative EIS Study of AlxCoCrFeNi Alloys in Ringer’s Solution for Medical Instruments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Samples Preparation
2.2. Test Environment
2.3. Microstructural Characterization
2.4. Microhardness
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. Microstructural Characterization
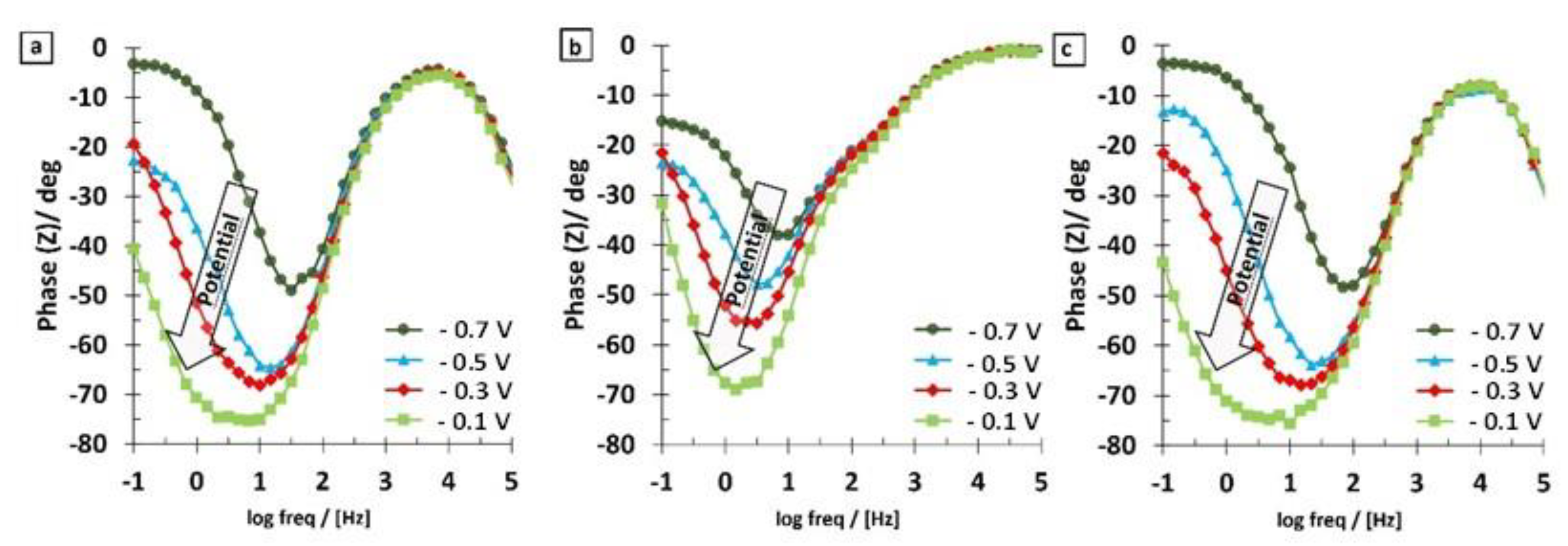
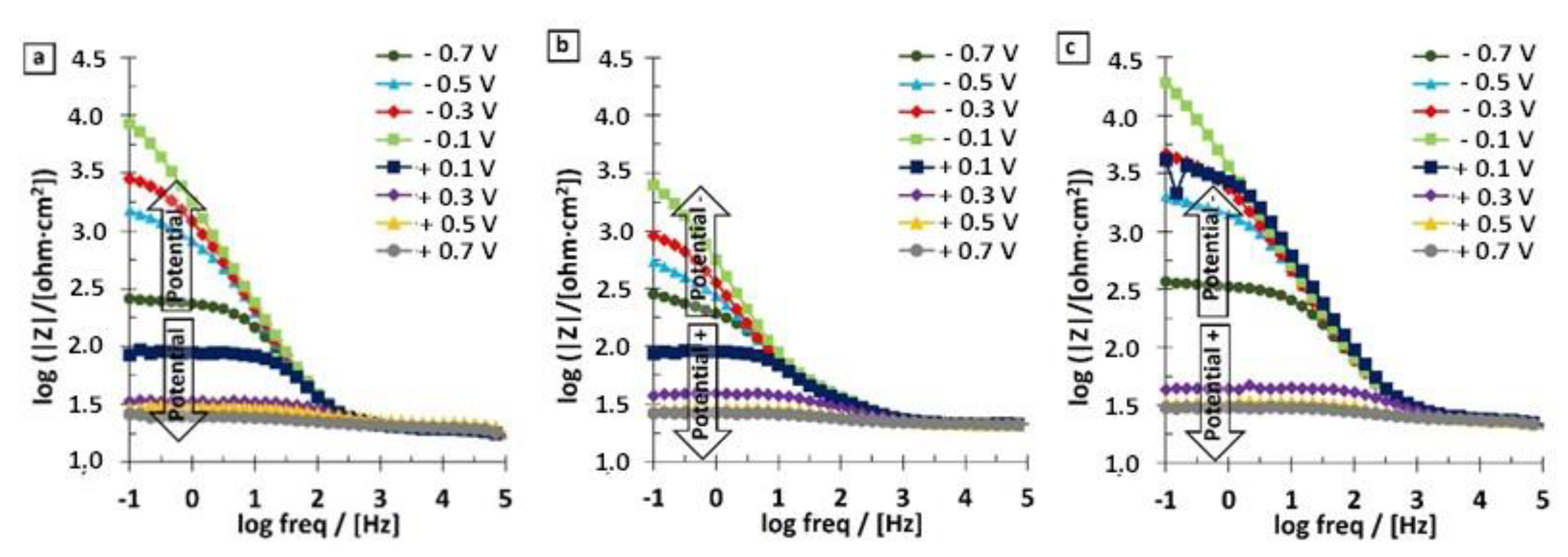
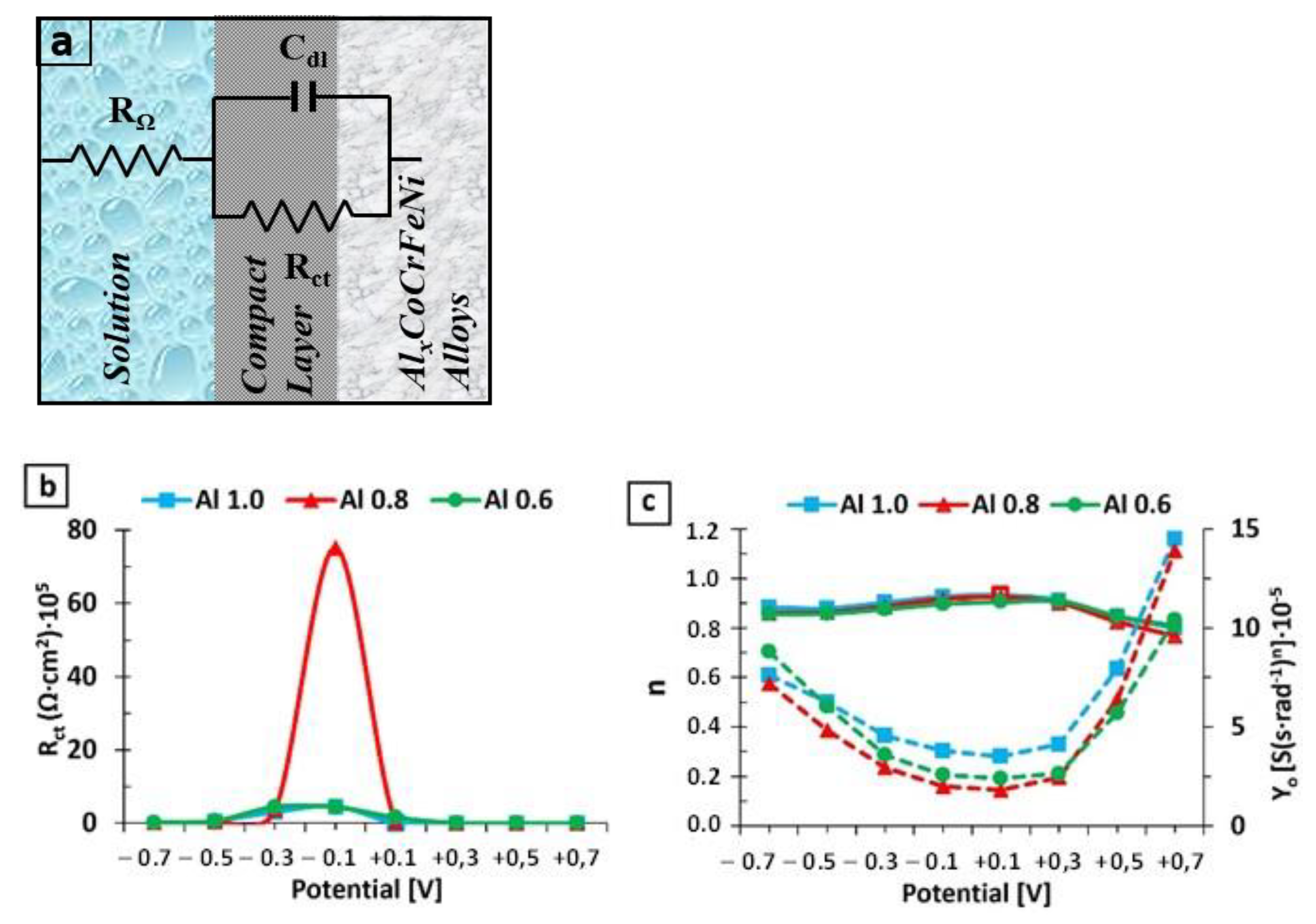
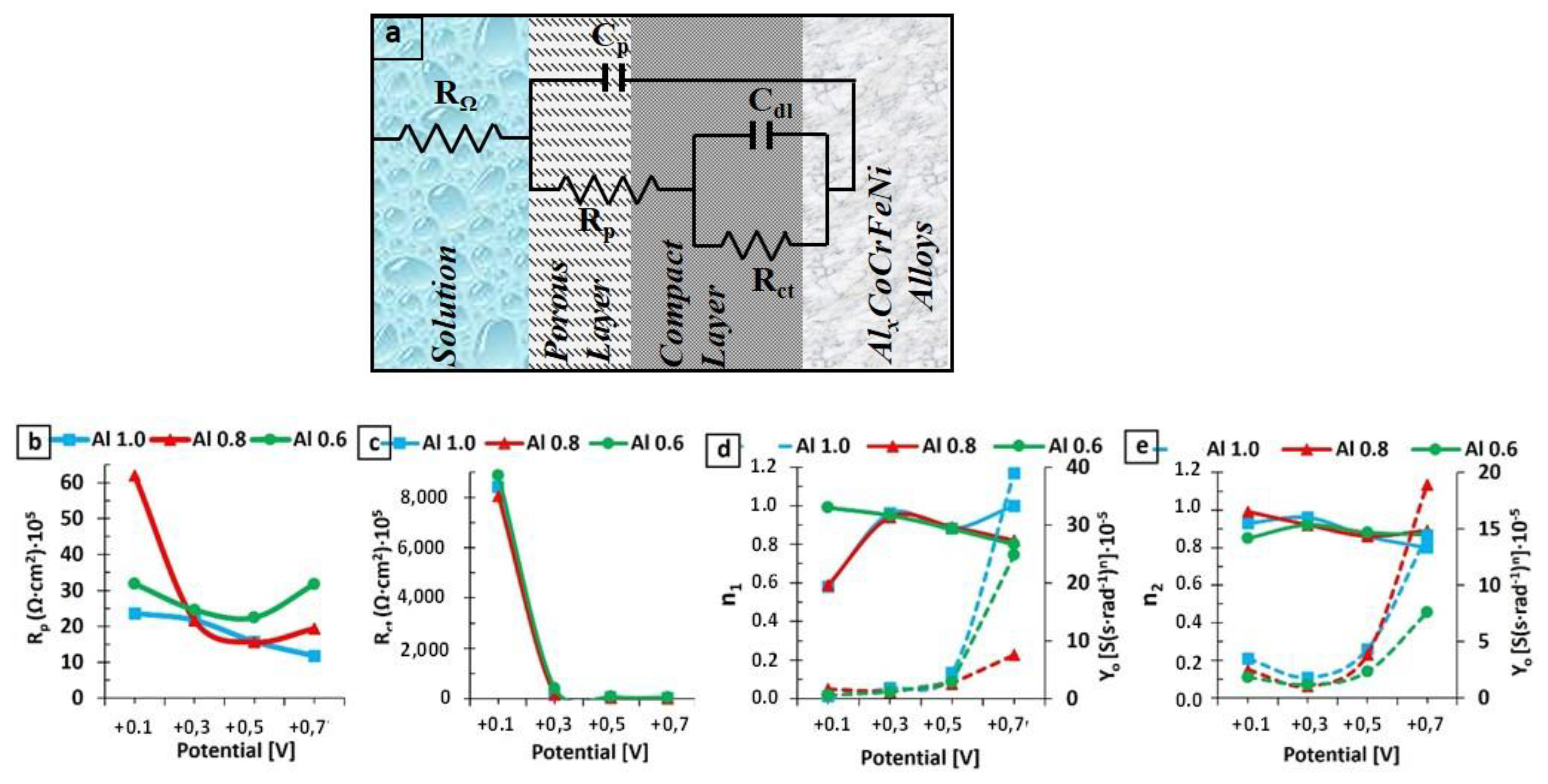
3.2. EIS Results
3.3. Microhardness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Chen, H.S.; Tsai, C.W.; Tung, C.C.; Yeh, L.W.; Shun, T.T.; Yang, C.C.; Chen, S.K. Effect of the substitution of Co by Mn in Al-Cr-Cu-Fe-Co-Ni high-entropy alloys. Ann. Chim. Sci. Mater. 2006, 31, 685–698. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.; Wang, T.; Wen, B.; Wang, Z.; Jie, J.; Cao, Z.; et al. A promising new class of high-temperature alloys: Eutectic high-entropy alloys. Sci. Rep. 2014, 4, 1–5. [Google Scholar] [CrossRef]
- Wang, C.; Yu, J.; Yu, Y.; Zhao, Y.; Zhang, Y.; Han, X. Comparison of the corrosion and passivity behavior between CrMnFeCoNi and CrFeCoNi coatings prepared by argon arc cladding. J. Mater. Res. Technol. 2020, 9, 8482–8496. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chang, Y.J.; Murakami, H.; Sasaki, T.; Hono, K.; Li, C.W.; Kakehi, K.; Yeh, J.W.; Yeh, A.C. Hierarchical microstructure strengthening in a single crystal high entropy superalloy. