Characterization Study of an Oxide Film Layer Produced under CO2/Steam Atmospheres on Two Different Maraging Steel Grades
Abstract
1. Introduction
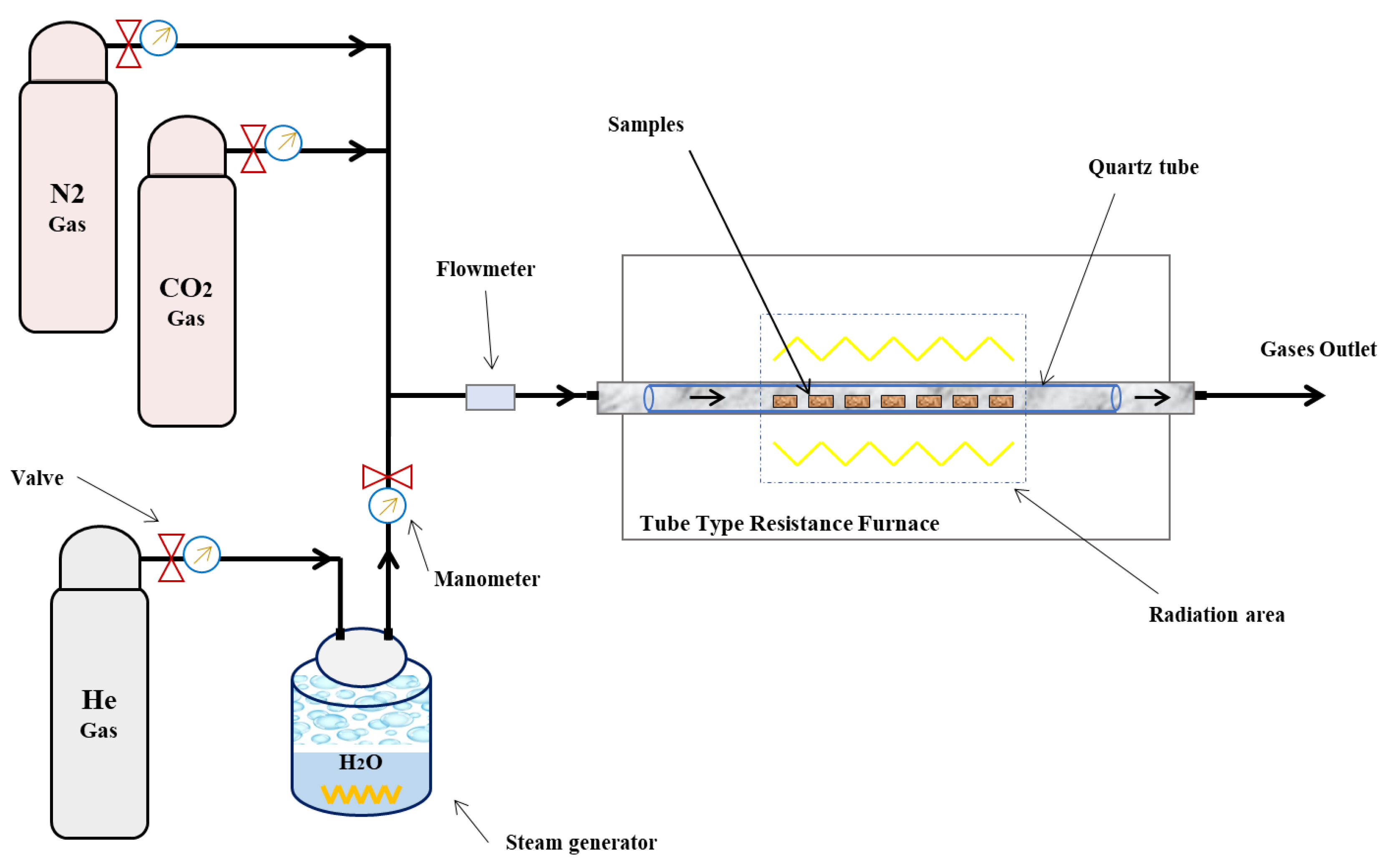
2. Materials and Methods
2.1. Materials
2.2. Microstructurally Characterization
2.3. Mechanical Properties: Scratch Tests
3. Results
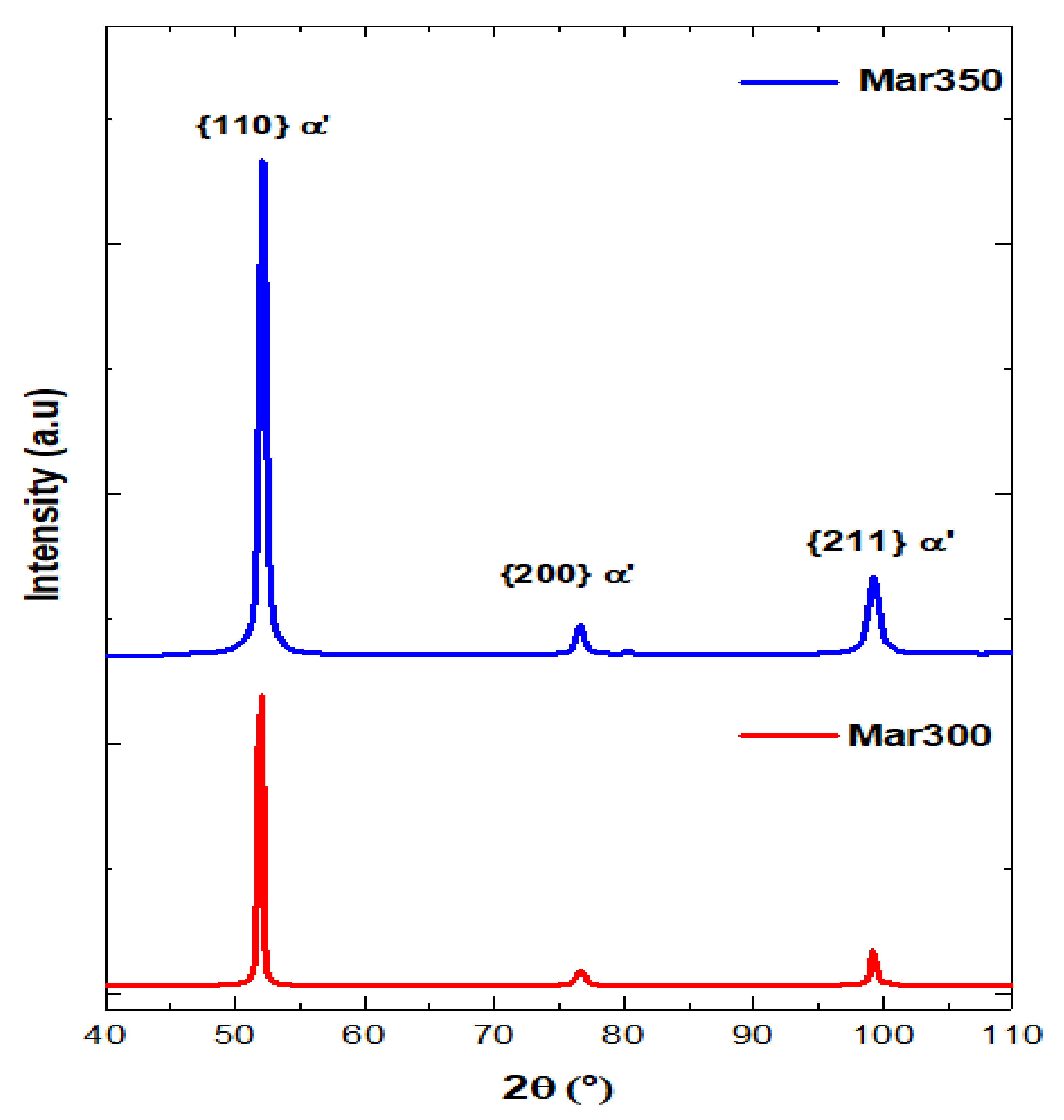
3.1. Steel Characterization
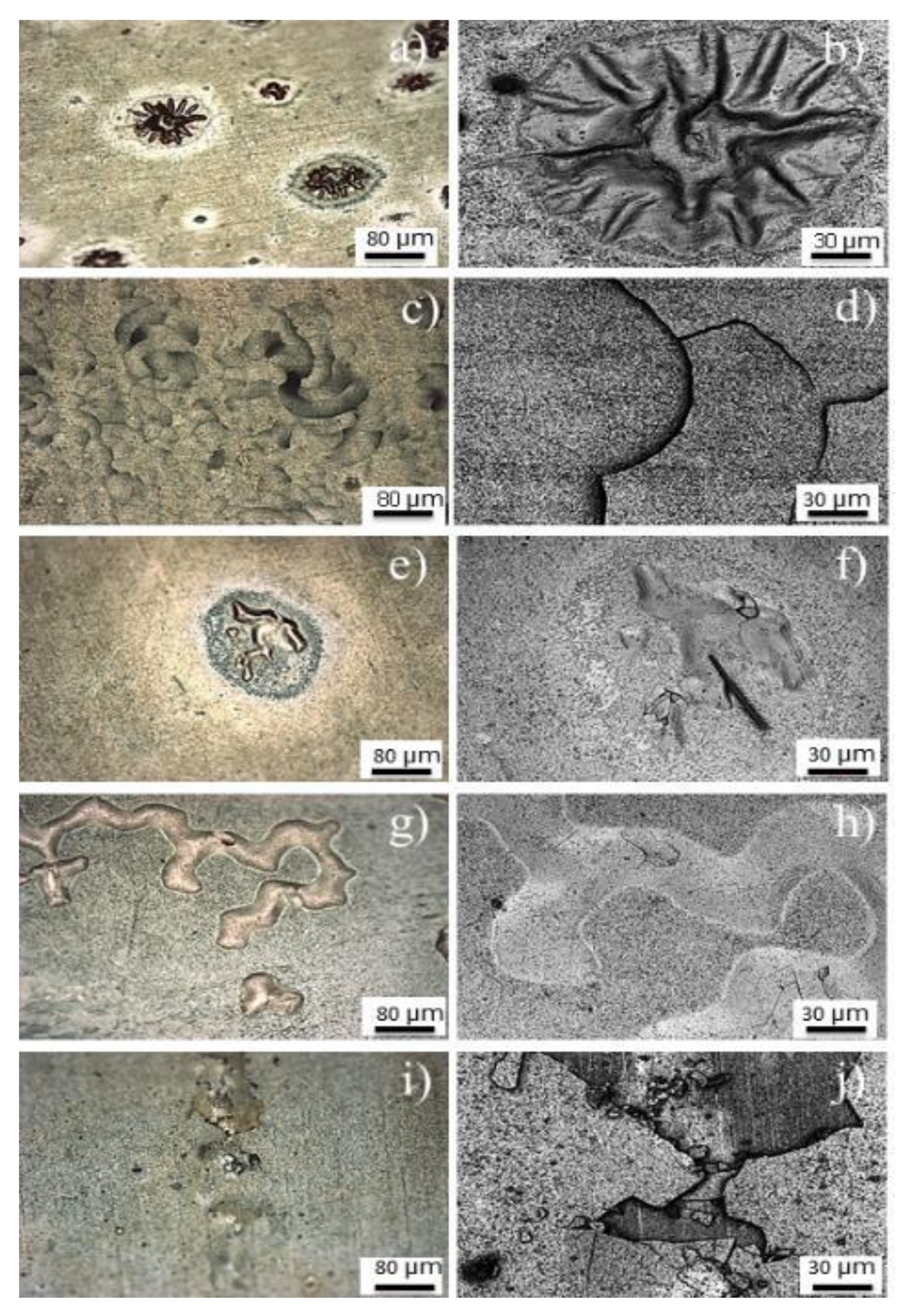
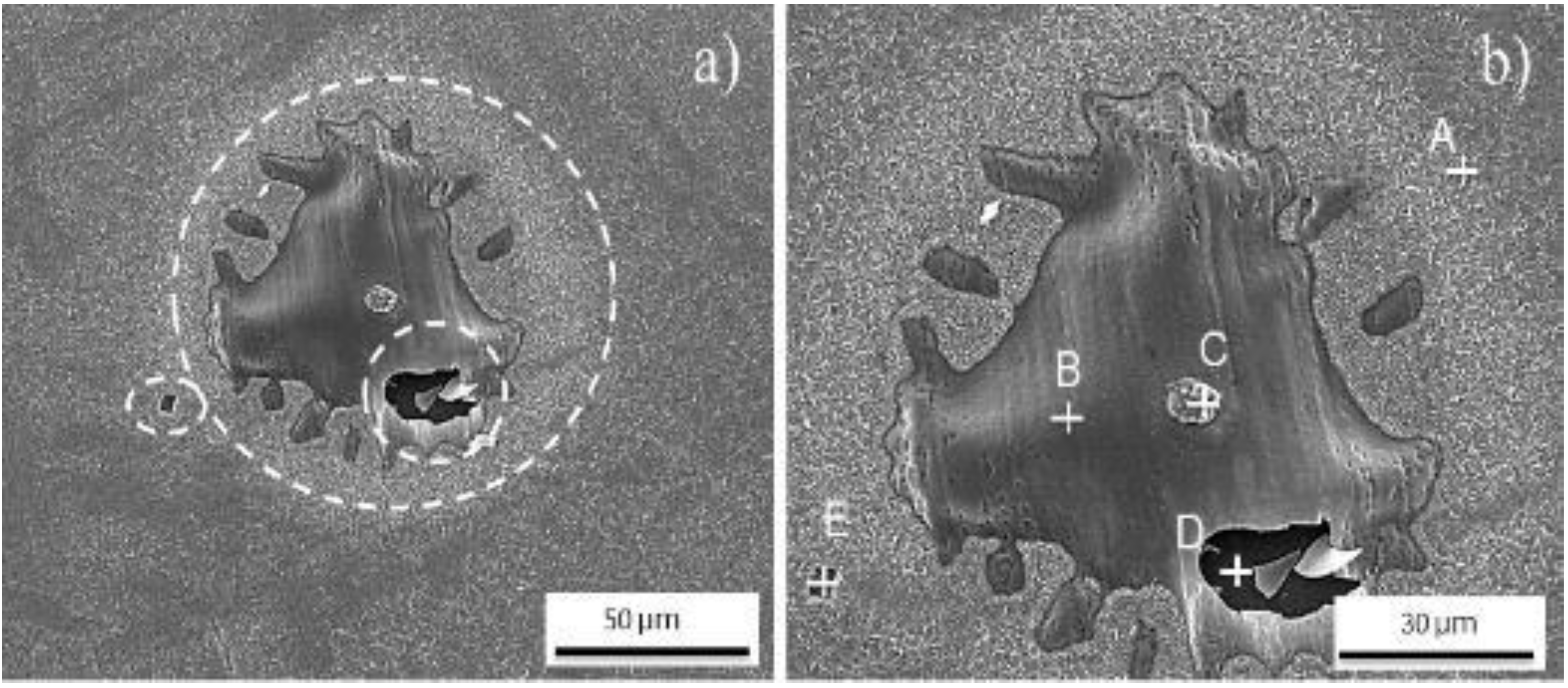
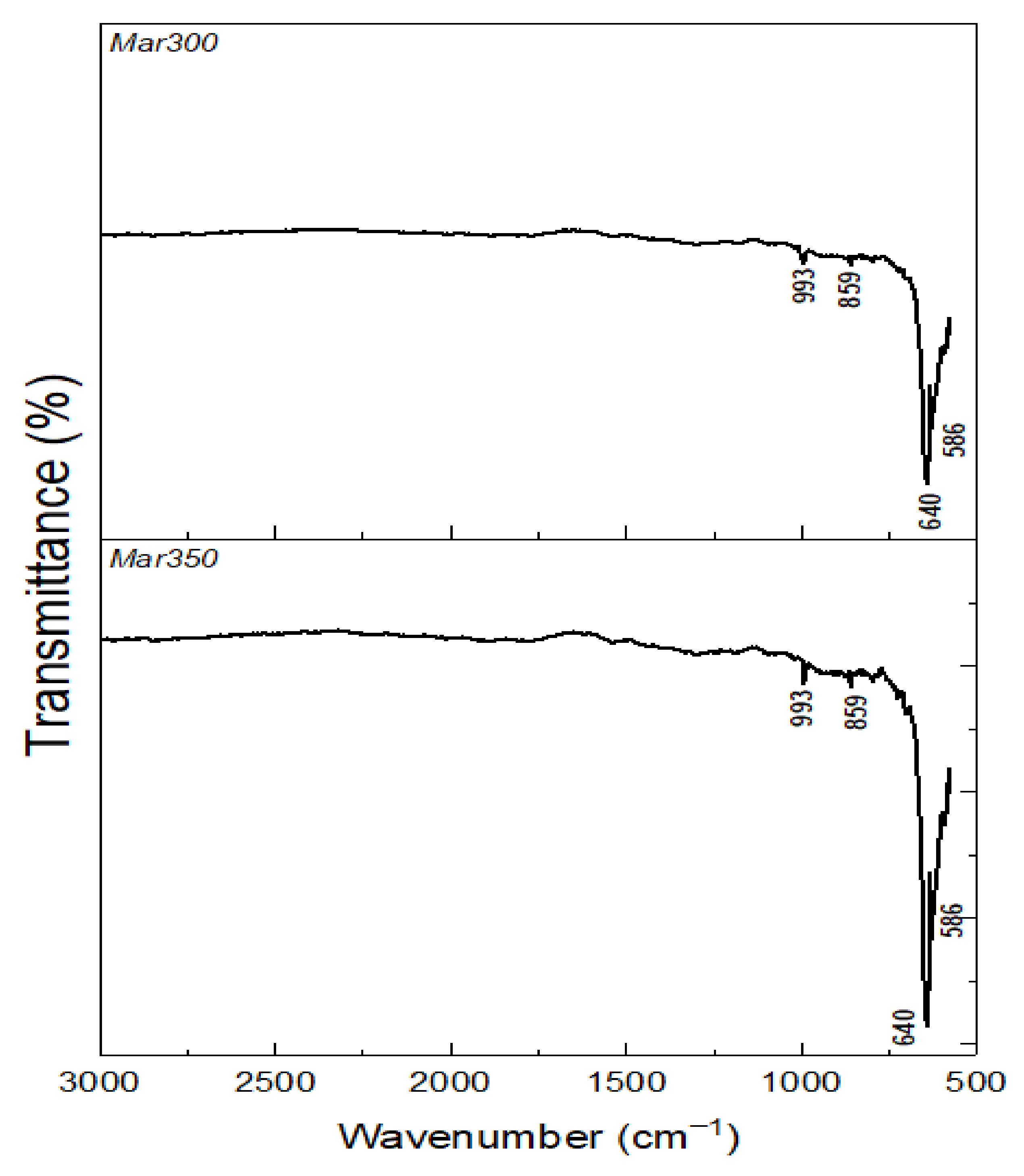
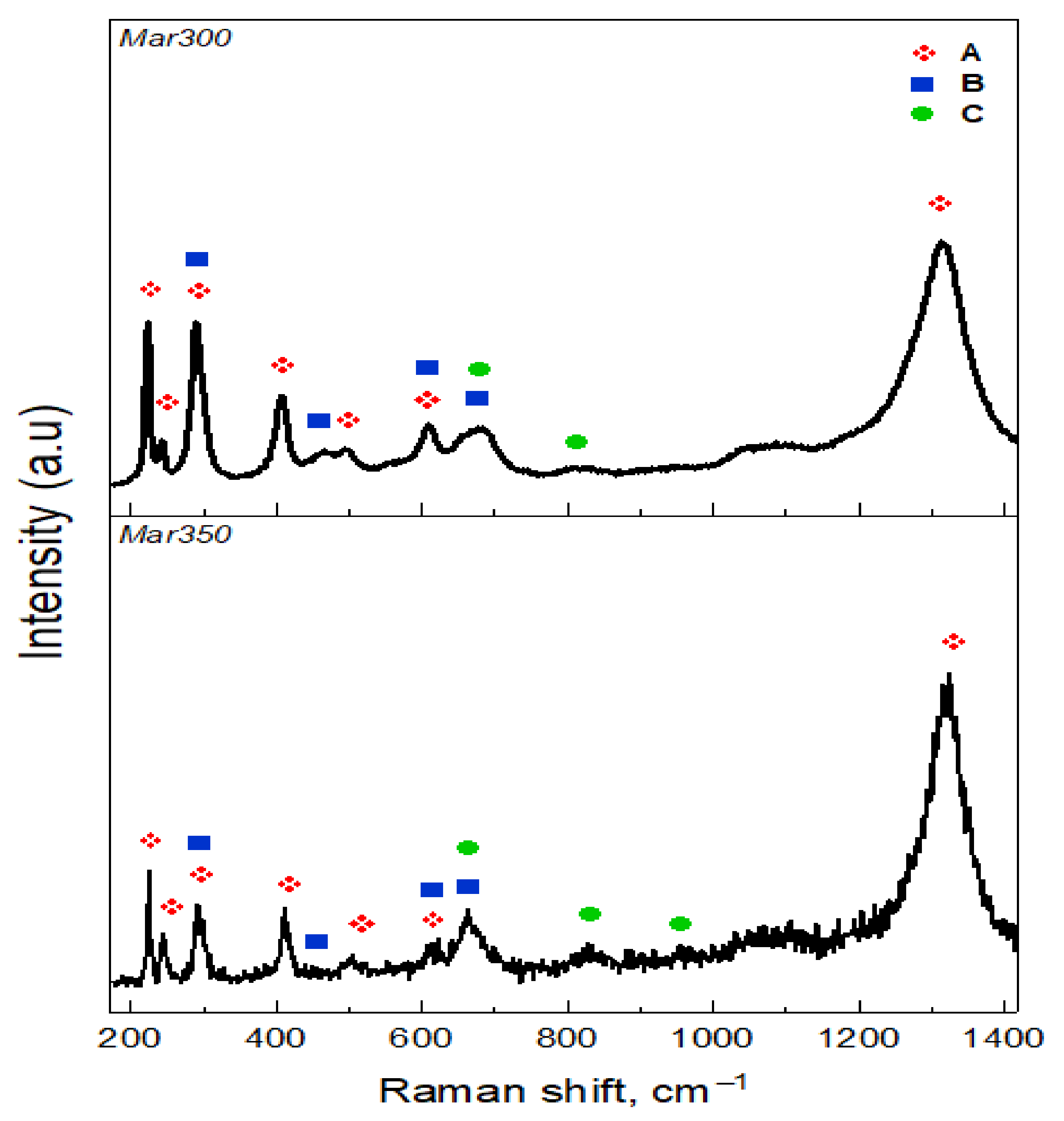
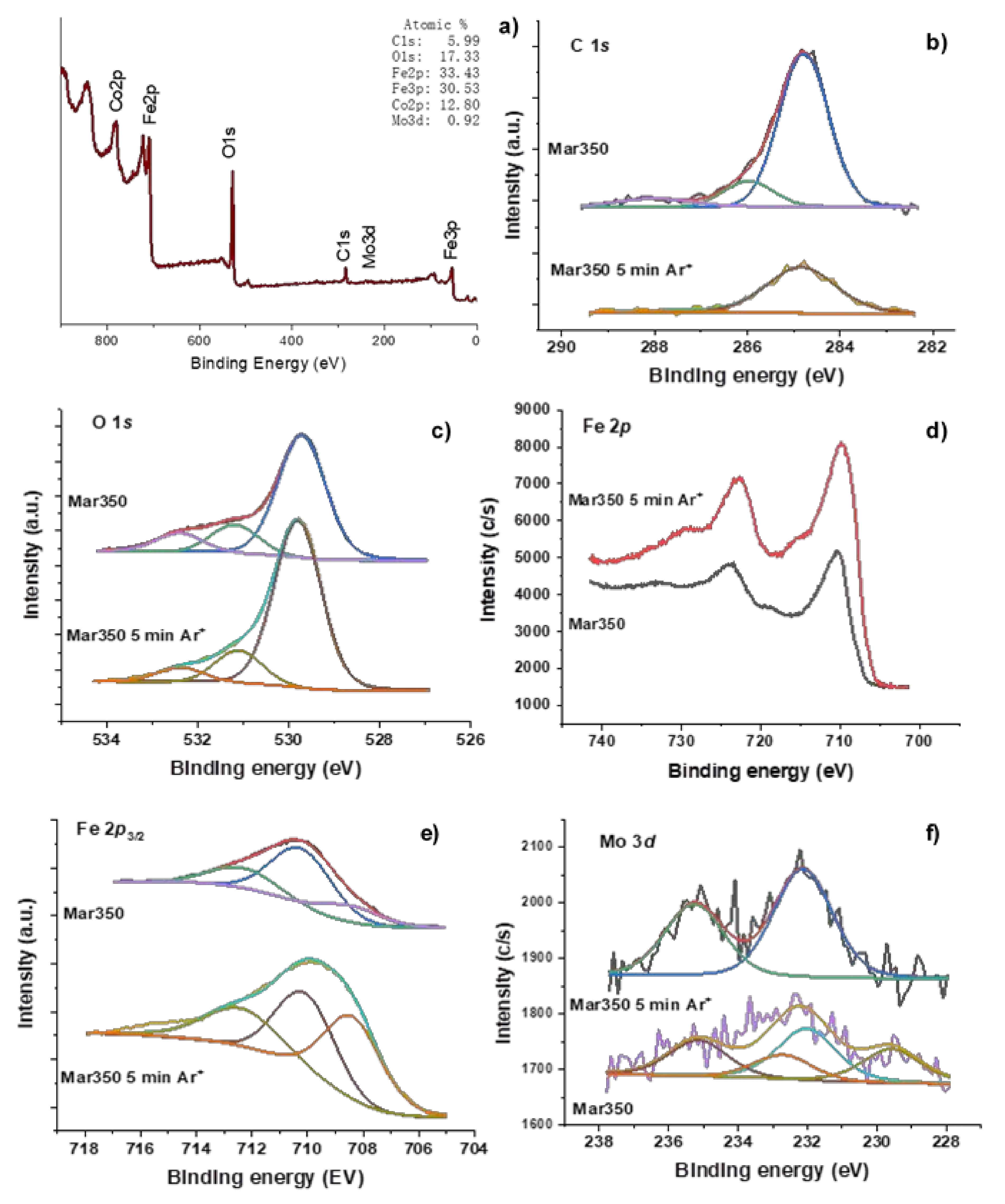
3.2. Oxide Characterization
3.3. Sliding Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, I.; Sharon, J.; Yaniv, A. A mechanism of oxidation of ferrous alloys by super-heat steam. Scr. Metall. 1981, 15, 141–144. [Google Scholar] [CrossRef]
- Klein, I.; Yaniv, A.; Sharon, J. The oxidation mechanism of Fe-Ni-Co alloys. Oxid. Met. 1981, 16, 1–2. [Google Scholar] [CrossRef]
- Klein, I.; Yaniv, A.; Sharon, J. The mechanism of oxidation of Fe-Ni-Co alloys; The role of Ti And Mo. Appl. Surf. Sci. 1983, 14, 351–358. [Google Scholar] [CrossRef]
