Investigation of the Cause-Effect Relationships between the Exothermic Reaction and the Microstructures of Reactive Ni-Al Particles Produced by High Energy Planetary Ball Milling
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactive Particle Manufacturing
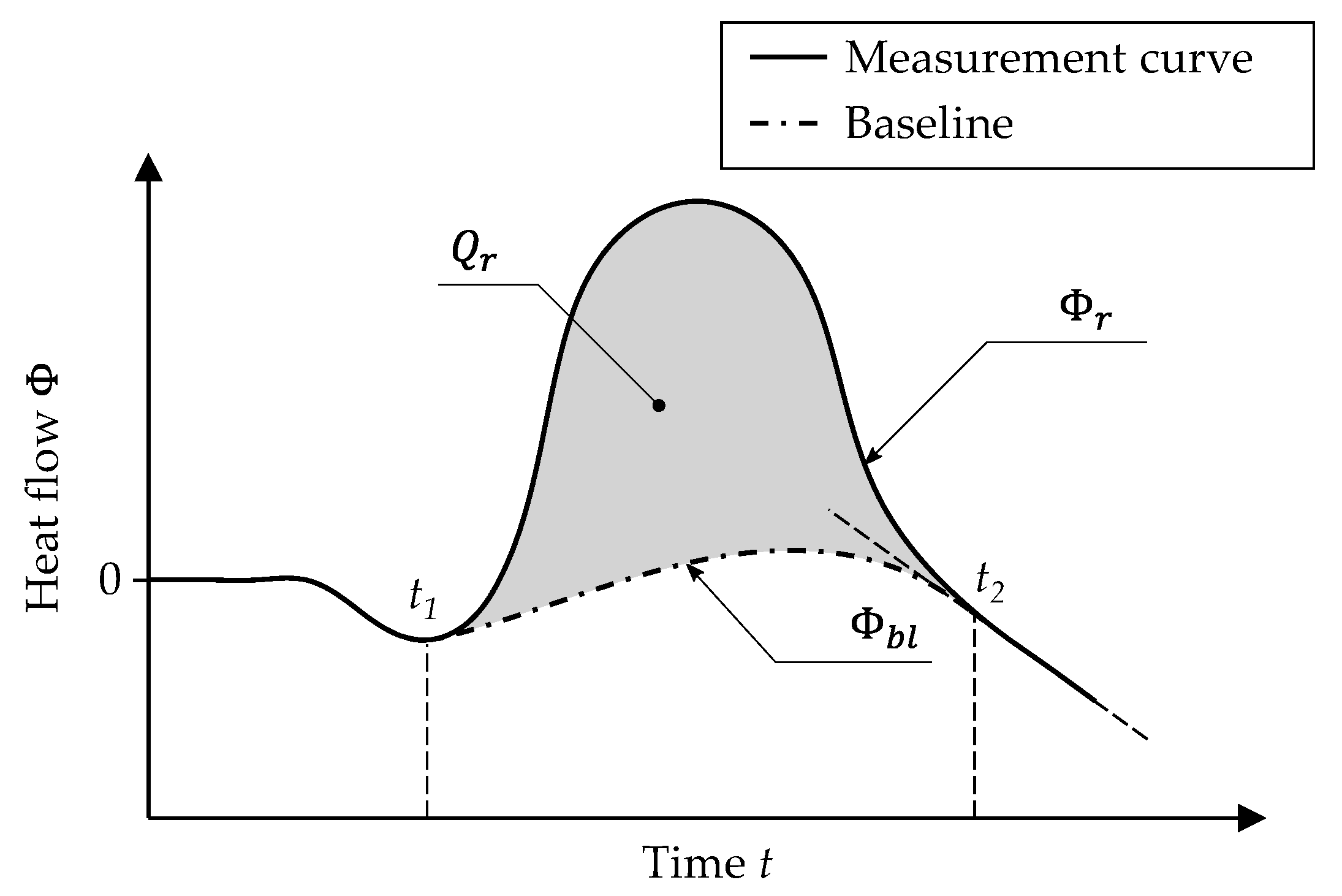
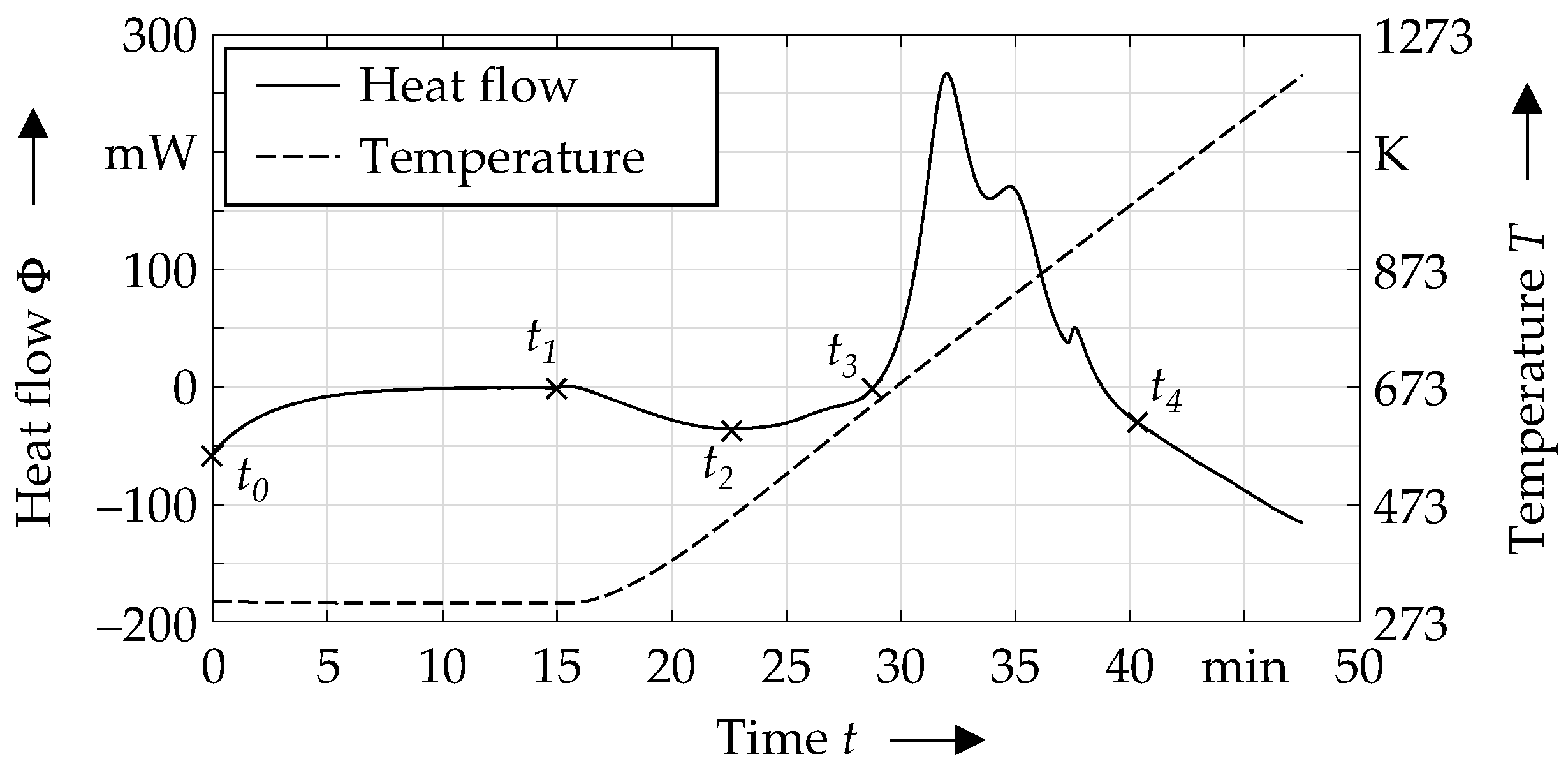
2.2. Differential Scanning Calorimetry
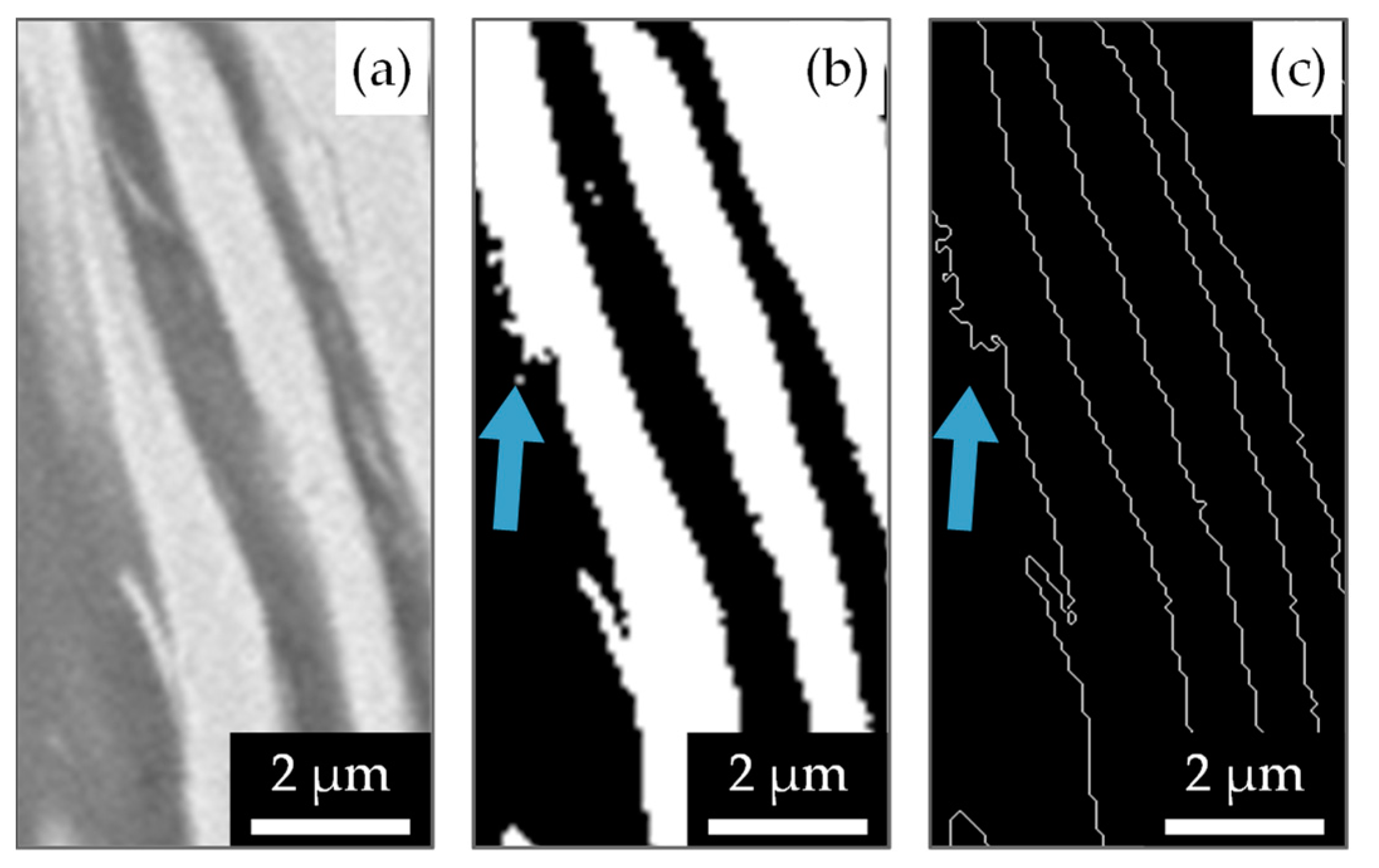
2.3. SEM Image Evaluation Algorithm
- The recorded SEM images had to be sharp.
- No epoxy resin could be visible.
- No outer edges of the reactive particles could be visible.
- The selection of the embedded reactive particles to be imaged was randomized.
3. Results and Discussion
3.1. Thermal Characterization by Means of DSC
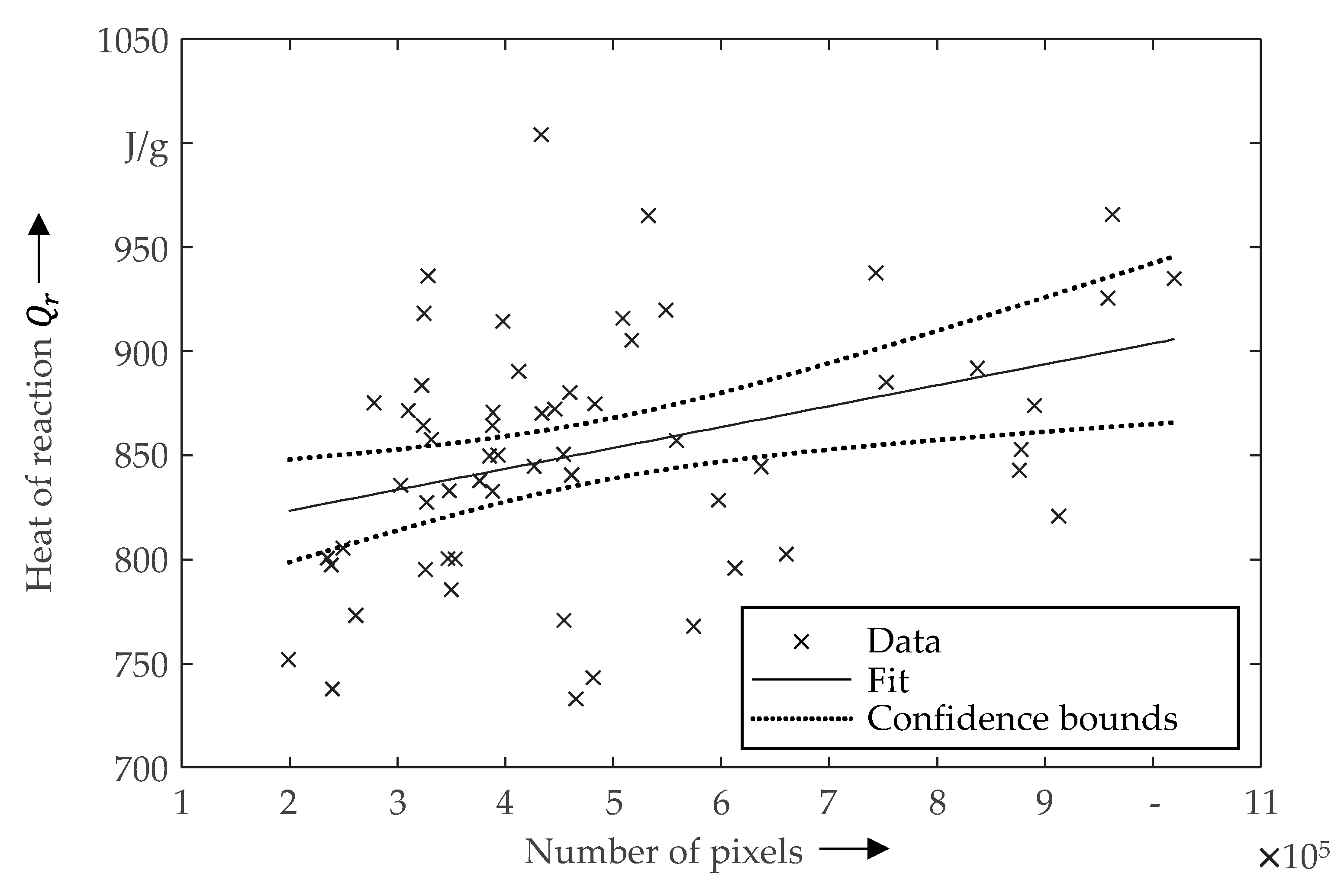
3.2. Correlation between the Microstructure and the Heat of Reaction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Exp. No. | n | t | rbtp | d | Exp. No. | n | t | rbtp | d | |
|---|---|---|---|---|---|---|---|---|---|---|
| in rpm | in min | in mm | in rpm | in min | in mm | |||||
| 1 | 725 | 22.5 | 7.5 | 5 | 31 | 800 | 30 | 7.5 | 3 | |
| 2 | 650 | 15 | 5 | 3 | 32 | 650 | 30 | 7.5 | 3 | |
| 3 | 800 | 15 | 5 | 3 | 33 | 725 | 30 | 5 | 5 | |
| 4 | 650 | 30 | 5 | 3 | 34 | 725 | 30 | 10 | 5 | |
| 5 | 800 | 30 | 5 | 3 | 35 | 800 | 30 | 7.5 | 5 | |
| 6 | 650 | 15 | 10 | 3 | 36 | 650 | 30 | 7.5 | 5 | |
| 7 | 800 | 15 | 10 | 3 | 37 | 800 | 15 | 5 | 5 | |
| 8 | 650 | 30 | 10 | 3 | 38 | 800 | 30 | 5 | 5 | |
| 9 | 800 | 30 | 10 | 3 | 39 | 800 | 15 | 10 | 5 | |
| 10 | 650 | 15 | 5 | 10 | 40 | 800 | 30 | 10 | 5 | |
