Effects of Slag Composition and Impurities of Alloys on the Inclusion Transformation during Industrial Ladle Furnace Refining
Abstract
1. Introduction
2. Experimental Method
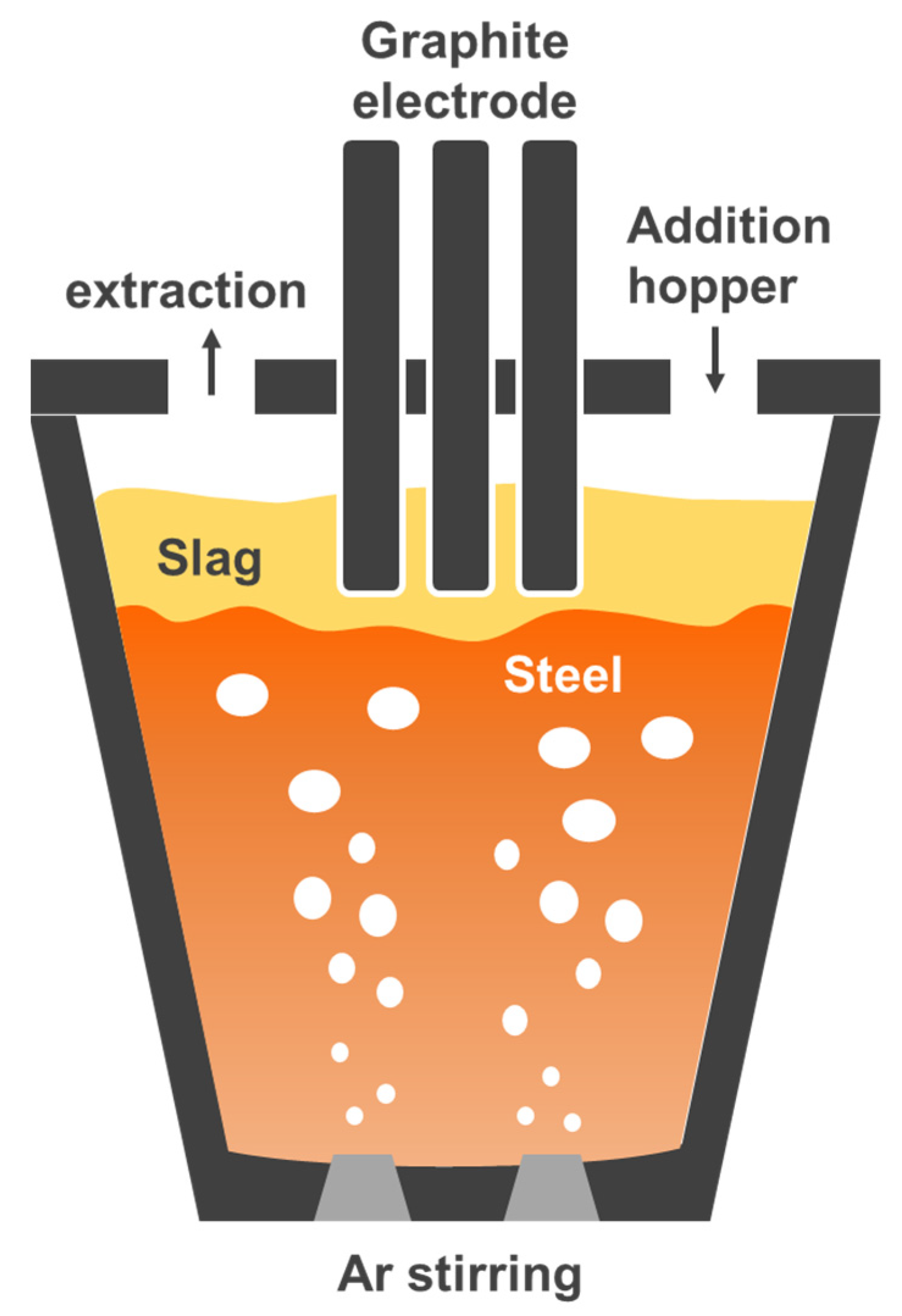