- Rezek, J.; Klein, I.; Yahalom, J. Structure and corrosion resistance of oxides grown on maraging steel in steam at elevated temperatures. Appl. Surf. Sci. 1997, 108, 159–165. [Google Scholar] [CrossRef]
- Greyling, C.; Kotzi, A.; Viljoen, P. The kinetics of oxide film growth on maraging steel as described by space-charge effects. Surf. Interface Anal. 1990, 16, 293–298. [Google Scholar] [CrossRef]
- Chung, F. Quantitative interpretation of X-ray diffraction patterns of mixtures. I. Matrix-Flushing method for quantitative multicomponent analysis. J. Appl. Cryst. 1974, 7, 519. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; Bu, H.; Deng, L.; Liu, H.; Yuan, P.; Du, P.; Song, H. XRD-based quantitative analysis of clay minerals using reference intensity ratios, mineral intensity factors, Rietveld, and full pattern summation methods: A critical review. Solid Earth Sci. 2018, 3, 16–29. [Google Scholar] [CrossRef]
- Flamant, Q.; García, F.M.; Roa, J.J.; Anglada, M. Hydrofluoric acid etching of dental zirconia. Part 1: Etching mechanism and surface characterization. J. Eur. Ceram. Soc. 2016, 36, 121–134. [Google Scholar] [CrossRef]