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ríos, M.L.; Perdomo, P.S.; Voiculescu, I.; Geanta, V.; Crăciun, V.; Boerasu, I.; Rosca, J.M. Effects of nickel content on the microstructure, microhardness and corrosion behavior of high-entropy AlCoCrFeNix alloys. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y. High-Entropy Materials—A Brief Introduction; Springer Nature: Singapore, 2019. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, B.S.; Ren, M.X.; Yang, C.; Fu, H.Z. Microstructure and compressive properties of AlCrFeCoNi high entropy alloy. Mater. Sci. Eng. A 2008, 491, 154–158. [Google Scholar] [CrossRef]
- Dasari, S.; Jagetia, A.; Chang, Y.J.; Soni, V.; Gwalani, B.; Gorsse, S.; Yeh, A.C.; Banerjee, R. Engineering multi-scale B2 precipitation in a heterogeneous FCC based microstructure to enhance the mechanical properties of a Al0.5Co1.5CrFeNi1.5 high entropy alloy. J. Alloys Compd. 2020, 830, 154707. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, M.C.; Diao, H. Aluminum Alloying Effects on Lattice Types, Microstructures, and Mechanical Behavior of High-Entropy Alloys SystemsNo Title. JOM 2013, 65, 1848–1858. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, M.; Yang, H.; Qiao, J.; Wu, Y. Surface strengthening in Al0.25CoCrFeNi high-entropy alloy by boronizing. Mater. Lett. 2019, 238, 258–260. [Google Scholar] [CrossRef]