2.1. Experimental Apparatus and Procedure
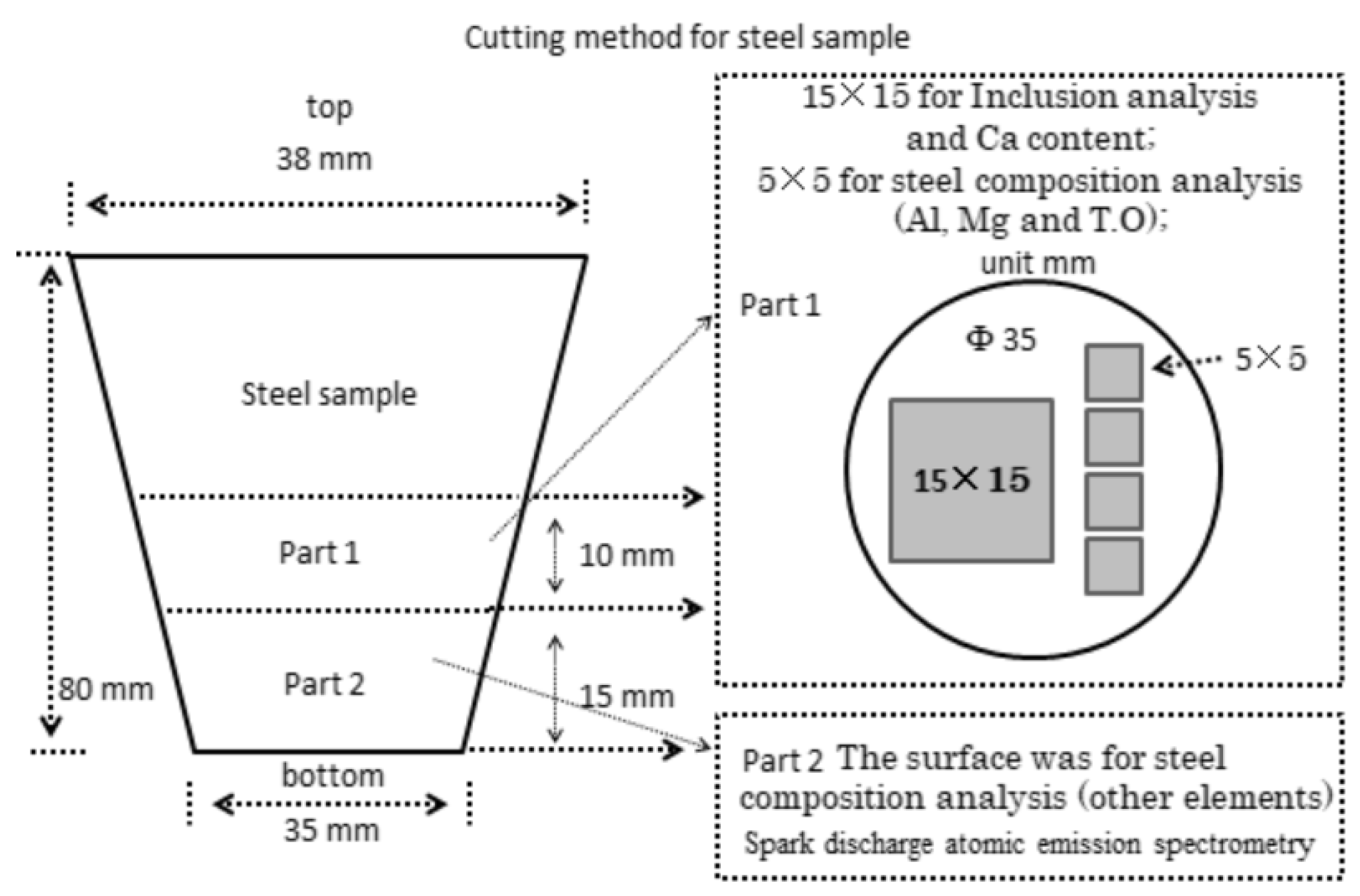
2.2. Analysis Method
2.3. Alloying Materials
3. Experimental Results
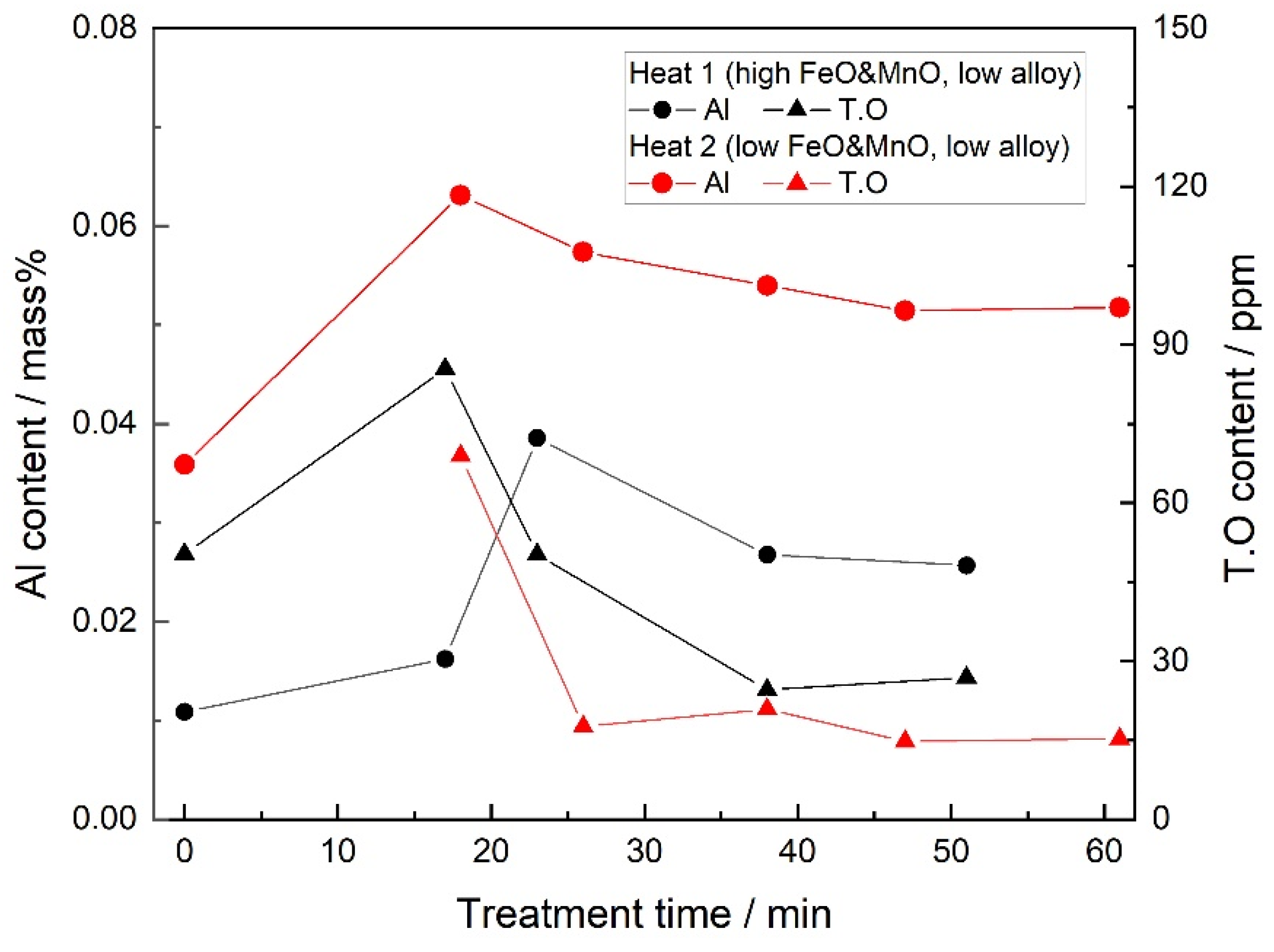
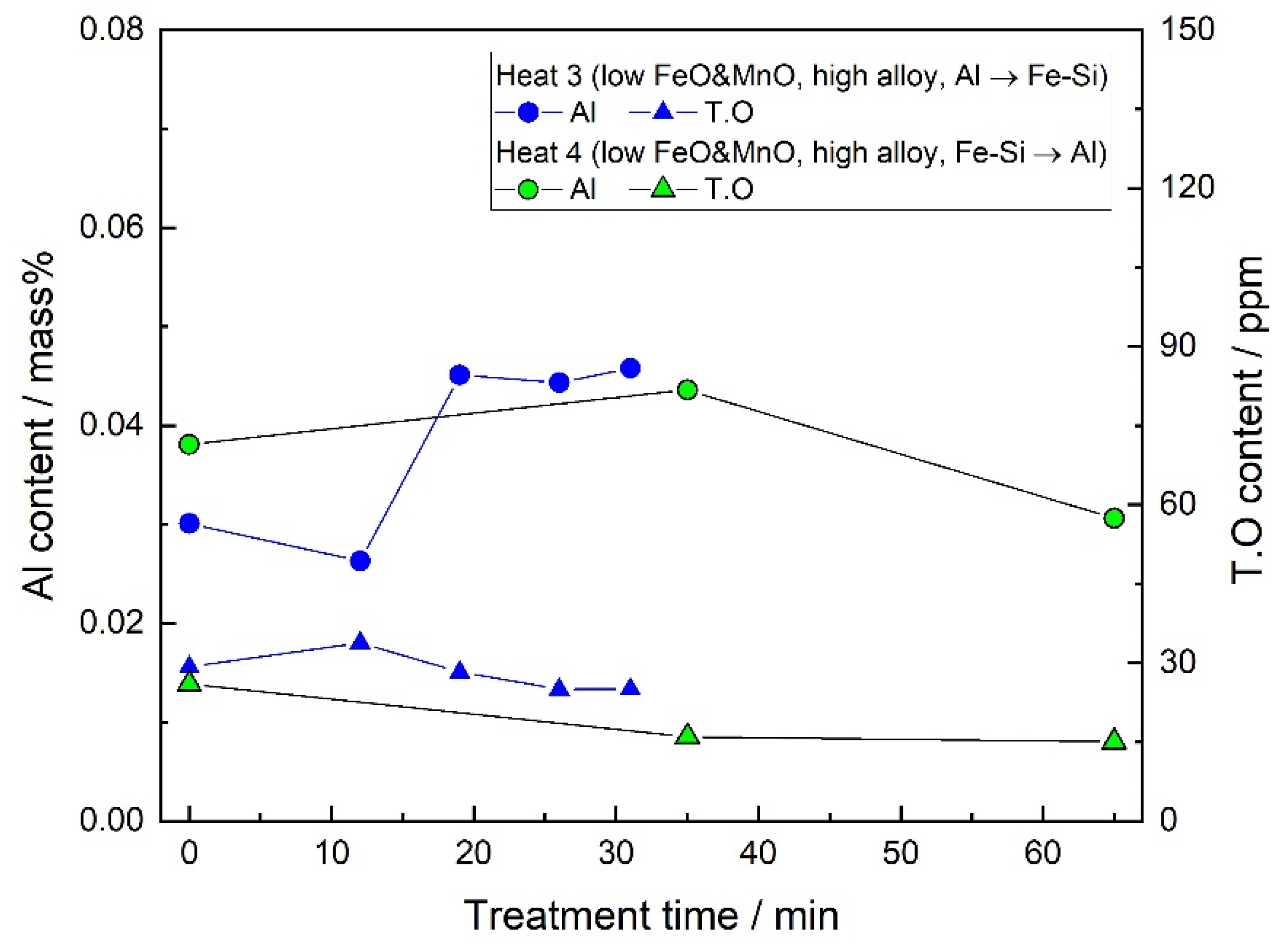
