Correlation of Morphology and Crystal Structure of Metal Powders Produced by Electrolysis Processes
Abstract
1. Introduction
- (a)
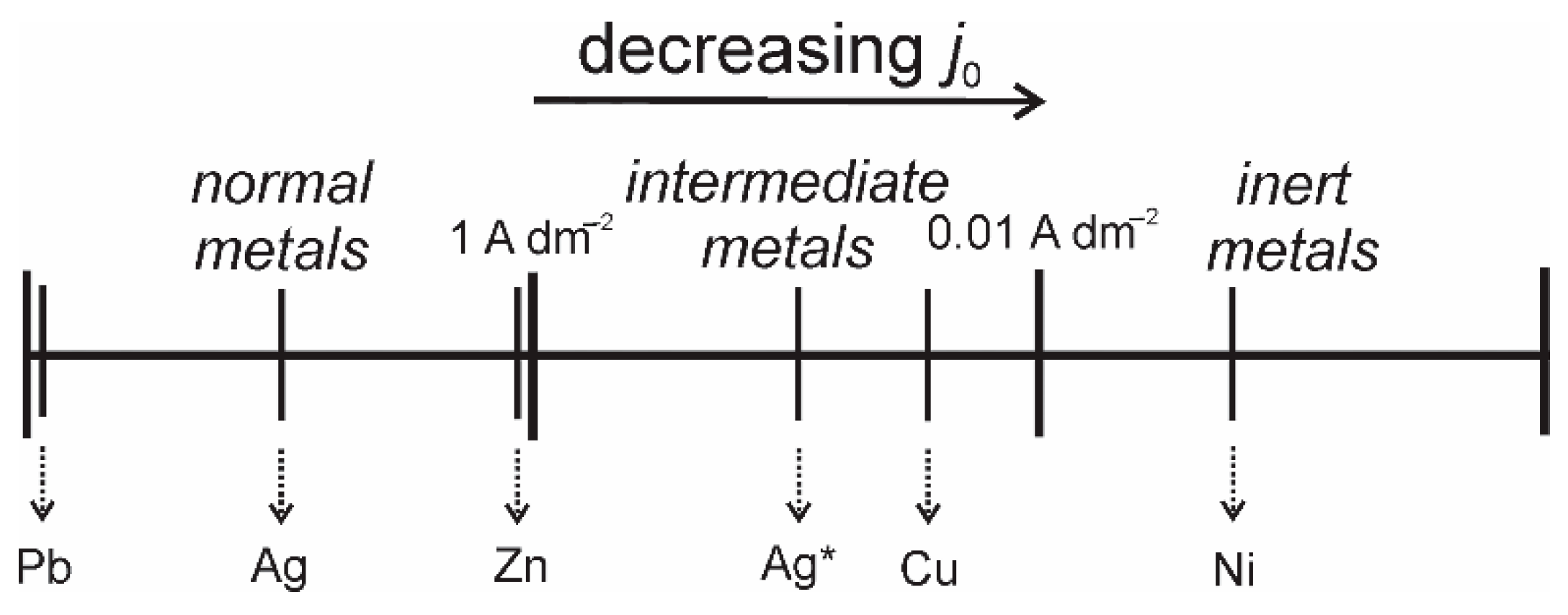
- Normal metals: metals such as Pb, Cd, Ag (basic electrolytes), Sn, and Zn. These metals are characterized by high j0 values (j0 > 1 A dm−2), low Tm, and high overpotentials for hydrogen evolution reaction.
- (b)
- Intermediate metals: metals such as Cu, Ag (ammonium electrolyte), and Au. These metals are characterized by medium j0 values (10−2 < j0 < 1 