- Shun, T.T.; Du, Y.C. Microstructure and tensile behaviors of FCC Al0.3CoCrFeNi high entropy alloy. J. Alloys Compd. 2009, 479, 157–160. [Google Scholar] [CrossRef]
- Gwalani, B.; Gorsse, S.; Choudhuri, D.; Zheng, Y.; Mishra, R.S.; Banerjee, R. Tensile yield strength of a single bulk Al 0.3 CoCrFeNi high entropy alloy can be tuned from 160 MPa to 1800 MPa. Scr. Mater. 2019, 162, 18–23. [Google Scholar] [CrossRef]
- Csaki, I.; Stefanoiu, R.; Geanta, V.; Voiculescu, I.; Sohaciu, M.G.; Soare, A.; Popescu, G.; Serghiuta, S. Researches regarding the processing technique impact on the chemical composition, microstructure and hardness of AlCrFeNiCo high entropy alloy. Rev. Chim. 2016, 67, 1373–1377. [Google Scholar]
- Lin, C.M.; Tsai, H.L. Evolution of microstructure, hardness, and corrosion properties of high-entropy Al0.5CoCrFeNi alloy. Intermetallics 2011, 19, 288–294. [Google Scholar] [CrossRef]
- Chen, M.; Lan, L.; Shi, X.; Yang, H.; Zhang, M.; Qiao, J. The tribological properties of Al0.6CoCrFeNi high-entropy alloy with the σ phase precipitation at elevated temperature. J. Alloys Compd. 2019, 777, 180–189. [Google Scholar] [CrossRef]