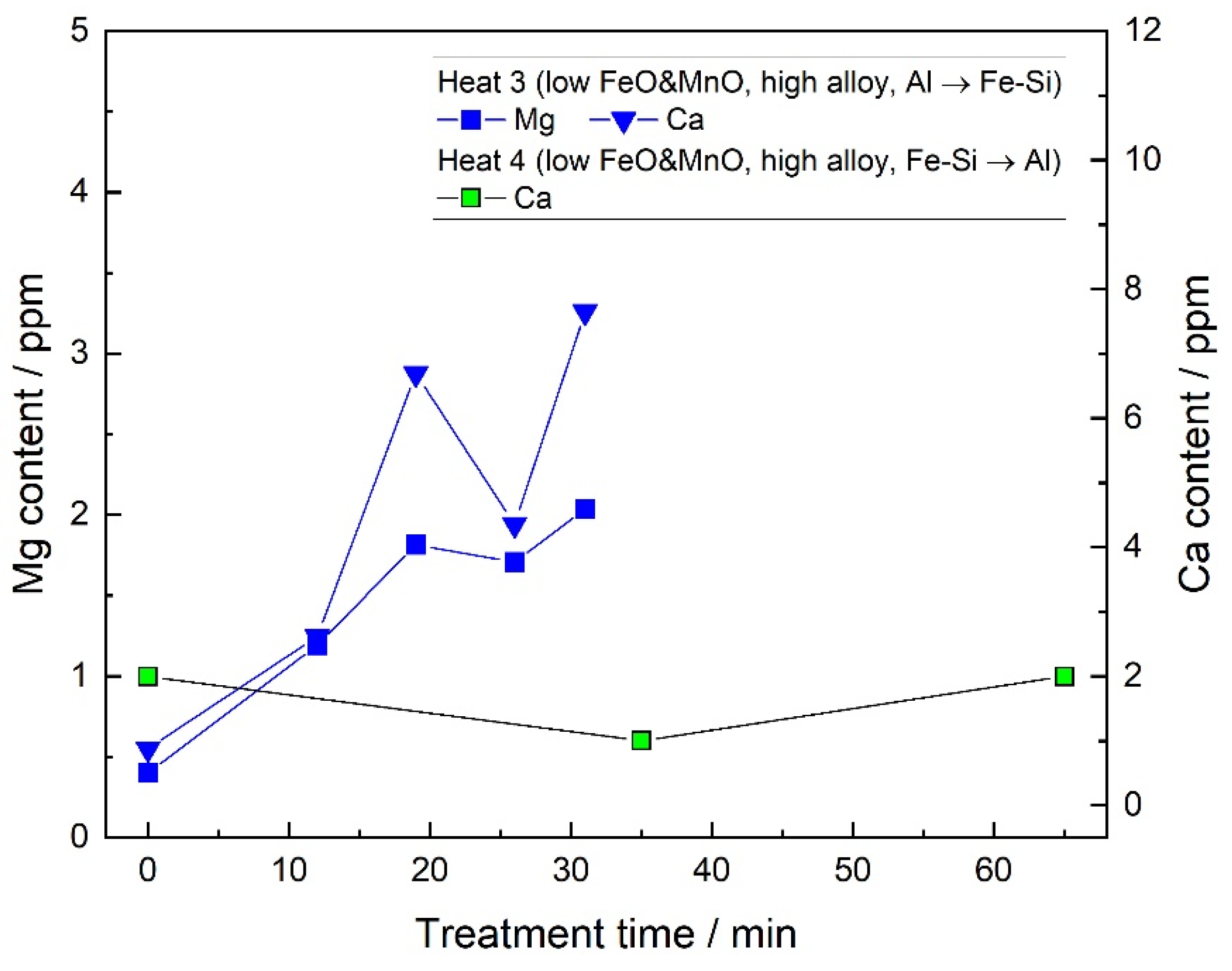
3.1. Steel Composition
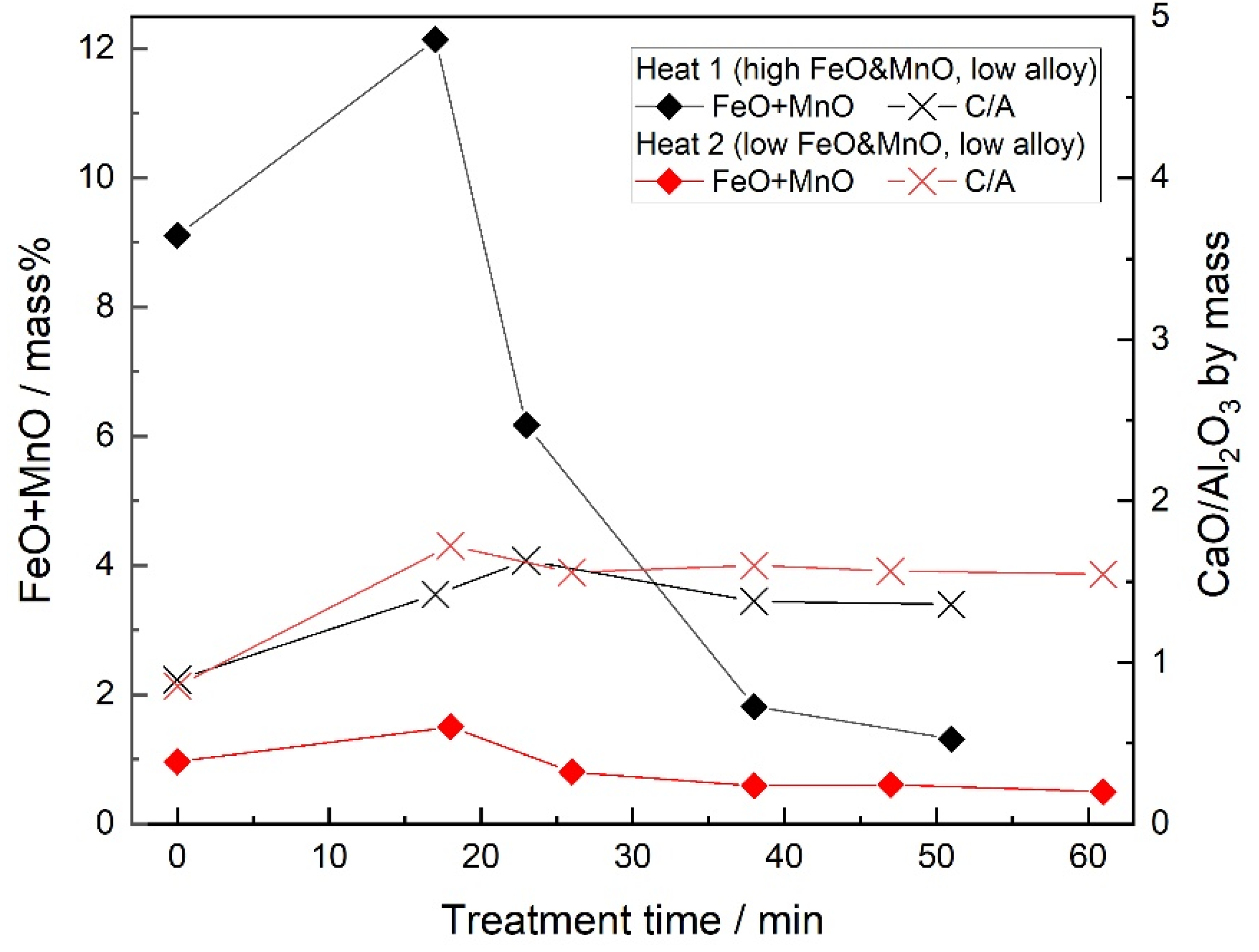
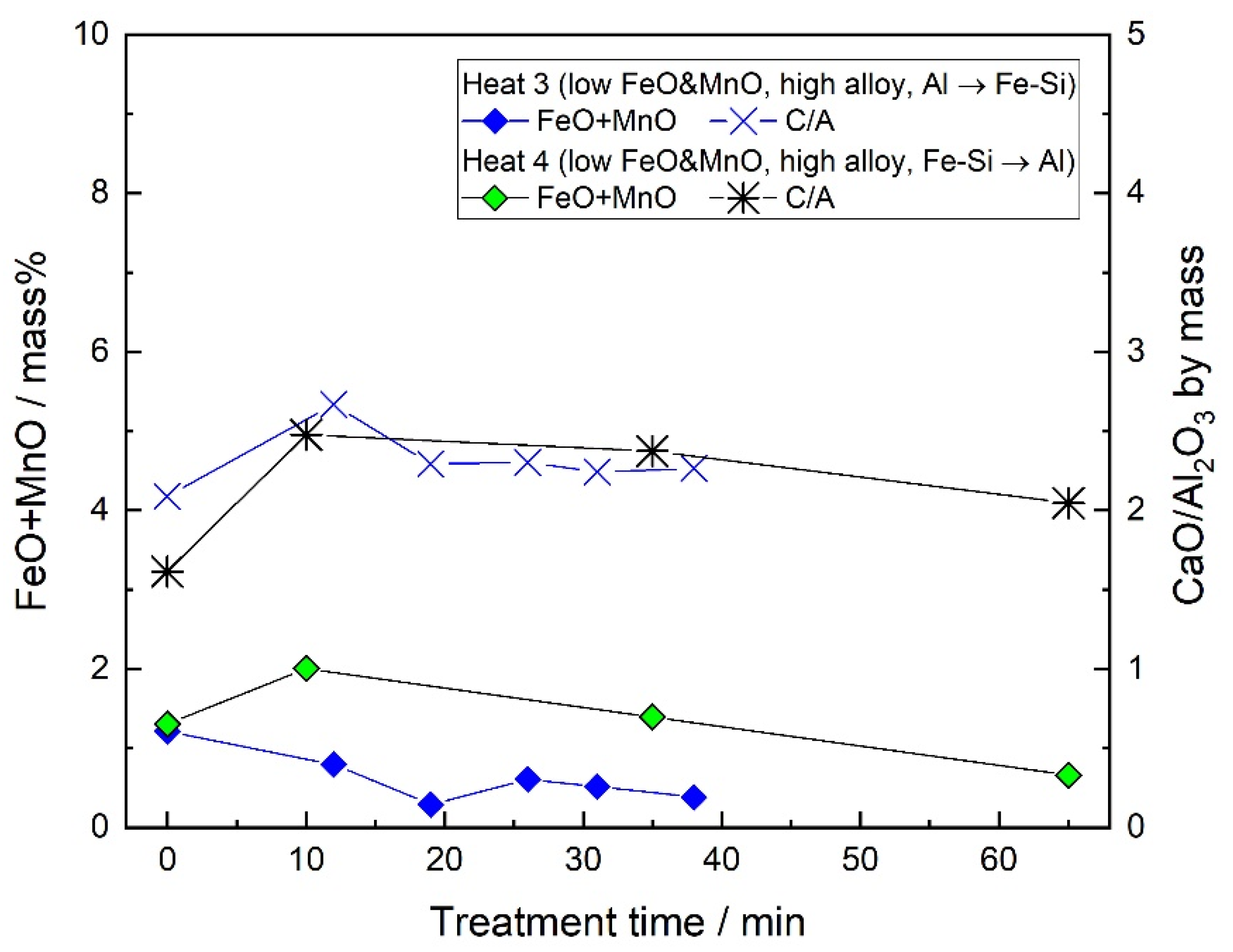
3.2. Slag Composition
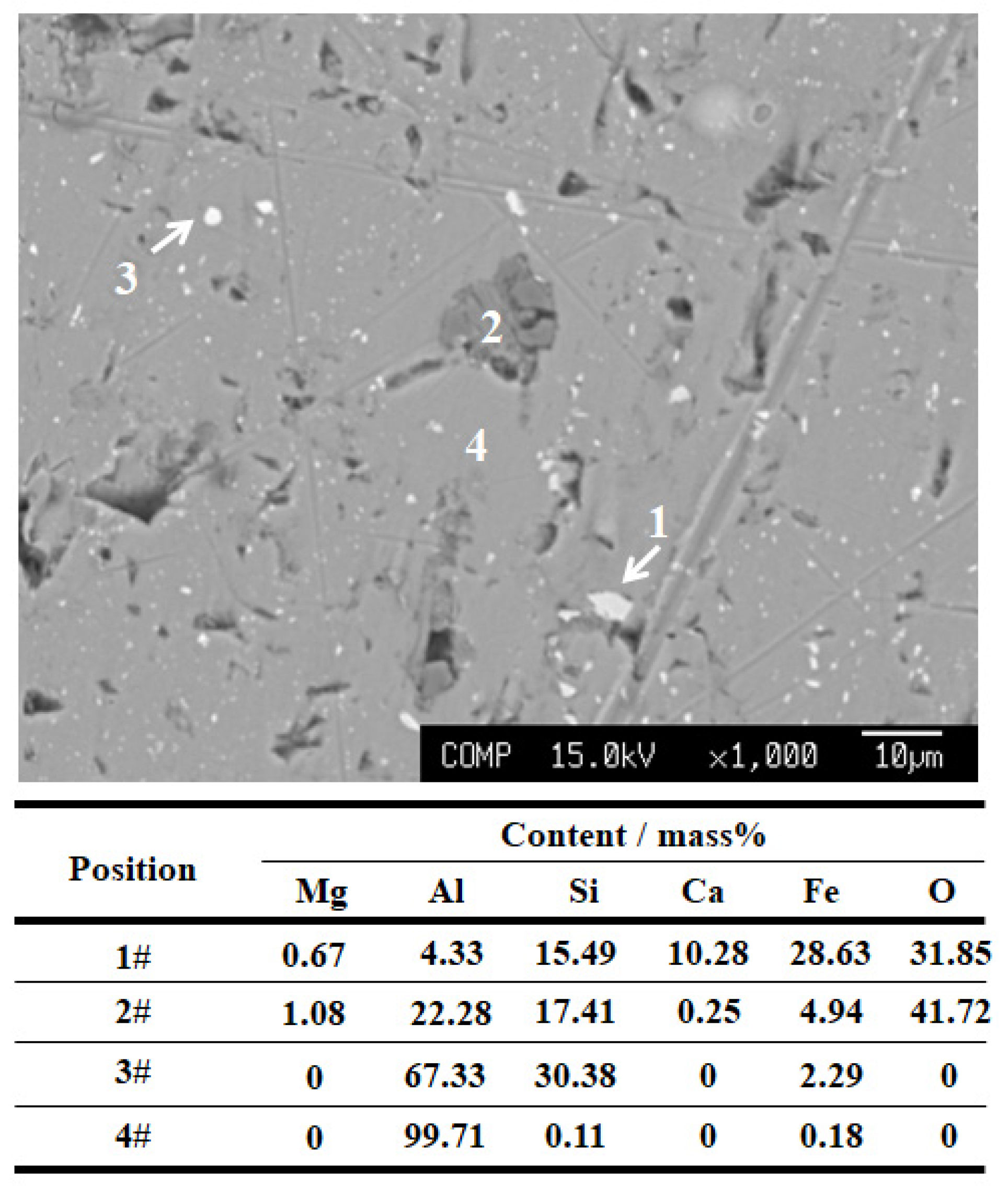
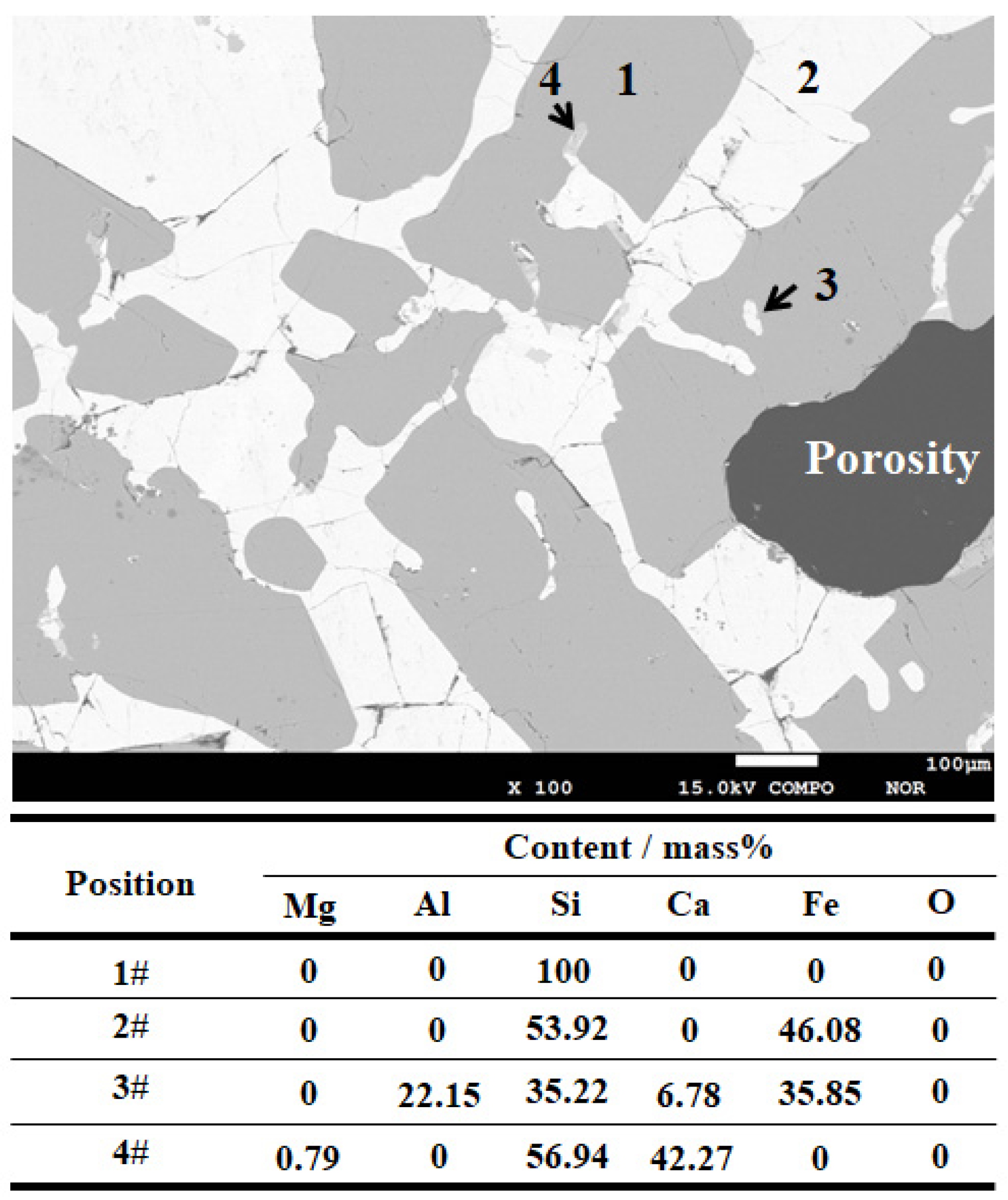
