Extraction of Manganese and Iron from a Refractory Coarse Manganese Concentrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Main Chemical Reagent and Equipment
2.3. Procedure Design
2.4. Analysis and Characterization
3. Results and Discussion
3.1. Mineral Composition Analysis of Coarse Manganese Concentrate
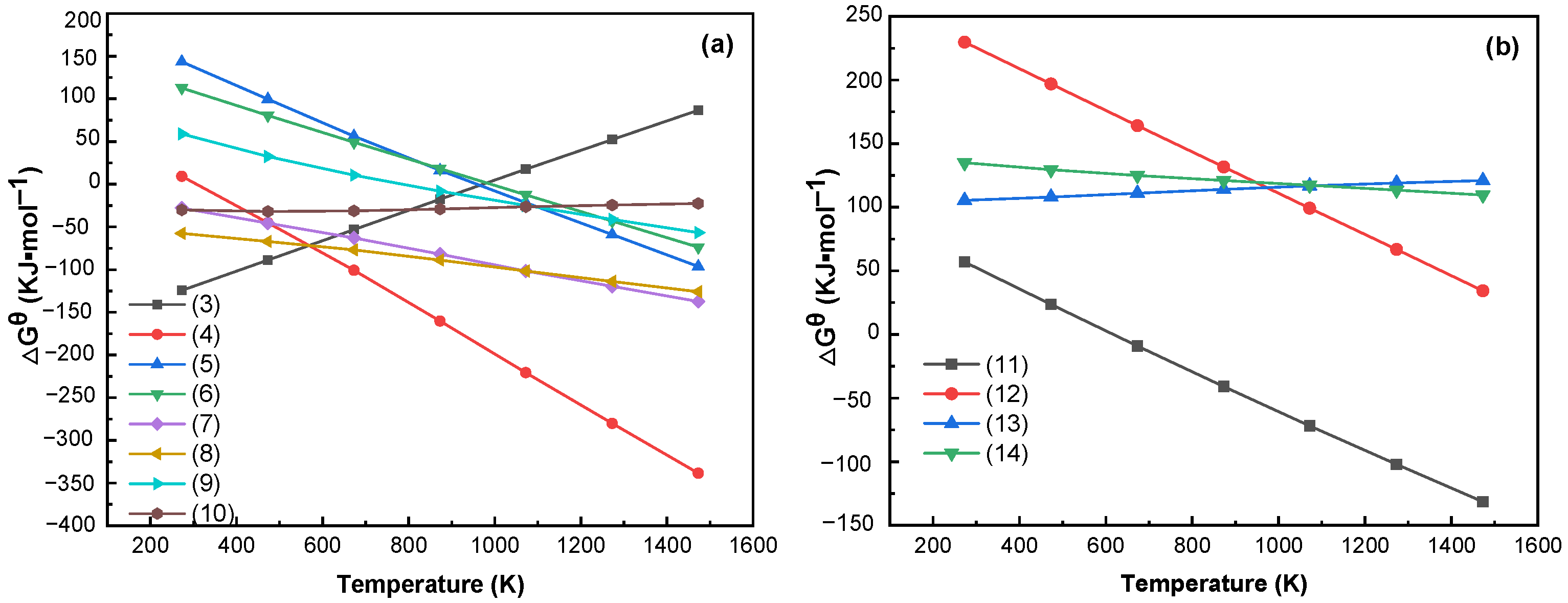
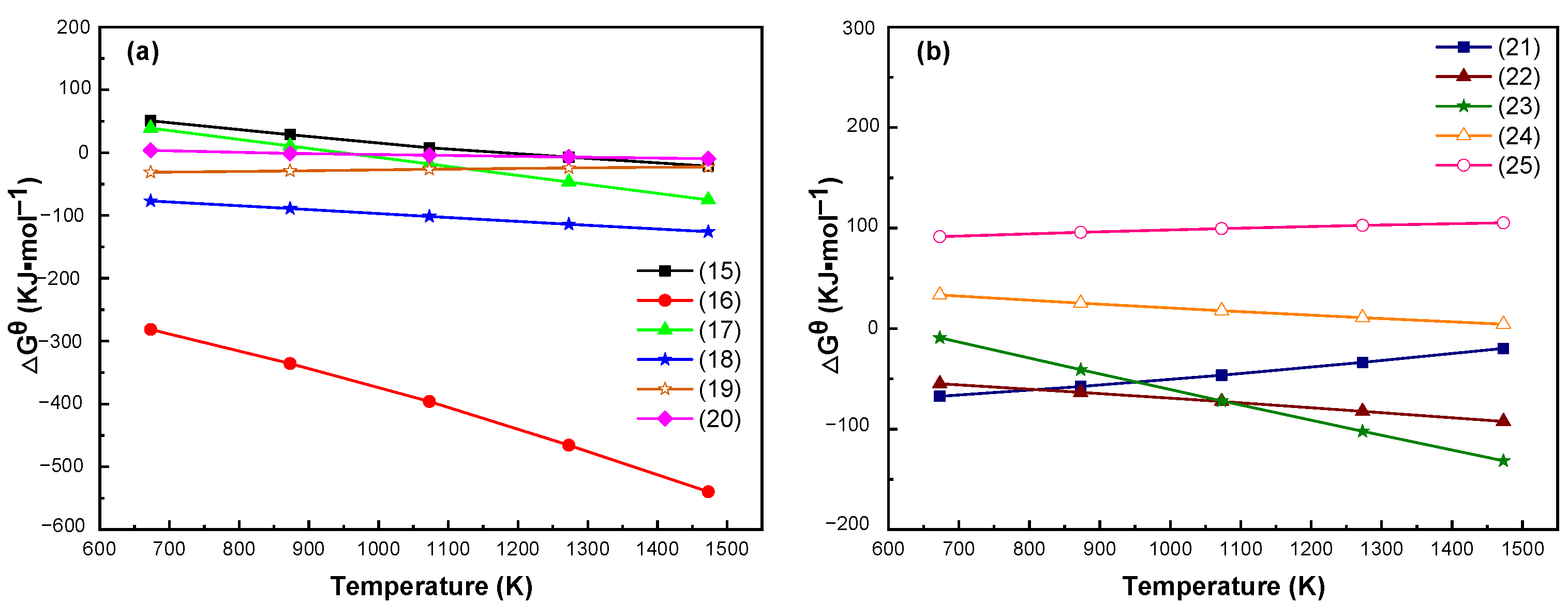
3.2. Thermodynamic Analysis of Limonite and Rhodochrosite
3.3. Iron Extraction from Coarse Manganese Concentrate
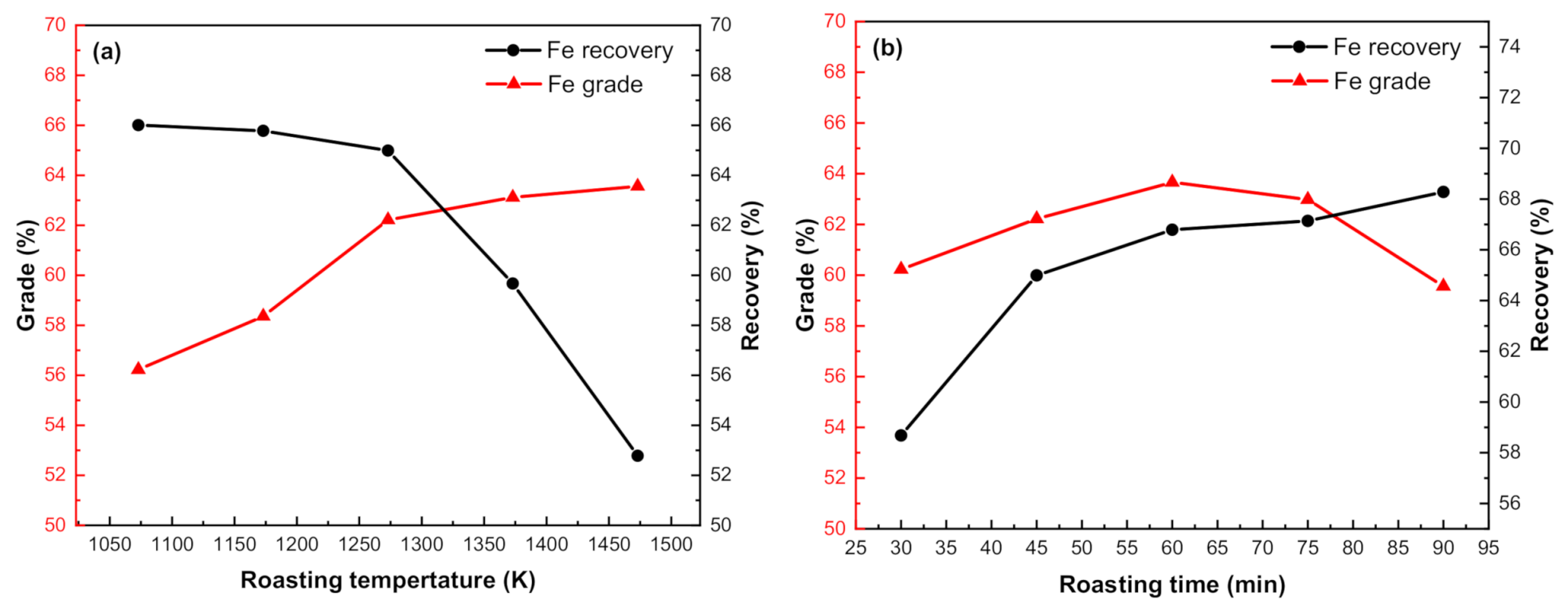
3.3.1. Effect of Roasting Temperature
3.3.2. Effect of Roasting Time