- Geantă, V.; Voiculescu, I.; Stefănoiu, R.; Chereches, T.; Zecheru, T.; Matache, L.; Rotariu, A. Dynamic Impact Behaviour of High Entropy Alloys Used in the Military Domain. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012041. [Google Scholar] [CrossRef]
- Li, C.; Li, J.C.; Zhao, M.; Jiang, Q. Effect of aluminum contents on microstructure and properties of Al xCoCrFeNi alloys. J. Alloys Compd. 2010, 504, 515–518. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Wang, S.C.; Tsai, Y.C.; Lai, C.H.; Yeh, J.W. Effects of Al addition on the microstructure and mechanical property of Al xCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Kao, Y.F.; Chen, T.J.; Chen, S.K.; Yeh, J.W. Microstructure and mechanical property of as-cast, -homogenized, and -deformed AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. J. Alloys Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, M.; Cui, H.; Zhao, Y.; Song, X.; Zeng, Y.; Gao, X.; Lu, F.; Wang, C.; Song, Q. Effects of Ti-to-Al ratios on the phases, microstructures, mechanical properties, and corrosion resistance of Al2-xCoCrFeNiTix high-entropy alloys. J. Alloys Compd. 2019, 805, 585–596. [Google Scholar] [CrossRef]
- Voiculescu, I.; Geanta, V.; Stefanoiu, R.; Patrop, D.; Binchiciu, H. Influence of the chemical composition on the microstructure and microhardness of alcrfeconi high entropy alloy. Rev. Chim. 2013, 64, 1441–1444. [Google Scholar]