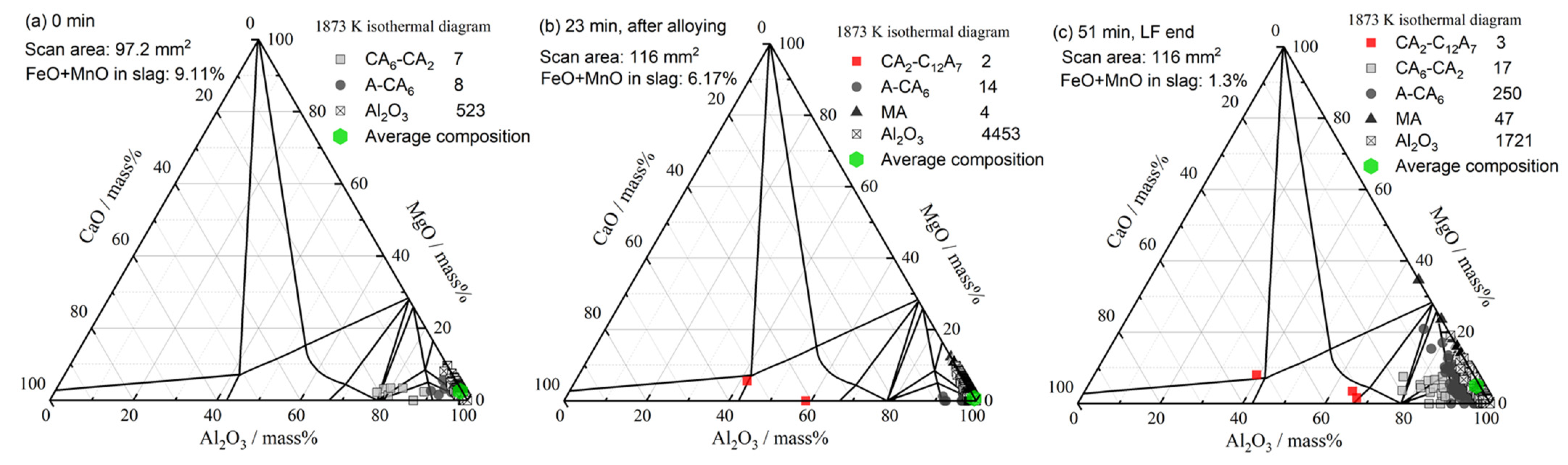
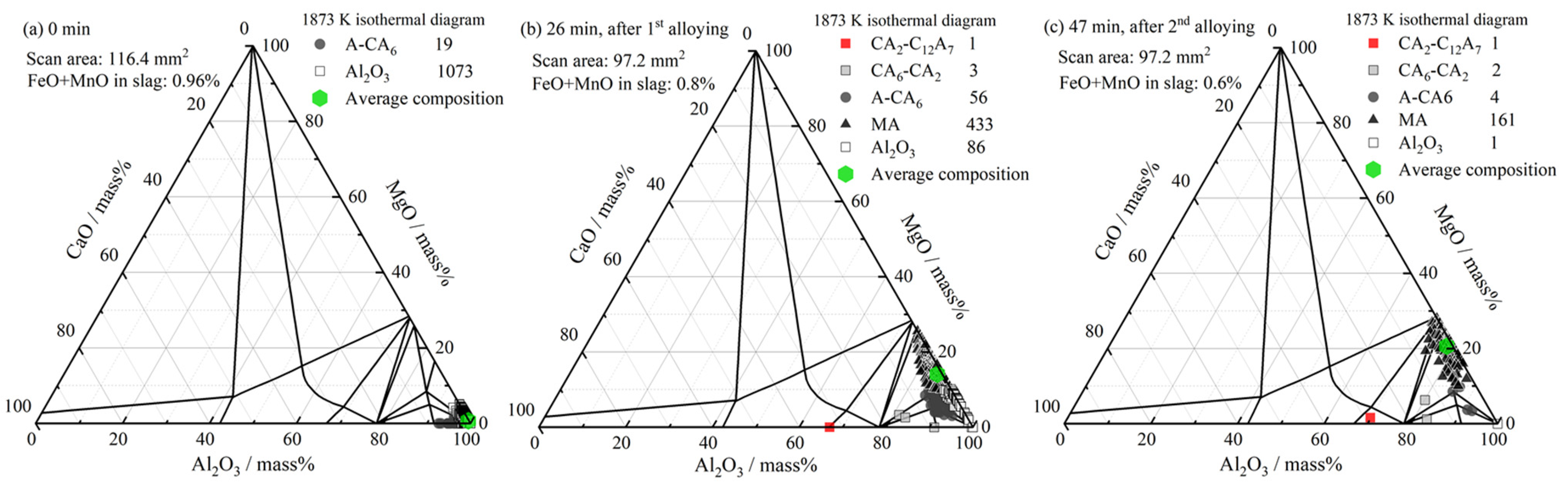
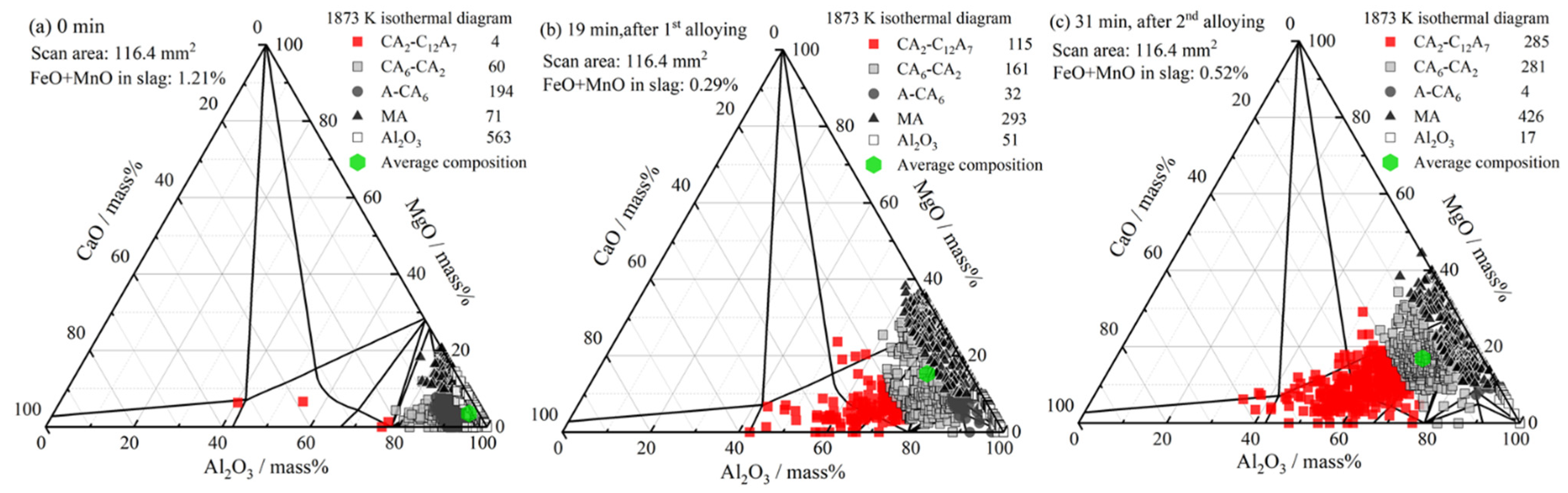
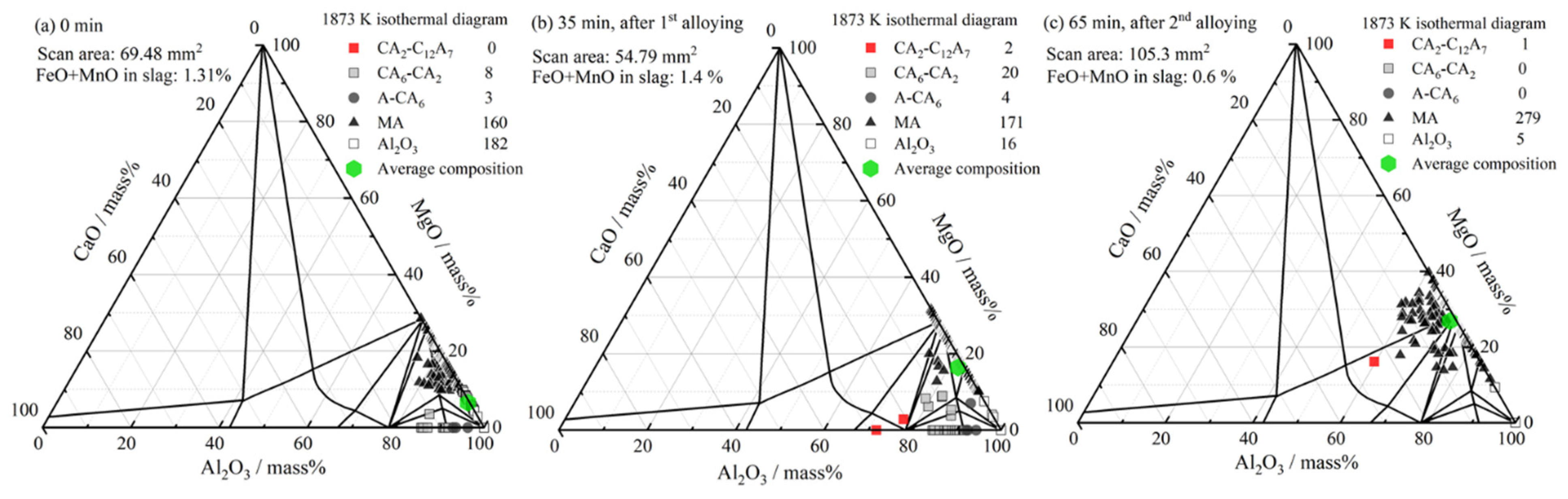
3.3. Inclusion Composition
4. Discussion