A dm−2) and lower overpotentials for hydrogen evolution reaction than the normal metals.
- (c)
- Inert metals: metals such as Co, Ni, Pt and Fe. These metals are characterized by low j0 values, high Tm, and low overpotentials for hydrogen evolution reaction.
The Crystallographic and Electrochemical Definitions of a Dendrite
2. Formation of Metal Powders by Electrolysis from Aqueous Electrolytes
2.1. The Normal Metals
2.1.1. Lead
2.1.2. Silver (The Nitrate Electrolyte)
2.2. The Intermediate Metals
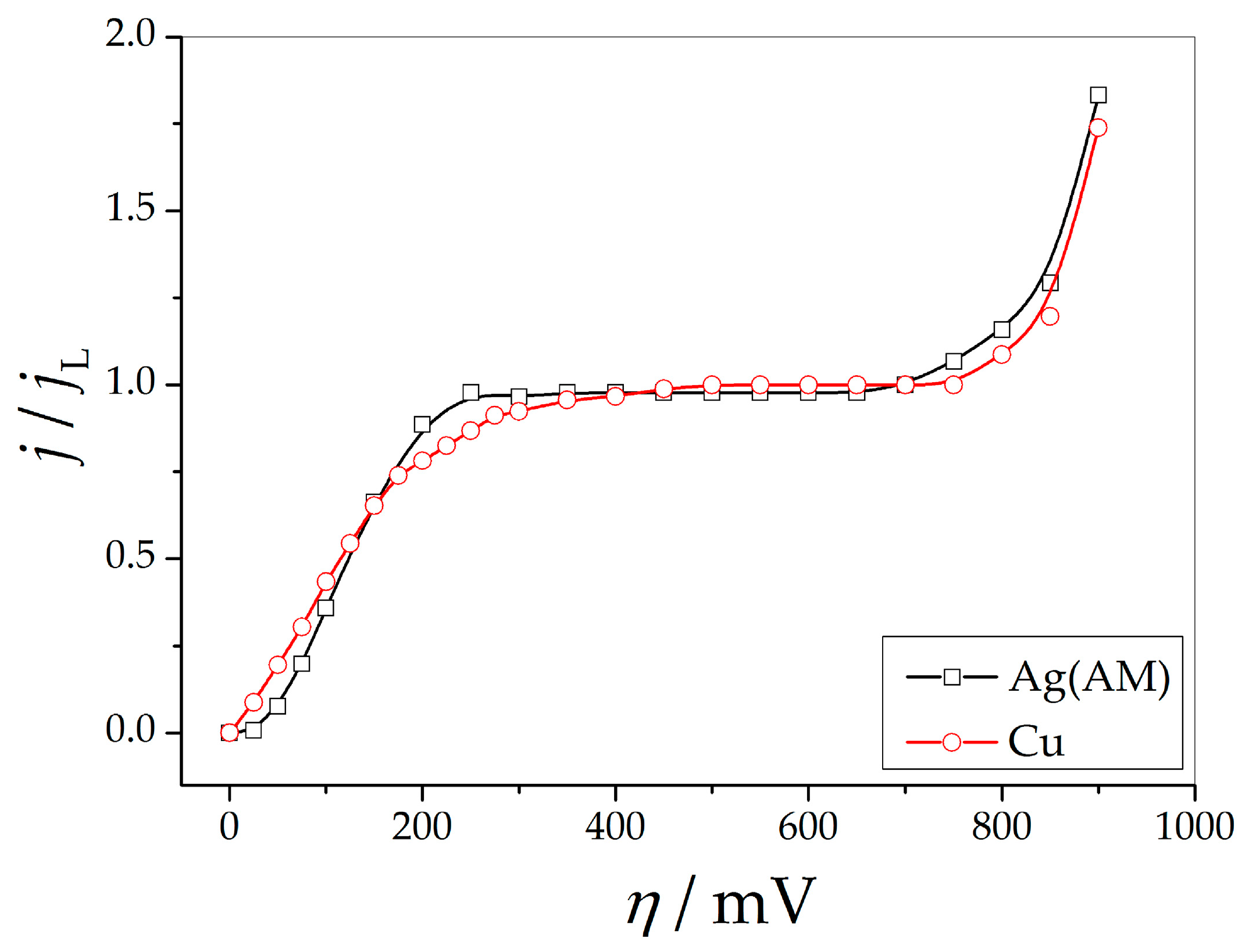