| 11 | 800 | 15 | 5 | 10 | 41 | 650 | 15 | 5 | 5 | |
| 12 | 650 | 30 | 5 | 10 | 42 | 650 | 30 | 5 | 5 | |
| 13 | 800 | 30 | 5 | 10 | 43 | 650 | 15 | 10 | 5 | |
| 14 | 650 | 15 | 10 | 10 | 44 | 800 | 15 | 10 | 10 | |
| 15 | 800 | 15 | 10 | 10 | 45 | 650 | 22.5 | 7.5 | 10 | |
| 16 | 650 | 30 | 10 | 10 | 46 | 725 | 30 | 5 | 10 | |
| 17 | 800 | 30 | 10 | 10 | 47 | 725 | 30 | 10 | 10 | |
| 18 | 650 | 22.5 | 7.5 | 5 | 48 | 725 | 15 | 7.5 | 10 | |
| 19 | 800 | 22.5 | 7.5 | 5 | 49 | 725 | 22.5 | 5 | 10 | |
| 20 | 725 | 15 | 7.5 | 5 | 50 | 800 | 30 | 7.5 | 10 | |
| 21 | 725 | 30 | 7.5 | 5 | 51 | 650 | 30 | 7.5 | 10 | |
| 22 | 725 | 22.5 | 5 | 5 | 52 | 800 | 15 | 7.5 | 10 | |
| 23 | 725 | 22.5 | 10 | 5 | 53 | 650 | 15 | 7.5 | 10 | |
| 24 | 725 | 22.5 | 7.5 | 3 | 54 | 725 | 15 | 10 | 10 | |
| 25 | 725 | 22.5 | 7.5 | 10 | 55 | 650 | 22.5 | 10 | 10 | |
| 26 | 800 | 22.5 | 7.5 | 3 | 56 | 800 | 22.5 | 5 | 10 | |
| 27 | 725 | 30 | 5 | 3 | 57 | 650 | 22.5 | 5 | 10 | |
| 28 | 725 | 30 | 10 | 3 | 58 | 725 | 15 | 5 | 10 | |
| 29 | 725 | 30 | 7.5 | 3 | 59 | 800 | 22.5 | 10 | 10 | |
| 30 | 725 | 22.5 | 10 | 3 |
References
- Morsi, K. Review: Reaction synthesis processing of Ni–Al intermetallic materials. Mater. Sci. Eng. A 2001, 299, 1–15. [Google Scholar] [CrossRef]
- Merzhanov, A.G. The chemistry of self-propagating high-temperature synthesis. J. Mater. Chem. 2004, 14, 1779–1786. [Google Scholar] [CrossRef]
- Merzhanov, A.G. History and recent developments in SHS. Ceram. Int. 1995, 21, 371–379. [Google Scholar] [CrossRef]
- Rogachev, A.S.; Mukasyan, A.S. Combustion for Material Synthesis; CISP; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-1-4822-3952-2. [Google Scholar]