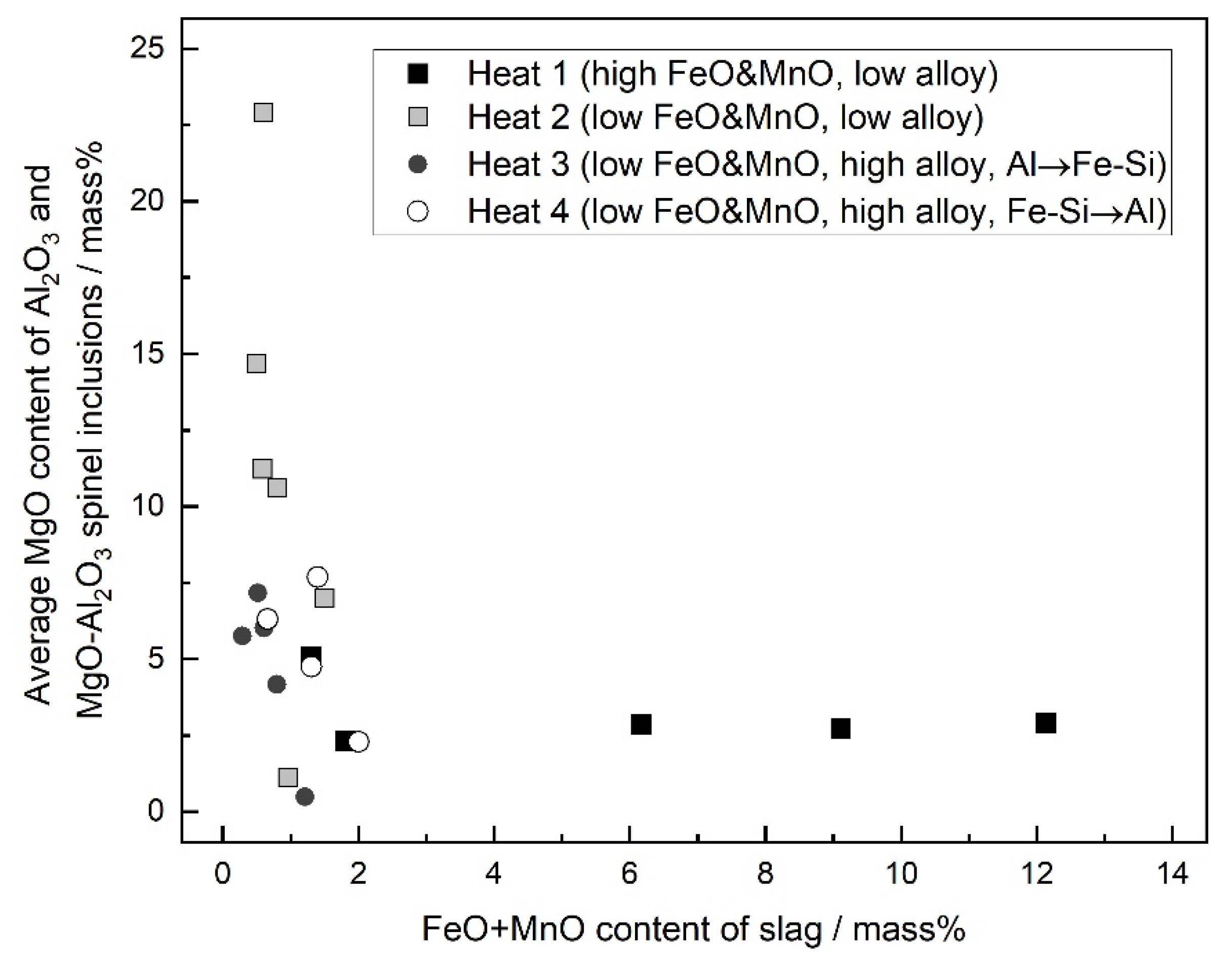
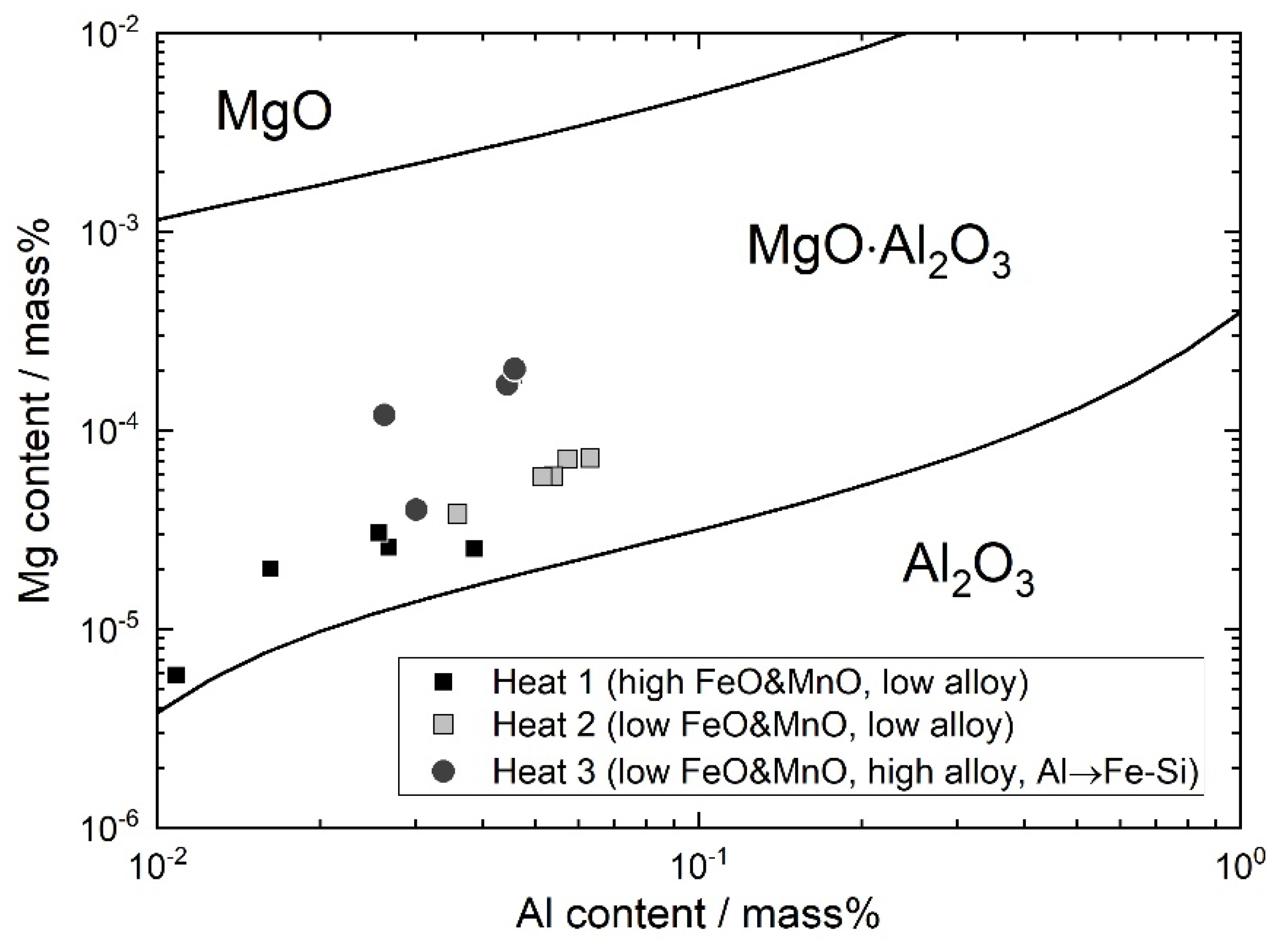
4.1. Formation of MgO–Al2O3 Spinel Inclusions
4.2. Formation of CaO–Al2O3 Inclusions
5. Summary
- When the FeO and MnO content in the slag was high (about 10 mass%) at the start of the LF treatment, the inclusions were maintained as Al2O3 throughout the LF treatment. When the FeO and MnO content in the slag was low (less than 1~2 mass%), the Al2O3 inclusions changed to a MA spinel inclusion.