2.2.1. Silver (The Ammonium Electrolyte)
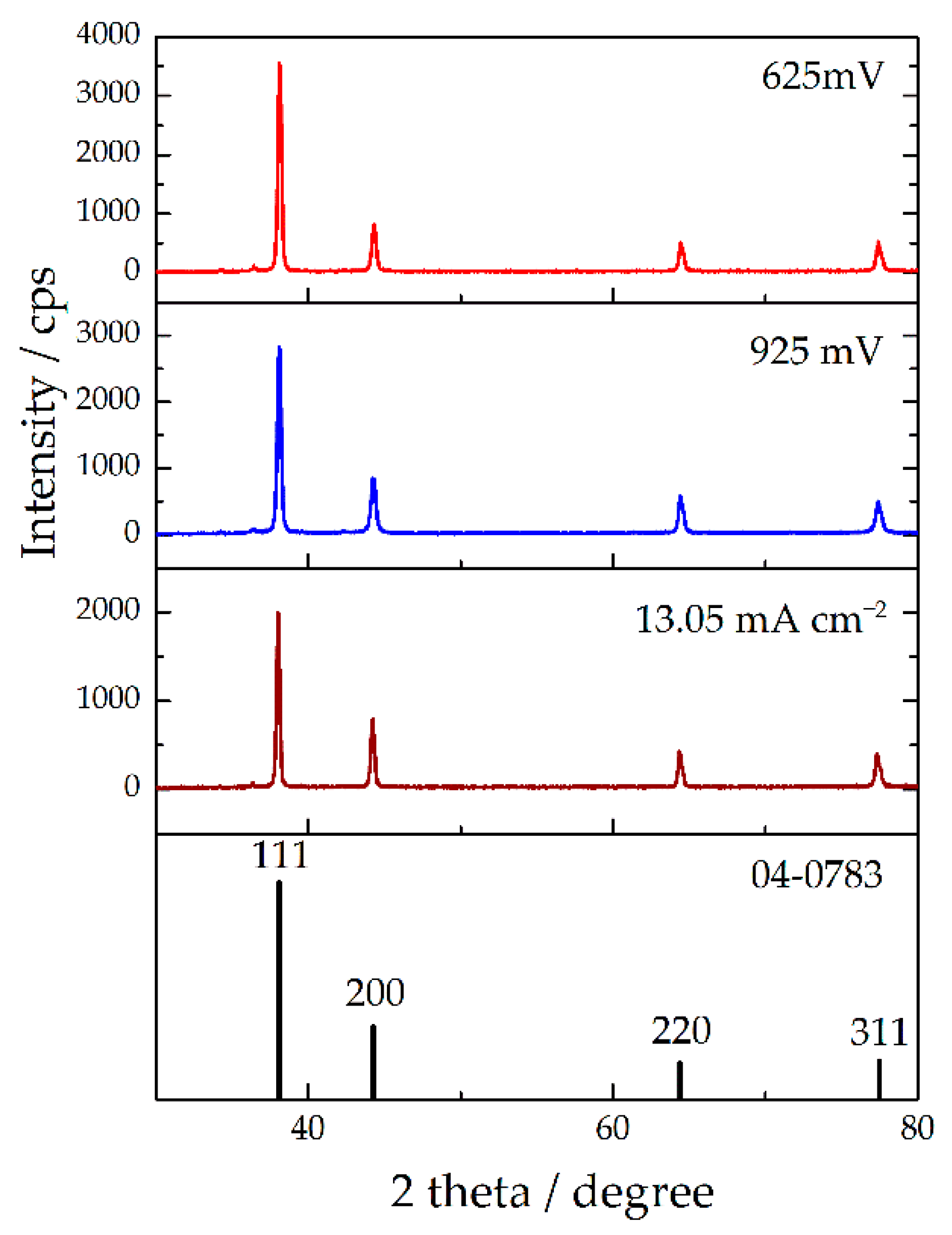
2.2.2. Copper
2.3. Inert Metals
Nickel
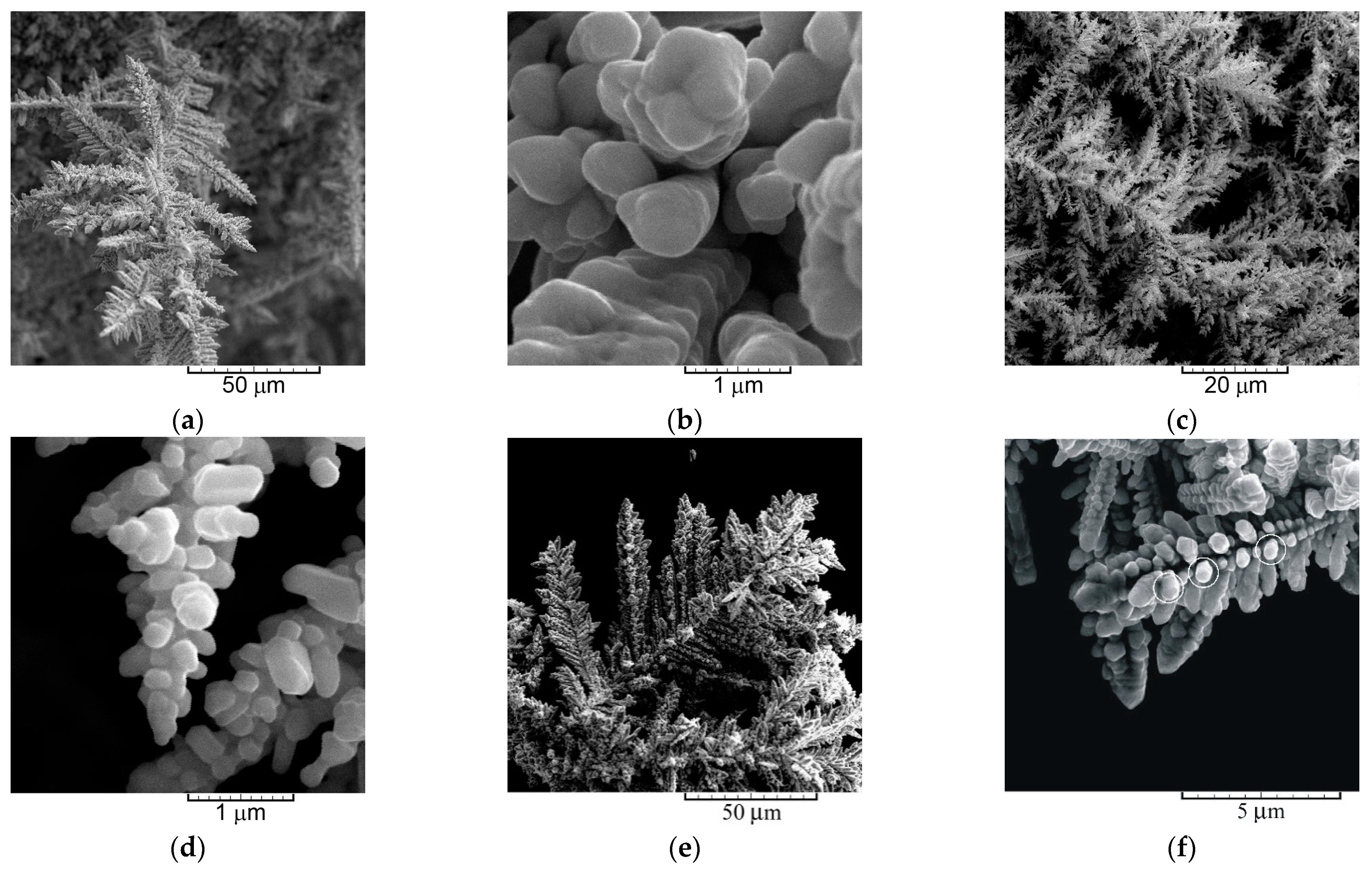
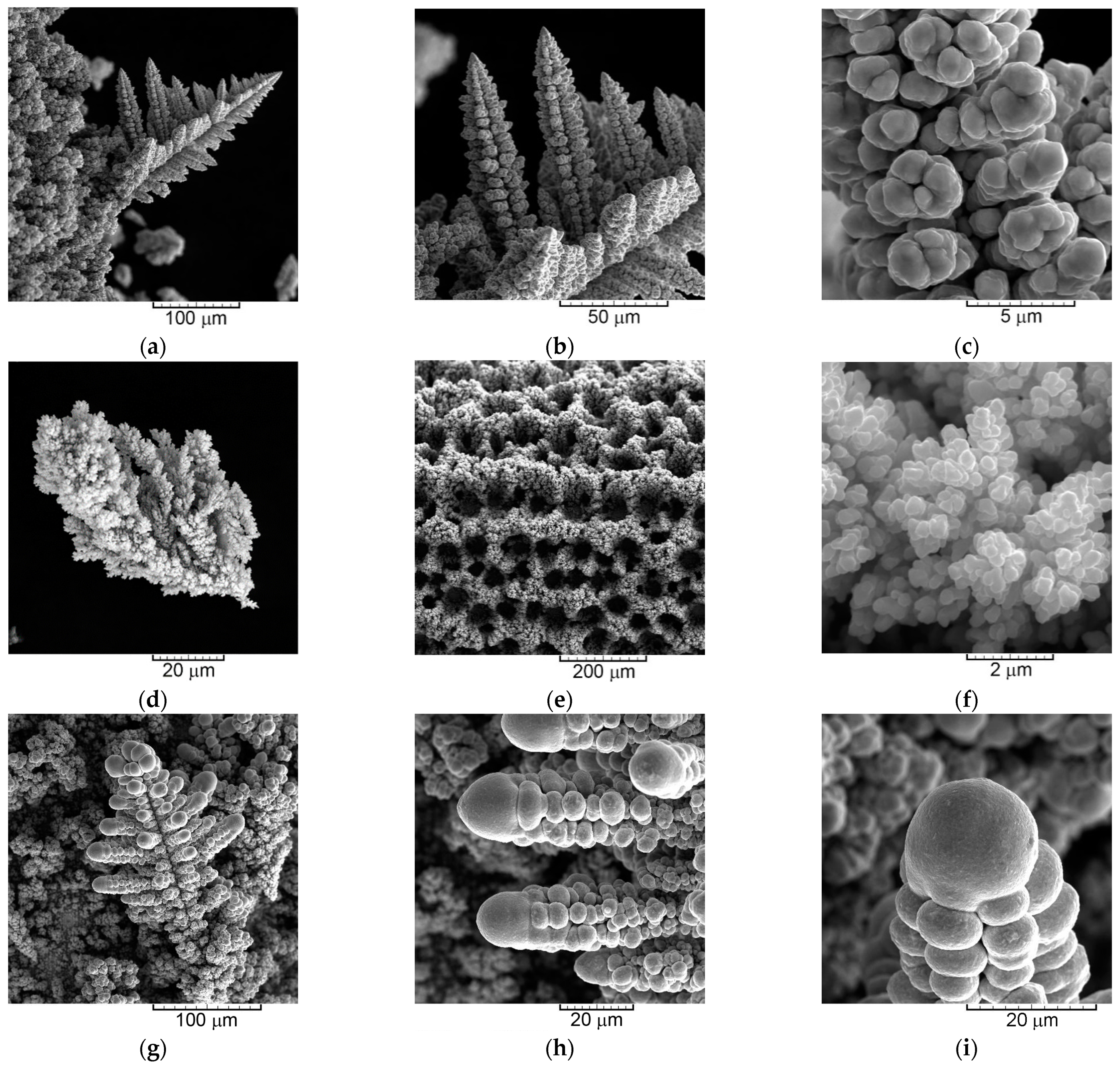
2.4. Correlation between Morphology and Crystal Structure of the Powder Particles
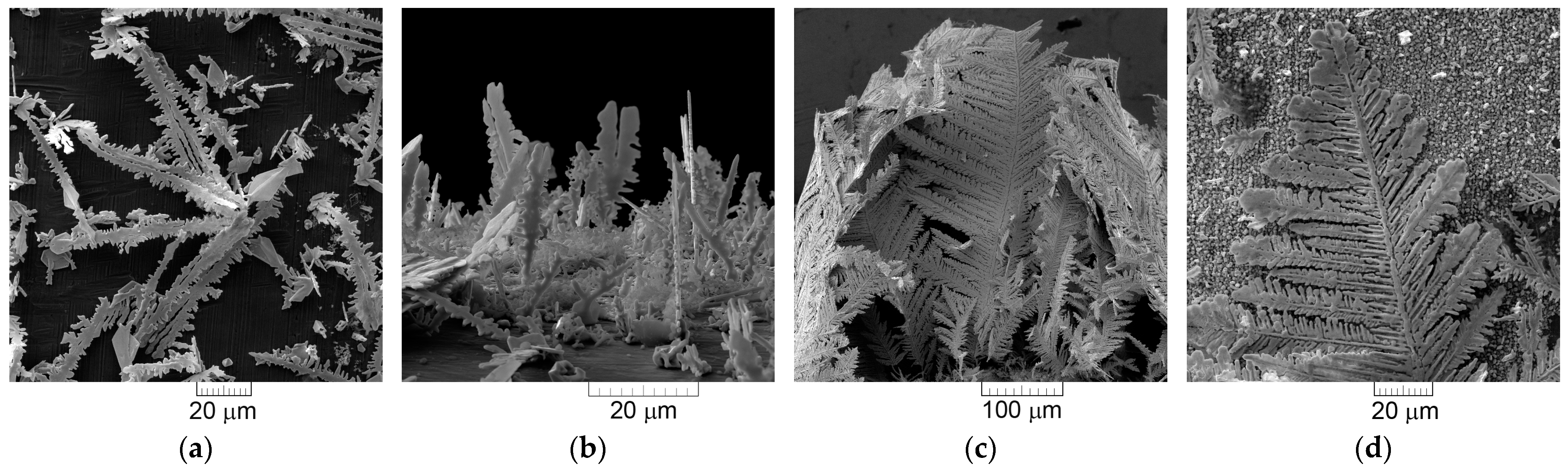
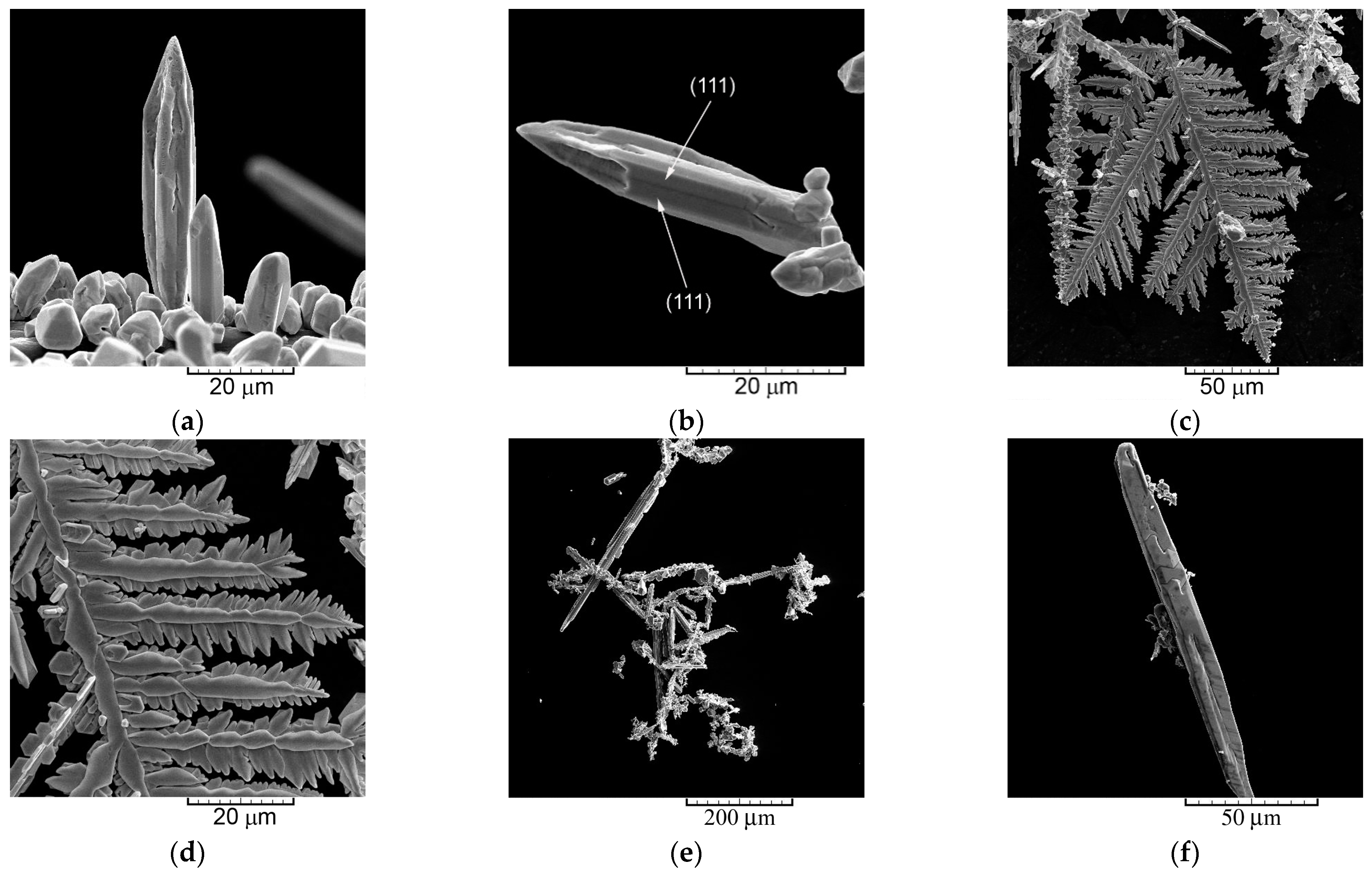
- (a)
- The dendrites of various shape—needle-like, 2D fern-like, and the 3D pine-like;
- (b)
- The cauliflower-like and the spongy-like particles.