- Deevi, S.C.; Sikka, V.K. Nickel and iron aluminides: An overview on properties, processing, and applications. Intermetallics 1996, 4, 357–375. [Google Scholar] [CrossRef]
- Theodossiadis, G.D.; Zaeh, M.F. Assessment of the technological potential and maturity of a novel joining technique based on reactive nanofoils. Prod. Eng. Res. Dev. 2017, 11, 237–243. [Google Scholar] [CrossRef]
- Grohmann, S.; Langhans, G.; Reindl, A.; Sidarava, V.; Zaeh, M.F. Investigation of reactive bimetallic Ni–Al particles as a heat source for microwave-assisted joining. J. Mater. Process. Technol. 2020, 282, 116637. [Google Scholar] [CrossRef]
- Schreiber, S.; Theodossiadis, G.D.; Zaeh, M.F. Combustion synthesis of reactive nickel-aluminum particles as an innovative approach for thermal joining applications. IOP Conf. Ser. Mater. Sci. Eng. 2017, 181, 1–8. [Google Scholar] [CrossRef]
- Schreiber, S.; Zaeh, M.F. Electroplating of aluminium microparticles with nickel to synthesise reactive core-shell structures for thermal joining applications. IOP Conf. Ser. Mater. Sci. Eng. 2018, 373, 1–9. [Google Scholar] [CrossRef]
- Rogachev, A.S.; Shkodich, N.F.; Vadchenko, S.G.; Baras, F.; Kovalev, D.Y.; Rouvimov, S.; Nepapushev, A.A.; Mukasyan, A.S. Influence of the high energy ball milling on structure and reactivity of the Ni+Al powder mixture. J. Alloy. Compd. 2013, 577, 600–605. [Google Scholar] [CrossRef]
- Hadjiafxenti, A.; Gunduz, I.E.; Kyratsi, T.; Doumanidis, C.C.; Rebholz, C. Exothermic reaction characteristics of continuously ball-milled Al/Ni powder compacts. Vacuum 2013, 96, 73–78. [Google Scholar] [CrossRef]
