The Effect of Thermal Annealing on the Microstructure and Mechanical Properties of Sn-0.7Cu-xZn Solder Joint
Abstract
1. Introduction
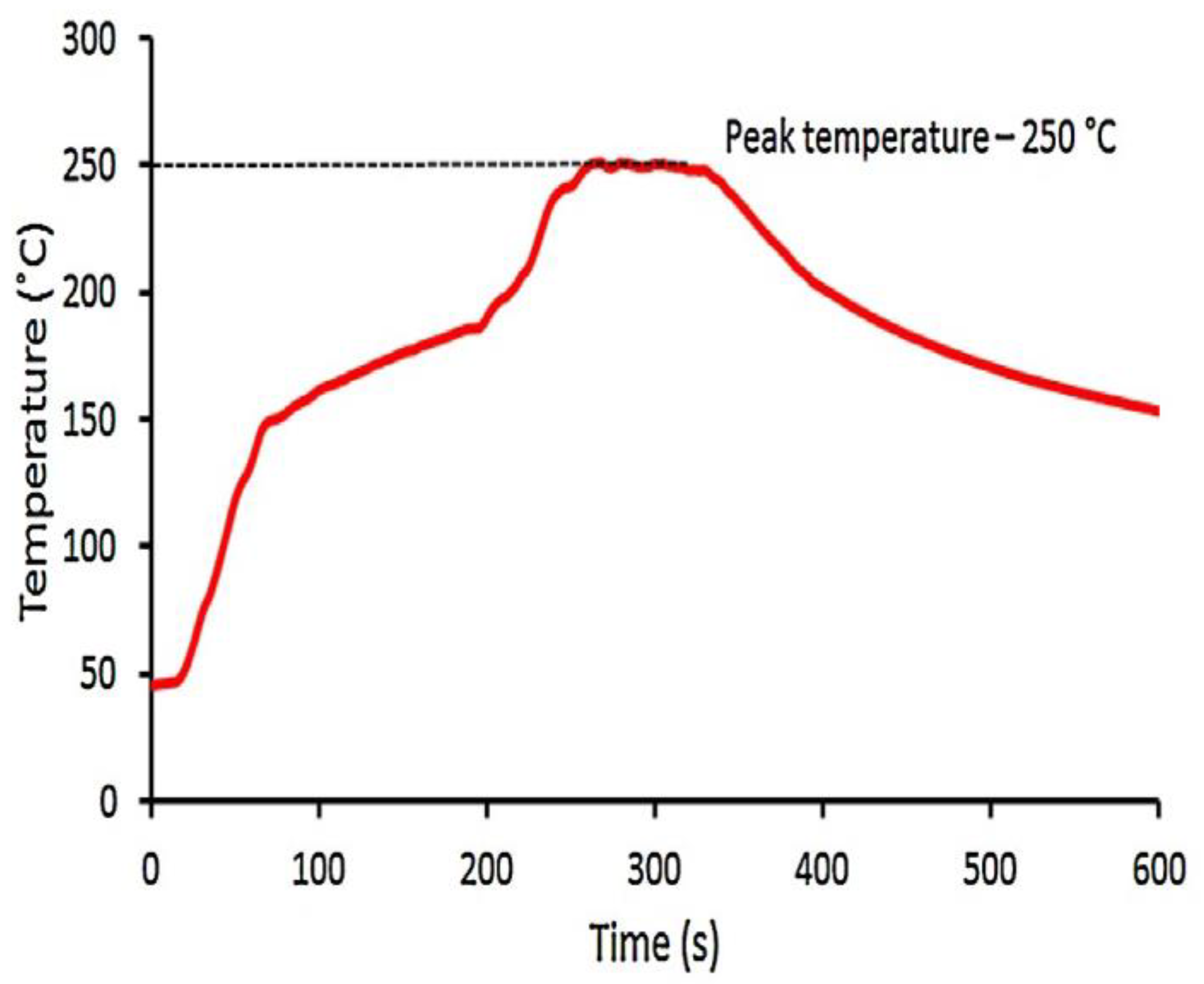