3. Formation of Powders by Molten Salt Electrolysis
4. Formation of Metal Powders by Electrolysis in Deep Eutectic Solvents (DESs)
5. Crystal Growth and Crystal Structure of Electrodeposited FCC Metals
6. Conclusions
- The needle-like and the 2D fern-like dendrites were obtained by electrolysis of metals characterized by the high j0 values (silver and lead). The powders composed of these particles possessed either the strong (111) (the needle-like Ag and the 2D fern-like Pb dendrites) or the (111) (the 2D fern-like Ag dendrites) preferred orientation.
- The 3D pine-like dendrites, including those built from globules, were produced by electrolysis of metals characterized by medium j0 values (Ag electrodeposited from the ammonium solution and Cu). The 3D pine-like dendrites were built from approximately spherical grains. The powders composed of these particles possessed a random orientation.
- The intensive hydrogen evolution as the parallel reaction strongly affected the form of the powder particles. The cauliflower-like (Cu) and the spongy-like (Ni) particles were obtained under vigorous hydrogen evolution. Both types of particles were built from approximately spherical grains. The powders composed of these particles showed a random orientation.
- Formation of metal powders by electrolysis from molten salt (Mg) and electrolysis in deep eutectic solvents (Pb, Sn, and Sb) was also presented. Special attention was given to the analysis of the morphology and crystal structure of powders obtained by these types of electrolysis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Popov, K.I.; Pavlović, M.G. Metal Powder Production by Electrolysis. In Electrochemistry Encyclopedia; The Electrochemical Society: Pennington, NJ, USA, 2005; Available online: https://knowledge.electrochem.org/encycl/art-p04-metalpowder.htm. (accessed on 17 March 2021).
- Making Metal Powder. Available online: https://www.mpif.org/IntrotoPM/MakingMetalPowder.aspx. (accessed on 17 March 2021).
- Stopić, S.; Dvorak, P.; Friedrich, B. Synthesis of Spherical Nanosized Copper Powder by Ultrasonic Spray Pyrolysis. World Metall. 2005, 58, 195–201. [Google Scholar]
- Meshram, P.; Sinha, M.K.; Sahu, S.K.; Khan, P.; Pandey, B.D.; Mankhand, T.R. Solvothermal Synthesis of High Value Copper Powder from Copper Bleed Solution of an Indian Copper Smelter. Powder Technol. 2013, 233, 335–340. [Google Scholar] [CrossRef]
- Jhajharia, R.; Jain, D.; Sengar, A.; Goyal, A.; Soni, P.R. Synthesis of Copper Powder by Mechanically Activated Cementation. Powder Technol. 2016, 301, 10–15. [Google Scholar] [CrossRef]
- Rosenband, V.; Gany, A. Preparation of Nickel and Copper Submicrometer Particles by Pyrolysis of their Formats. J. Mater. Process. Technol. 2004, 153, 1058–1061. [Google Scholar] [CrossRef]
- Djokić, S.S. Production of Metallic Powders from Aqueous Solutions without an External Current Source. In Electrochemical Production of Metal Powders, Series: Modern Aspects of Electrochemistry; Djokić, S.S., Ed.; Springer: New York, NY, USA, 2012; Volume 54, pp. 369–398. [Google Scholar]
- Antony, L.V.M.; Reddy, R.G. Processes for Production of High-Purity Metal Powders. JOM 2003, 55, 14–18. [Google Scholar] [CrossRef]
- Chaubey, A.K.; Scudino, S.; Khoshkhoo, M.S.; Prashanth, K.G.; Mukhopadhyay, N.K.; Mishra, B.K.; Eckert, J. Synthesis and Characterization of Nanocrystalline Mg-7.4%Al Powders Produced by Mechanical Alloying. Metals 2013, 3, 58–68. [Google Scholar] [CrossRef]
- Popov, K.I.; Djokić, S.S.; Nikolić, N.D.; Jović, V.D. Morphology of Electrochemically and Chemically Deposited Metals; Springer: New York, NY, USA, 2016; pp. 1–368. [Google Scholar]
- Amiri, M.; Nouhi, S.; Azizian-Kalandaragh, Y. Facile Synthesis of Silver Nanostructures by Using Various Deposition Potential and Time: A Nonenzymatic Sensors for Hydrogen Peroxide. Mater. Chem. Phys. 2015, 155, 129–135. [Google Scholar] [CrossRef]
- Calusaru, A. Electrodeposition of Metal Powders; Elsevier: New York, NY, USA, 1979; pp. 1–544. [Google Scholar]
- Ru, J.; Hua, Y.; Xu, C.; Li, J.; Li, Y.; Wang, D.; Gong, K.; Zhou, Z. Preparation of Sub-micrometer Lead Wires from PbO by Electrodeposition in Choline Chloride-urea Deep Eutectic Solvent. Adv. Powder Technol. 2015, 26, 91–97. [Google Scholar] [CrossRef]
- Winand, R. Electrodeposition of Metals and Alloys–New Results and Perspectives. Electrochim. Acta 1994, 39, 1091–1105. [Google Scholar] [CrossRef]
- Nikolić, N.D. Influence of the Exchange Current Density and Overpotential for Hydrogen Evolution Reaction on the Shape of Electrolytically Produced Disperse Forms. J. Electrochem. Sci. Eng. 2020, 10, 111–126. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Popov, K.I.; Pavlović, L.J.; Pavlović, M.G. The Effect of Hydrogen Codeposition on the Morphology of Copper Electrodeposits. I. The Concept of Effective Overpotential. J. Electroanal. Chem. 2006, 588, 88–98. [Google Scholar] [CrossRef]
- Berube, L.P.; Esperance, G.L. A Quantitative Method of Determining of the Degree of Texture of Zinc Electrodeposits. J. Electrochem. Soc. 1989, 136, 2314–2315. [Google Scholar] [CrossRef]
- Avramović, L.; Pavlović, M.M.; Maksimović, V.M.; Vuković, M.; Stevanović, J.S.; Bugarin, M.; Nikolić, N.D. Comparative Morphological and Crystallographic Analysis of Electrochemically- and Chemically-Produced Silver Powder Particles. Metals 2017, 7, 160. [Google Scholar] [CrossRef]
- Avramović, L.; Maksimović, V.M.; Baščarević, Z.; Ignjatović, N.; Bugarin, M.; Marković, R.; Nikolić, N.D. Influence of the Shape of Copper Powder Particles on the Crystal Structure and Some Decisive Characteristics of the Metal Powders. Metals 2019, 9, 56. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Maksimović, V.M.; Branković, G.; Živković, P.M.; Pavlović, M.G. Correlation between Crystal Orientation and Morphology of Electrolytically Produced Powder Particles: Analysis of the Limiting Cases. Mater. Prot. 2018, 59, 256–264. [Google Scholar] [CrossRef]
- Wranglen, G. Dendrites and Growth Layers in the Electrocrystallization of Metals. Electrochim. Acta 1960, 2, 130–146. [Google Scholar] [CrossRef]
- Popov, K.I.; Nikolić, N.D. General Theory of Disperse Metal Electrodeposits Formation. In Electrochemical Production of Metal Powders, Series: Modern Aspects of Electrochemistry; Djokić, S.S., Ed.; Springer: New York, NY, USA, 2012; Volume 54, pp. 1–62. [Google Scholar]