- Hadjiafxenti, A.; Gunduz, I.E.; Tsotsos, C.; Kyratsi, T.; Doumanidis, C.C.; Rebholz, C. Synthesis of reactive Al/Ni structures by ball milling. Intermetallics 2010, 18, 2219–2223. [Google Scholar] [CrossRef]
- Cherukara, M.J.; Germann, T.C.; Kober, E.M.; Strachan, A. Shock loading of granular Ni/Al composites. Part 2: Shock-induced chemistry. J. Phys. Chem. C 2016, 120, 6804–6813. [Google Scholar] [CrossRef]
- Rogachev, A.S.; Moskovskikh, D.O.; Nepapushev, A.A.; Sviridova, T.A.; Vadchenko, S.G.; Rogachev, S.A.; Mukasyan, A.S. Experimental investigation of milling regimes in planetary ball mill and their influence on structure and reactivity of gasless powder exothermic mixtures. Powder Technol. 2015, 274, 44–52. [Google Scholar] [CrossRef]
- White, J.D.E.; Reeves, R.V.; Son, S.F.; Mukasyan, A.S. Thermal explosion in Al-Ni system: Influence of mechanical activation. J. Phys. Chem. A 2009, 113, 13541–13547. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; White, J.D.E.; Kovalev, D.Y.; Kochetov, N.A.; Ponomarev, V.I.; Son, S.F. Dynamics of phase transformation during thermal explosion in the Al–Ni system: Influence of mechanical activation. Phys. B Condens. Matter 2010, 405, 778–784. [Google Scholar] [CrossRef]
- Shuck, C.E.; Mukasyan, A.S. Reactive Ni/Al nanocomposites: Structural characteristics and activation energy. J. Phys. Chem. A 2017, 121, 1175–1181. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Mason, B.A.; Groven, L.J.; Lin, Y.-C.; Cherukara, M.; Son, S.F.; Strachan, A.; Mukasyan, A.S. Tailored reactivity of Ni+Al nanocomposites: Microstructural correlations. J. Phys. Chem. C 2012, 116, 21027–21038. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Al-Aqeeli, N. Mechanically alloyed nanocomposites. Prog. Mater. Sci. 2013, 58, 383–502. [Google Scholar] [CrossRef]