- Dong, W.P.; Sullivan, P.J.; Stout, K.J. Comprehensive study of parameters for characterising three-dimensional surface topography: III: Parameters for characterising amplitude and some functional properties. Wear 1994, 178, 29–43. [Google Scholar] [CrossRef]
- Dong, W.P.; Sullivan, P.J.; Stout, K.J. Comprehensive study of parameters for characterising three-dimensional surface topography: IV: Parameters for characterising spatial and hybrid properties. Wear 1994, 178, 45–60. [Google Scholar] [CrossRef]
- Viana, N.F.; Dos Santos, N.C.; De Abreu, H.F.G. The variant selection in the transformation from austenite to martensite in samples of maraging-350 steel. J. Mater. Res. Technol. 2013, 2, 298–302. [Google Scholar] [CrossRef][Green Version]
- Conde, F.F.; Escobar, J.D.; Oliveira, J.P.; Béreš, M.; Jardini, A.L.; Bose, W.W.; Avila, J.A. Effect of thermal cycling and aging stages on the microstructure and bending strength of a selective laser melted 300-grade maraging steel. Mater. Sci. Eng. A 2019, 758, 192–201. [Google Scholar] [CrossRef]
- Capurro, C.; Cicutti, C. Analysis of titanium nitrides precipitated during medium carbon steels solidification. J. Mater. Res. Technol. 2018, 7, 342–349. [Google Scholar] [CrossRef]
