Synthesis of θ-Al2O3 Whiskers with Twins
Abstract
1. Introduction
2. Experimental Procedure
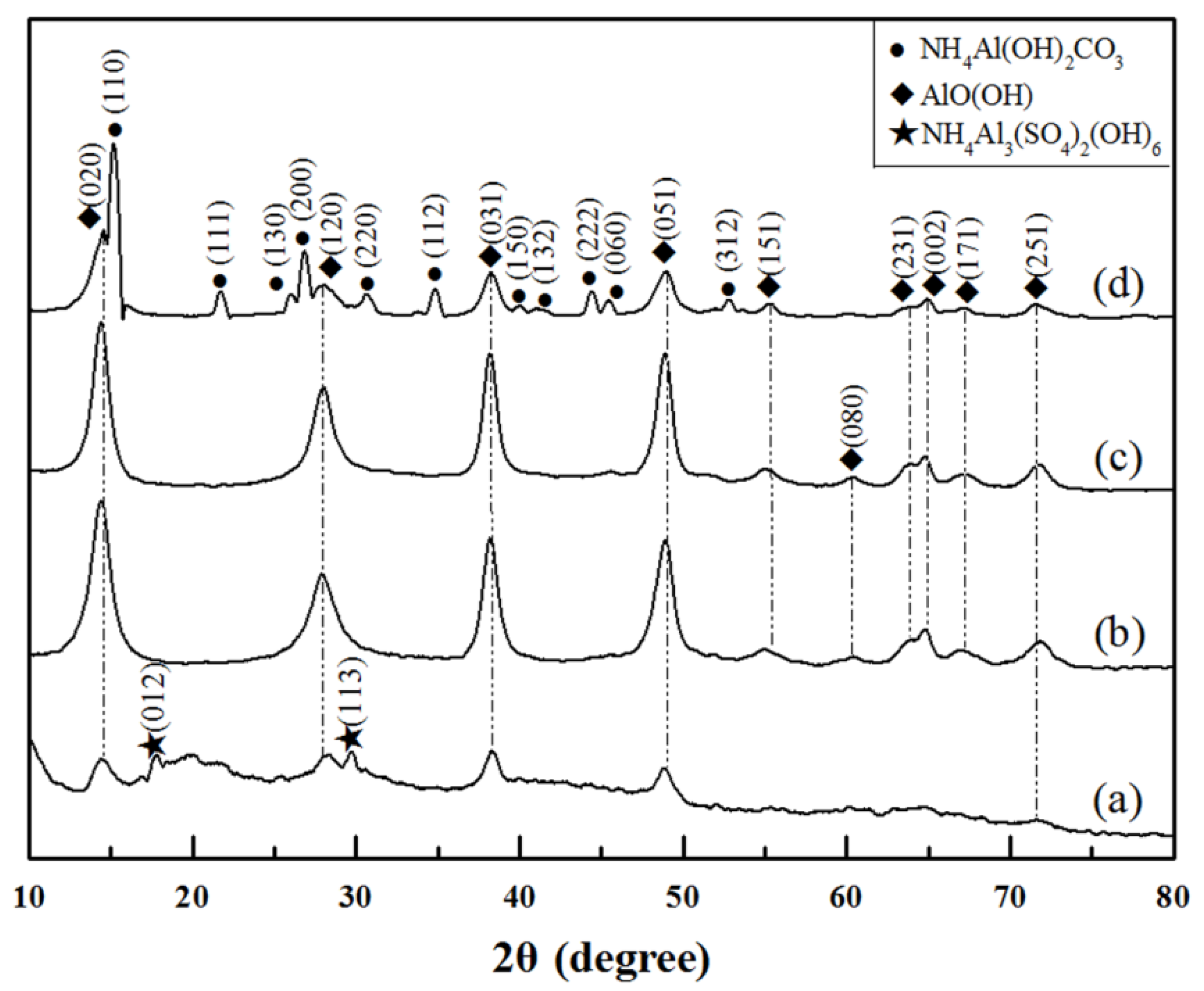