- Diggle, J.W.; Despic, A.R.; Bockris, J.O.’M. The Mechanism of the Dendritic Electrocrystallization of Zinc. J. Electrochem. Soc. 1969, 116, 1503–1514. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Živković, P.M.; Branković, G.; Pavlović, M.G. Estimation of the Exchange Current Density and Comparative Analysis of Morphology of Electrochemically Produced Lead and Zinc Deposits. J. Serb. Chem. Soc. 2017, 82, 539–550. [Google Scholar] [CrossRef]
- Fetter, K. Electrochemical Kinetics; Khimiya: Moscow, Russia, 1967. (In Russian) [Google Scholar]
- Nikolić, N.D.; Branković, G.; Lačnjevac, U. Formation of Two-dimensional (2D) Lead Dendrites by Application of Different Regimes of Electrolysis. J. Solid State Electrochem. 2012, 16, 2121–2126. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Vaštag, D.D.; Živković, P.M.; Jokić, B.; Branković, G. Influence of the Complex Formation on the Morphology of Lead Powder Particles Produced by the Electrodeposition Processes. Adv. Powder Technol. 2013, 24, 674–682. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Popov, K.I.; Živković, P.M.; Branković, G. A New Insight into the Mechanism of Lead Electrodeposition: Ohmic–diffusion Control of the Electrodeposition Process. J. Electroanal. Chem. 2013, 691, 66–76. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Popov, K.I.; Ivanović, E.R.; Branković, G.; Stevanović, S.I.; Živković, P.M. The Potentiostatic Current Transients and the Role of Local Diffusion Fields in Formation of the 2D Lead Dendrites from the Concentrated Electrolyte. J. Electroanal. Chem. 2015, 739, 137–148. [Google Scholar] [CrossRef]
- Wong, S.M.; Abrantes, L.M. Lead Electrodeposition from Very Alkaline Media. Electrochim. Acta 2005, 51, 619–626. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Vaštag, D.D.; Maksimović, V.M.; Branković, G. Morphological and Crystallographic Characteristics of Lead Powder Obtained by Electrodeposition from an Environmentally Friendly Electrolyte. Trans. Nonferrous Met. Soc. China 2014, 24, 884–892. [Google Scholar] [CrossRef]
- Ni, Y.; Zhang, Y.; Hong, J. Hierarchical Pb Microstructures: A Facile Electrochemical Synthesis, Shape Evolution and Influencing Factors. CrystEngComm 2011, 13, 934–940. [Google Scholar] [CrossRef]
- Sivasubramanian, R.; Sangaranarayanan, M.V. Electrodeposition of Silver Nanostructures: From Polygons to Dendrites. CrystEngComm 2013, 15, 2052–2056. [Google Scholar] [CrossRef]
- Avramović, L.; Ivanović, E.R.; Maksimović, V.M.; Pavlović, M.M.; Vuković, M.; Stevanović, J.S.; Nikolić, N.D. Correlation between Crystal Structure and Morphology of Potentiostatically Electrodeposited Silver Dendritic Nanostructures. Trans. Nonferrous Met. Soc. China 2018, 28, 1903–1912. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Popov, K.I. A New Approach to the Understanding of the Mechanism of Lead Electrodeposition. In Electrodeposition and Surface Finishing; Series: Modern Aspects of Electrochemistry; Djokić, S.S., Ed.; Springer: New York, NY, USA, 2014; Volume 57, pp. 85–132. [Google Scholar]
- Popov, K.I.; Krstajić, N.V.; Popov, S.R. Fundamental Aspects of Plating Technology II: Morphological Aspects of Metal Electrodeposition from Complex Salt Solutions. Surf. Technol. 1983, 20, 203–208. [Google Scholar] [CrossRef]
- Popov, K.I.; Grgur, B.N.; Stojilković, E.R.; Pavlović, M.G.; Nikolić, N.D. The Effect of Deposition Process Exchange Current Density on the Thin Metal Film Formation on Inert Substrate. J. Serb. Chem. Soc. 1997, 62, 433–442. [Google Scholar]
- Nikolić, N.D.; Avramović, L.; Ivanović, E.R.; Maksimović, V.M.; Baščarević, Z.; Ignjatović, N. Comparative Morphological and Crystallographic Analysis of Copper Powders Obtained under Different Electrolysis Conditions. Trans. Nonferrous Met. Soc. China 2019, 29, 1275–1284. [Google Scholar] [CrossRef]
- Jović, V.D.; Jović, B.M.; Pavlović, M.G. Electrodeposition of Ni, Co and Ni-Co Alloy Powders. Electrochim. Acta 2006, 51, 5468–5477. [Google Scholar] [CrossRef]
- Jović, V.D.; Nikolić, N.D.; Lačnjevac, U.Č.; Jović, B.M.; Popov, K.I. Morphology of Different Electrodeposited Pure Metal Powders. In Electrochemical Production of Metal Powders; Series: Modern Aspects of Electrochemistry; Djokić, S.S., Ed.; Springer: New York, NY, USA, 2012; Volume 54, pp. 63–123. [Google Scholar]
- Nikolić, N.D.; Pavlović, L.J.; Pavlović, M.G.; Popov, K.I. Morphologies of Electrochemically Formed Copper Powder Particles and their Dependence on the Quantity of Evolved Hydrogen. Powder Technol. 2008, 185, 195–201. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Branković, G.; Pavlović, M.G. Correlate Between Morphology of Powder Particles Obtained by the Different Regimes of Electrolysis and the Quantity of Evolved Hydrogen. Powder Technol. 2012, 221, 271–277. [Google Scholar] [CrossRef]
- Han, J.; Liu, J. Electrodeposition of Crystalline Dendritic Silver in 12-Tungstosilicate Acid System. J. Nanoeng. Nanomanuf. 2012, 2, 171–174. [Google Scholar] [CrossRef]
- Mandke, M.V.; Han, S.-H.; Pathan, H.M. Growth of Silver Dendritic Nanostructures via Electrochemical Route. CrystEngComm 2012, 14, 86–89. [Google Scholar] [CrossRef]
- Orhan, G.; Hapci, G. Effect of Electrolysis Parameters on the Morphologies of Copper Powder Obtained in a Rotating Cylinder Electrode Cell. Powder Technol. 2010, 201, 57–63. [Google Scholar] [CrossRef]
- Ostanina, T.N.; Rudoi, V.M.; Patrushev, A.V.; Darintseva, A.B.; Farlenkov, A.S. Modelling the Dynamic Growth of Copper and Zinc Dendritic Deposits under the Galvanostatic Electrolysis Conditions. J. Electroanal. Chem. 2015, 750, 9–18. [Google Scholar] [CrossRef]
- Nikitin, V.S.; Ostanina, T.N.; Rudoi, V.M.; Kuloshvili, T.S.; Darintseva, A.B. Features of Hydrogen Evolution during Electrodeposition of Loose Deposits of Copper, Nickel and Zinc. J. Electroanal. Chem. 2020, 870, 114230. [Google Scholar] [CrossRef]
- Nekouei, R.K.; Rashchi, F.; Ravanbakhsh, A. Copper Nanopowder Synthesis by Electrolysis Method in Nitrate and Sulfate Solutions. Powder Technol. 2013, 250, 91–96. [Google Scholar] [CrossRef]
- Copper Powder. Available online: http://www.cnpcpowder.com/products/copper/ (accessed on 17 March 2021).