- By the slag/steel reaction, the CaO–Al2O3 inclusions did not form under the current industrial conditions. The only Ca source for the formation of CaO–Al2O3 inclusions was confirmed to be the Ca–containing Fe–Si alloy under the current conditions. When the dissolved oxygen of the molten steel was low and a large amount of Fe–Si was added, the Al2O3 inclusions transformed into CaO–Al2O3 inclusions. However, when the dissolved oxygen was high at the LF start and less Fe–Si was added, the Ca of the Fe–Si alloy could not change the compositions of the inclusions.
Author Contributions
Funding
Conflicts of Interest
References
- Park, J.H.; Todoroki, H. Control of MgO·Al2O3 Spinel Inclusions in Stainless Steels. ISIJ Int. 2010, 50, 1333–1346. [Google Scholar] [CrossRef]
- Liu, C.; Gao, X.; Ueda, S.; Guo, M.; Kitamura, S.-Y. Composition Changes of Inclusions by Reaction with Slag and Refractory: A Review. ISIJ Int. 2020, 60, 1835–1848. [Google Scholar] [CrossRef]
- Nishi, T.; Shinme, K. Formation of Spinel Inclusions in Molten Stainless Steel under Al Deoxidation with Slags. Tetsuto Hagane 1998, 84, 837–843. [Google Scholar] [CrossRef]
- Okuyama, G.; Yamaguchi, K.; Takeuchi, S.; Sorimachi, K.-I. Effect of Slag Composition on the Kinetics of Formation of Al2O3 MgO Inclusions in Aluminum Killed Ferritic Stainless Steel. ISIJ Int. 2000, 40, 121–128. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.; Chen, B.; Wang, W. Formation of MgO·Al2O3 Inclusions in High Strength Alloyed Structural Steel Refined by CaO–SiO2–Al2O3–MgO Slag. ISIJ Int. 2008, 48, 885–890. [Google Scholar] [CrossRef]
- Harada, A.; Maruoka, N.; Shibata, H.; Zeze, M.; Asahara, N.; Huang, F.; Kitamura, S.-Y. Kinetic Analysis of Compositional Changes in Inclusions during Ladle Refining. ISIJ Int. 2014, 54, 2569–2577. [Google Scholar] [CrossRef]
- Harada, A.; Matsui, A.; Nabeshima, S.; Kikuchi, N.; Miki, Y. Effect of Slag Composition on MgO·Al2O3 Spinel-Type Inclusions in Molten Steel. ISIJ Int. 2017, 57, 1546–1552. [Google Scholar] [CrossRef]
- Harada, A.; Matsui, A.; Nabeshima, S. Composition Change of Inclusions in High Carbon Steel before and after Addition of Aluminum. ISIJ Int. 2021, 61, 715–723. [Google Scholar] [CrossRef]
- Brabie, V. Mechanism of Reaction between Refractory Materials and Aluminum Deoxidised Molten Steel. ISIJ Int. 1996, 36, S109–S112. [Google Scholar] [CrossRef]
- Jansson, S.; Brabie, V.; Jonsson, P. Magnesia–carbon refractory dissolution in Al killed low carbon steel. Ironmak. Steelmak. 2006, 33, 389–397. [Google Scholar] [CrossRef]
- Liu, C.; Gao, X.; Kim, S.-J.; Ueda, S.; Kitamura, S.-Y. Dissolution Behavior of Mg from MgO–C Refractory in Al-killed Molten Steel. ISIJ Int. 2018, 58, 488–495. [Google Scholar] [CrossRef]
- Liu, C.; Huang, F.; Suo, J.; Wang, X. Effect of Magnesia-Carbon Refractory on the Kinetics of MgO·Al2O3 Spinel Inclusion Generation in Extra-Low Oxygen Steels. Met. Mater. Trans. A 2016, 47, 989–998. [Google Scholar] [CrossRef]
- Harada, A.; Miyano, G.; Maruoka, N.; Shibata, H.; Kitamura, S.-Y. Dissolution Behavior of Mg from MgO into Molten Steel Deoxidized by Al. ISIJ Int. 2014, 54, 2230–2238. [Google Scholar] [CrossRef]
- Liu, C.; Yagi, M.; Gao, X.; Kim, S.-J.; Huang, F.; Ueda, S.; Kitamura, S.-Y. Dissolution Behavior of Mg from Magnesia-Chromite Refractory into Al-killed Molten Steel. Met. Mater. Trans. A 2018, 49, 2298–2307. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, C.; Gao, X.; Zhang, L.; Ueda, S.; Kitamura, S.-Y. Dissolution Behavior of Mg and Ca from Dolomite Refractory into Al-killed Molten Steel. ISIJ Int. 2021. [Google Scholar] [CrossRef]
- Todoroki, H.; Mizuno, K. Variation of Inclusion Composition in 304 Stainless Steel Deoxidized with Aluminum. Iron Steelmak. 2003, 30, 60–67. [Google Scholar]
- Shin, J.H.; Park, J.H. Modification of Inclusions in Molten Steel by Mg-Ca Transfer from Top Slag: Experimental Confirmation of the ‘Refractory-Slag-Metal-Inclusion (ReSMI)’ Multiphase Reaction Model. Met. Mater. Trans. A 2017, 48, 2820–2825. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.; Chen, B.; Wang, W. Laboratory Study on Evolution Mechanisms of Non-metallic Inclusions in High Strength Alloyed Steel Refined by High Basicity Slag. ISIJ Int. 2010, 50, 95–104. [Google Scholar] [CrossRef]
- Deng, Z.; Zhu, M. Evolution Mechanism of Non-metallic Inclusions in Al-Killed Alloyed Steel during Secondary Refining Process. ISIJ Int. 2013, 53, 450–458. [Google Scholar] [CrossRef]
- Yoshioka, T.; Nakahata, K.; Kawamura, T.; Ohba, Y. Factors to Determine Inclusion Compositions in Molten Steel during the Secondary Refining Process of Case-Hardening Steel. ISIJ Int. 2016, 56, 1973–1981. [Google Scholar] [CrossRef]
- Liu, C.; Gao, X.; Ueda, S.; Kitamura, S.-Y. Change in Composition of Inclusions through the Reaction between Al-killed Steel and the Slag of CaO and MgO Saturation. ISIJ Int. 2019, 59, 268–276. [Google Scholar] [CrossRef]
- Kumar, D.; Piva, S.P.T.; Pistorius, P.C. Transfer of Calcium to Oxide Inclusions in Steel without Calcium Treatment. In Proceedings of the 10th International Conference on Clean Steel. OMBKE, Budapest, Hungary, 18–20 September 2018. [Google Scholar]
- Shin, J.H.; Park, J.H. Effect of CaO/Al2O3 Ratio of Ladle Slag on Formation Behavior of Inclusions in Mn and V Alloyed Steel. ISIJ Int. 2018, 58, 88–97. [Google Scholar] [CrossRef]