- Gu, Z.; Cui, Q.; Chen, J.; Buckley, J.; Ando, T.; Erdeniz, D.; Wong, P.; Hadjiafxenti, A.; Epaminonda, P.; Gunduz, I.E.; et al. Fabrication, characterization and applications of novel nanoheater structures. Surf. Coat. Technol. 2013, 215, 493–502. [Google Scholar] [CrossRef]
- Kovalev, D.Y.; Kochetov, N.A.; Ponomarev, V.I.; Mukasyan, A.S. Effect of mechanical activation on thermal explosion in Ni-Al mixtures. Int. J. Self-Propag. High-Temp. Synth. 2010, 19, 120–125. [Google Scholar] [CrossRef]
- Gunduz, I.E.; Kyriakou, A.; Vlachos, N.; Kyratsi, T.; Doumanidis, C.C.; Son, S.; Rebholz, C. Spark ignitable Ni–Al ball-milled powders for bonding applications. Surf. Coat. Technol. 2014, 260, 396–400. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Kim, T.-J.; Cho, Y.-J. Application of ball-milled powder mixture to intermetallics coating through reaction synthesis. Met. Mater. Int. 2013, 19, 1289–1293. [Google Scholar] [CrossRef]
- Höhne, G.W.H.; Hemminger, W.; Flammersheim, H.-J. Differential Scanning Calorimetry, 2nd ed.; Springer: Berlin, Germany, 2003; ISBN 354000467x. [Google Scholar]
- Otsu, N. A threshold selection method from gray-Level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Thiers, L.; Mukasyan, A.S.; Varma, A. Thermal explosion in Ni-Al system: Influence of reaction medium microstructure. Combust. Flame 2002, 131, 198–209. [Google Scholar] [CrossRef]