- Silva, C.; Farias, J.; Miranda, H.; Guimarães, R.; Menezes, J.A.M.; Neto, M. Microstructural characterization of the HAZ in AISI 444 ferritic stainless steel welds. Mater. Charact. 2008, 59, 528–533. [Google Scholar] [CrossRef]
- JCPDS. X-Ray Diffraction Data Cards of the Joint Committee on Powder Diffraction Standards; International Center for Diffraction Data: Swarthmore, PA, USA, 1975. [Google Scholar]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Martin, M. Synthesis and microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 high entropy oxide characterized by spinel structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Zhao, N.; Fan, H.; Zhang, M.; Ma, J.; Du, Z.; Yan, B.; Li, H.; Jiang, X. Simple electrodeposition of MoO3 film on carbon cloth for high-performance aqueous symmetric supercapacitors. Chem. Eng. J. 2020, 390, 124477. [Google Scholar] [CrossRef]
- Magnée, A.; Drapier, J.M.; Dumont, J.; Coutsouradis, D.; Habraken, L. Cobalt Containing High Strength Steels; Centre d’Information du Cobalt: Brussels, Belgium, 1974; p. 128. Available online: http://refhub.elsevier.com/S0360-3199(19)31930-5/sref1 (accessed on 10 March 2020).