3.3.3. Effect of Calcium Chloride Dosage
3.3.4. Effect of Coke Dosage
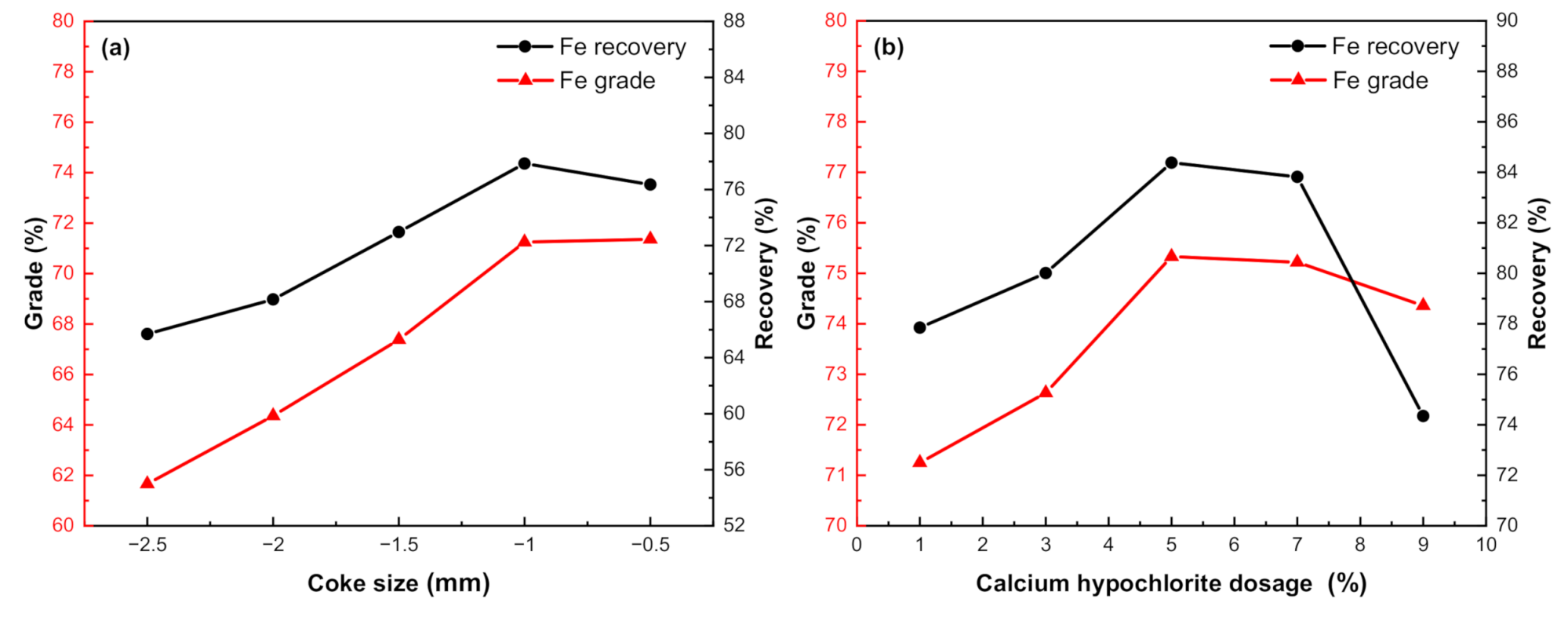
3.3.5. Effect of Coke Size
3.3.6. Effect of Additives Dosage
3.3.7. Grinding Fineness
3.3.8. Low-Intensity Magnetic Field Intensity
3.4. Manganese Extraction from Low-Intensity Magnetic Separation Tailings
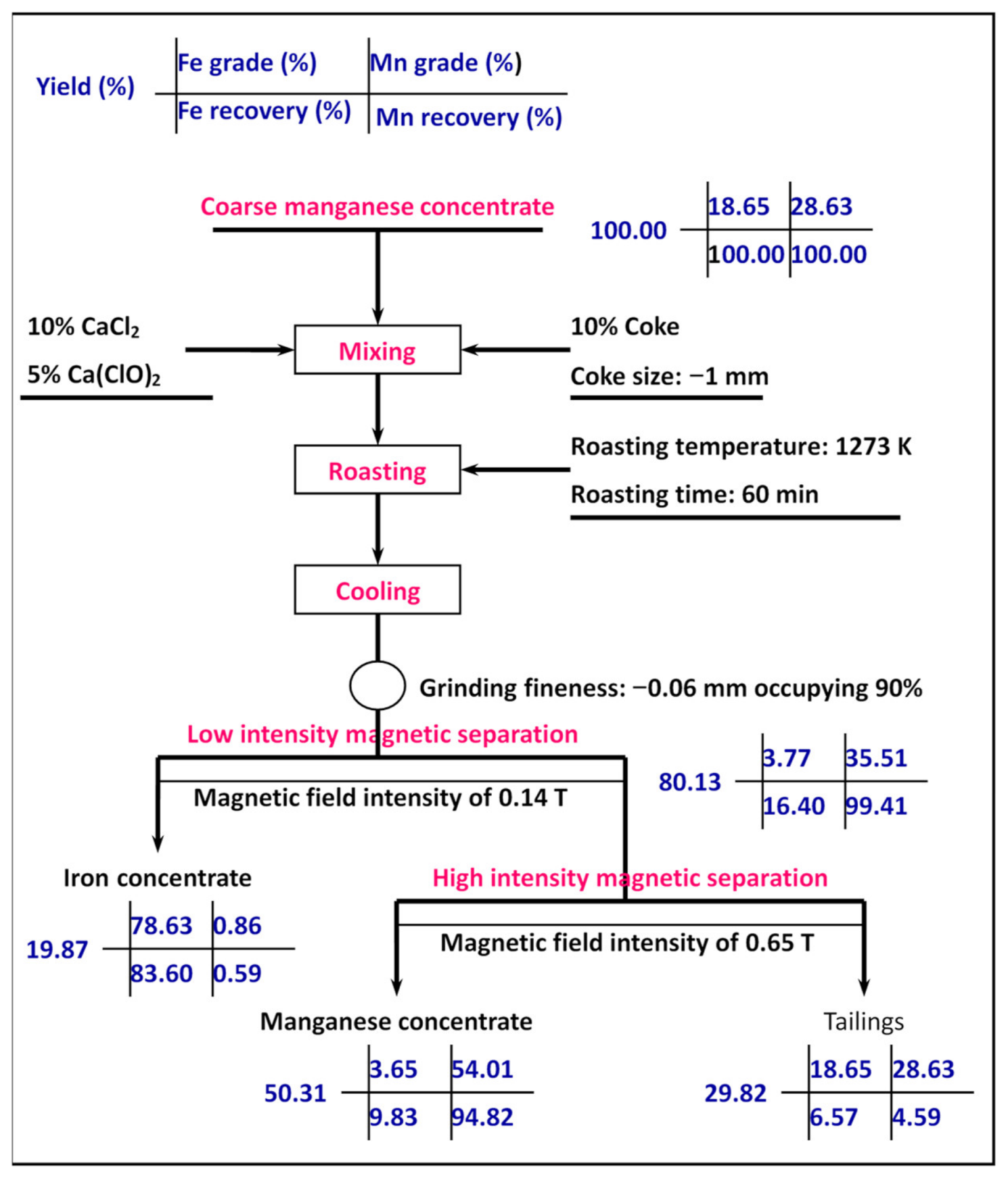
3.5. The Scale-Up Test of Iron and Manganese Extraction
3.6. Phase Transformation Mechanism in Roasting Process
4. Conclusions
- (1)
- The coarse manganese concentrate contained 28.63% Mn and 18.65% Fe. Rhodochrosite, limonite, quartz, and olivine are the main minerals in coarse manganese concentrate, and the rhodochrosite and limonite have a close symbiotic relationship. Separation of manganese and iron was difficult to achieve with magnetic separation, gravity separation, or flotation.
- (2)
- A novel process of segregation roasting with two stages of magnetic separation was used to treat the coarse manganese concentrate. The addition of calcium chloride and calcium hypochlorite synergistically enhanced the transformation of iron from a weakly magnetic mineral to a strongly magnetic mineral dominated by metallic iron and magnetite, and manganese changed from manganese carbonate to manganese oxide. Test results show that iron concentrate with an iron grade of 78.63% and iron recovery of 83.60%, and manganese concentrate with a manganese grade of 54.04% and manganese recovery of 94.82% were achieved under the following comprehensive conditions: roasting temperature of 1273 K, roasting time of 60 min, calcium chloride dosage of 10%, calcium hypochlorite dosage of 5%, coke dosage of 10%, coke size of −1 mm, grinding fineness of −0.06 mm occupying 90%, low-intensity magnetic field intensity of 0.14 T, and high-intensity magnetic field intensity of 0.65 T. Extraction of manganese and iron was straightforward.