- Knepper, R.; Snyder, M.R.; Fritz, G.; Fisher, K.; Knio, O.M.; Weihs, T.P. Effect of varying bilayer spacing distribution on reaction heat and velocity in reactive Al/Ni multilayers. J. Appl. Phys. 2009, 105, 083504-1–083504-9. [Google Scholar] [CrossRef]
- Simões, S.; Viana, F.; Ramos, A.S.; Vieira, M.T.; Vieira, M.F. Anisothermal solid-state reactions of Ni/Al nanometric multilayers. Intermetallics 2011, 19, 350–356. [Google Scholar] [CrossRef]
- Baláž, P. Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin, Germany, 2008; ISBN 978-3-540-74855-7. [Google Scholar]
- Stover, A.K.; Krywopusk, N.M.; Fritz, G.M.; Barron, S.C.; Gibbins, J.D.; Weihs, T.P. An analysis of the microstructure and properties of cold-rolled Ni:Al laminate foils. J. Mater. Sci. 2013, 48, 5917–5929. [Google Scholar] [CrossRef]

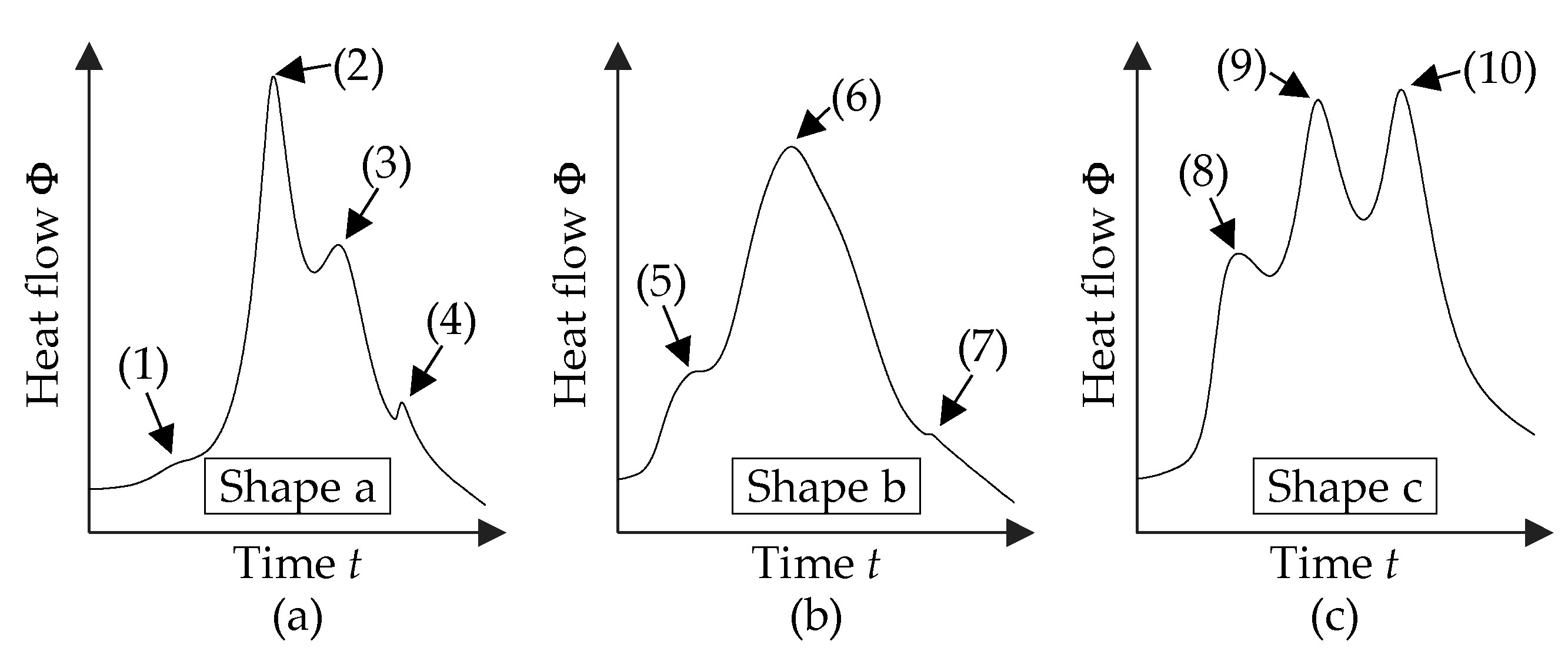
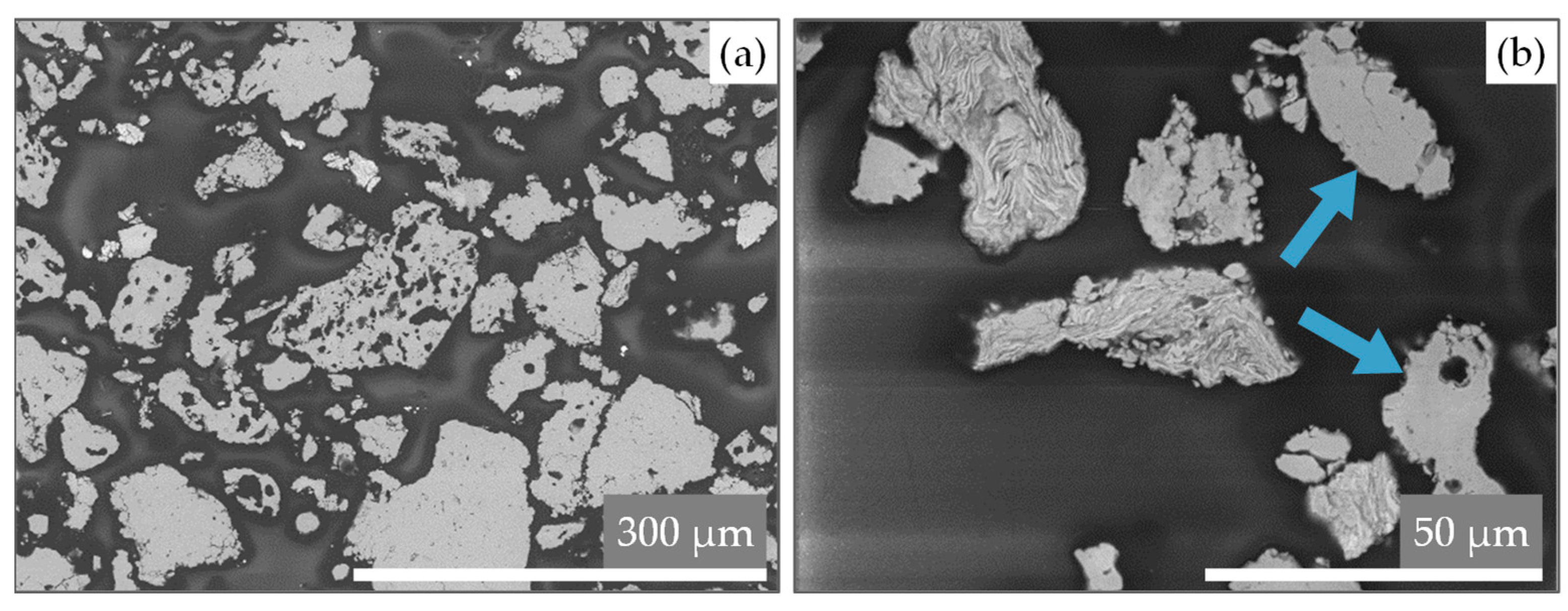

| Exp. No. | Shape | Exp. No. | Shape | Exp. No. | Shape | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| in J/g | in J/g | in J/g | ||||||||
| 1 | 914 | b | 21 | 920 | b | 41 | 740 | a | ||
| 2 | 801 | a | 22 | 918 | a-b | 42 | 872 | a-b | ||
| 3 | 858 | a-b | 23 | 829 | b-c | 43 | 865 | a-b | ||
| 4 | 864 | a-b | 24 | 800 | b | 44 | 823 | c | ||
| 5 | 890 | b | 25 | 880 | a | 45 | 837 | a | ||
| 6 | 771 | b | 26 | 872 | b | 46 | 840 | a | ||
| 7 | 743 | b | 27 | 831 | b | 47 | 854 | c | ||
| 8 | 733 | b | 28 | 894 | b | 48 | 796 | a | ||
| 9 | 796 | b | 29 | 850 | b | 49 | 839 | a | ||
| 10 | 752 | a | 30 | 851 | b | 50 | 925 | c | ||
| 11 | 875 | a | 31 | 876 | a-b | 51 | 872 | a | ||
| 12 | 827 | a-b | 32 | 871 | b | 52 | 874 | a | ||