- Lou, W.; Cai, W.; Li, P.; Su, J.; Zheng, S.; Zhang, Y.; Jin, W. Additives-assisted Electrodeposition of Fine Spherical Copper Powder from Sulfuric Acid Solution. Powder Technol. 2018, 326, 84–88. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Živković, P.M.; Pavlović, M.G.; Baščarević, Z. Overpotential Controls a Morphology of Electrolytically Produced Copper Dendritic Forms. J. Serb. Chem. Soc. 2019, 84, 1209–1220. [Google Scholar] [CrossRef]
- Bockris, J.O.’M.; Reddy, A.K.N.; Gamboa-Aldeco, M.E. Modern Electrochemistry 2A, Fundamentals of Electrodics; Kluwer Academic/Plenum Publishers: New York, NY, USA; Springer: Boston, MA, USA, 2000; p. 1333. [Google Scholar]
- Zhang, J.M.; Ma, F.; Xu, K.W. Calculation of the Surface Energy of FCC Metals with Modified Embedded-atom Method. Appl. Surf. Sci. 2004, 229, 34–42. [Google Scholar] [CrossRef]
- Wang, S.G.; Tian, E.K.; Lung, C.W. Surface Energy of Arbitrary Crystal Plane of bcc and fcc Metals. J. Phys. Chem. Solids 2000, 61, 1295–1300. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Maksimović, V.M.; Branković, G. Morphological and Crystallographic Characteristics of Electrodeposited Lead from the Concentrated Electrolyte. RSC Adv. 2013, 3, 7466–7471. [Google Scholar] [CrossRef]
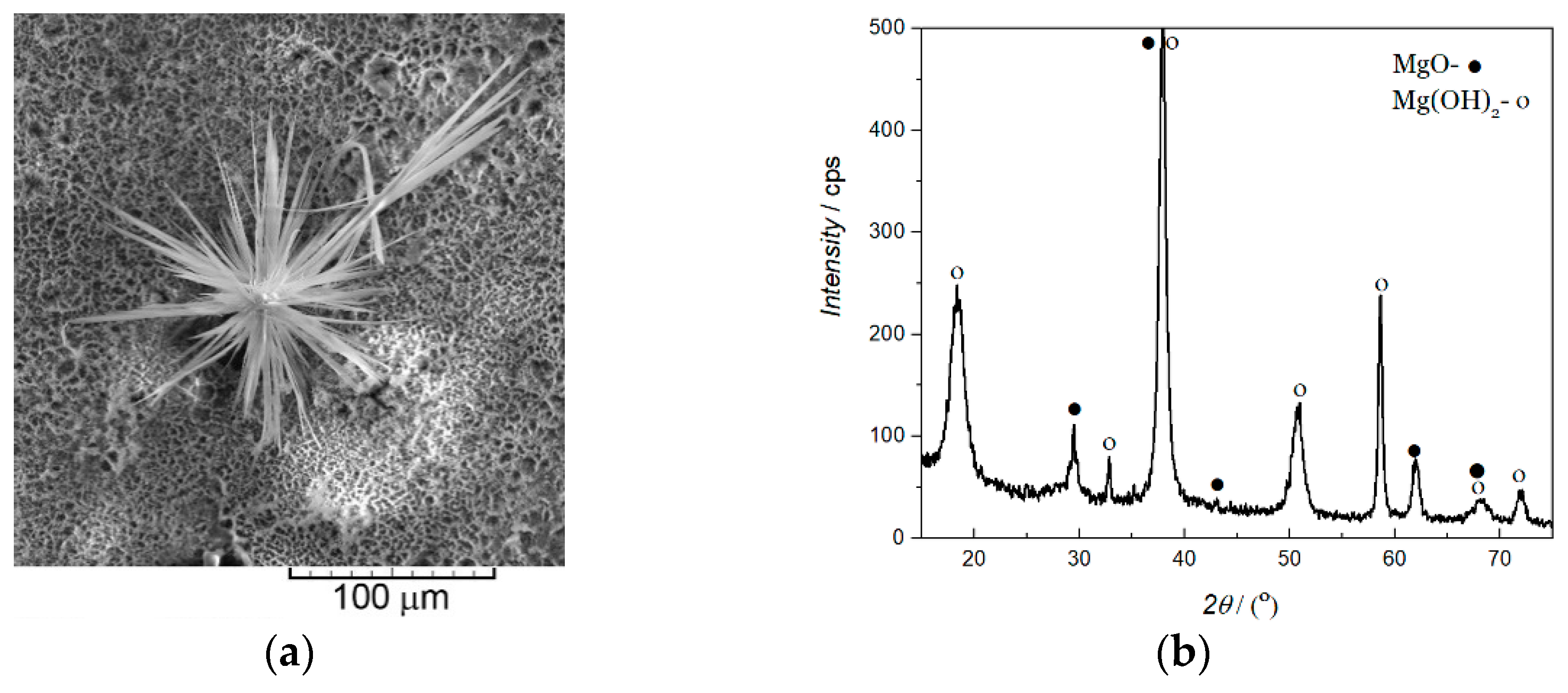
- Cvetković, V.S.; Vukićević, N.M.; Nikolić, N.D.; Branković, G.; Barudžija, T.S.; Jovićević, J.N. Formation of Needle-like and Honeycomb-like Magnesium Oxide/Hydroxide Structures by Electrodeposition from Magnesium Nitrate Melts. Electrochim. Acta 2018, 268, 494–502. [Google Scholar] [CrossRef]
- Cvetković, V.S.; Vukićević, N.M.; Nikolić, N.D.; Baščarević, Z.; Barudžija, T.S.; Jovićević, J.N. A Possible Mechanism of Formation of Flower-like MgO/Mg(OH)2 Structures by Galvanostatic Molten Salt Electrolysis: The Concept of Local Diffusion Fields. J. Electroanal. Chem. 2019, 842, 168–175. [Google Scholar] [CrossRef]
- Ru, J.; Hua, Y.; Xu, C.; Li, J.; Li, Y.; Wang, D.; Qi, C.; Jie, Y. Morphology-controlled Preparation of Lead Powders by Electrodeposition from Different PbO-containing Choline Chloride-urea Deep Eutectic Solvent. Appl. Surf. Sci. 2015, 335, 153–159. [Google Scholar] [CrossRef]
- Ru, J.; Hua, Y.; Xu, C.; Li, J.; Li, Y.; Wang, D.; Qi, C.; Gong, K. Electrochemistry of Pb(II)/Pb during Preparation of Lead Wires from PbO in Choline Chloride–Urea Deep Eutectic Solvent. Russ. J. Electrochem. 2015, 51, 773–781. [Google Scholar] [CrossRef]
- Ru, J.; Bu, J.; Wang, Z.; Hua, Y.; Wang, D. Eco-friendly and Facile Electrochemical Synthesis of Sub-micrometer Lead Powders in Deep Eutectic Solvents Using Galena as a Raw Material. J. Appl. Electrochem. 2019, 49, 369–377. [Google Scholar] [CrossRef]