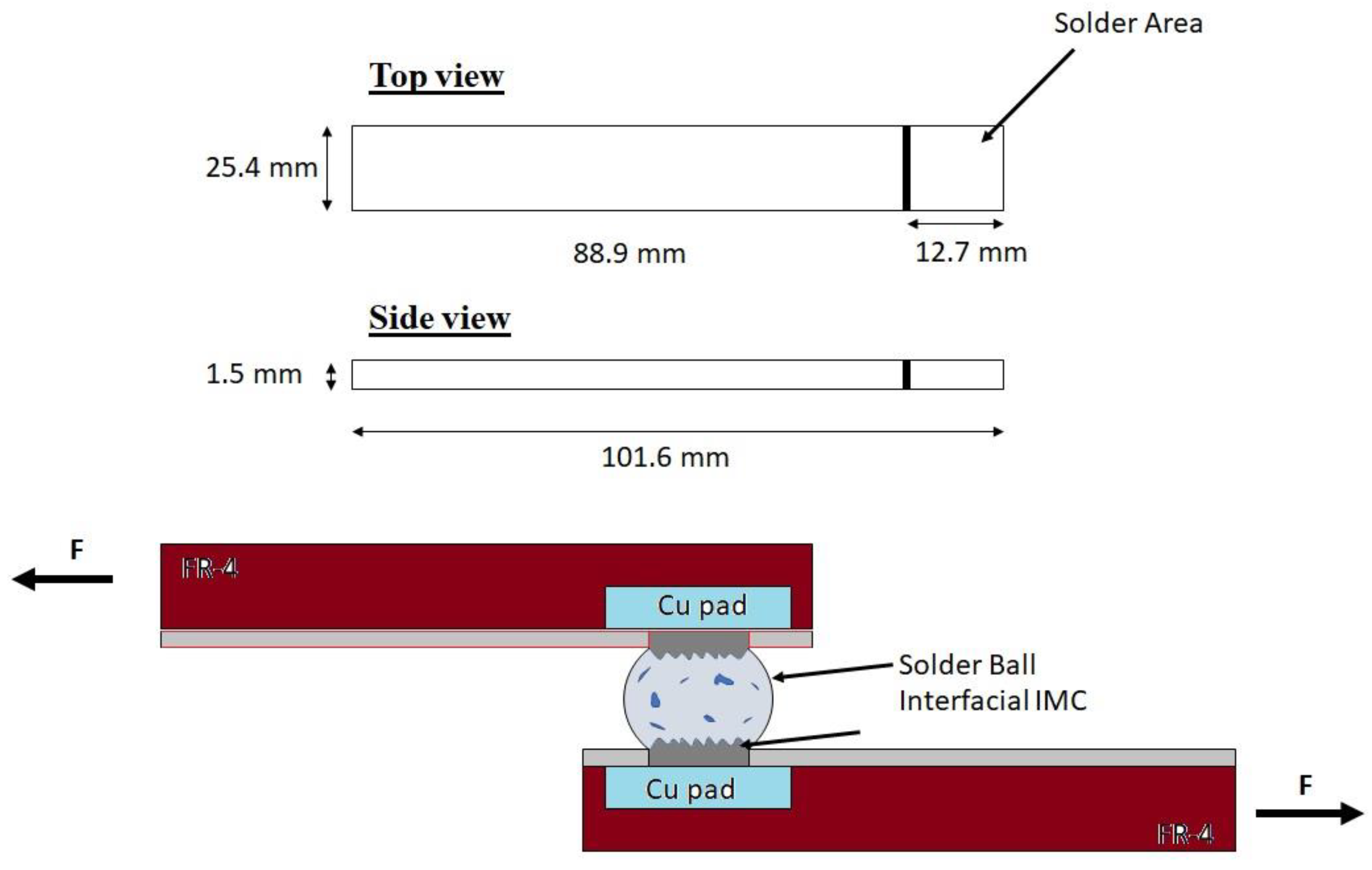
2. Materials and Methods