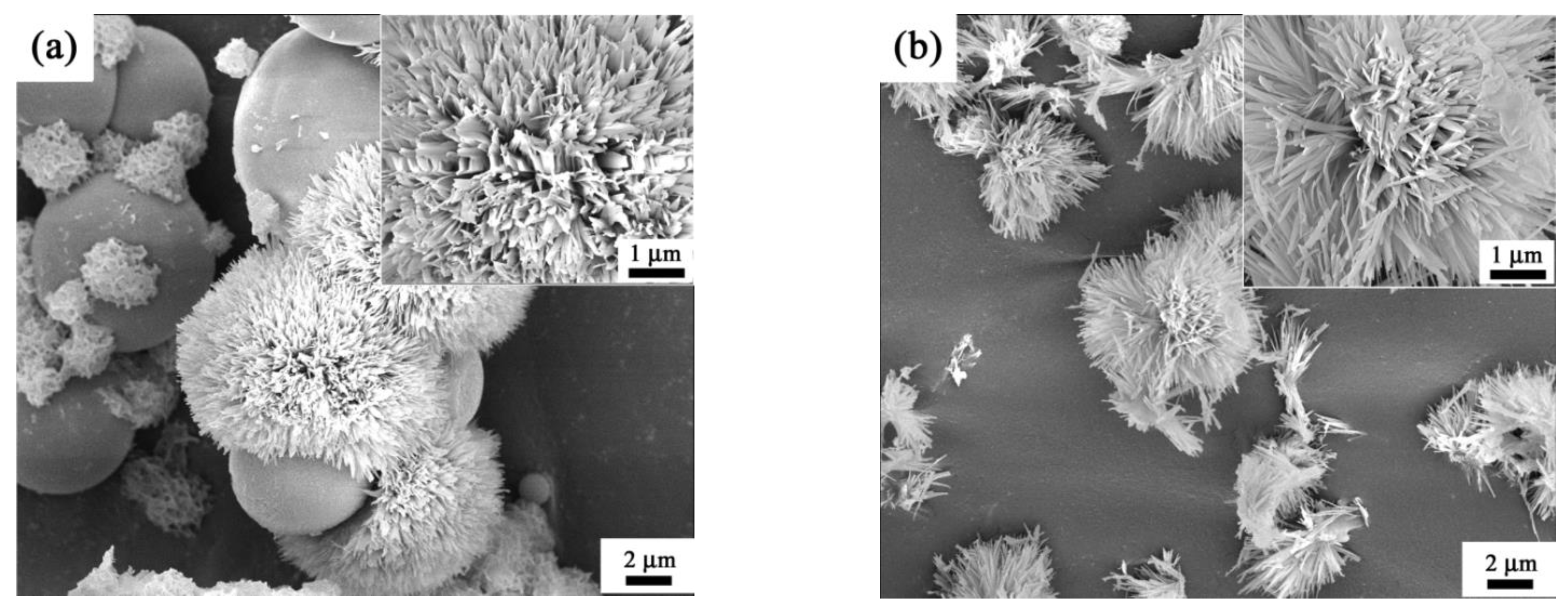
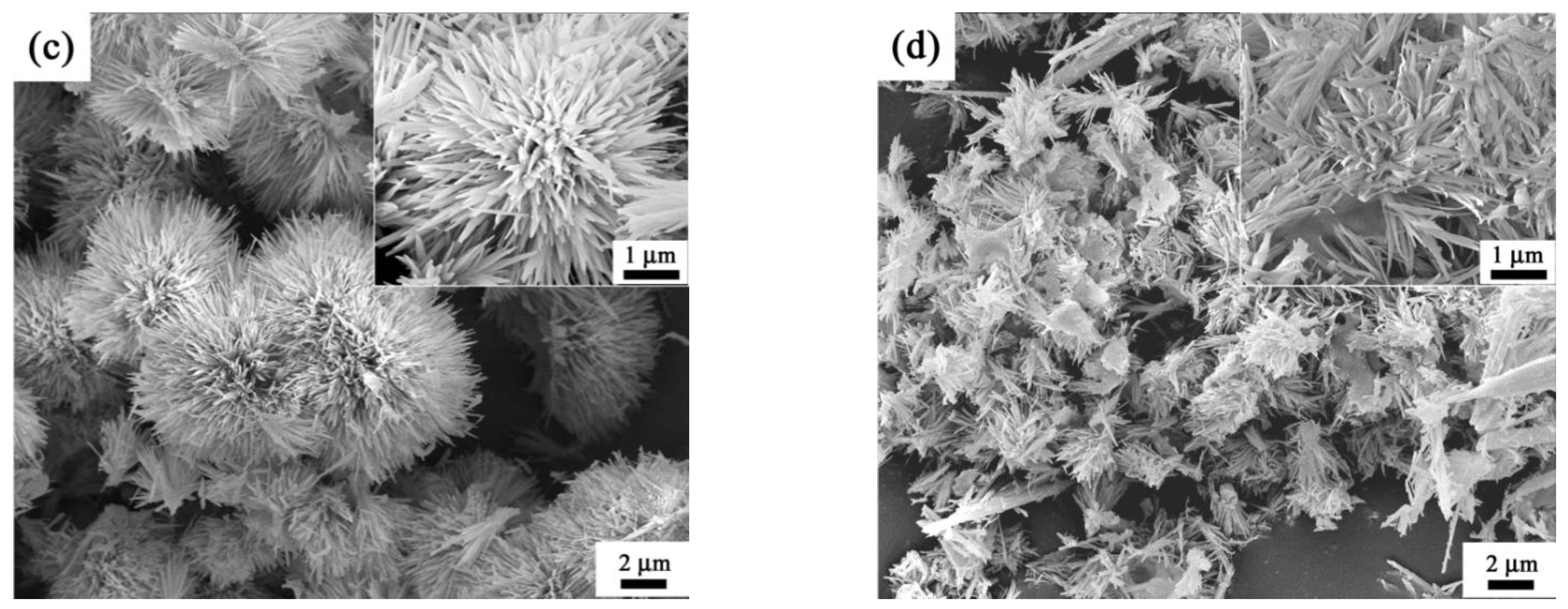
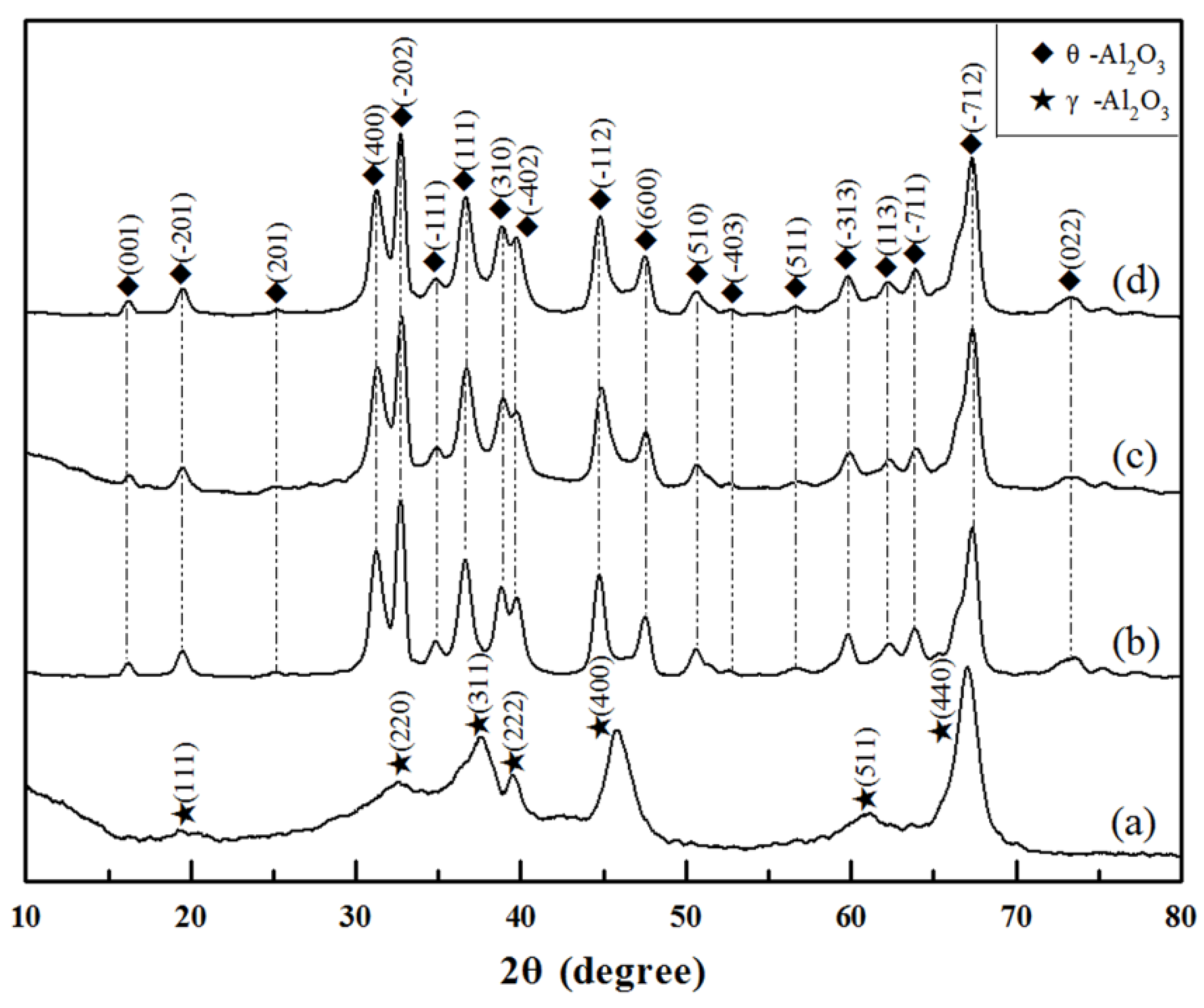
