Mo–Nb–Si–B Alloy: Synthesis, Composition, and Structure
Abstract
1. Introduction
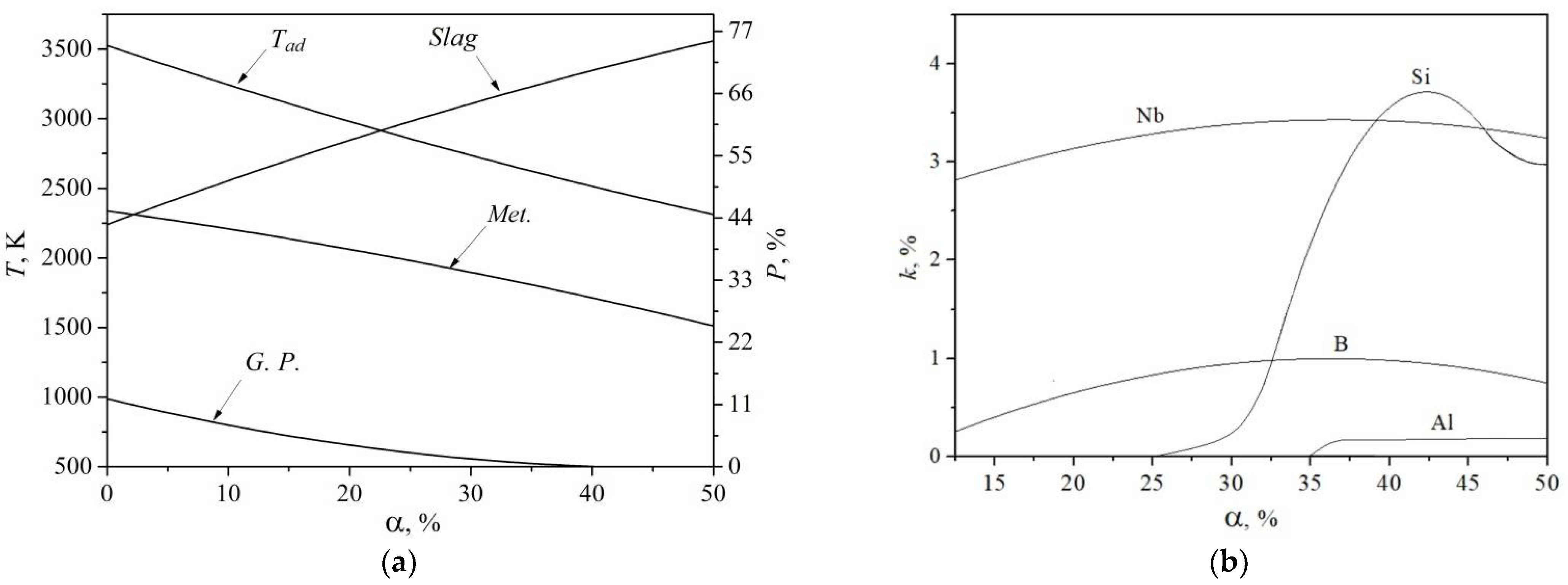
2. Thermodynamic Calculation
3. Experimental
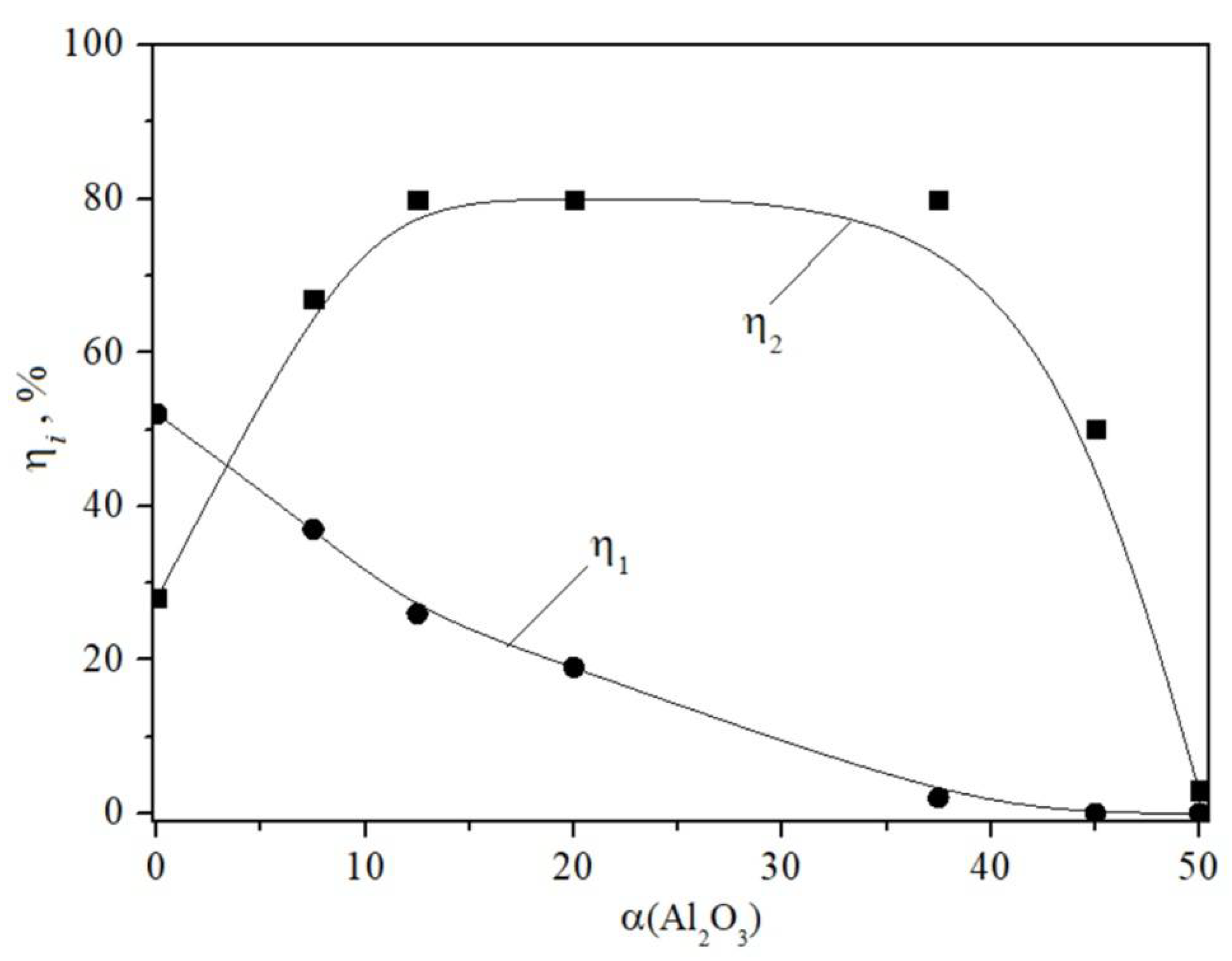
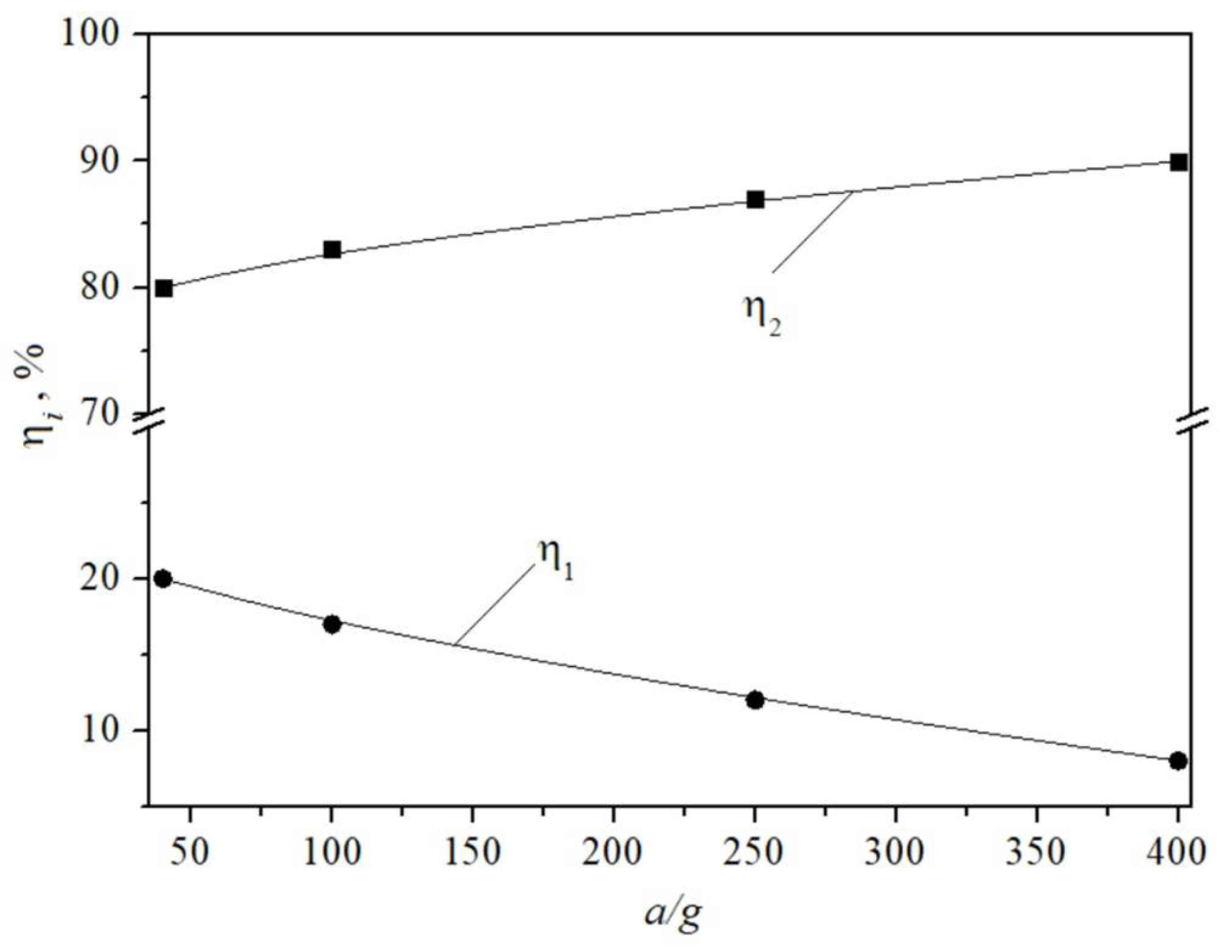
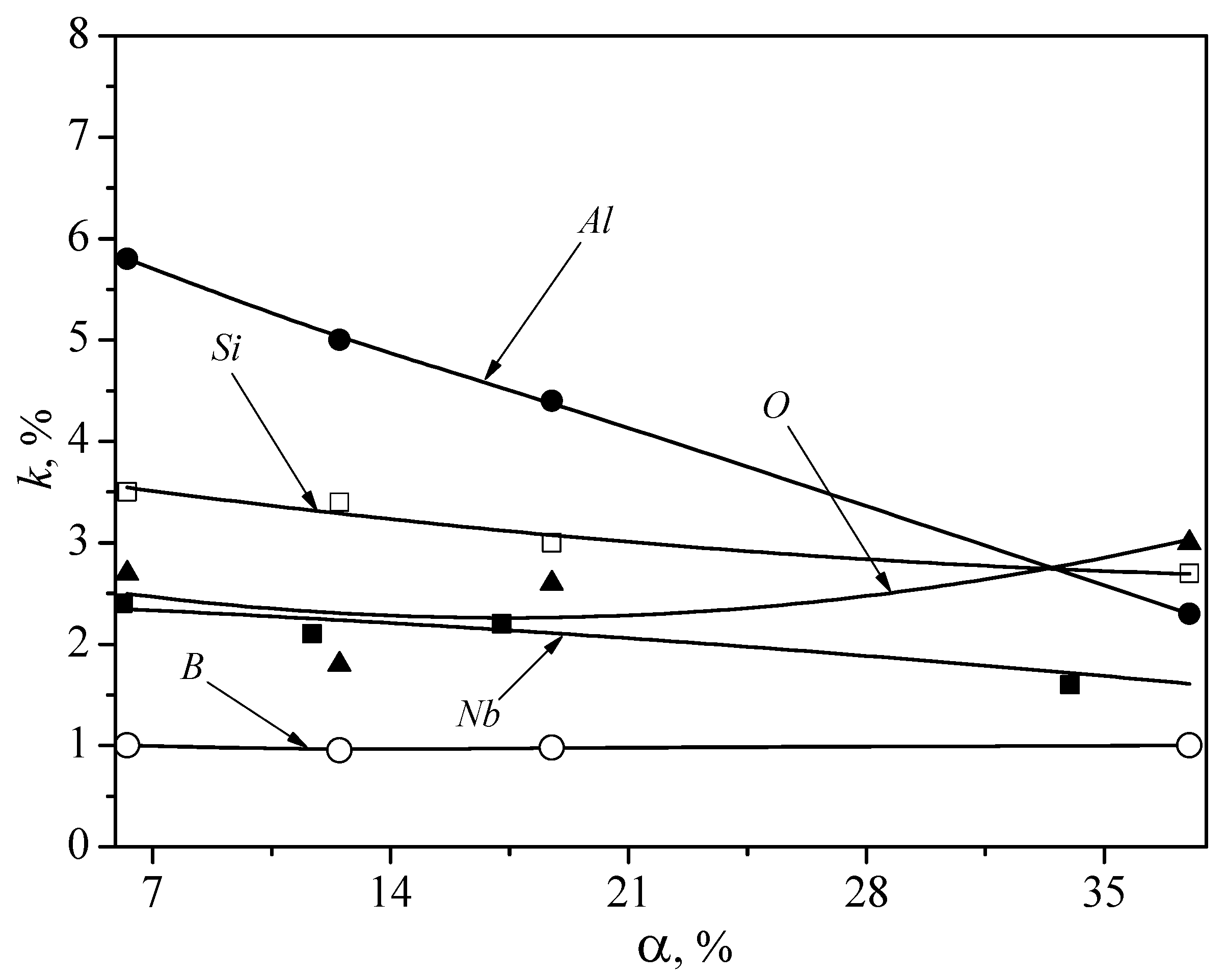
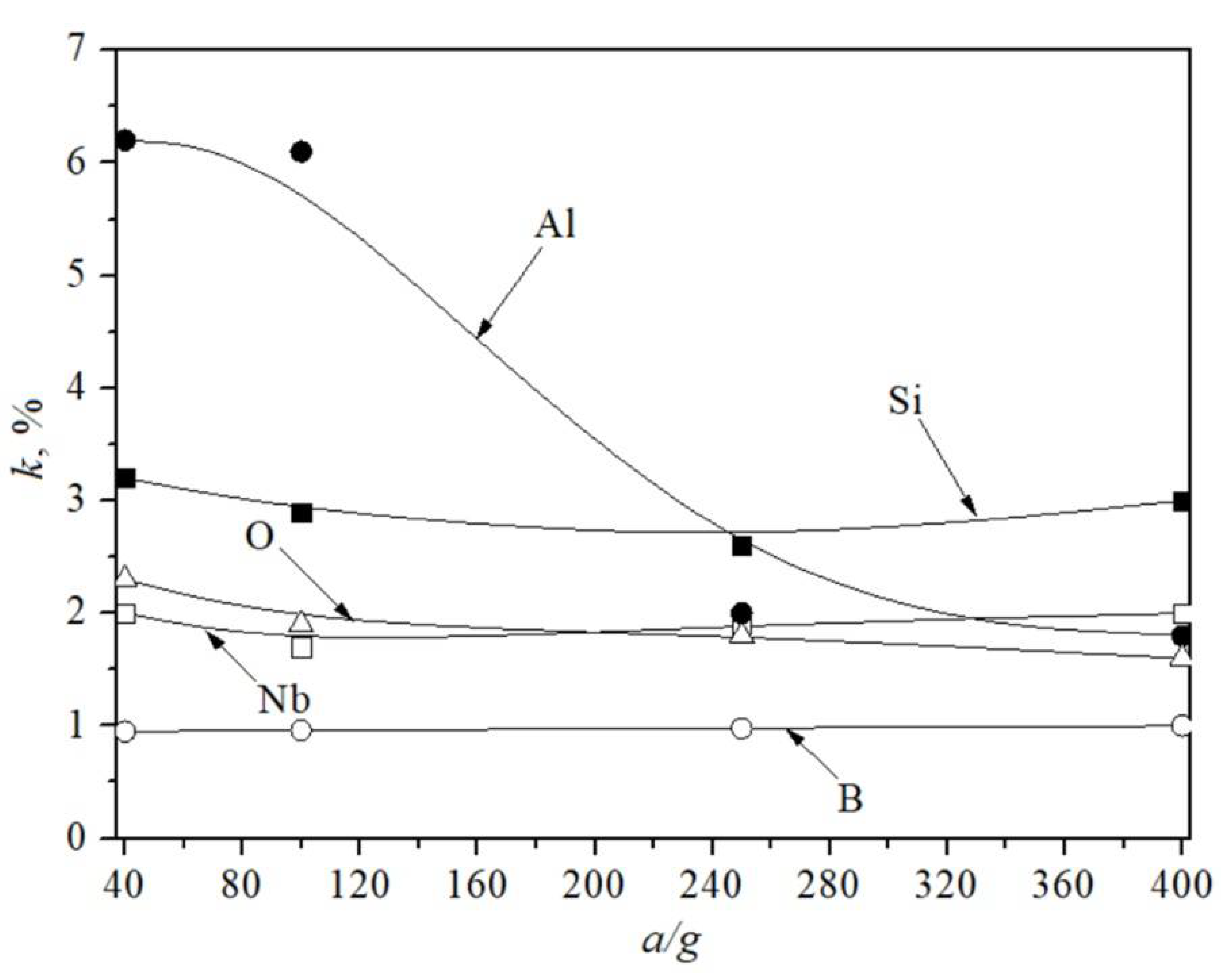
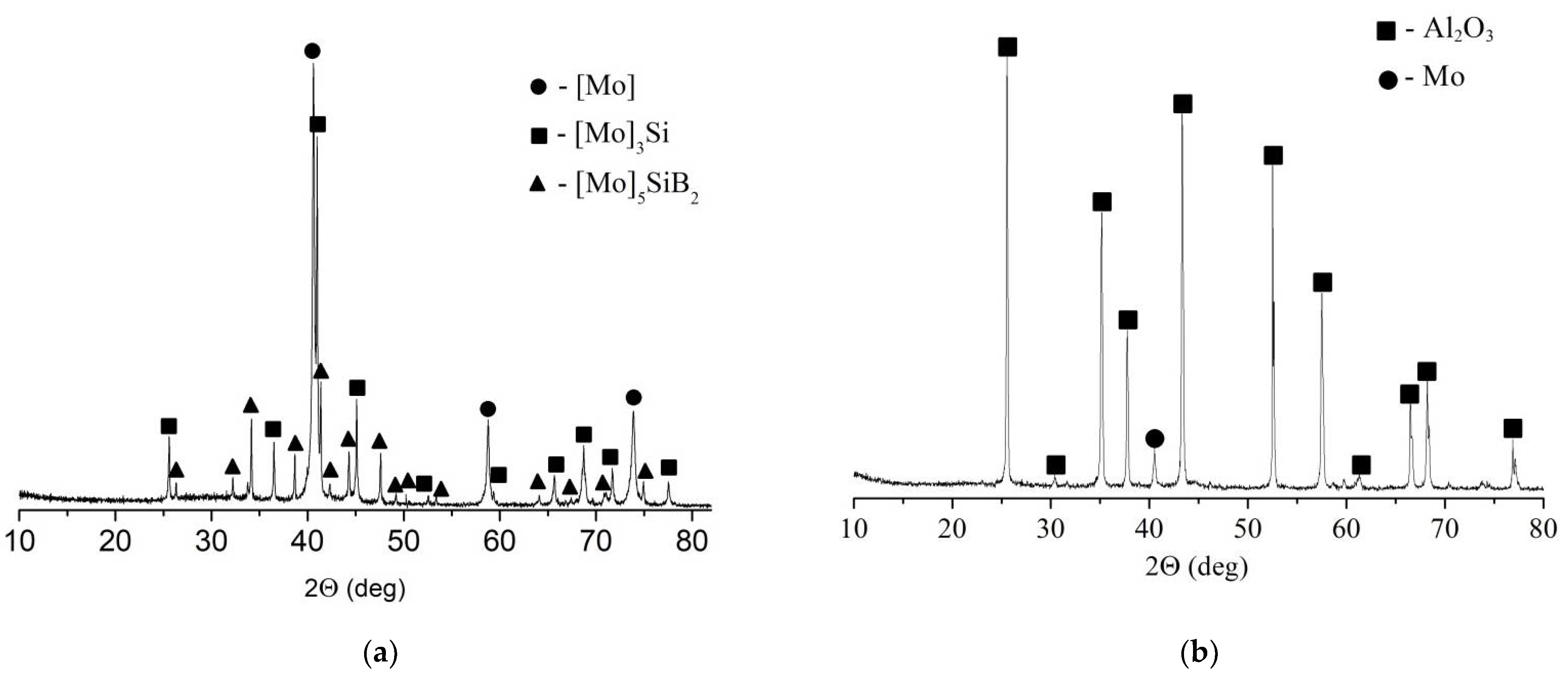
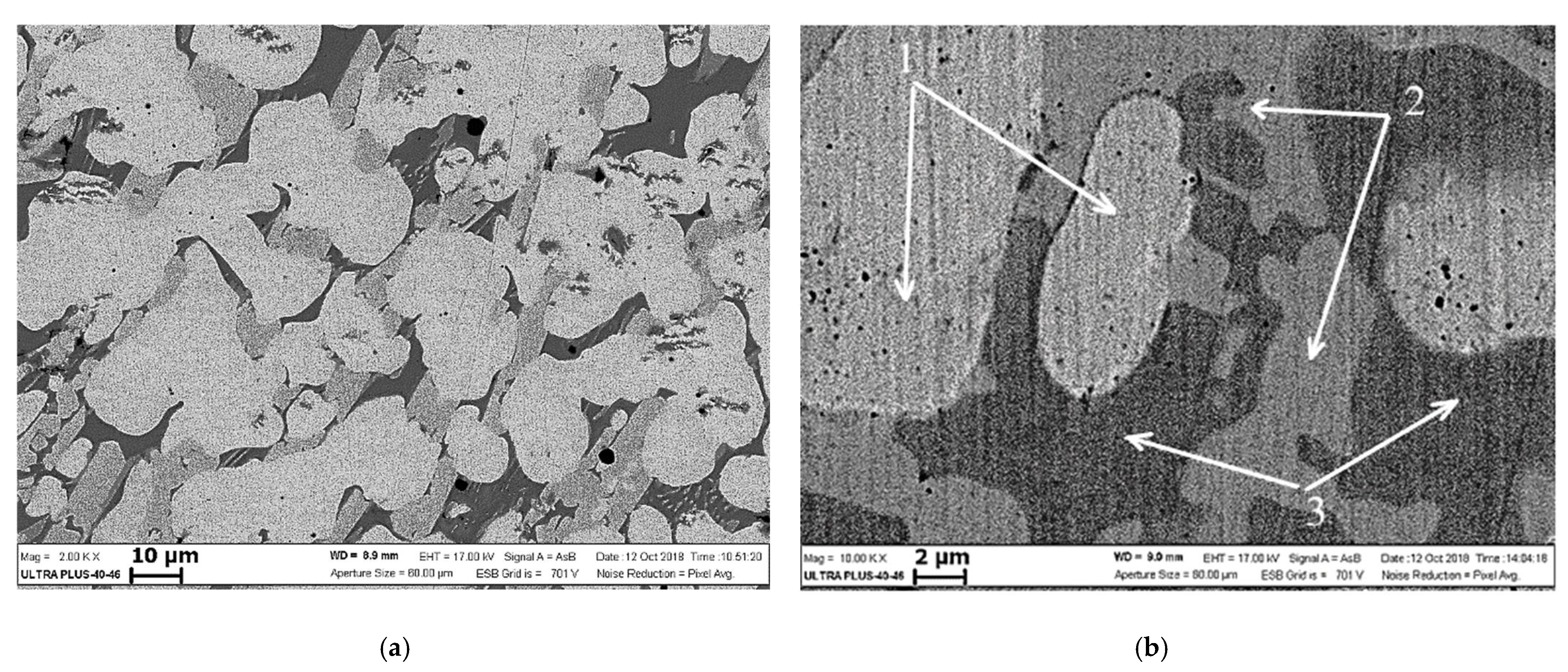
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jéhanno, P.; Heilmaier, M.; Saage, H.; Böning, M.; Kestler, H.; Freudenberger, J.; Drawin, S. Assessment