3. Results and Discussion
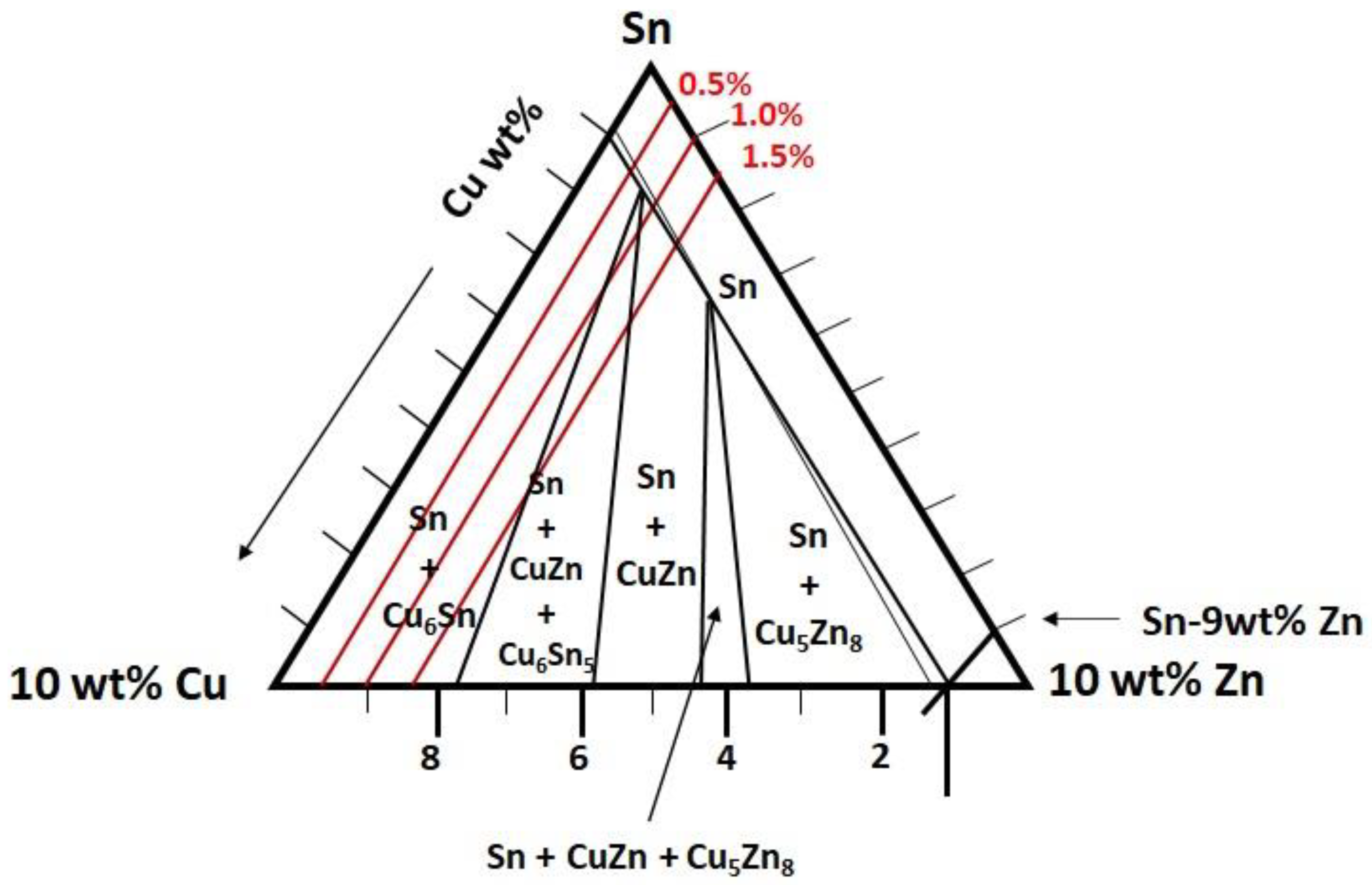
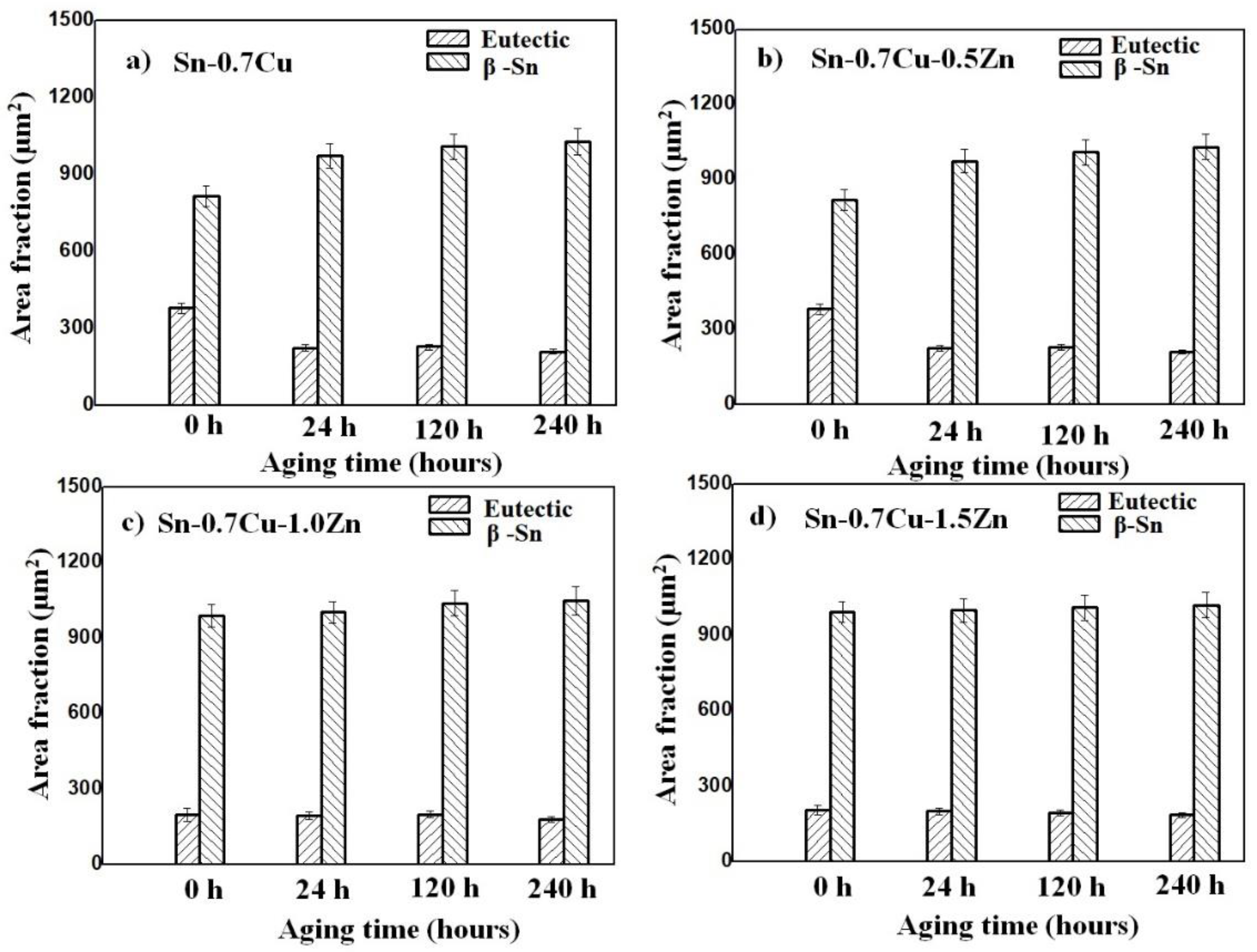