- Kao, Y.F.; Lee, T.D.; Chen, S.K.; Chang, Y.S. Electrochemical passive properties of AlxCoCrFeNi (x = 0, 0.25, 0.50, 1.00) alloys in sulfuric acids. Corros. Sci. 2010, 52, 1026–1034. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, L.; He, W.; Liaw, P.K. Alloying and processing effects on the aqueous corrosion behavior of high-entropy alloys. Entropy 2014, 16, 895–911. [Google Scholar] [CrossRef]
- Kao, Y.F.; Chen, S.K.; Chen, T.J.; Chu, P.C.; Yeh, J.W.; Lin, S.J. Electrical, magnetic, and Hall properties of AlxCoCrFeNi high-entropy alloys. J. Alloys Compd. 2011, 509, 1607–1614. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Ritchie, R.O.; Meyers, M.A. Mechanical properties of high-entropy alloys with emphasis on face-centered cubic alloys. Prog. Mater. Sci. 2019, 102, 296–345. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Levine, D.L.; Staehle, R.W. Crevice Corrosion in Orthopedic Implant Metals. J. Biomed. Mater. Res. 1977, 11, 553–561. [Google Scholar] [CrossRef]
- ASTM B214-16, Standard Test Method for Sieve Analysis of Metal Powders; ASTM International: West Conshohocken, PA, USA, 2016. [CrossRef]
- ISO 14577-1:2015 Metallic Materials—Instrumented Indentation Test for Hardness and Materials Parameters—Part 1: Test Method n.d; ISO: Geneva, Switzerland, 2015.
- ISO 16773-1-4:2016 Electrochemical Impedance Spectroscopy (EIS) on Coated and Uncoated Metallic Specimens n.d; ISO: Geneva, Switzerland, 2015.