- Vasudevan, V.; Kim, S.; Wayman, C. Precipitation reactions and strengthening behavior in 18 Wt Pct nickel maraging steels. Metall. Trans. A 1990, 21, 2655–2668. [Google Scholar] [CrossRef]
- Sha, W.; Cerezo, A.; Smith, G.D.W. Phase chemistry and precipitation reactions in maraging steels: Part 4. Discussion and conclusion. Metall. Trans. A 1993, 24, 1251–1256. [Google Scholar] [CrossRef]
- Lima Filho, V.; Barrosa, I.; Abreu, H. Influence of solution annealing on microstructure and mechanical properties of maraging 300 steel. Mater. Res. 2017, 20, 10–14. [Google Scholar] [CrossRef]
- Ding, M.; Chen, W.; Xu, H.; Shen, Z.; Lin, T.; Hu, K.; Hui Lu, C.; Xie, Z. Novel α-Fe2O3/MXene nanocomposite as heterogeneous activator of peroxymonosulfate for the degradation of salicylic acid. J. Hazard. Mater. 2020, 382, 121064. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, A.; Zhang, D.; Yang, J.; Li, H.; Meng, F.; Sun, Z.; Chen, G. Ultra-low loading of Ag2CrO4 on BiOI/CoFe2O4 microsphere with p-n heterojunction: Highly improved photocatalytic performance for Hg0 removal and mechanism insight. J. Photochem. Photobiol. A Chem. 2020, 396, 112543. [Google Scholar] [CrossRef]
- Routray, K.L.; Saha, S.; Behera, D. Nanosized CoFe2O4-graphene nanoplatelets with massive dielectric enhancement for high frequency device application. Mater. Sci. Eng. B 2020, 257, 114548. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Lu, H.; Li, X. Preparation of CoFe2O4eP4VP@Ag NPs as effective and recyclable catalysts for the degradation of organic pollutants with NaBH4 in water. Int. J. Hydrog. Energy 2020, 45, 16080–16093. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Xhang, X.; Cui, X.; He, S.; Liang, H.; Ding, A. Sludge activated carbon-based CoFe2O4-SAC nanocomposites used as heterogeneous catalysts for degrading antibiotic norfloxacin through activating peroxymonosulfate. Chem. Eng. J. 2020, 384, 123319. [Google Scholar] [CrossRef]
- Sharma, P.K.; Raghubanshi, A.S.; Shah, K. Examining dye degradation and antibacterial properties of organically induced α-MoO3 nanoparticles, their uptake and phytotoxicity in rice seedlings. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100315. [Google Scholar] [CrossRef]
- Ramar, V.; Balasubramanian, K. Optical and highly enhanced solar light-driven photocatalytic activity of reduced graphene oxide wrapped α-MoO3 nanoplates. Sol. Energy 2019, 194, 1–10. [Google Scholar] [CrossRef]
- Guo, R.; Dang, L.; Liu, Z.; Lei, Z. Incorporation of electroactive NiCo2S4 and Fe2O3 into graphene aerogel for high-energy asymmetric supercapacitor. Colloids Surf. A 2020, 602, 125110. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Lopes, F.N. Heated goethite and natural hematite: Can Raman spectroscopy be used to differentiate them? Vib. Spectrosc. 2007, 45, 117–121. [Google Scholar] [CrossRef]
- Wang, W.; Ding, Z.; Zhao, X.; Wu, S.; Li, F.; Yue, M.; Liu, J.P. Microstructure and magnetic properties of MFe2O4 (M = Co, Ni, and Mn) ferrite nanocrystals prepared using colloid mill and hydrothermal method. J. Appl. Phys. 2015, 117, 17A328. [Google Scholar] [CrossRef]
- De La Figuera, J.; Quesada, A.; Martín-García, L.; Sanz, M.; Oujja, M.; Rebollar, E.; Castillejo, M.; Prieto, P.; Muñoz-Martín, A.; Aballe, L.; et al. Self-organized single crystal mixed magnetite/cobalt ferrite films grown by infrared pulsed-laser deposition. Appl. Surf. Sci. 2015, 359, 480–485. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, X.; Chen, P.; Zhu, K.; Cheng, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. Creating oxygen-vacancies in MoO3-x nanobelts toward high volumetric energy-density asymmetric supercapacitors with long lifespan. Nano Energy 2019, 58, 455–465. [Google Scholar] [CrossRef]