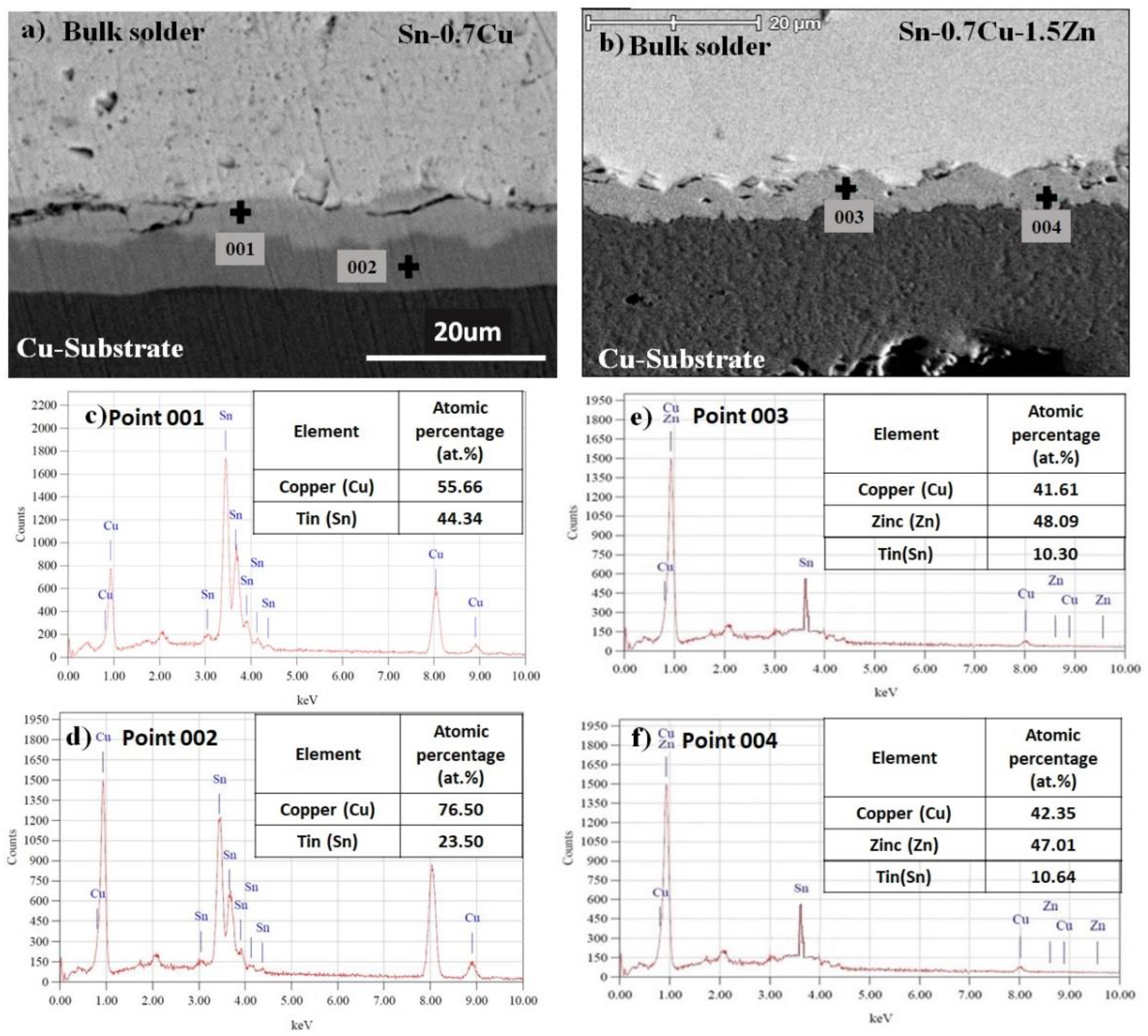
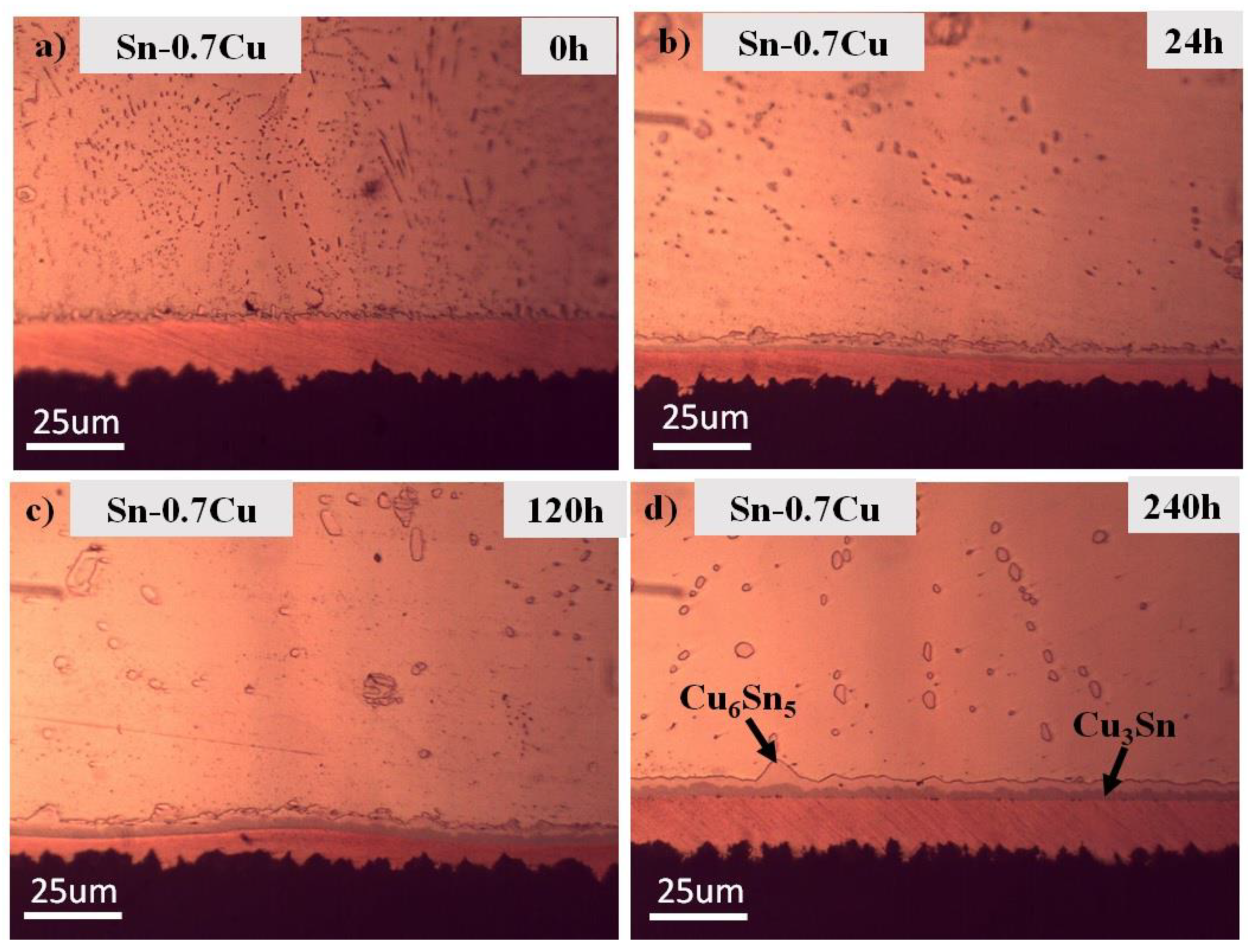