of the high temperature deformation behavior of molybdenum silicide alloys. Mater. Sci. Eng. A 2007, 463, 216–223. [Google Scholar] [CrossRef]
- Jéhanno, P.; Heilmaier, M.; Kestler, H.; Böning, M.; Venskutonis, A.; Bewlay, B.P.; Jackson, M.R. Assessment of a powder metallurgical processing route for refractory metal silicide alloys. Metall. Mater. Trans. 2005, 36A, 515–523. [Google Scholar] [CrossRef]
- Berczik, D.M. Method for enhancing the oxidation resistance of a molybdenum alloy and a method of making a molybdenum alloy. U.S. Patent 5595616, 21 January 1997. [Google Scholar]
- Meyer, M.K.; Thom, A.J.; Akinc, M. Oxide scale formation and isothermal oxidation behavior of Mo–Si–B intermetallics at 600–1000 °C. Intermetallics 1999, 7, 153–162. [Google Scholar] [CrossRef]
- Schneibel, J.H.; Kruzic, J.J.; Ritchie, R.O. Mo-Si-B Alloy Development. In Proceedings of the 17th Annual Conference on Fossil Energy Materials, Baltimore, MD, USA, 22–24 April 2003. [Google Scholar]
- Takata, N.; Sekido, N.; Takeyama, M.; Perepezko, J.H.; Follett-Figueroa, M.; Zhang, C. Solidification of BCC/T1/T2 three-phase microstructure