- (3)
- Phase transformation mechanism analysis results show that limonite (Fe2O3·nH2O) was heated and lost crystalline water to form hematite (Fe2O3); hematite reacted with carbonic oxide (CO) and was reduced to magnetite in the reducing atmosphere. Some magnetite was reduced to ferrous oxide (FeO) by carbonic oxide (CO). Ferrous oxide reacted with hydrogen chloride and chlorine gas to form ferrous chloride. Ferrous chloride was reduced by carbon or hydrogen to become metallic iron (Fe) and was adsorbed onto the coke surface. Rhodochrosite (MnCO3) is mainly a decomposition reaction to produce manganese oxide (MnO) in the roasting process. The thermodynamic calculation results and XRD and SEM–EDS analysis characterization results also further verified the phase transition mechanism of iron and manganese and the reliability of the test results.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garnit, H.; Kraemer, D.; Bouhlel, S.; Davoli, M.; Barca, D. Manganese Ores in Tunisia: Genetic Constraints from Trace Element Geochemistry and Mineralogy. Ore Geol. Rev. 2020, 120, 103451. [Google Scholar] [CrossRef]
- Steenkamp, J.D.; Chetty, D.; Singh, A.; Hockaday, S.A.C.; Denton, G.M. From Ore Body to High Temperature Processing of Complex Ores: Manganese-A South African Perspective. JOM 2020, 72, 3422–3435. [Google Scholar] [CrossRef]
- Shaif, M.; Siddiquie, F.N.; Ahmad, M. Mineralogical Studies of the Manganese Ores in Banswara Manganese Belt, Banswara District, Rajasthan. J. Geol. Soc. India 2020, 96, 189–198. [Google Scholar] [CrossRef]
- Singh, V.; Ghosh, T.K.; Ramamurthy, Y.; Tathavadkar, V. Beneficiation and agglomeration process to utilize low-grade ferruginous manganese ore fines. Int. J. Miner. Process. 2011, 99, 84–86. [Google Scholar] [CrossRef]
- Liu, B.B.; Zhang, Y.B.; Lu, M.M.; Su, Z.J.; Li, G.H.; Jiang, T. Extraction and separation of manganese and iron from ferruginous manganese ores: A review. Miner. Eng. 2019, 131, 286–303. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, B.; Ge, W.; Yan, C.J.; Yang, X. Magnetic Separation and Magnetic Properties of Low-Grade Manganese Carbonate Ore. JOM 2015, 67, 361–368. [Google Scholar] [CrossRef]
- Zou, S.; Wang, S.; Zhong, H.; Qin, W.Q. Hydrophobic agglomeration of rhodochrosite fines in aqueous suspensions with sodium oleate. Powder Technol. 2021, 377, 186–193. [Google Scholar] [CrossRef]
- Luo, N.; Wei, D.Z.; Shen, Y.B.; Liu, W.G.; Gao, S.L. Effect of calcium ion on the separation of rhodochrosite and calcite. J. Mater. Res. Technol. 2018, 7, 96–101. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.B.; Yan, F.; Zhang, Z.; Shen, X.H.; Zhang, Z.T. Recycling of spent lithium-ion batteries: Selective ammonia leaching of valuable metals and simultaneous synthesis of high-purity manganese carbonate. Waste Manag. 2020, 114, 253–262. [Google Scholar] [CrossRef]