- Scully, R.; Silverman, D.C.; Kendig, M. Electrochemical Impedance: Analysis and Interpretation; ASTM: Philadelphia, PA, USA, 1993. [Google Scholar] [CrossRef]
- González, J.E.G.; Mirza-Rosca, J.C. Study of the corrosion behavior of titanium and some of its alloys for biomedical and dental implant applications. J. Electroanal. Chem. 1999, 471, 109–115. [Google Scholar] [CrossRef]
- Boukamp, B.A. A nonlinear least squares fit procedure for analysis of immittance data of electrochemical systems. Solid State Ionics 1986, 20, 31–44. [Google Scholar] [CrossRef]
- Nascimento, C.B.; Donatus, U.; Ríos, C.T.; Antunes, R.A. Electronic properties of the passive films formed on CoCrFeNi and CoCrFeNiAl high entropy alloys in sodium chloride solution. J. Mater. Res. Technol. 2020, 9, 13879–13892. [Google Scholar] [CrossRef]
- Fan, Q.C.; Li, B.S.; Zhang, Y. Influence of Al and Cu elements on the microstructure and properties of (FeCrNiCo)AlxCuy high-entropy alloys. J. Alloys Compd. 2014, 614, 203–210. [Google Scholar] [CrossRef]
- Rios, M.L.; Baldevenites, V.L.; Voiculescu, I.; Rosca, J.M. AlCoCrFeNi High Entropy Alloys as Possible Nuclear Materials. Microsc. Microanal. 2020, 26, 406–407. [Google Scholar] [CrossRef]
- Li, B.; Peng, K.; Hu, A.; Zhou, L.; Zhu, J.; Li, D. Structure and properties of FeCoNiCrCu0.5Alx high-entropy alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 735–741. [Google Scholar] [CrossRef]