in Mo–Nb–Si–B alloys. Intermetallics 2016, 72, 1–8. [Google Scholar] [CrossRef]
- Byan, J.M.; Bang, S.-R.; Kim, S.H.; Choi, W.J.; Kim, Y.D. Mechanical properties of Mo–Nb–Si–B quaternary alloys fabricated by powder metallurgical method. Int. J. Refract. Met. Hard Mater. 2017, 65, 14–18. [Google Scholar] [CrossRef]
- Drawin, S.; Justin, J.F. Advanced lightweight silicide and nitride based materials for turbo-engine applications. AerospaceLab 2011, 3, 1–13. [Google Scholar]
- Vdovin, Y.S.; Andreev, D.E.; Yukhvid, V.I. Mo-Based composites reinforced with Nb, Si, and B by metallothermic SHS under artificial gravity. Int. J. Self-Propag. High.-Temp. Synth. 2019, 28, 274–275. [Google Scholar] [CrossRef]
- Shiryaev, A. Thermodynamics of SHS processes: An advanced approach. Int. J. Self-Propag. High.-Temp. Synth. 1995, 4, 351–362. [Google Scholar]
- Yukhvid, V.I.; Alymov, M.I.; Sanin, V.N.; Andreev, D.E.; Sachkova, N.V. Synthesis of composition materials based on niobium silicides by metallothermic SHS. Inorg. Mater. 2015, 51, 1347–1354. [Google Scholar] [CrossRef]