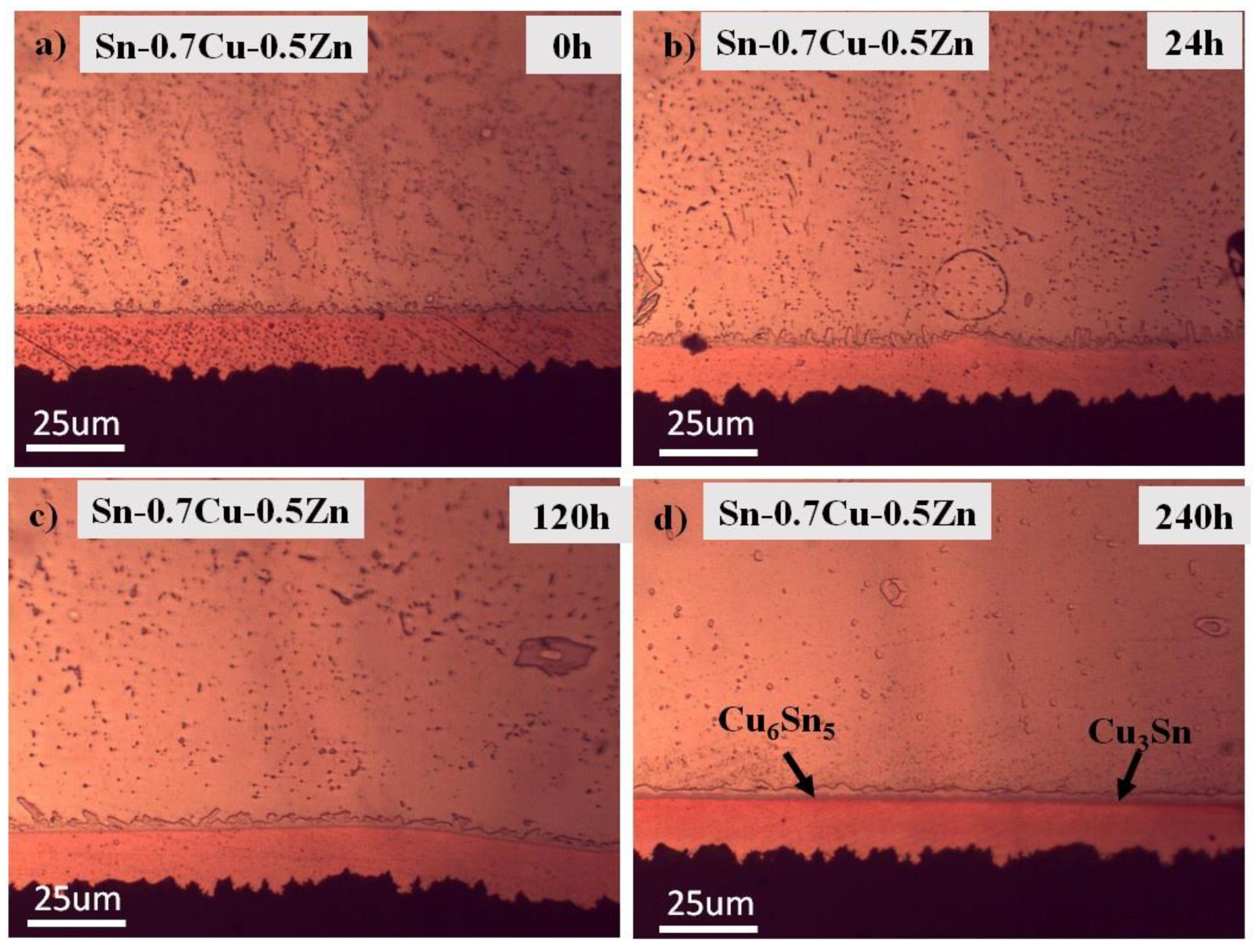
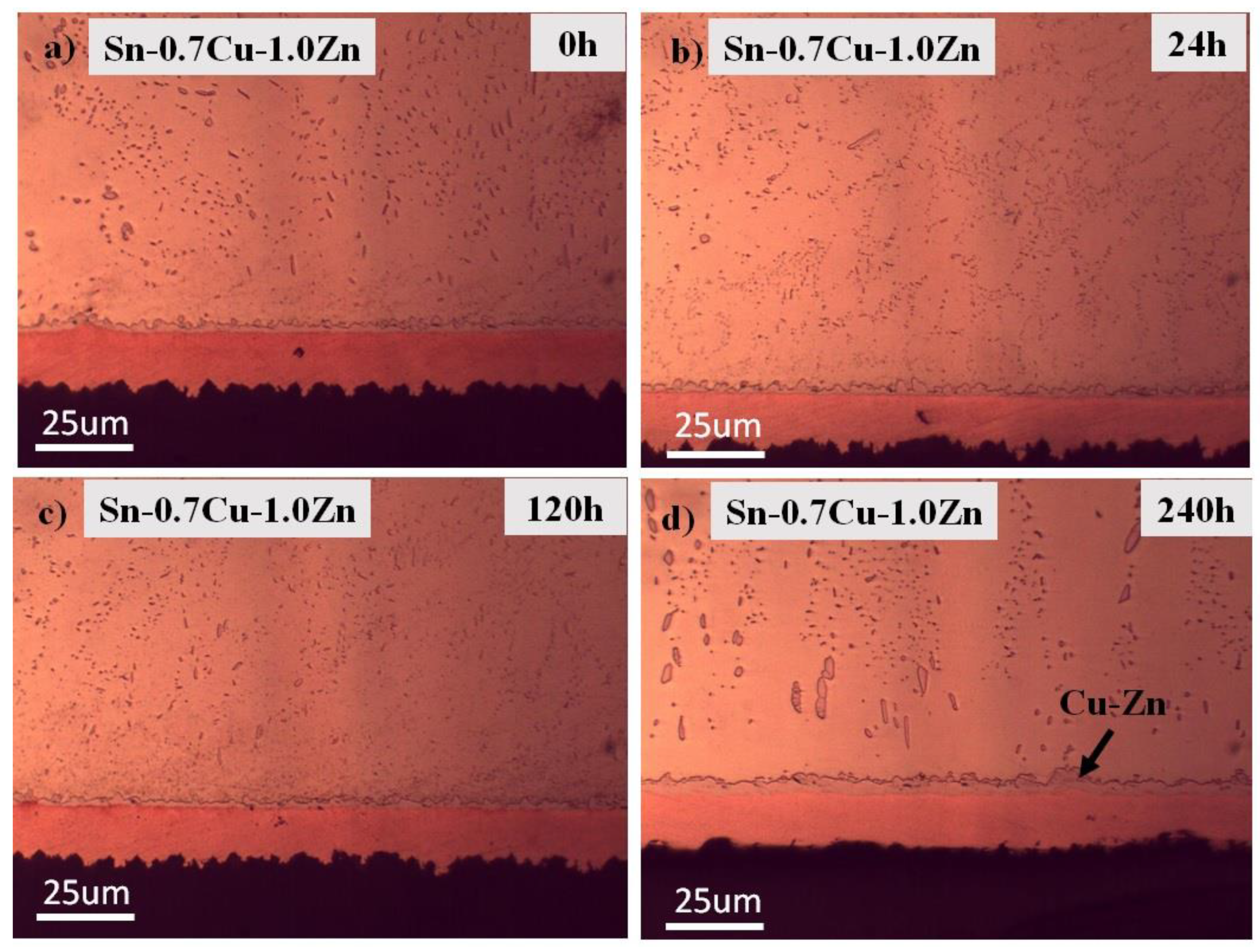