- Long, Y.F.; Su, J.; Ye, X.J.; Su, H.F.; Wen, Y.X. Reduction-roast leaching of low grade pyrolusite using bagasse as a reducing agent. Adv. Mater. Res. 2013, 699, 28–33. [Google Scholar] [CrossRef]
- Lu, J.M.; Dreisinger, D.; Gluck, T. Manganese electrodeposition—A literature review. Hydrometallurgy 2014, 141, 105–116. [Google Scholar] [CrossRef]
- You, Z.X.; Li, G.H.; Zhang, Y.B.; Peng, Z.W.; Jiang, T. Extraction of manganese from iron rich MnO2 ores via selective sulfation roasting with SO2 followed by water leaching. Hydrometallurgy 2015, 156, 225–231. [Google Scholar] [CrossRef]
- Elliott, R.; Barati, M. A Review of the Beneficiation of Low-Grade Manganese Ores by Magnetic Separation. Can. Metall. Q. 2020, 59, 1–16. [Google Scholar] [CrossRef]
- Singh, V.; Chakraborty, T.; Tripathy, S.K. A Review of Low Grade Manganese Ore Upgradation Processes. Miner. Process. Extr. Metall. Rev. 2020, 41, 417–438. [Google Scholar] [CrossRef]
- Yu, W.C.; Polgari, M.; Gyollai, I.; Fintor, K.; Szabo, M.; Kovacs, I.; Fekete, J.; Du, Y.S.; Zhou, Q. Microbial metallogenesis of Cryogenian manganese ore deposits in South China. Precambrian Res. 2019, 322, 122–135. [Google Scholar] [CrossRef]
- Gao, J.B.; Yang, R.D.; Xu, H.; Zhang, X.; Feng, K.N.; Zheng, L.L. Genesis of Permian sedimentary manganese deposits in Zunyi, Guizhou Province, SW China: Constraints from geology and elemental geochemistry. J. Geochem. Explor. 2018, 192, 142–154. [Google Scholar] [CrossRef]
- Li, S.; Kang, Z.; Liu, W.; Lian, Y.C.; Yang, H.S. Reduction behavior and direct reduction kinetics of red mud-biomass composite pellets. J. Sustain. Metall. 2021. [Google Scholar] [CrossRef]
- Cheraghi, A.; Yoozbashizadeh, H.; Safarian, J. Gaseous Reduction of Manganese Ores: A Review and Theoretical Insight. Miner. Process. Extr. Metall. Rev. 2020, 41, 198–215. [Google Scholar] [CrossRef]
- Yu, J.W.; Han, Y.X.; Li, Y.J.; Gao, P. Recent advances in magnetization roasting of refractory iron ores: A technological review in the past decade. Miner. Process. Extr. Metall. Rev. 2019, 41, 349–359. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.S.; Han, Y.X.; Li, Y.J.; Gao, P. Effect of Thermal Oxidation Pretreatment on the Magnetization Roasting and Separation of Refractory Iron Ore. Miner. Process. Extr. Metall. Rev. 2020. [Google Scholar] [CrossRef]
- Mpho, M.; Samson, B.; Ayo, A. Evaluation of reduction roasting and magnetic separation for upgrading Mn/Fe ratio of fine ferromanganese. Int. J. Min. Sci. Technol. 2013, 23, 537–541. [Google Scholar] [CrossRef]
- Xiao, J.H.; Zou, K.; Ding, W.; Peng, Y.; Chen, T. Extraction of Lead and Zinc from a Rotary Kiln Oxidizing Roasting Cinder. Metals 2020, 10, 465. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Banerjee, P.K.; Suresh, N. Effect of desliming on the magnetic separation of low-grade ferruginous manganese ore. Int. J. Miner. Metall. Mater. 2015, 22, 661–673. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Mallick, M.K.; Singh, V.; Murthy, Y.R. Preliminary studies on teeter bed separator for separation of manganese fines. Powder Technol. 2013, 239, 284–289. [Google Scholar] [CrossRef]