| 13 | 1004 | b-c | 33 | 906 | a-b | 53 | 769 | a | ||
| 14 | 785 | a | 34 | 938 | c | 54 | 851 | a | ||
| 15 | 803 | c | 35 | 969 | c | 55 | 845 | a | ||
| 16 | 768 | a-b | 36 | 858 | a | 56 | 844 | a | ||
| 17 | 843 | c | 37 | 834 | a-b | 57 | 803 | a | ||
| 18 | 884 | a-b | 38 | 935 | b-c | 58 | 800 | a | ||
| 19 | 965 | b | 39 | 918 | c | 59 | 937 | c | ||
| 20 | 800 | b | 40 | 886 | b-c |
| Shape acc. to Figure 5 | Quantity | in J/g | Standard Deviation |
|---|---|---|---|
| all | 59 | 852 | 60 |
| a | 19 | 824 | 42 |
| b | 15 | 842 | 66 |
| c | 9 | 890 | 56 |
| a-b | 11 | 861 | 39 |
| b-c | 4 | 913 | 65 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| n | 40,682 | 2 | 20,341 | 11.55 | 0.0001 |
| t | 38,110 | 2 | 19,055 | 10.82 | 0.0001 |
| rbtp | 4980 | 2 | 2490 | 1.41 | 0.2529 |
| d | 26,049 | 2 | 13,025 | 7.39 | 0.0015 |
| Error | 88,076 | 50 | 1762 | ||
| Total | 203,812 | 58 |
| Exp. No. | NOP (Mean) | Exp. No. | NOP (Mean) | Exp. No. | NOP (Mean) | ||
|---|---|---|---|---|---|---|---|
| 1 | 3.98 | 21 | 5.49 | 41 | 2.40 | ||
| 2 | 2.35 | 22 | 3.25 | 42 | 3.10 | ||
| 3 | 3.31 | 23 | 5.98 | 43 | 3.88 | ||
| 4 | 3.24 | 24 | 3.54 | 44 | 9.13 | ||
| 5 | 4.12 | 25 | 4.60 | 45 | 3.03 | ||
| 6 | 4.54 | 26 | 4.34 | 46 | 4.61 | ||
| 7 | 4.82 | 27 | 3.88 | 47 | 8.78 | ||
| 8 | 4.65 | 28 | 8.37 | 48 | 3.26 | ||
| 9 | 6.13 | 29 | 4.54 | 49 | 3.76 | ||
| 10 | 1.99 | 30 | 3.85 | 50 | 9.59 | ||
| 11 | 2.79 | 31 | 8.90 | 51 | 4.46 | ||
| 12 | 3.27 | 32 | 3.89 | 52 | 4.83 | ||
| 13 | 4.33 | 33 | 5.17 | 53 | 2.61 | ||
| 14 | 3.50 | 34 | 10.20 | 54 | 3.93 | ||
| 15 | 6.61 | 35 | 9.63 | 55 | 6.37 | ||