- Reed, B.W.; Williams, D.R.; Moser, B.P.; Koski, K.J. Chemically tuning quantized acoustic phonons in 2D layered MoO3 nanoribbons. Nano Lett. 2019, 19, 4406–4412. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, P.; Sobola, D.; Dallaev, R.; Ramazanov, S.; Nebojsa, A.; Rezaee, S.; Grmela, L. Characterization of Fe2O3 thin film on highly oriented pyrolytic graphite by AFM, Ellipsometry and XPS. Appl. Surf. Sci. 2019, 493, 673–678. [Google Scholar] [CrossRef]
- Barreca, D.; Battiston, G.A.; Berto, D.; Gerbasi, R.; Tondello, E. Chemical vapor deposited Fe2O3 thin films analyzed by XPS. Surf. Sci. Spectra 2001, 8, 240. [Google Scholar] [CrossRef]
- Galtayries, A.; Sporken, R.; Riga, J.; Blanchard, G.; Caudano, R. XPS comparative study of ceria/zirconia mixed oxides: Powders and thin film characterization. J. Electron Spectrosc. 1998, 88, 951–956. [Google Scholar] [CrossRef]
- Barr, T.L. An ESCA study of the termination of the passivation of elemental metals. J. Phys. Chem. 1978, 82, 1801–1810. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; Mcintyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Lin, T.C.; Seshadri, G.; Kelber, J.A. A consistent method for quantitative XPS peak analysis of thin oxide films on clean polycrystalline iron surfaces. Appl. Surf. Sci. 1997, 119, 83–92. [Google Scholar] [CrossRef]
- Sanchis, R.; Alonso-Domínguez, D.; Dejoz, A.; Picom, M.P.; Álvarez-Serrano, I.; García, T.; López, M.L.; Solsona, B. Eco-friendly cavity-containing iron oxides prepared by mild routes as very efficient catalysts for the total oxidation of VOCs. Materials 2018, 11, 1387. [Google Scholar] [CrossRef]
- Lu, L.; Ai, Z.; Li, J.; Zheng, Z.; Li, Q.; Zhang, L. Synthesis and characterization of Fe–Fe2O3 core–shell nanowires and nanoneckaces. Cryst. Growth Des. 2007, 7, 459–464. [Google Scholar] [CrossRef]
- Yi, Y.; Wu, Q.; Wang, W.; Cui, C. In situ depositing an ultrathin CoOxHy layer on hematite in alkaline media for photoelectrochemical water oxidation. Appl. Catal. B Environ. 2020, 263, 118334. [Google Scholar] [CrossRef]
- Hong, H.; Memon, N.K.; Dong, Z.; Kear, B.H.; Tse, S.D. Flame synthesis of gamma-iron-oxide (γ-Fe2O3) nanocrystal films and carbon nanotubes on stainless-steel substrates. Proc. Combust. Inst. 2019, 37, 1249–1256. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.C.; Gong, J.; Li, Q.; Li, G. α-Fe2O3 Nanorings Prepared by a Microwave-Assisted Hydrothermal Process and Their Sensing Properties. Adv. Mater. 2007, 19, 2324–2329. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, B.; Miao, T.; Xie, J.; Liu, L.; Si, J.; Zhou, G.; Qin, H.; Hu, J. Room temperature electric field control of magnetic properties for the α-Fe2O3/Fe3O4 composite structure. J. Magn. Magn. Mater. 2019, 491, 165500. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Wang, L.; Zhang, Z.; Wang, S. Spinel CoFe2O4 supported by three dimensional graphene as high-performance bi-functional electrocatalysts for oxygen reduction and evolution reaction. Int. J. Hydrog. Energy 2019, 44, 1610–1619. [Google Scholar] [CrossRef]
- Yan, W.; Cao, X.; Tian, J.; Jin, C.; Ke, K.; Yang, R. Nitrogen/sulfur dual-doped 3D reduced graphene oxide networks-supported CoFe2O4 with enhanced electrocatalytic activities for oxygen reduction and evolution reactions. Carbon 2016, 99, 195–202. [Google Scholar] [CrossRef]
- Yan, W.; Bian, W.; Jin, C.; Tian, J.H.; Yang, R. An Efficient Bi-functional electrocatalyst based on strongly coupled CoFe2O4/Carbon nanotubes hybrid for Oxygen reduction and Oxygen evolution. Electrochim. Acta 2015, 177, 65–72. [Google Scholar] [CrossRef]
- Zhao, Y.; Nie, G.; Ma, X.; Xu, P.; Zhao, X. Peroxymonosulfate catalyzed by rGO assisted CoFe2O4 catalyst for removing Hg0 from flue gas in heterogeneous system. Environ. Pollut. 2019, 249, 868–877. [Google Scholar] [CrossRef]
- Fantauzzi, M.; Secci, F.; Angotzi, M.S.; Passiu, C.; Cannas, C.; Rossi, A. Nanostructured spinel cobalt ferrites: Fe and Co chemical state, cation distribution and size effects by X-ray photoelectron spectroscopy. RSC Adv. 2019, 9, 19171–19179. [Google Scholar] [CrossRef]