- Lee, K.; Park, J.; Chung, J.; Choi, S.; Ahn, S. The secondary refining technologies for improving the cleanliness of ultra-low carbon steel at Kwangyang Works. Revue Métallurgie 1996, 93, 503–509. [Google Scholar] [CrossRef]
- Kitamura, M.; Soejima, T.; Koyama, S.; Matsuda, Y.; Abu, J.; Nimiya, Y.; Yao, Y. Improvement of surface quality of continuously cast slab for conditioning-free rolling. Trans. Iron Steel Inst. Jpn. 1984, 24, 966–972. [Google Scholar] [CrossRef]
- Mizuno, K.; Todoroki, H.; Noda, M.; Tohge, T. Effects of Al and Ca in ferrosilicon alloys for deoxidation on inclusion composition in type 304 stainless steel. ISS Transact. 2001, 28, 93–101. [Google Scholar]
- Wijk, O.; Brabie, V. The Purity of Ferrosilicon and Its Influence on Inclusion Cleanliness of Steel. ISIJ Int. 1996, 36, S132–S135. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Li, Q.; Huang, F.; Li, H.; Yang, J. Control of Stringer Shaped Non-Metallic Inclusions of CaO-Al2O3 System in API X80 Linepipe Steel Plates. Steel Res. Int. 2014, 85, 155–163. [Google Scholar] [CrossRef]
- Verein Deutscher Eisenhuttenleute (VDEh). Slag Atlas, 2nd ed.; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1995; Volume 104. [Google Scholar]
- Graham, K.; Irons, G. The Behavior of Non-Metallic Inclusions During Ladle Refining; AISTech2009: St. Louis, MO, USA, 2009; Volume 1, pp. 1003–1014. [Google Scholar]
- Mendez, J.; Gomez, A.; Capurro, C.; Donayo, R.; Cicutti, C. Effect of process conditions on the evolution of MgO content of inclusions during the production of calcium treated, aluminum killed steels. In Proceedings of the 8th International Conference on Clean Steel, Budapest, Hungary, 14–16 May 2012. [Google Scholar]
- Cicutti, C.; Capurro, C.; Cerrutti, G. Development of a kinetic model to predict the evolution of inclusions composition during the production of aluminum killed, calcium treated steels. In Proceedings of the 9th International Conference on Clean Steel, Budapest, Hungary, 8–10 September 2015. [Google Scholar]
- Chen, G.; Guo, Y.; He, S. Effect of FeO content in Slag on formation of MgOfflAl2O3inclusion for Al-killed steel. Met. Res. Technol. 2016, 113, 204. [Google Scholar] [CrossRef]
- Hino, M.; Ito, K. Thermodynamic Data for Steelmaking; Tohoku University Press: Sendai, Japan, 2010; Volume 16, p. 251. [Google Scholar]
- Itoh, H.; Hino, M.; Ban-Ya, S. Thermodynamics on the formation of spinel nonmetallic inclusion in liquid steel. Met. Mater. Trans. A 1997, 28, 953–956. [Google Scholar] [CrossRef]
- Itoh, H.; Hino, M.; Ban-Ya, S. Deoxidation Equilibrium of Magnesium in Liquid Iron. Tetsuto Hagane 1997, 83, 623–628. [Google Scholar] [CrossRef]
- Itoh, H.; Hino, M.; Ban-Ya, S. Deoxidation Equilibrium of Calcium in Liquid Iron. Tetsuto Hagane 1997, 83, 695–700. [Google Scholar] [CrossRef]
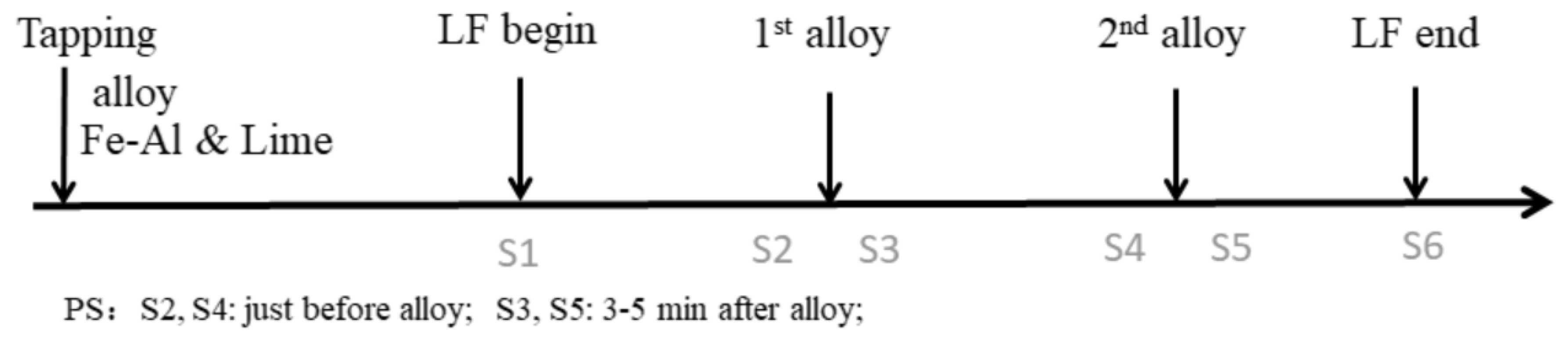



| Experiment NO. | Slag Condition | Alloy Amount | Alloy Time/Min | Deoxidizer Added during Alloying |
|---|---|---|---|---|
| Heat 1 | High FeO content at the start of treatment; MgO & CaO sat. | Small | 1st alloy: 20 | Al |
| Heat 2 | Low FeO content at the start of treatment; MgO & CaO sat. | Small | 1st alloy: 20 | Al only |
| 2nd alloy: 40 | No deoxidizer | |||
| Heat 3 | Low FeO content at the start of treatment; MgO & CaO sat. | Large | 1st alloy: 13 | Al |
| 2nd alloy: 28 | Fe–Si | |||
| Heat 4 | Low FeO content at the start of treatment; MgO & CaO sat. | Large | 1st alloy: 30 | Fe–Si |
| 2nd alloy: 60 | Fe–Al |
| Experiment No. | During BOF Tapping kg/ton | During 1st Alloy kg/ton | During 2nd Alloy kg/ton |
|---|---|---|---|
| Heat 1 | Fe–Al 3.37 Fe–Mn 1.64 | Fe–Al 0.85 C 0.10 | – |
| Heat 2 | Fe–Al 4.92 | Fe–Al 0.91 | Fe–Mn 1.20 |
| Heat 3 | C 0.17 Fe–Mn 3.92 Fe–Si 3.29 Fe–P 1.67 Al 1.97 Fe–Cr 5.93 Ni 0.66 Cu 2.79 | C 0.08 Al 0.53 Fe–Si 0.66 Fe–Cr 1.48 Fe–P 0.64 | Fe–P 0.28 Fe–Mn 0.23 Fe–Si 0.43 Al 0.20 |
| Heat 4 | C 6.41 Fe–Al 3.43 Fe–Si 4.21 Fe–Mn 13.16 | Fe–Mn 2.02 Fe–Si 0.25 C 0.38 | Fe–Mn 0.48 Fe–Al 0.35 C 0.23 |
| Alloy Brand | Mg Content/Mass% | Ca Content/Mass% |
|---|---|---|
| Fe–Al | 0.0003 | 0.0010 |
| Fe–Mn–high C | 0 | 0.0010 |
| Al (lump) | 0.0628 | 0.1910 |
| Fe–Mn–medium C | 0.0003 | 0.0018 |
| Fe–Cr | 0 | 0 |
| Fe–P | 0 | 0.0008 |
| Fe–Si | 0.0261 | 0.3195 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Jia, Y.; Hao, L.; Han, S.; Huang, F.; Yu, H.; Gao, X.; Ueda, S.; Kitamura, S.-y. Effects of Slag Composition and Impurities of Alloys on the Inclusion Transformation during Industrial Ladle Furnace Refining. Metals 2021, 11, 763. https://doi.org/10.3390/met11050763
Liu C, Jia Y, Hao L, Han S, Huang F, Yu H, Gao X, Ueda S, Kitamura S-y. Effects of Slag Composition and Impurities of Alloys on the Inclusion Transformation during Industrial Ladle Furnace Refining. Metals. 2021; 11(5):763. https://doi.org/10.3390/met11050763
Chicago/Turabian StyleLiu, Chunyang, Yi Jia, Lixia Hao, Shaowei Han, Fuxiang Huang, Huixiang Yu, Xu Gao, Shigeru Ueda, and Shin-ya Kitamura. 2021. "Effects of Slag Composition and Impurities of Alloys on the Inclusion Transformation during Industrial Ladle Furnace Refining" Metals 11, no. 5: 763. https://doi.org/10.3390/met11050763
APA StyleLiu, C., Jia, Y., Hao, L., Han, S., Huang, F., Yu, H., Gao, X., Ueda, S., & Kitamura, S.-y. (2021). Effects of Slag Composition and Impurities of Alloys on the Inclusion Transformation during Industrial Ladle Furnace Refining. Metals, 11(5), 763. https://doi.org/10.3390/met11050763