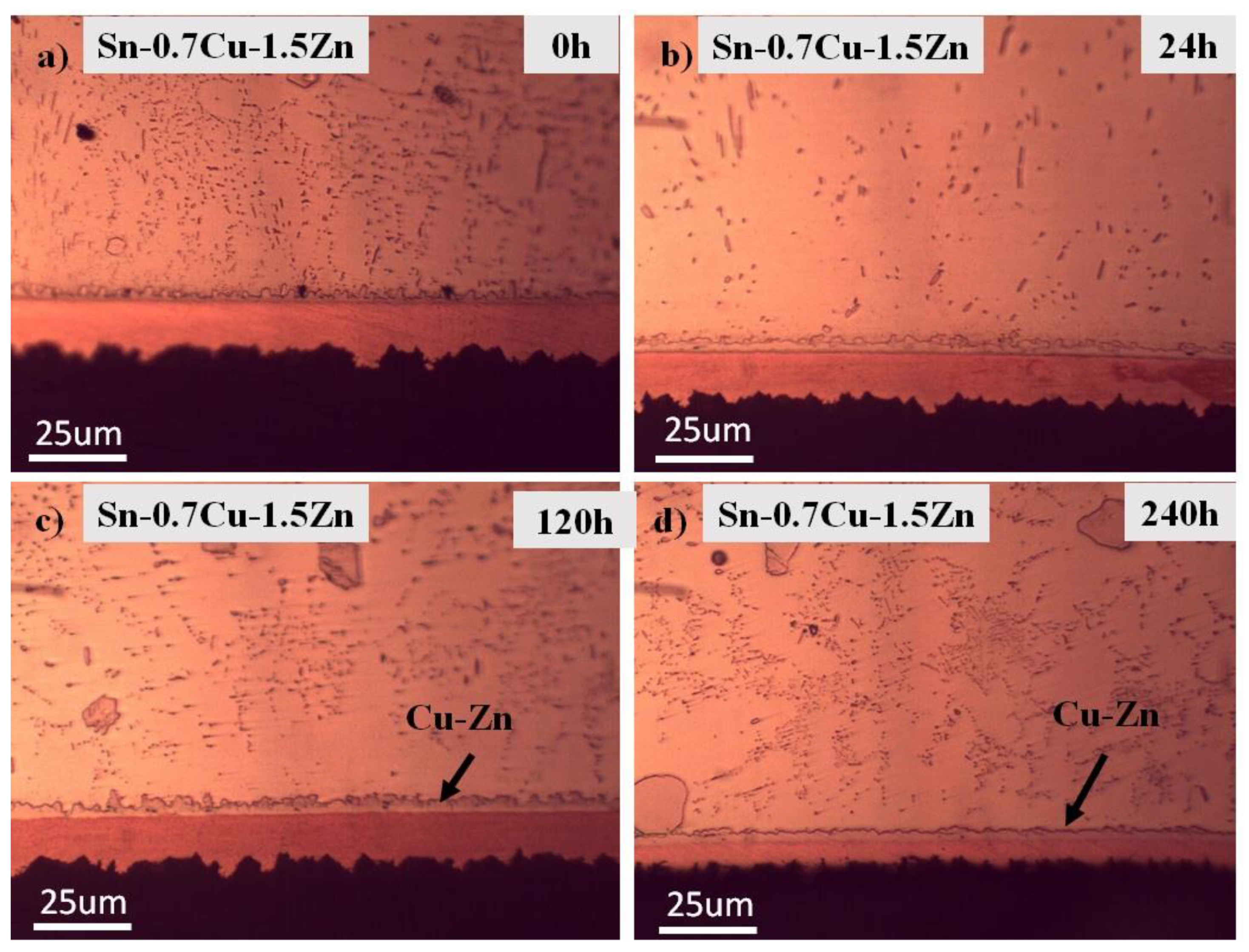
3.1. Microstructure Analysis