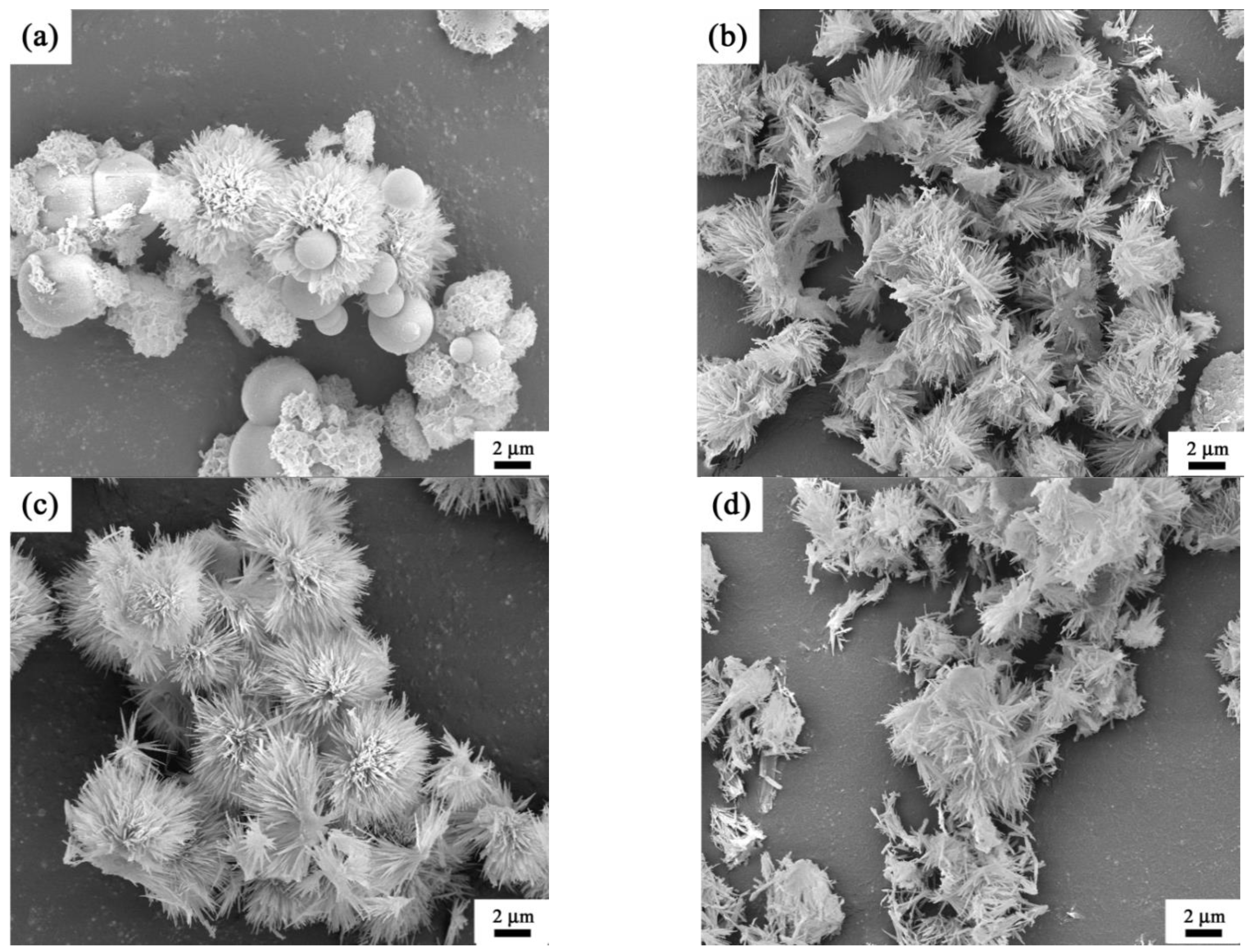
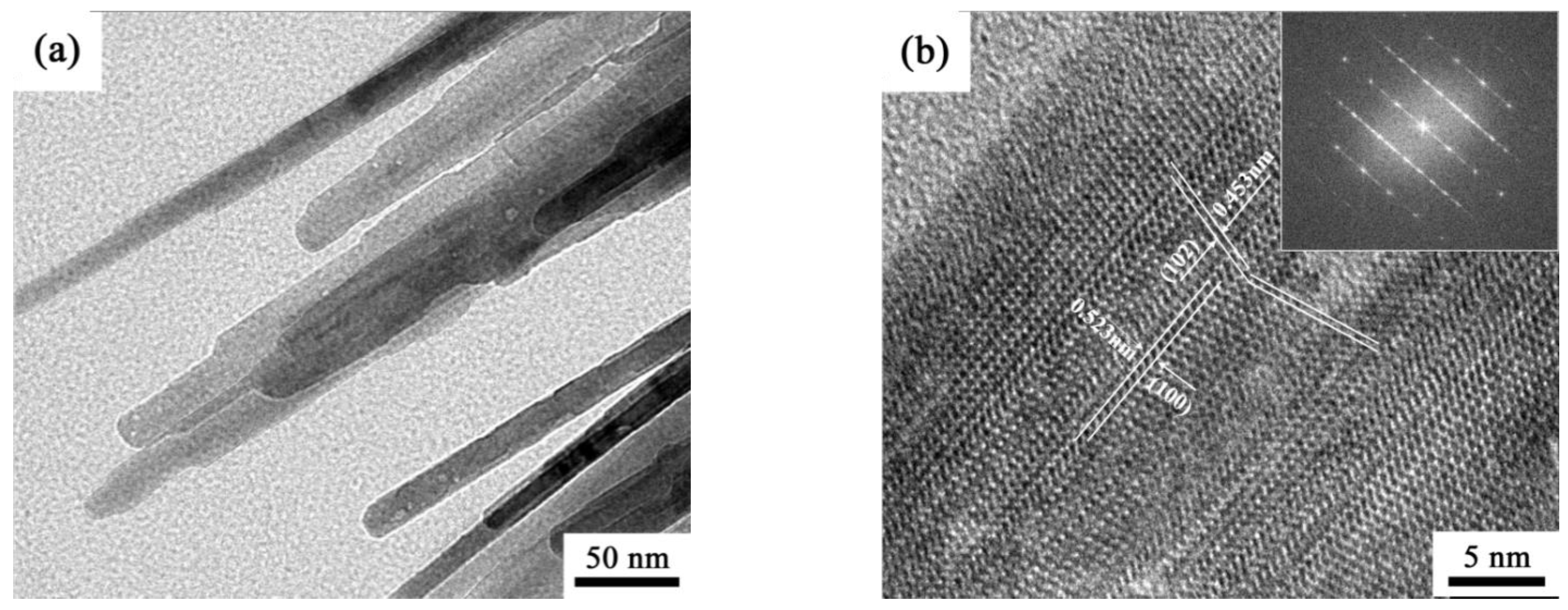
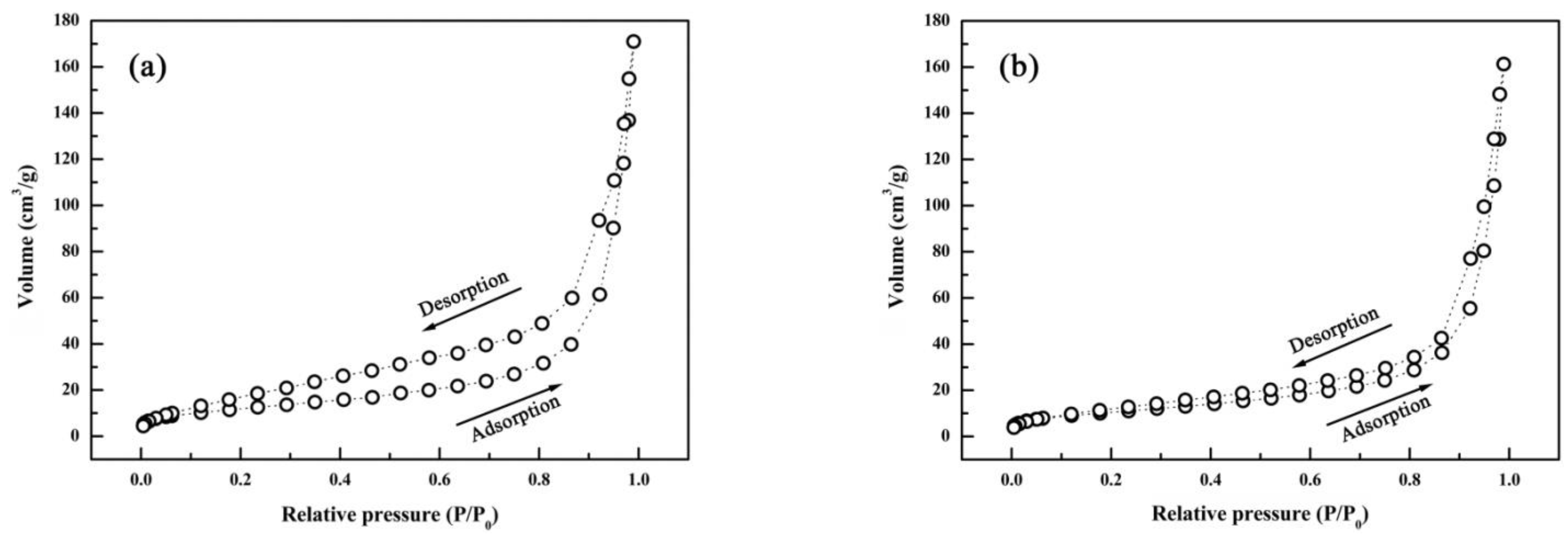
3. Results and Discussion
4. Conclusions
- θ-Al2O3 whiskers were successfully fabricated by using the hydrothermal method followed by annealing at 1000 °C in the flowing argon using Al2(SO4)3·18H2O, CO(NH2)2 and PEG2000 as the initial materials.