| 16 | 5.74 | 36 | 5.59 | 56 | 4.27 | ||
| 17 | 8.76 | 37 | 3.48 | 57 | 2.50 | ||
| 18 | 3.23 | 38 | 3.29 | 58 | 2.39 | ||
| 19 | 5.33 | 39 | 5.09 | 59 | 7.44 | ||
| 20 | 3.47 | 40 | 7.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernauer, C.; Grohmann, S.; Angermann, P.; Dickes, D.; Holzberger, F.; Amend, P.; Zaeh, M.F. Investigation of the Cause-Effect Relationships between the Exothermic Reaction and the Microstructures of Reactive Ni-Al Particles Produced by High Energy Planetary Ball Milling. Metals 2021, 11, 876. https://doi.org/10.3390/met11060876
Bernauer C, Grohmann S, Angermann P, Dickes D, Holzberger F, Amend P, Zaeh MF. Investigation of the Cause-Effect Relationships between the Exothermic Reaction and the Microstructures of Reactive Ni-Al Particles Produced by High Energy Planetary Ball Milling. Metals. 2021; 11(6):876. https://doi.org/10.3390/met11060876
Chicago/Turabian StyleBernauer, Christian, Sandra Grohmann, Philipp Angermann, Daniel Dickes, Florian Holzberger, Pierre Amend, and Michael F. Zaeh. 2021. "Investigation of the Cause-Effect Relationships between the Exothermic Reaction and the Microstructures of Reactive Ni-Al Particles Produced by High Energy Planetary Ball Milling" Metals 11, no. 6: 876. https://doi.org/10.3390/met11060876
APA StyleBernauer, C., Grohmann, S., Angermann, P., Dickes, D., Holzberger, F., Amend, P., & Zaeh, M. F. (2021). Investigation of the Cause-Effect Relationships between the Exothermic Reaction and the Microstructures of Reactive Ni-Al Particles Produced by High Energy Planetary Ball Milling. Metals, 11(6), 876. https://doi.org/10.3390/met11060876