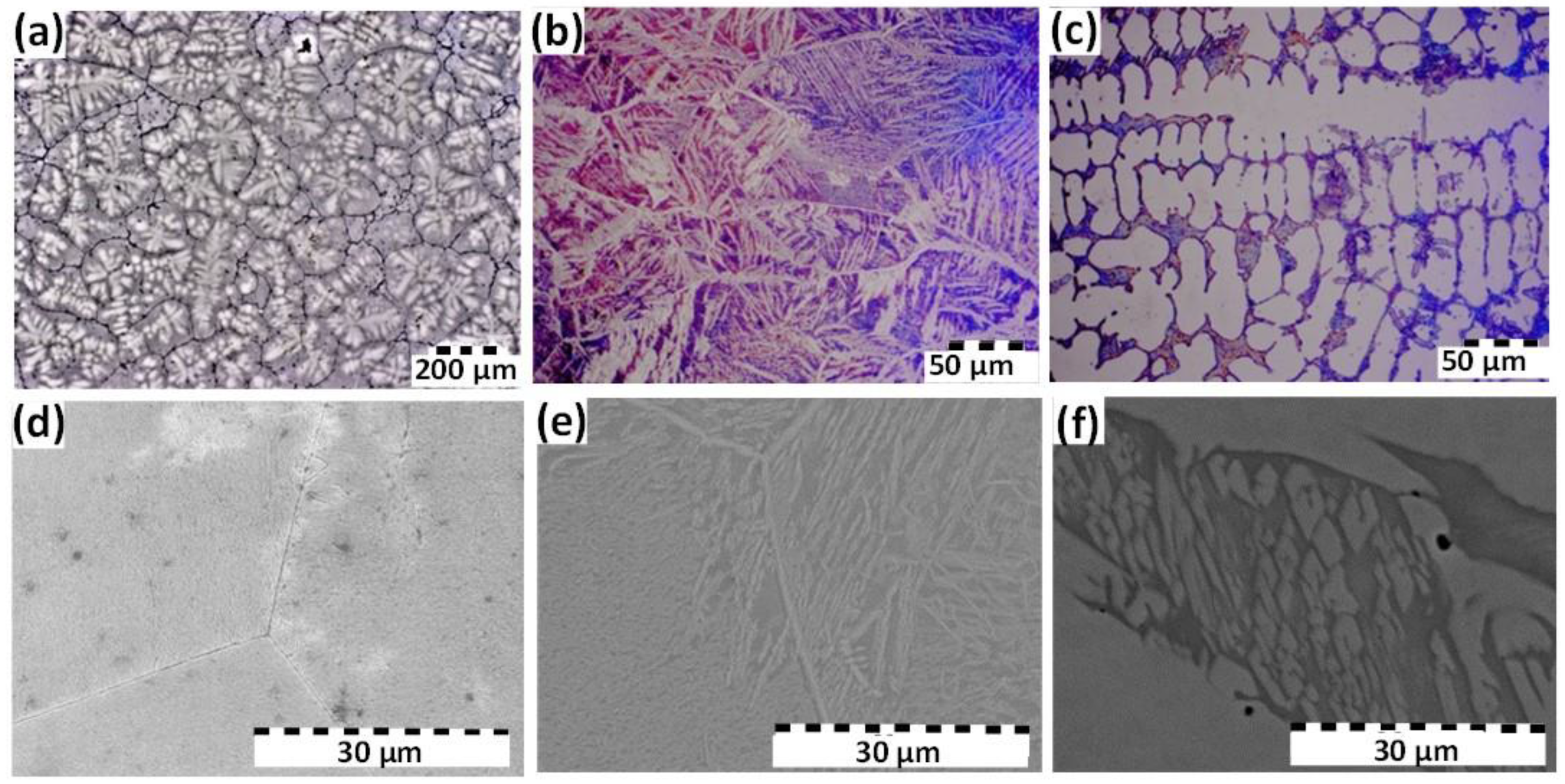


| Composition (wt %) | Al | Cr | Fe | Co | Ni |
|---|---|---|---|---|---|
| Al1.0CrFeCoNi (Al1.0) | 10.67 | 20.55 | 22.13 | 23.32 | 23.33 |
| Al0.8CrFeCoNi (Al0,8) | 8.72 | 21.00 | 22.61 | 23.82 | 23.85 |
| Al0.6CrFeCoNi (Al0,6) | 6.68 | 21.47 | 23.12 | 24.36 | 24.36 |
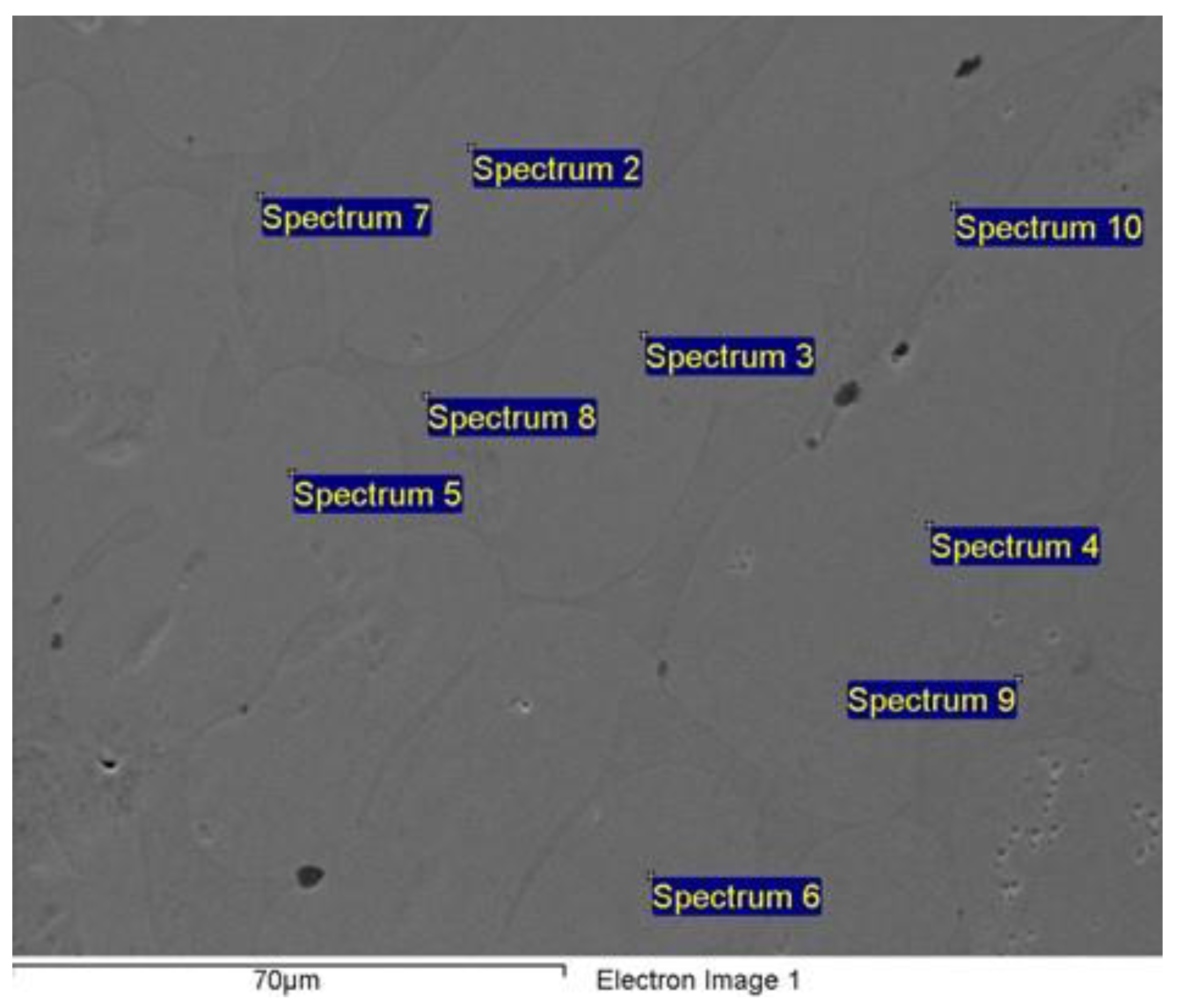
| Spectrum | Al | Cr | Fe | Co | Ni |
|---|---|---|---|---|---|
| Sum Spectrum | 6.87 | 20.66 | 24.18 | 24.04 | 24.26 |
| Spectrum 2 | 5.74 | 20.90 | 26.23 | 25.04 | 22.09 |
| Spectrum 3 | 5.86 | 20.78 | 26.07 | 24.82 | 22.47 |
| Spectrum 4 | 6.02 | 20.93 | 25.43 | 24.71 | 22.91 |
| Spectrum 5 | 5.74 | 20.89 | 26.10 | 24.88 | 22.38 |
| Spectrum 6 | 6.12 | 20.71 | 25.70 | 24.63 | 22.84 |
| Spectrum 7 | 8.75 | 22.12 | 22.70 | 22.48 | 23.95 |
| Spectrum 8 | 8.16 | 21.08 | 23.08 | 23.45 | 24.24 |
| Spectrum 9 | 8.57 | 22.28 | 22.99 | 22.55 | 23.61 |
| Spectrum 10 | 9.04 | 21.48 | 22.59 | 22.38 | 24.52 |
| Mean | 7.09 | 21.18 | 24.51 | 23.90 | 23.33 |
| Standard deviation | 1.38 | 0.58 | 1.55 | 1.09 | 0.89 |
| Alloy | Parameters | Al | Cr | Fe | Co | Ni |
|---|---|---|---|---|---|---|
| Al0.6CrFeCoNi | D | 5.74 | 20.9 | 26.23 | 25.04 | 22.09 |
| ID | 9.04 | 21.48 | 22.59 | 22.38 | 24.52 | |
| SR | 0.63 | 0.97 | 1.16 | 1.12 | 0.90 | |