- The optimized molar ratio of Al3+:CO(NH2)2 was determined as 1:6 to obtain the homogeneous AlO(OH) precursor.
- The final morphology of θ-Al2O3 whiskers was derived from the initial microstructure of AlO(OH) precursor.
- θ-Al2O3 whiskers contained twins owing to the phase transformation from tetragonal system to monoclinic system during annealing. The high specific surface area of θ-Al2O3 whiskers was measured as 38.2 m2/g.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, W.; Zhu, S.; Bai, T.; Luo, Y. Influence of Al2O3 whisker concentration on mechanical properties of WC–Al2O3 whisker composite. Ceram. Int. 2015, 41, 13685–13691. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, Z.; Shen, J.; Zhu, L.; Xu, L.; Zhang, Y.; Wang, S.; Liu, T. Fabrication and catalytic growth mechanism of mullite ceramic whiskers using molybdenum oxide as catalyst. Ceram. Int. 2017, 43, 2871–2875. [Google Scholar] [CrossRef]
- Lv, X.; Ye, F.; Cheng, L.; Fan, S.; Liu, Y. Fabrication of SiC whisker-reinforced SiC ceramic matrix composites based on 3D printing and chemical vapor infiltration technology. J. Eur. Ceram. Soc. 2019, 39, 3380–3386. [Google Scholar] [CrossRef]
- Zou, D.; Ke, X.; Qiu, M.; Chen, X.; Fan, Y. Design and fabrication of whisker hybrid ceramic membranes with narrow pore size distribution and high permeability via co-sintering process. Ceram. Int. 2018, 44, 21159–21169. [Google Scholar] [CrossRef]
- Ding, J.; Deng, C.; Yuan, W.; Zhu, H.; Li, J. The synthesis of titanium nitride whiskers on the surface of graphite by molten salt media. Ceram. Int. 2013, 39, 2995–3000. [Google Scholar] [CrossRef]
- Xiong, H.; Li, Z.; Zhou, K. TiC whisker reinforced ultra-fine TiC-based cermets: Microstructure and mechanical properties. Ceram. Int. 2016, 42, 6858–6867. [Google Scholar] [CrossRef]
- Chen, X.; Li, T.; Ren, Q.; Wu, X.; Dang, A.; Li, H.; Zhao, T. Fabrication and morphology control of high strength lightweight mullite whisker network. J. Alloys Compd. 2017, 729, 285–292. [Google Scholar] [CrossRef]
- Zuo, F.; Meng, F.; Lin, D.-T.; Yu, J.-J.; Wang, H.-J.; Xu, S.; Guo, W.-M.; Cerecedo, C.; Valcárcel, V.; Lin, H.-T. Influence of whisker-aspect-ratio on densification, microstructure and mechanical properties of Al2O3 whiskers-reinforced CeO2-stabilized ZrO2 composites. J. Eur. Ceram. Soc. 2018, 38, 1796–1801. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, N.; Du, G.; Zhang, M.; Zhao, X.; Wu, J. Effect of Y2O3-stabilized ZrO2 whiskers on the microstructure, mechanical and wear resistance properties of Al2O3 based ceramic composites. Ceram. Int. 2019, 45, 16504–16511. [Google Scholar] [CrossRef]
- Akatsu, T.; Takashima, H.; Shinoda, Y.; Wakai, F.; Wakayama, S. Thermal-Shock Fracture and Damage Resistance Improved by Whisker Reinforcement in Alumina Matrix Composite. Int. J. Appl. Ceram. Technol. 2016, 13, 653–661. [Google Scholar] [CrossRef]
- Zhao, Z.; Johnsson, M.; Shen, Z. Microstructure and mechanical properties of titanium carbonitride whisker reinforced β-sialon matrix composites. Mater. Res. Bull. 2002, 37, 1175–1187. [Google Scholar] [CrossRef]