- Ding, W.; Xiao, J.H.; Peng, Y.; Shen, S.Y.; Chen, T. Iron Extraction from Red Mud using Roasting with Sodium Salt. Miner. Process. Extr. Metall. Rev. 2021, 42, 153–161. [Google Scholar]
- Li, G.H.; Zhang, S.H.; Rao, M.J.; Zhang, Y.B.; Jiang, T. Effects of Sodium Salts on Reduction Roasting and Fe-P Separation of High-Phosphorus Oolitic Hematite Ore. Int. J. Miner. Process. 2013, 124, 26–34. [Google Scholar] [CrossRef]
- Ding, W.; Xiao, J.H.; Yang, P.; Shen, S.Y.; Chen, T.; Zou, K.; Wang, Z. Extraction of Scandium and Iron from Red Mud. Miner. Process. Extr. Metall. Rev. 2020. [Google Scholar] [CrossRef]
- Aleksandrov, P.V.; Medvedev, A.S.; Kadirov, A.A.; Imideev, V.A. Processing Molybdenum Concentrates Using Low-Temperature Oxidizing-Chlorinating Roasting. Russ. J. Non-Ferr. Met. 2014, 55, 114–119. [Google Scholar] [CrossRef]
- Xiao, J.H.; Ding, W.; Peng, Y.; Wu, Q.; Chen, Z.Q.; Wang, Z.; Wang, J.M.; Peng, T. Upgrading Iron and Removing Phosphorus of High Phosphorus Oolitic Iron Ore by Segregation Roasting with Calcium Chloride and Calcium Hypochlorite. J. Min. Metall. Sect. B 2019, 55, 305–314. [Google Scholar] [CrossRef]
- Xiao, J.H.; Ding, W.; Peng, Y.; Chen, T.; Zou, K.; Wang, Z. Extraction of Nickel from Garnierite Laterite Ore Using Roasting and Magnetic Separation with Calcium Chloride and Iron Concentrate. Minerals 2020, 10, 352. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, Y.B.; Huang, Z.C.; Li, G.H.; Fan, X.H. Preheating and Roasting Characteristics of Hematite-Magnetite (H-M) Concentrate Pellets. Ironmak. Steelmak. 2008, 35, 21–26. [Google Scholar] [CrossRef]
- Xiao, J.H.; Zhang, Y.S. Extraction of Cobalt and Iron from Refractory Co-Bearing Sulfur Concentrate. Processes 2020, 8, 200. [Google Scholar] [CrossRef]
- Liu, M.X.; Wang, C.G.; Luo, J.; Rao, M.J.; Li, G.H.; Jiang, T. Sodium-Salt-Assisted Reductive Roasting for Separation and Enrichment of Valuable Components from Lateritic Iron Ore. JOM 2019, 71, 3181–3189. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, Q.; Zhang, Y.H.; Geng, C.Y.; Yu, B.; Chi, J. Preparation of magnetically separable mesoporous activated carbons from brown coal with Fe3O4. Int. J. Min. Sci. Technol. 2019, 2, 513–519. [Google Scholar] [CrossRef]
- Larssen, T.A.; Senk, D.; Tangstad, M. Reduction of Manganese Ores in CO-CO2 Atmospheres. Metall. Mater. Trans. B 2020. [Google Scholar] [CrossRef]
- Sahu, S.N.; Baskey, P.K.; Barma, S.D.; Sahoo, S.; Meikap, B.C.; Biswal, S.K. Pelletization of Synthesized Magnetite Concentrate Obtained By Magnetization Roasting of Indian Low-Grade BHQ Iron Ore. Powder Technol. 2020, 374, 190–200. [Google Scholar] [CrossRef]