- Wang, Z.; Ru, J.; Hua, Y.; Bu, J.; Geng, X.; Zhang, W. Electrodeposition of Sn Powders with Pyramid Chain and Dendrite Structures in Deep Eutectic Solvent: Roles of Current Density and SnCl2 Concentration. J. Solid State Electrochem. 2021, 25, 1111–1120. [Google Scholar] [CrossRef]
- Wang, Z.; Ru, J.; Hua, Y.; Wang, D.; Bu, J. Morphology-Controlled Preparation of Sn Powders by Electrodeposition in Deep Eutectic Solvent as Anodes for Lithium Ion Batteries. J. Electrochem. Soc. 2020, 167, 082504. [Google Scholar] [CrossRef]
- Bu, J.; Ru, J.; Wang, Z.; Hua, Y.; Xu, C.; Zhang, Y.; Wang, Y. Controllable Preparation of Antimony Powders by Electrodeposition in Choline Chloride-ethylene Glycol. Adv. Powder Technol. 2019, 30, 2859–2867. [Google Scholar] [CrossRef]
- Radmilovic, V.V.; Kacher, J.; Ivanovic, E.R.; Minor, A.M.; Radmilovic, V.R. Multiple Twinning and Stacking Faults in Silver Dendrites. Cryst. Growth Des. 2016, 16, 467–474. [Google Scholar] [CrossRef]
- Smallman, R.; Dillamore, I.; Dobson, P. The Measurement of Stacking Fault Energy. J. Phys. Colloq. 1966, 27, C3-86–C3-93. [Google Scholar] [CrossRef]
- Liu, F.; Yuan, H.; Yin, J.; Wang, J.T. Influence of Stacking Fault Energy and Temperature on Microstructures and Mechanical Properties of fcc Pure Metals Processed by Equal-channel Angular Pressing. Mat. Sci. Eng. A Struct. 2016, 662, 578–587. [Google Scholar] [CrossRef]
- Cabrera, N.; Vermilyea, D.A. The Growth of Crystals from Solution. In Growth and Perfection of Crystals; Doremus, R.H., Roberts, B.W., Turnbull, D., Eds.; John Wiley & Sons: New York, NY, USA; Chapman & Hall: London, UK, 1958; pp. 393–410. [Google Scholar]
- Ivanović, E.R.; Nikolić, N.D.; Radmilović, V.R. Randomly Oriented Twin Domains in Electrodeposited Silver Dendrites. J. Serb. Chem. Soc. 2015, 80, 107–113. [Google Scholar] [CrossRef]
- Smit, J.; Ogburn, F.; Bechtoldt, C.J. Multiple Twin Structures in Electrodeposited Silver Dendrites. J. Electrochem. Soc. 1968, 115, 371–374. [Google Scholar] [CrossRef]
- Hamilton, D.R.; Seidensticker, R.G.J. Propagation Mechanism of Germanium Dendrites. Appl. Phys. 1960, 31, 1165–1168. [Google Scholar] [CrossRef]
- Tian, M.L.; Wang, J.G.; Kurtz, J.; Mallouk, T.E.; Chan, M.H.W. Electrochemical Growth of Single-Crystal Metal Nanowires via a Two-Dimensional Nucleation and Growth Mechanism. Nano Lett. 2003, 3, 919–923. [Google Scholar] [CrossRef]


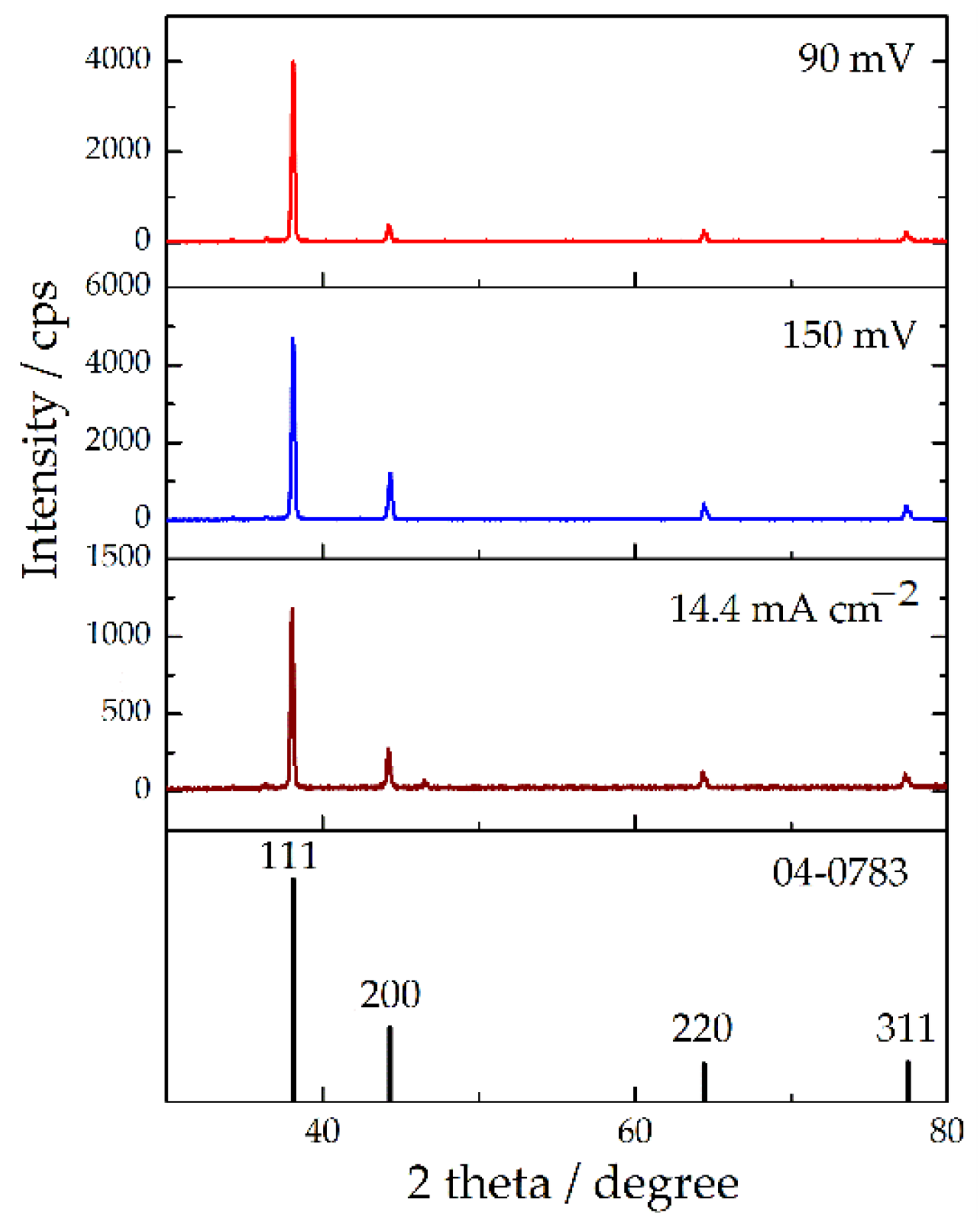
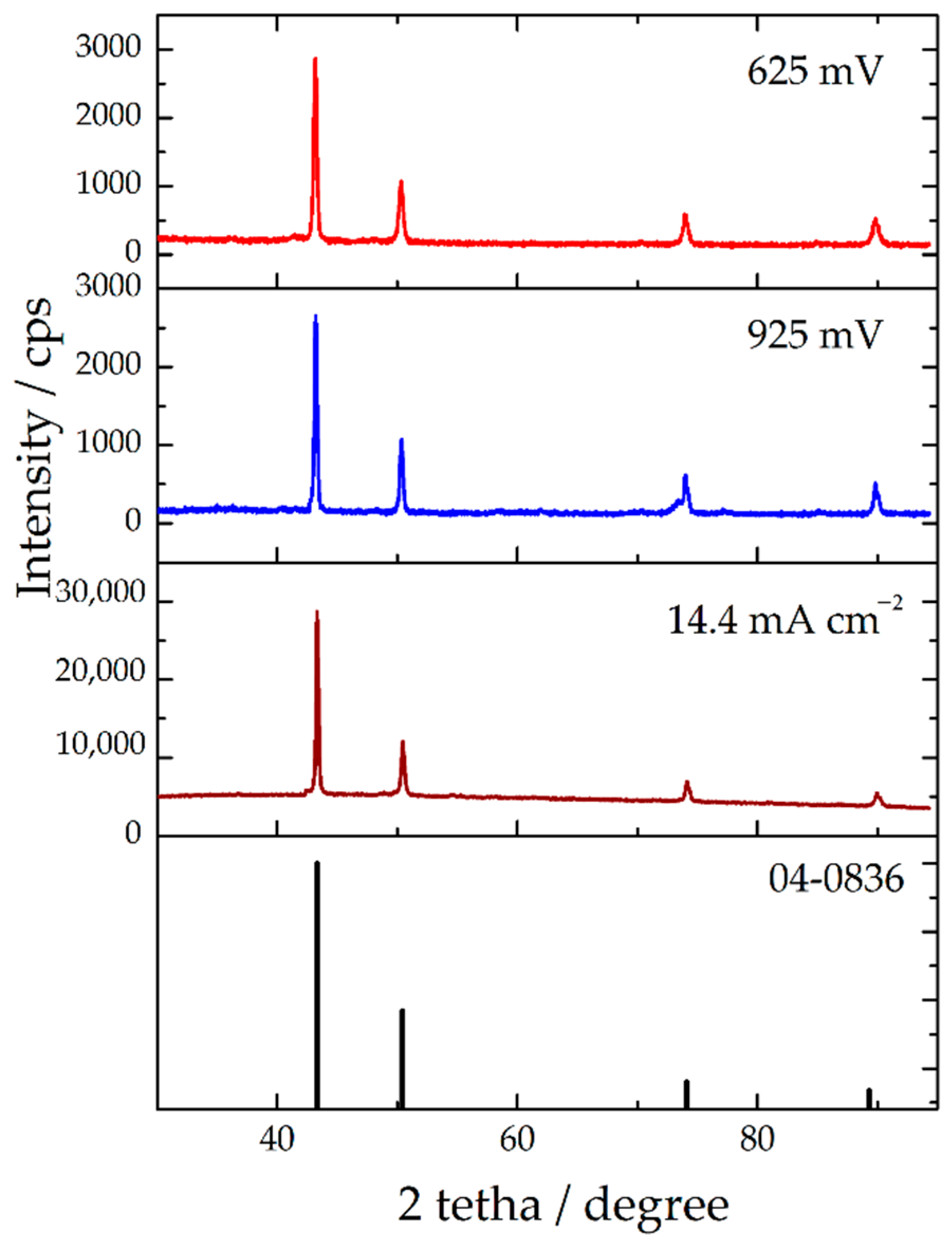

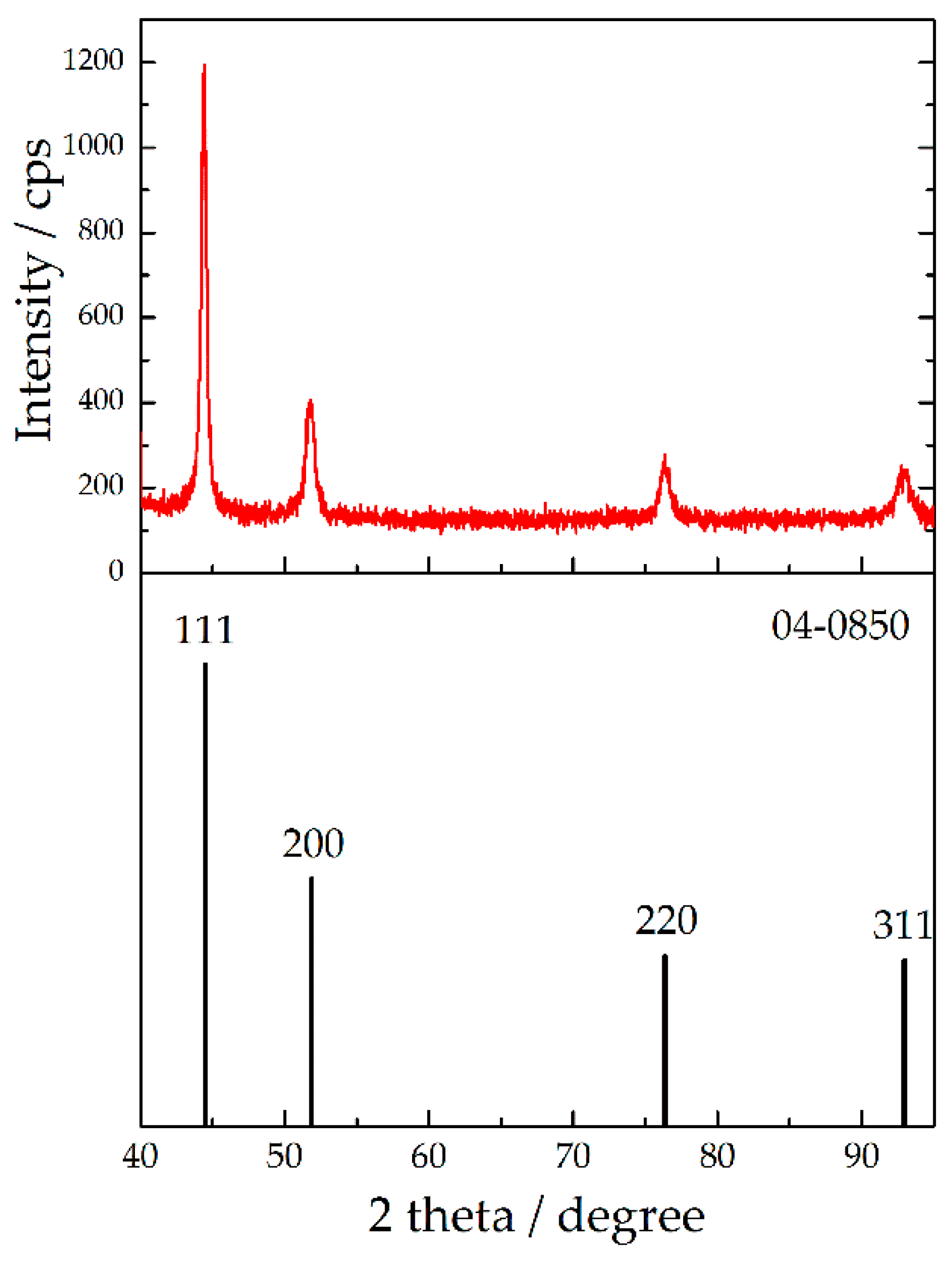
| Plane | R (in%) | Rs | TC | RTC (in%) | |||
|---|---|---|---|---|---|---|---|
| (hkl) | RNIT | RAC | (in%) | TCNIT | TCAC | RTCNIT | RTCAC |
| (111) | 89.7 | 83.5 | 46.9 | 1.91 | 1.78 | 75.5 | 63.8 |
| (200) | 3.1 | 4.4 | 23.5 | 0.13 | 0.19 | 5.2 | 6.8 |