- Abdullah, M.; Mehmood, M.; Ahmad, J. Single step hydrothermal synthesis of 3D urchin like structures of AACH and aluminum oxide with thin nano-spikes. Ceram. Int. 2012, 38, 3741–3745. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, S.L.; Ma, S.H.; Liu, C.L.; Wang, X.H. Mullite-bonded SiC-whisker-reinforced SiC matrix composites: Preparation, characterization, and toughening mechanisms. J. Eur. Ceram. Soc. 2018, 38, 5282–5293. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Zeng, D.J.; Wang, B.; Yang, J.F. Effect of heating parameters on sintering behaviors and properties of mullite whisker frameworks. Nanotechnology 2018, 29, 164001. [Google Scholar] [CrossRef]
- Qu, X.Y.; Wang, F.C.; Shi, C.S.; Zhao, N.Q.; Liu, E.Z.; He, C.N.; He, F. In situ synthesis of a gamma-Al2O3 whisker reinforced aluminum matrix composite by cold pressing and sintering. Mater. Sci. Eng. A 2018, 709, 223–231. [Google Scholar] [CrossRef]
- Tamura, Y.; Moshtaghioun, B.M.; Zapata-Solvas, E.; Gomez-Garcia, D.; Domínguez-Rodríguez, A.; Cerecedo-Fernández, C.; Valcárcel-Juárez, V. Is an alumina-whisker-reinforced alumina composite the most efficient choice for an oxidation-resistant high-temperature ceramic? J. Eur. Ceram. Soc. 2018, 38, 1812–1818. [Google Scholar] [CrossRef]
- Ali, M.; Mujtaba-Ul-Hassan, S.; Ahmad, J.; Khurshid, A.; Shahzad, F.; Iqbal, Z.; Mehmood, M.; Waheed, K. Fabrication of PEGylated Porous Alumina Whiskers (PAW) for drug delivery applications. Mater. Lett. 2019, 241, 23–26. [Google Scholar] [CrossRef]
- Valcárcel, V.; Souto, A.; Guitián, F. Development of single-crystal α-Al2O3 fibers by vapor-liquid-solid deposition (VLS) from aluminum and powdered silica. Adv. Mater. 1998, 10, 138–140. [Google Scholar] [CrossRef]
- Chu, Y.; Jing, S.; Liu, D.; Ye, B. Screw dislocation assisted spontaneous growth of single-crystalline α-Al2O3 microrods. Compos. Commun. 2018, 10, 93–96. [Google Scholar] [CrossRef]
- Ahmad, J.; Tariq, M.I.; Ahmad, R.; Ul-Hassan, S.M.; Mehmood, M.; Khan, A.F.; Waseem, S.; Mehboob, S.; Tanvir, M.T. Formation of porous α-alumina from ammonium aluminum carbonate hydroxide whiskers. Ceram. Inter. 2019, 45, 4645–4652. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Nai, X.; Bian, S.; Liu, X.; Wei, M. Synthesis and formation of alumina whiskers from hydrothermal solution. J. Mater. Sci. 2009, 45, 177–181. [Google Scholar] [CrossRef]
- Mirzajany, R.; Alizadeh, M.; Saremi, M.; Rahimipour, M.-R. Suspension preparation of alumina whiskers for spray granulation. Mater. Lett. 2018, 228, 344–347. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, H.; Liu, H.; Yang, D. PEG-directed hydrothermal synthesis of alumina nanorods with mesoporous structure via AACH nanorod precursors. J. Mater. Sci. 2010, 45, 46–50. [Google Scholar] [CrossRef]
- Morinaga, K.; Torikai, T.; Nakagawa, K.; Fujino, S. Fabrication of fine α-alumina powders by thermal decomposition of ammonium aluminum carbonate hydroxide (AACH). Acta Mater. 2000, 48, 4735–4741. [Google Scholar] [CrossRef]
- Deng, Z.-Y.; Fukasawa, T.; Ando, M.; Zhang, G.-J.; Ohji, T. High-Surface-Area Alumina Ceramics Fabricated by the Decomposition of Al(OH)3. J. Am. Ceram. Soc. 2001, 84, 485–491. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, D.; Grasso, S.; Hu, C. Tunable morphology of aluminum oxide whiskers grown by hydrothermal method. Ceram. Int. 2018, 44, 14967–14973. [Google Scholar] [CrossRef]
- Bradford, S.C. On the Theory of Gels. Biochem. J. 1921, 15, 553–562.1. [Google Scholar] [CrossRef] [PubMed]
- Digne, M.; Sautet, P.; Raybaud, P.; Toulhoat, H.; Artacho, E. Structure and Stability of Aluminum Hydroxides: A Theoretical Study. J. Phys. Chem. B 2002, 106, 5155–5162. [Google Scholar] [CrossRef]