- Roy, S.K.; Nayak, D.; Rath, S.S. A Review on the Enrichment of Iron Values of Low-Grade Iron Ore Resources Using Reduction Roasting-Magnetic Separation. Powder Technol. 2020, 367, 796–808. [Google Scholar] [CrossRef]
- Ponomar, V.P. Thermomagnetic Properties of the Goethite Transformation during High-Temperature Treatment. Miner. Eng. 2018, 127, 143–152. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Liu, B.B.; You, Z.X.; Su, Z.J.; Luo, W.; Li, G.H.; Jiang, T. Consolidation behavior of high-Fe manganese ore sinters with natural basicity. Miner. Process. Extr. Metall. Rev. 2016, 37, 333–341. [Google Scholar] [CrossRef]




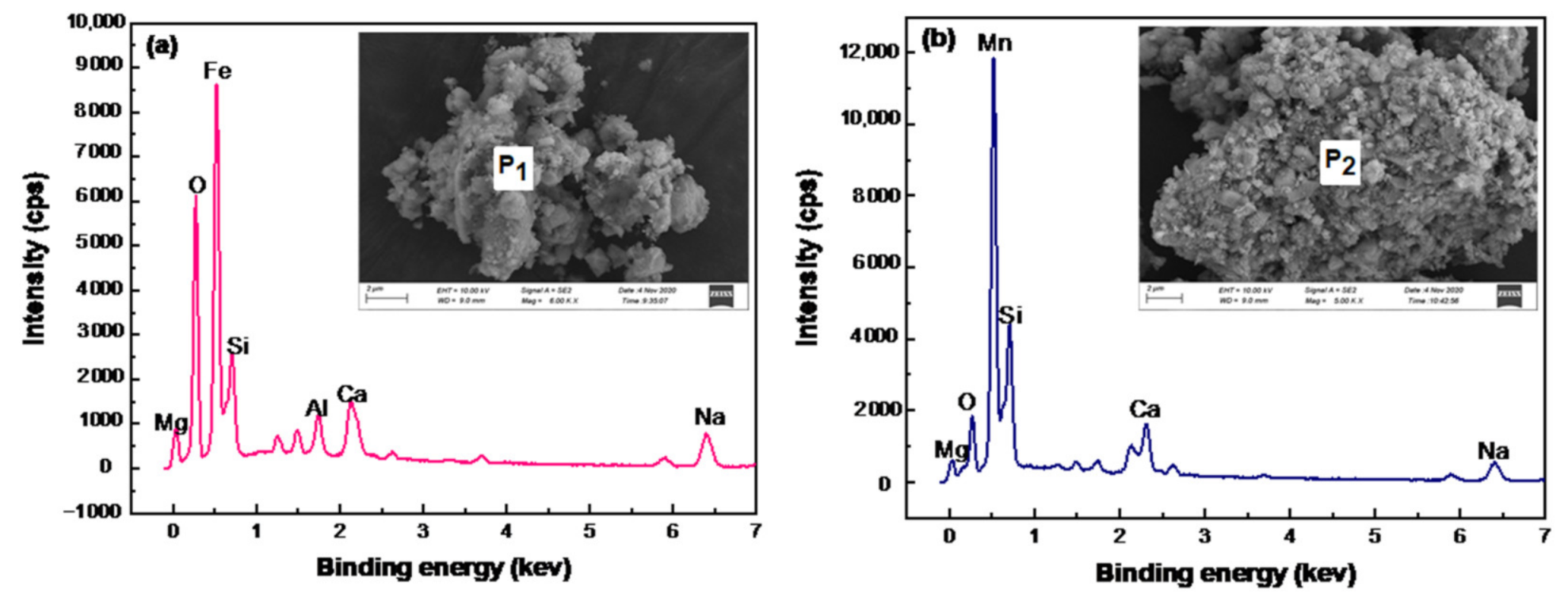
| Mn | Fe | C | S | P | As | Al2O3 | SiO2 | MgO | CaO | Na2O |
|---|---|---|---|---|---|---|---|---|---|---|
| 28.63 | 18.65 | 6.88 | 0.15 | 0.23 | 0.005 | 5.36 | 24.34 | 3.93 | 3.27 | 0.52 |
| Mad (%) | Ad (%) | FCd (%) | Vdaf (%) | Characteristic of Char Residue |
|---|---|---|---|---|
| 0.45 | 1.68 | 97.12 | 1.06 | 2 |
| Fe | Mn | Al2O3 | SiO2 | MgO | CaO | Na2O |
|---|---|---|---|---|---|---|
| 4.36 | 32.25 | 6.86 | 28.66 | 4.49 | 3.03 | 0.59 |
| Magnetic Filed Intensity (T) | Products | Yield | Mn Grade | Mn Recovery |
|---|---|---|---|---|
| 0.45 | Mn concentrate | 47.68 | 55.63 | 82.25 |
| Tailings | 52.32 | 10.94 | 17.25 | |
| Totals | 100.00 | 32.25 | 100.00 | |
| 0.55 | Mn concentrate | 53.16 | 54.22 | 89.33 |
| Tailings | 46.84 | 7.35 | 10.07 | |
| Totals | 100.00 | 32.27 | 100.00 | |
| 0.65 | Mn concentrate | 57.29 | 53.42 | 94.49 |
| Tailings | 42.71 | 3.88 | 5.51 | |
| Totals | 100.00 | 32.26 | 100.00 | |
| 0.75 | Mn concentrate | 81.22 | 38.84 | 97.78 |
| Tailings | 18.78 | 3.81 | 2.22 | |
| Totals | 100.00 | 32.26 | 100.00 |
| Products | Fe | Mn | S | P | Al2O3 | SiO2 | MgO | CaO | Na2O |
|---|---|---|---|---|---|---|---|---|---|
| Fe concentrate | 78.63 | 0.86 | 0.06 | 0.05 | 1.22 | 6.88 | 1.66 | 4.22 | 0.23 |
| Mn concentrate | 4.12 | 53.55 | 0.08 | 0.04 | 0.98 | 14.86 | 2.87 | 6.23 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Zou, K.; Chen, T.; Xiong, W.; Deng, B. Extraction of Manganese and Iron from a Refractory Coarse Manganese Concentrate. Metals 2021, 11, 563. https://doi.org/10.3390/met11040563
Xiao J, Zou K, Chen T, Xiong W, Deng B. Extraction of Manganese and Iron from a Refractory Coarse Manganese Concentrate. Metals. 2021; 11(4):563. https://doi.org/10.3390/met11040563
Chicago/Turabian StyleXiao, Junhui, Kai Zou, Tao Chen, Wenliang Xiong, and Bing Deng. 2021. "Extraction of Manganese and Iron from a Refractory Coarse Manganese Concentrate" Metals 11, no. 4: 563. https://doi.org/10.3390/met11040563
APA StyleXiao, J., Zou, K., Chen, T., Xiong, W., & Deng, B. (2021). Extraction of Manganese and Iron from a Refractory Coarse Manganese Concentrate. Metals, 11(4), 563. https://doi.org/10.3390/met11040563