- Ji, G.B.; Tang, S.L.; Ren, S.K.; Zhang, F.M.; Gu, B.X.; Du, Y.W. Simplified synthesis of single-crystalline magnetic CoFe2O4 nanorods by a surfactant-assisted hydrothermal process. J. Cryst. Growth 2004, 270, 156–161. [Google Scholar] [CrossRef]
- Srinivasan, P.; Ravappan, J.B.B. Growth of α-MoO3 Golf Ball Architectures with Interlocking Loops for Selective Probing of Trimethylamine at Room Temperature. Mater. Res. Bull. 2020, 130, 110944. [Google Scholar] [CrossRef]
- Rajagopal, S.; Nataraj, D.; Khyzhum, O.Y.; Djaoued, Y.; Robichaud, J.; Senthil, K.; Mangalaraj, D. Systematic synthesis and analysis of change in morphology, electronic structure and photoluminescence properties of pyrazine intercalated MoO3 hybrid nanostructures. CrystEngComm 2011, 13, 2358–2368. [Google Scholar] [CrossRef]
- Surman, P.L. The oxidation of iron at controlled Oxygen partial pressures—I. Hydrogen/Water vapour. Corros. Sci. 1973, 13, 113–124. [Google Scholar] [CrossRef]
- Subbaraman, R.; Deshmukh, S.A.; Sankaranarayanan, S.K. Atomistic insights into early stage oxidation and nanoscale oxide growth on Fe (100), Fe (111) and Fe (110) surfaces. J. Phys. Chem. C 2013, 117, 5195–5207. [Google Scholar] [CrossRef]
- Luo, D.W.; Shen, Z.S. Oxidation behavior of Kovar alloy in controlled atmosphere. Acta Metall. Sin. Engl. Lett. 2008, 21, 409–418. [Google Scholar] [CrossRef]
- Tsukimura, K.; Sasaki, S.; Kimizuka, N. Cation distributions in Nickel ferrites. Jpn. J. Appl. Phys. 1997, 36, 3609. [Google Scholar] [CrossRef]
- Rodrigues, A.P.G.; Gomes, D.K.S.; Araújo, J.H.; Melo, D.M.A.; Oliveira, N.A.S.; Braga, R.M. Nanoferrites of nickel doped with cobalt: Influence of Co2+ on the structural and magnetic properties. J. Magn. Magn. Mater. 2015, 374, 748–754. [Google Scholar] [CrossRef]
- Bliem, R.; Pavelec, J.; Gamba, O.; Mcdermott, E.; Wang, Z.; Gerhold, S.; Wagner, M.; Osiecki, J.; Schulte, K.; Schmid, M.; et al. Adsorption and incorporation of transition metals at the magnetite Fe3O4 (001) surface. Phys. Rev. B 2015, 92, 75440. [Google Scholar] [CrossRef]
- Pardavi-Horvath, M. Microwave applications of soft ferrites. J. Magn. Magn. Mater. 2000, 215–216, 171–183. [Google Scholar] [CrossRef]
- Genuzio, F.; Sala, A.; Schmidt, T.; Menzel, D.; Freund, H.-J. Phase transformations in thin iron oxide films: Spectromicroscopic study of velocity and shape of the reaction fronts. Surf. Sci. 2016, 648, 177–187. [Google Scholar] [CrossRef]
- Silva, M.J.G.; Cardoso, J.L.; Carvalho, D.S.; Santos, L.P.M.; Herculano, L.F.G.; Abreu, H.F.G.; Pardal, J.M. The effect of prior austenite grain size on hydrogen embrittlement of Co-containing 18Ni 300 maraging steel. Int. J. Hydrog. Energy 2019, 44, 18606–18615. [Google Scholar] [CrossRef]
- Jeon, B.; Van Overmeere, Q.; Van Duin, A.C.; Ramanathan, S. Nanoscale oxidation and complex oxide growth on single crystal iron surfaces and external electric field effects. Phys. Chem. Chem. Phys. 2013, 15, 1821–1830. [Google Scholar] [CrossRef]
- Parkinson, G.S. Iron oxide surfaces. Surf. Sci. Rep. 2016, 71, 272–365. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Yang, Z.; Xu, X. Kinetics and intermediate phases in epitaxial growth of Fe3O4 films from deposition and thermal reduction. J. Appl. Phys. 2016, 120, 085313. [Google Scholar] [CrossRef]
- Genuzio, F.; Sala, A.; Schmidt, T.; Menzel, D.; Freund, H.-J. Interconversion of α-Fe2O3 and Fe3O4 thin films: Mechanisms, morphology, and evidence for unexpected substrate participation. J. Phys. Chem. C 2014, 118, 29068–29076. [Google Scholar] [CrossRef]


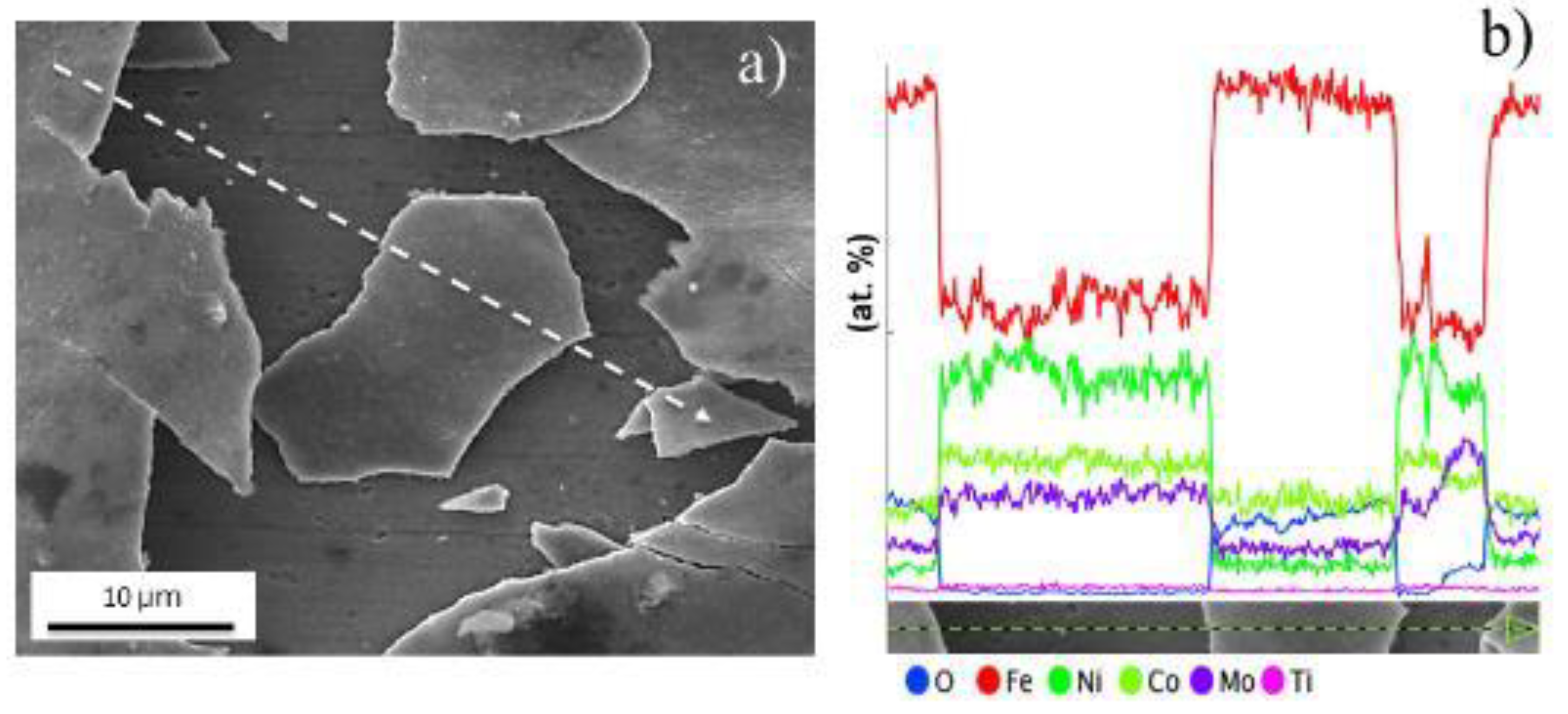
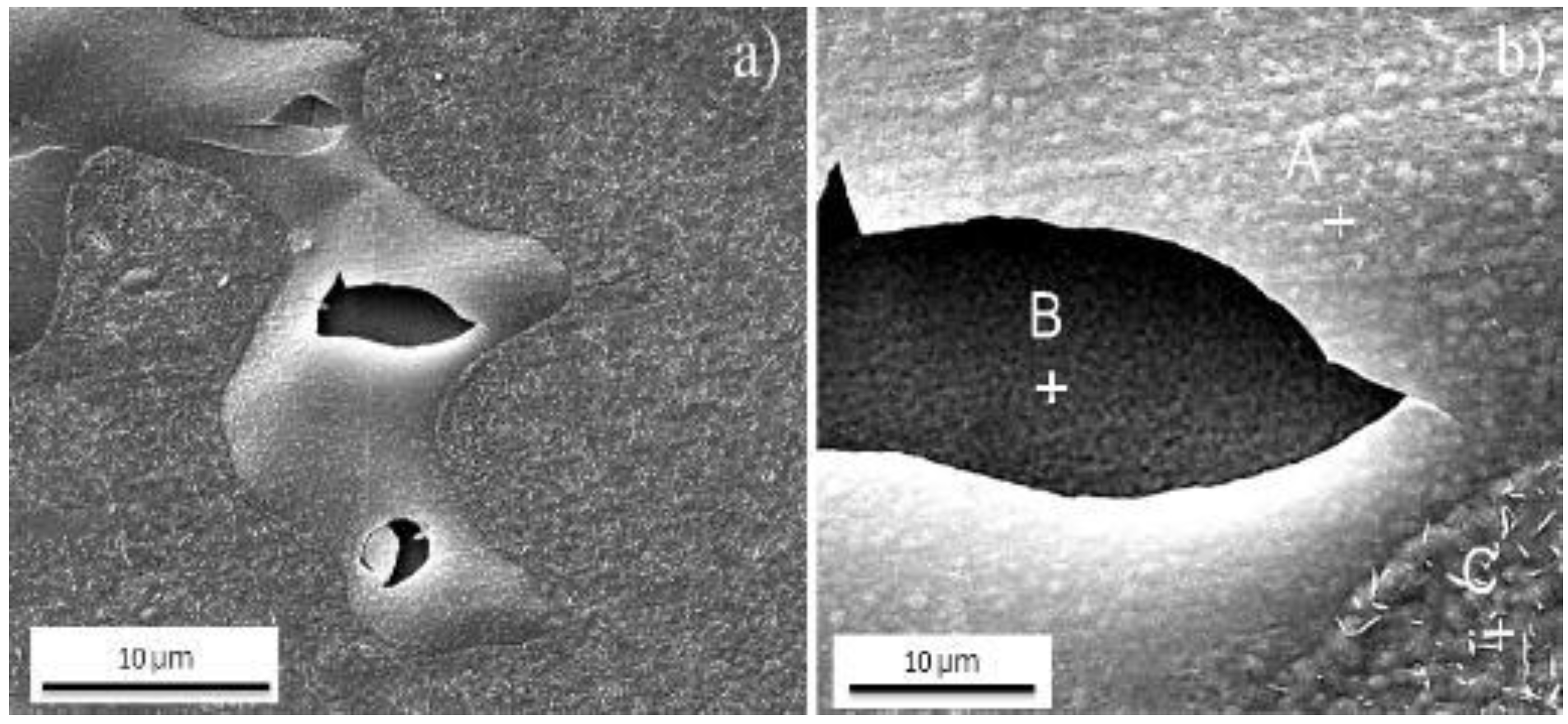

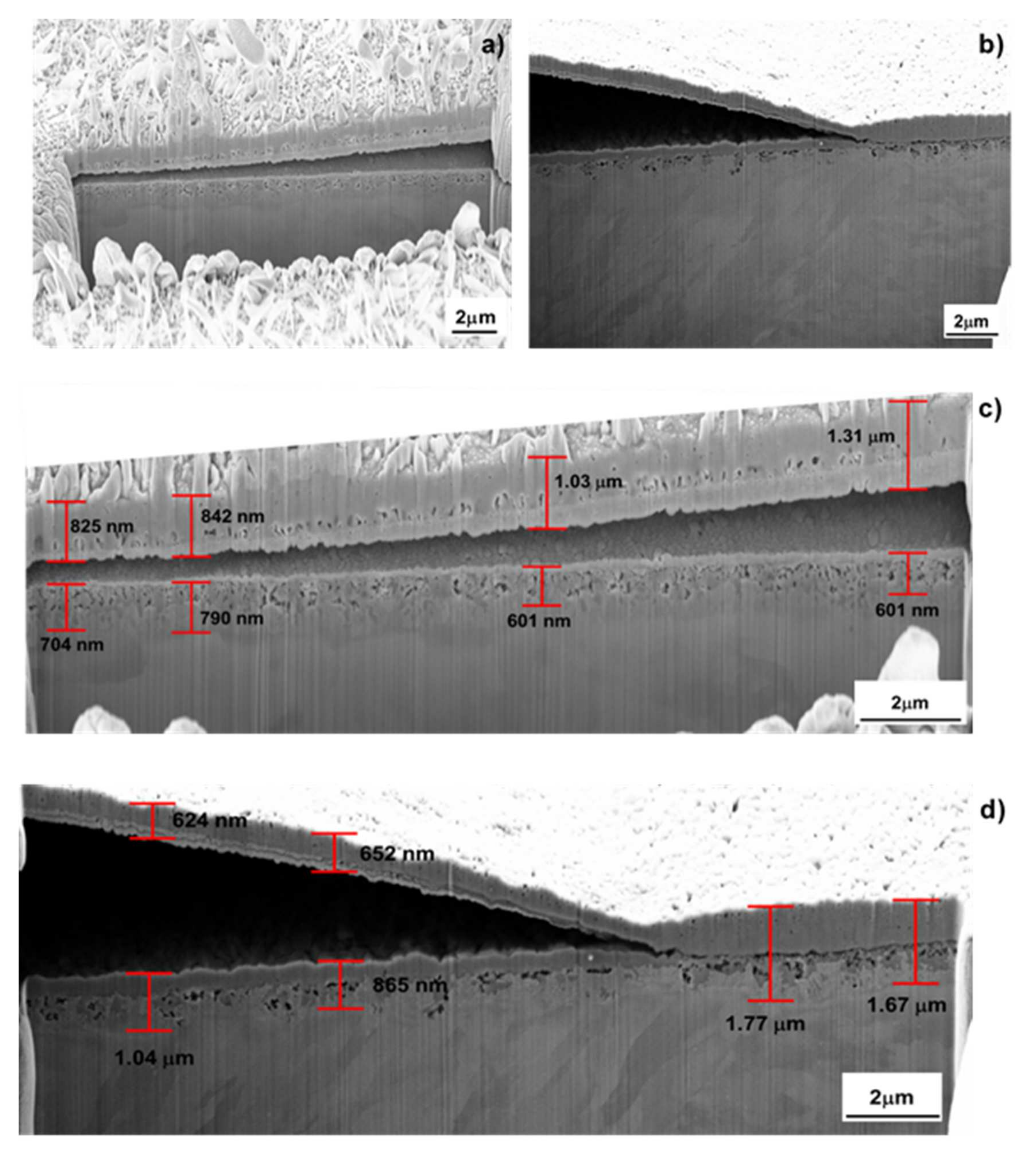
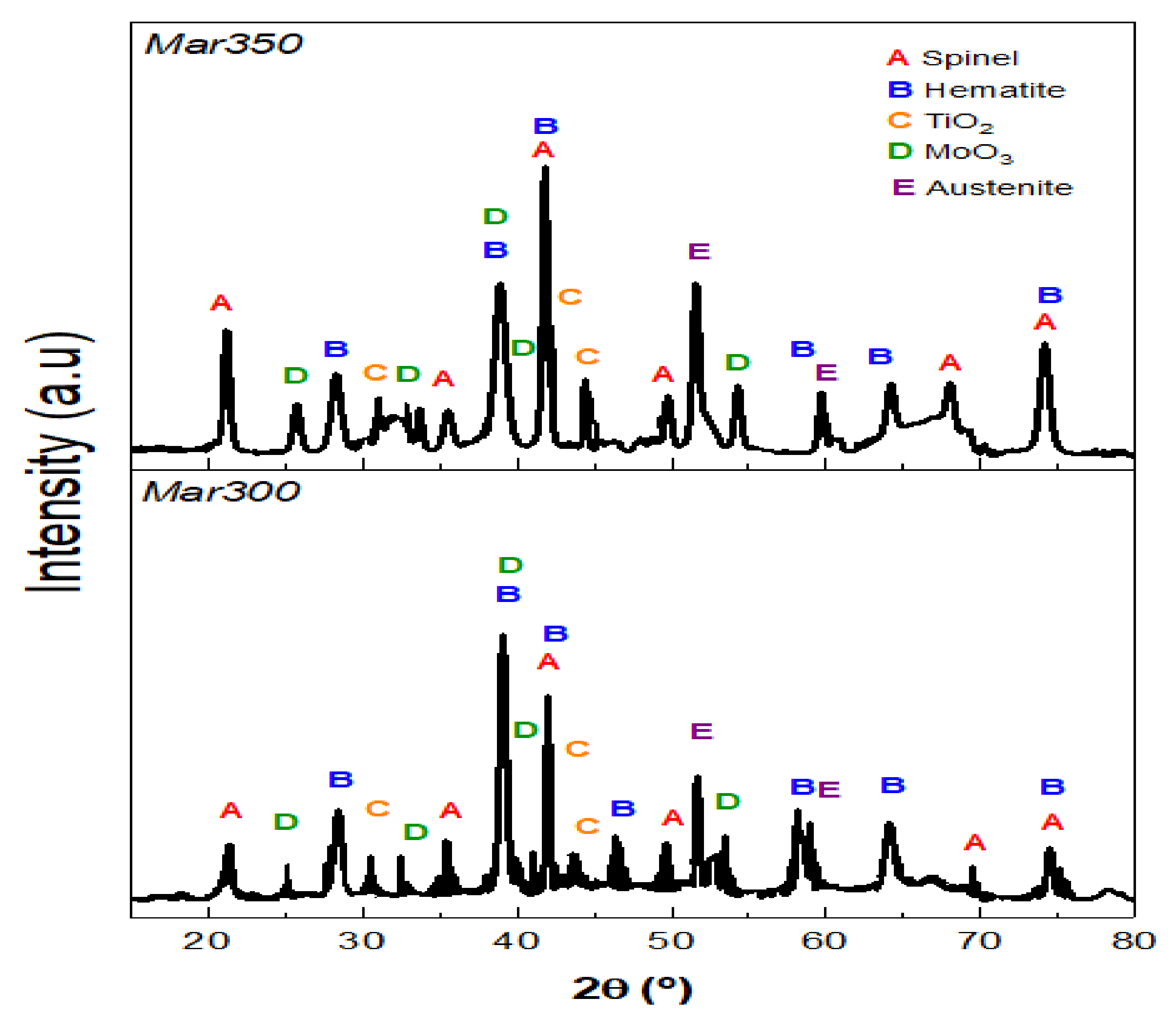


| Samples | Fe | Ni | Co | Mo | Ti | Cr | V | Si | Al | C |
|---|---|---|---|---|---|---|---|---|---|---|
| Mar300 | Bal. | 18.28 | 9.51 | 4.80 | 0.73 | 0.12 | 0.10 | 0.08 | 0.07 | <0.01 |
| Mar350 | Bal. | 17.65 | 11.65 | 4.69 | 1.44 | 0.05 | 0.10 | 0.04 | 0.06 | 0.002 |