- Mirzajany, R.; Alizadeh, M.; Rahimipour, M.-R.; Saremi, M. Seed-assisted hydrothermally synthesized AACH as the alumina precursors. Mater. Chem. Phys. 2019, 221, 188–196. [Google Scholar] [CrossRef]
- Łodziana, Z.; Topsøe, N.-Y.; Nørskov, J.K. A negative surface energy for alumina. Nat. Mater. 2004, 3, 289–293. [Google Scholar] [CrossRef]
- Wang, Y.G.; Bronsveld, P.M.; DeHosson, J.T.M.; Djuricic, B.; McGarry, D.; Pickering, S. Ordering of Octahedral Vacancies in Transition Aluminas. J. Am. Ceram. Soc. 2005, 81, 1655–1660. [Google Scholar] [CrossRef]
- Wang, Y.; Bronsveld, P.; De Hosson, J.; Djuricic, B.; McGarry, D.; Pickering, S. Twinning in θ Alumina Investigated with High Resolution Transmission Electron Microscopy. J. Eur. Ceram. Soc. 1998, 18, 299–304. [Google Scholar] [CrossRef]
- Xue, Y.; Lin, J.; Fan, Y.; Li, J.; Elsanousi, A.; Xu, X.; Liu, N.; Huang, Y.; Hu, L.; Liu, Y.; et al. Synthesis and hydrogen absorption of high-specific-surface ultrafine theta-Al2O3 nanowires. J. Cryst. Growth 2013, 382, 52–55. [Google Scholar] [CrossRef]
- Shen, P.; Lee, W.H. (111)-Specific Coalescence Twinning and Martensitic Transformation of Tetragonal ZrO2 Condensates. Nano Lett. 2001, 1, 707–711. [Google Scholar] [CrossRef]
- Lam, K.Y.; Zhang, J.M. Transformation twinning in monoclinic zirconia particles. Acta Met. Mater. 1992, 40, 1395–1401. [Google Scholar] [CrossRef]
- Lin, G.; Lei, T.; Zhou, Y. In-situ TEM observations of tetragonal to monoclinic phase transformation in ZrO2-2 mol% Y2O3 ceramics. Ceram. Int. 1998, 24, 307–312. [Google Scholar] [CrossRef]
- Simha, N. Twin and habit plane microstructures due to the tetragonal to monoclinic transformation of zirconia. J. Mech. Phys. Solids 1997, 45, 261–263. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, J.; Mao, X.; Zhang, F.; Sun, F.; Chen, F.; Wang, J.; Tian, R.; Liu, J.; Wang, S. Strengthening mechanism of twin lamellas in transparent AlON ceramics. J. Eur. Ceram. Soc. 2018, 38, 3235–3239. [Google Scholar] [CrossRef]
- Zhao, B.; Song, J.; Fang, T.; Liu, P.; Jiao, Z.; Zhang, H.; Jiang, Y. Hydrothermal method to prepare porous NiO nanosheet. Mater. Lett. 2012, 67, 24–27. [Google Scholar] [CrossRef]
- Wang, L.; An, L.; Zhao, J.; Shimai, S.; Mao, X.; Zhang, J.; Liu, J.; Wang, S. High-strength porous alumina ceramics prepared from stable wet foams. J. Adv. Ceram. 2021, 1–8. [Google Scholar] [CrossRef]
- Krivoshapkina, E.F.; Krivoshapkin, P.V.; Vedyagin, A.A. Synthesis of Al2O3-SiO2-MgO ceramics with hierarchical porous structure. J. Adv. Ceram. 2017, 6, 11–19. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, N.; Su, X.; Zhang, H.; Feng, Q.; Grasso, S.; Hu, C. Synthesis of θ-Al2O3 Whiskers with Twins. Metals 2021, 11, 895. https://doi.org/10.3390/met11060895
Liao N, Su X, Zhang H, Feng Q, Grasso S, Hu C. Synthesis of θ-Al2O3 Whiskers with Twins. Metals. 2021; 11(6):895. https://doi.org/10.3390/met11060895
Chicago/Turabian StyleLiao, Nan, Xiaojia Su, Haiwen Zhang, Qingguo Feng, Salvatore Grasso, and Chunfeng Hu. 2021. "Synthesis of θ-Al2O3 Whiskers with Twins" Metals 11, no. 6: 895. https://doi.org/10.3390/met11060895
APA StyleLiao, N., Su, X., Zhang, H., Feng, Q., Grasso, S., & Hu, C. (2021). Synthesis of θ-Al2O3 Whiskers with Twins. Metals, 11(6), 895. https://doi.org/10.3390/met11060895