| Al0.8CrFeCoNi | D | 7.98 | 26.63 | 23.32 | 22.15 | 19.92 |
| ID | 10.85 | 20.99 | 20.63 | 22.39 | 25.15 | |
| SR | 0.74 | 1.27 | 1.13 | 0.99 | 0.79 |
| Property | Al1.0CrFeCoNi (Al1.0) | Al0.8CrFeCoNi (Al0,8) | Al0.6CrFeCoNi (Al0,6) |
|---|---|---|---|
| HV (MPa) | 562 ± 33 | 427 ± 17 | 245 ± 11 |
| σy (MPa) | 187.5 ± 11 | 142.3 ± 6 | 81.9 ± 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Socorro-Perdomo, P.P.; Florido-Suárez, N.R.; Voiculescu, I.; Mirza-Rosca, J.C. Comparative EIS Study of AlxCoCrFeNi Alloys in Ringer’s Solution for Medical Instruments. Metals 2021, 11, 928. https://doi.org/10.3390/met11060928
Socorro-Perdomo PP, Florido-Suárez NR, Voiculescu I, Mirza-Rosca JC. Comparative EIS Study of AlxCoCrFeNi Alloys in Ringer’s Solution for Medical Instruments. Metals. 2021; 11(6):928. https://doi.org/10.3390/met11060928
Chicago/Turabian StyleSocorro-Perdomo, Pedro P., Néstor R. Florido-Suárez, Ionelia Voiculescu, and Julia C. Mirza-Rosca. 2021. "Comparative EIS Study of AlxCoCrFeNi Alloys in Ringer’s Solution for Medical Instruments" Metals 11, no. 6: 928. https://doi.org/10.3390/met11060928
APA StyleSocorro-Perdomo, P. P., Florido-Suárez, N. R., Voiculescu, I., & Mirza-Rosca, J. C. (2021). Comparative EIS Study of AlxCoCrFeNi Alloys in Ringer’s Solution for Medical Instruments. Metals, 11(6), 928. https://doi.org/10.3390/met11060928