- Lim, K.S.; Kim, Y.S.; Hong, S.H.; Song, G.; Kim, K.B. Influence of N2 gas flow ratio and working pressure on amorphous Mo–Si–N coating during magnetron sputtering. Coatings 2020, 10, 34. [Google Scholar] [CrossRef]
| Mo | Si | Nb | B | MoO3 | Nb2O5 | Al | |
|---|---|---|---|---|---|---|---|
| Green mixture | – | 1.5 | – | 0.5 | 68.9 | 2.4 | 26.7 |
| Combustion product | 92.5 | 3.0 | 3.4 | 1.0 | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreev, D.; Vdovin, Y.; Yukhvid, V.; Golosova, O. Mo–Nb–Si–B Alloy: Synthesis, Composition, and Structure. Metals 2021, 11, 803. https://doi.org/10.3390/met11050803
Andreev D, Vdovin Y, Yukhvid V, Golosova O. Mo–Nb–Si–B Alloy: Synthesis, Composition, and Structure. Metals. 2021; 11(5):803. https://doi.org/10.3390/met11050803
Chicago/Turabian StyleAndreev, Dmitrii, Yurii Vdovin, Vladimir Yukhvid, and Olga Golosova. 2021. "Mo–Nb–Si–B Alloy: Synthesis, Composition, and Structure" Metals 11, no. 5: 803. https://doi.org/10.3390/met11050803
APA StyleAndreev, D., Vdovin, Y., Yukhvid, V., & Golosova, O. (2021). Mo–Nb–Si–B Alloy: Synthesis, Composition, and Structure. Metals, 11(5), 803. https://doi.org/10.3390/met11050803