| (220) | 3.6 | 5.8 | 14.6 | 0.25 | 0.40 | 9.9 | 14.3 |
| (311) | 3.6 | 6.3 | 15.0 | 0.24 | 0.42 | 9.4 | 15.1 |
| Plane (hkl) | R (in%) | Rs (in%) | TC | RTC (in%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R90 | R150 | R14.4 | TC90 | TC150 | TC14.4 | RTC90 | RTC150 | RTC14.4 | ||
| (111) | 82.2 | 70.1 | 69.4 | 52.4 | 1.57 | 1.34 | 1.32 | 58.1 | 43.4 | 41.6 |
| (200) | 7.7 | 18.1 | 16.3 | 20.9 | 0.37 | 0.87 | 0.78 | 13.7 | 28.1 | 24.6 |
| (220) | 5.2 | 6.3 | 7.4 | 13.1 | 0.40 | 0.48 | 0.56 | 14.8 | 15.6 | 17.7 |
| (311) | 4.9 | 5.5 | 6.9 | 13.6 | 0.36 | 0.40 | 0.51 | 13.4 | 12.9 | 16.1 |
| Plane (hkl) | R (in%) | Rs (in%) | TC | RTC (in%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R625 | R925 | R13.05 | TC625 | TC925 | TC13.05 | RTC625 | RTC925 | RTC13.05 | ||
| (111) | 65.9 | 59.2 | 54.9 | 52.4 | 1.26 | 1.13 | 1.05 | 37.0 | 30.5 | 27.2 |
| (200) | 15.1 | 17.8 | 21.9 | 20.9 | 0.72 | 0.85 | 1.05 | 21.1 | 23.0 | 27.2 |
| (220) | 9.4 | 12.2 | 11.9 | 13.1 | 0.72 | 0.93 | 0.93 | 21.1 | 25.1 | 24.1 |
| (311) | 9.6 | 10.8 | 11.3 | 13.6 | 0.71 | 0.79 | 0.83 | 20.8 | 21.4 | 21.5 |
| Plane (hkl) | R (in%) | Rs (in%) | TC | RTC (in%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R625 | R925 | R14.4 | TC625 | TC925 | TC14.4 | RTC625 | RTC925 | RTC14.4 | ||
| (111) | 56.9 | 54.7 | 54.1 | 54.6 | 1.04 | 1.00 | 0.99 | 25.7 | 24.0 | 23.6 |
| (200) | 21.2 | 22.0 | 22.5 | 25.1 | 0.845 | 0.876 | 0.90 | 20.1 | 21.0 | 21.5 |
| (220) | 11.6 | 12.7 | 13.1 | 10.9 | 1.06 | 1.16 | 1.20 | 26.2 | 27.8 | 28.6 |
| (311) | 10.3 | 10.6 | 10.3 | 9.4 | 1.10 | 1.13 | 1.10 | 27.2 | 27.1 | 26.3 |
| Plane (hkl) | R (in%) | Rs (in%) | TC | RTC (in%) |
|---|---|---|---|---|
| (111) | 56.9 | 54.6 | 1.04 | 26.1 |
| (200) | 19.6 | 23.0 | 0.85 | 21.3 |
| (220) | 12.2 | 11.5 | 1.06 | 26.5 |
| (311) | 11.3 | 10.9 | 1.04 | 26.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolić, N.D.; Maksimović, V.M.; Avramović, L. Correlation of Morphology and Crystal Structure of Metal Powders Produced by Electrolysis Processes. Metals 2021, 11, 859. https://doi.org/10.3390/met11060859
Nikolić ND, Maksimović VM, Avramović L. Correlation of Morphology and Crystal Structure of Metal Powders Produced by Electrolysis Processes. Metals. 2021; 11(6):859. https://doi.org/10.3390/met11060859
Chicago/Turabian StyleNikolić, Nebojša D., Vesna M. Maksimović, and Ljiljana Avramović. 2021. "Correlation of Morphology and Crystal Structure of Metal Powders Produced by Electrolysis Processes" Metals 11, no. 6: 859. https://doi.org/10.3390/met11060859
APA StyleNikolić, N. D., Maksimović, V. M., & Avramović, L. (2021). Correlation of Morphology and Crystal Structure of Metal Powders Produced by Electrolysis Processes. Metals, 11(6), 859. https://doi.org/10.3390/met11060859