| Roughness Parameter | Oxide of the Mar300 (μm) | Oxide of the Mar350 (μm) |
|---|---|---|
| Arithmetic Mean Height (Sa) | 0.21 | 0.20 |
| Maximum Height (Sz) | 2.22 | 2.19 |
| Maximum Peak Height (Sp) | 0.90 | 0.85 |
| Maximum Pit Height (Sv) | 1.32 | 1.34 |
| Root-Mean-Square Height (Sq) | 0.31 | 0.25 |
| EDS Quantitative Analysis from A and B Points (Weight Conc.) | |||
|---|---|---|---|
| Element Number | Element Symbol | Point A | Point B |
| 26 | Fe | 52.57 | 60.25 |
| 8 | O | 32.46 | 27.70 |
| 27 | Co | 13.64 | 9.47 |
| 28 | Ni | 0.69 | 1.48 |
| 42 | Mo | 0.59 | 0.95 |
| 22 | Ti | 0.05 | 0.15 |
| EDS Quantitative Analysis at Points A and B (Weight Conc.) | ||||||
|---|---|---|---|---|---|---|
| Element Number | Element Symbol | Point A | Point B | Point C | Point D | Point E |
| 26 | Fe | 62.65 | 60.22 | 62.50 | 60.98 | 3.92 |
| 8 | O | 32.68 | 38.10 | 32.34 | 19.11 | 31.43 |
| 27 | Co | 3.21 | 0.64 | 3.39 | 9.14 | 0.63 |
| 28 | Ni | 0.35 | 0.53 | 0.90 | 4.90 | - |
| 42 | Mo | 0.94 | 0.29 | 0.71 | 4.66 | - |
| 22 | Ti | 0.17 | 0.22 | 0.16 | 1.21 | 44.54 |
| 7 | N | - | - | - | - | 19.48 |
| EDS Quantitative Analysis from Points A and B (Weight Conc.) | ||||
|---|---|---|---|---|
| Elem. Number | Elem. Symbol | Point A | Point B | Point C |
| 26 | Fe | 62.61 | 55.23 | 59.43 |
| 8 | O | 34.84 | 27.97 | 34.01 |
| 27 | Co | 1.25 | 4.41 | 4.24 |
| 28 | Ni | 0.67 | 5.23 | 0.79 |
| 42 | Mo | 0.42 | 5.19 | 1.30 |
| 22 | Ti | 0.21 | 1.98 | 0.23 |
| Sample | C 1s | O 1s | Fe 2p3/2 | Co 2p3/2 | Mo 3d5/2 | Fe2+/(Fe2+ + Fe3+) |
|---|---|---|---|---|---|---|
| Mar300 | 284.8 (82) 285.6 (8) 286.4 (5) 288.1 (5) | 529.3 (63) 531.2 (24) 533.4 (13) | 709.2 (43) 710.8 (48) 712.9 (9) | 781.3 | 232.1 | 43 |
| Mar300 5 min Ar+ | 284.8 (95) 286.0 (5) | 529.7 (78) 531.2 (14) 532.2 (8) | 708.9 (50) 710.5 (37) 712.6 (13) | 780.7 782.0 | 53 | |
| Mar350 | 284.8 (80) 286.0 (13) 288.0 (7) | 529.7 (72) 531.2 (16) 532.4 (12) | 708.5 (17) 710.2 (60) 712.4 (23) | 780.7 782.7 | 232 (59) 232.7 (41) | 17 |
| Ma350 5 min Ar+ | 284.8 (96) 287.4 (4) | 529.8 (78) 531.1 (15) 532.4 (7) | 708.4 (40) 710.0 (35) 712.4 (25) | 782.3 | 232.1 | 40 |
| Sample | C | O | Fe | Co | Mo |
|---|---|---|---|---|---|
| Mar300 | 47.94 | 38.57 | 10.13 | 3.24 | 0.12 |
| Mar300 5 min Ar+ | 18.41 | 42.35 | 31.12 | 8.14 | 0.00 |
| Mar350 | 37.49 | 42.58 | 14.83 | 4.90 | 0.20 |
| Mar350 5 min Ar+ | 10.82 | 41.60 | 36.64 | 18.19 | 0.38 |
| Maraging Steel | Pc2 (mN) |
|---|---|
| Mar300 | 2 |
| Mar350 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florez, M.A.C.; Fargas Ribas, G.; Rovira, J.J.R.; Vilarrasa-Garcia, E.; Rodríguez-Castellon, E.; Sousa, A.B.F.; Cardoso, J.L.; Gomes da Silva, M.J. Characterization Study of an Oxide Film Layer Produced under CO2/Steam Atmospheres on Two Different Maraging Steel Grades. Metals 2021, 11, 746. https://doi.org/10.3390/met11050746
Florez MAC, Fargas Ribas G, Rovira JJR, Vilarrasa-Garcia E, Rodríguez-Castellon E, Sousa ABF, Cardoso JL, Gomes da Silva MJ. Characterization Study of an Oxide Film Layer Produced under CO2/Steam Atmospheres on Two Different Maraging Steel Grades. Metals. 2021; 11(5):746. https://doi.org/10.3390/met11050746
Chicago/Turabian StyleFlorez, Mauro Andres Cerra, Gemma Fargas Ribas, Joan Josep Roa Rovira, Enrique Vilarrasa-Garcia, Enrique Rodríguez-Castellon, Ana Beatriz Ferreira Sousa, Jorge Luiz Cardoso, and Marcelo José Gomes da Silva. 2021. "Characterization Study of an Oxide Film Layer Produced under CO2/Steam Atmospheres on Two Different Maraging Steel Grades" Metals 11, no. 5: 746. https://doi.org/10.3390/met11050746
APA StyleFlorez, M. A. C., Fargas Ribas, G., Rovira, J. J. R., Vilarrasa-Garcia, E., Rodríguez-Castellon, E., Sousa, A. B. F., Cardoso, J. L., & Gomes da Silva, M. J. (2021). Characterization Study of an Oxide Film Layer Produced under CO2/Steam Atmospheres on Two Different Maraging Steel Grades. Metals, 11(5), 746. https://doi.org/10.3390/met11050746