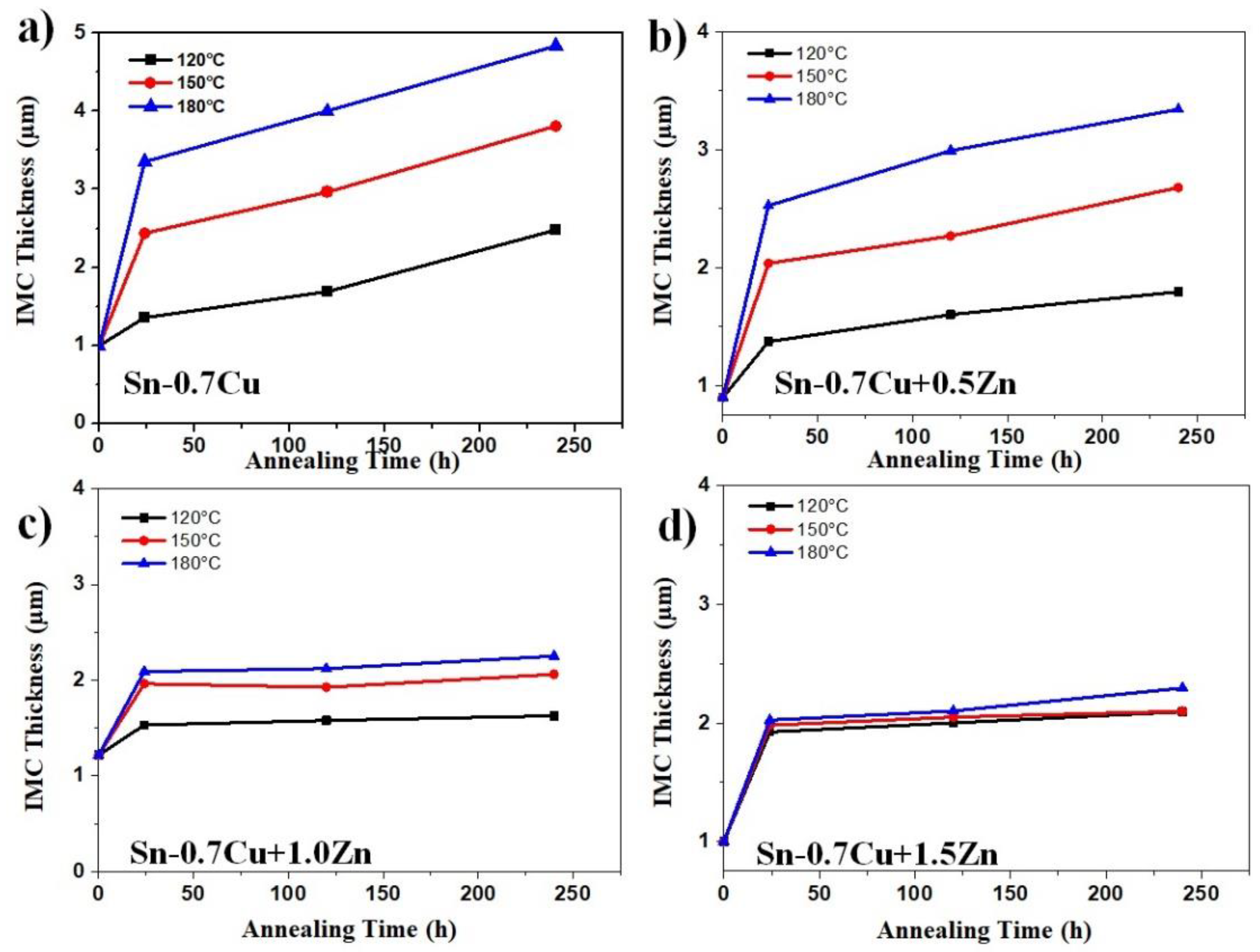
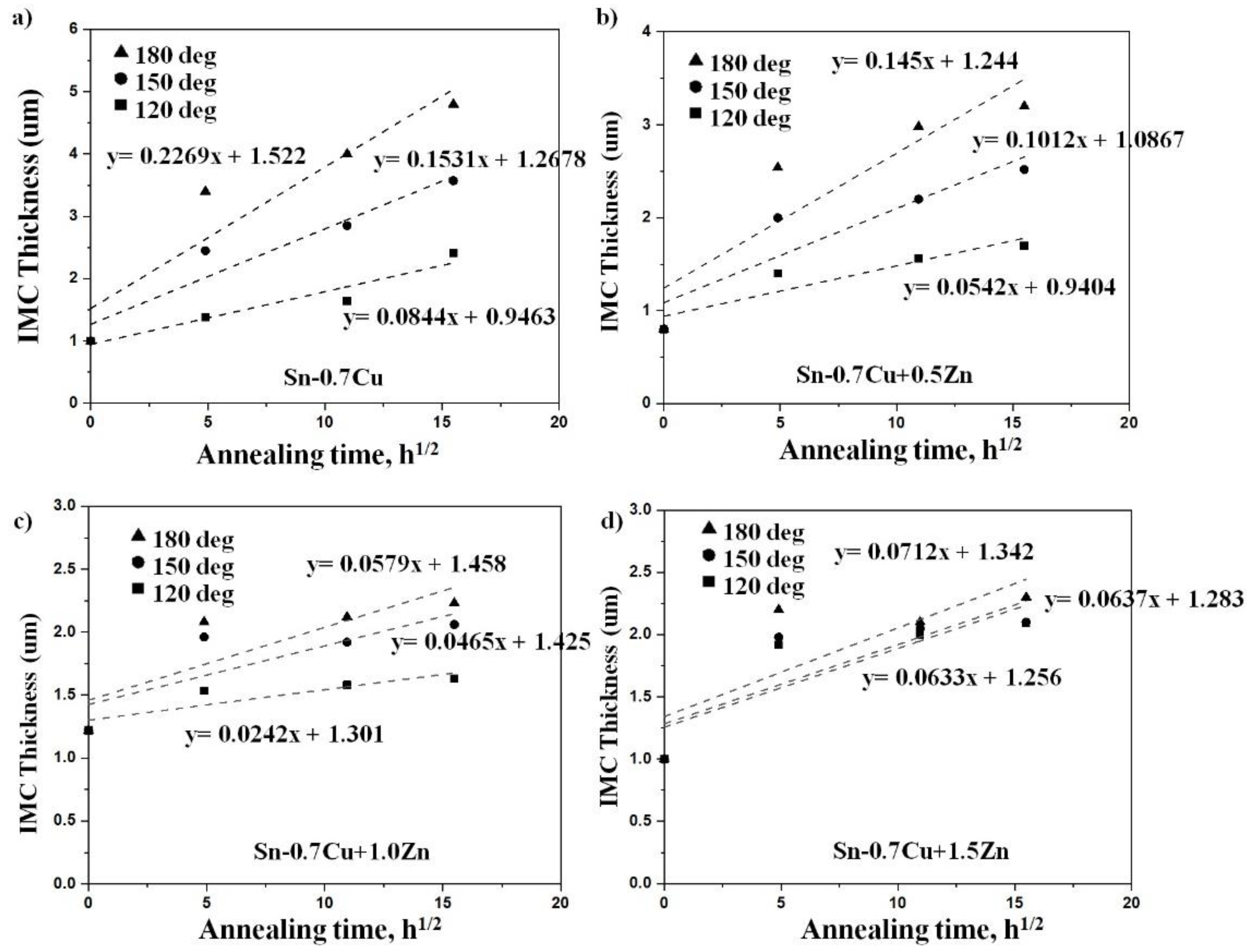
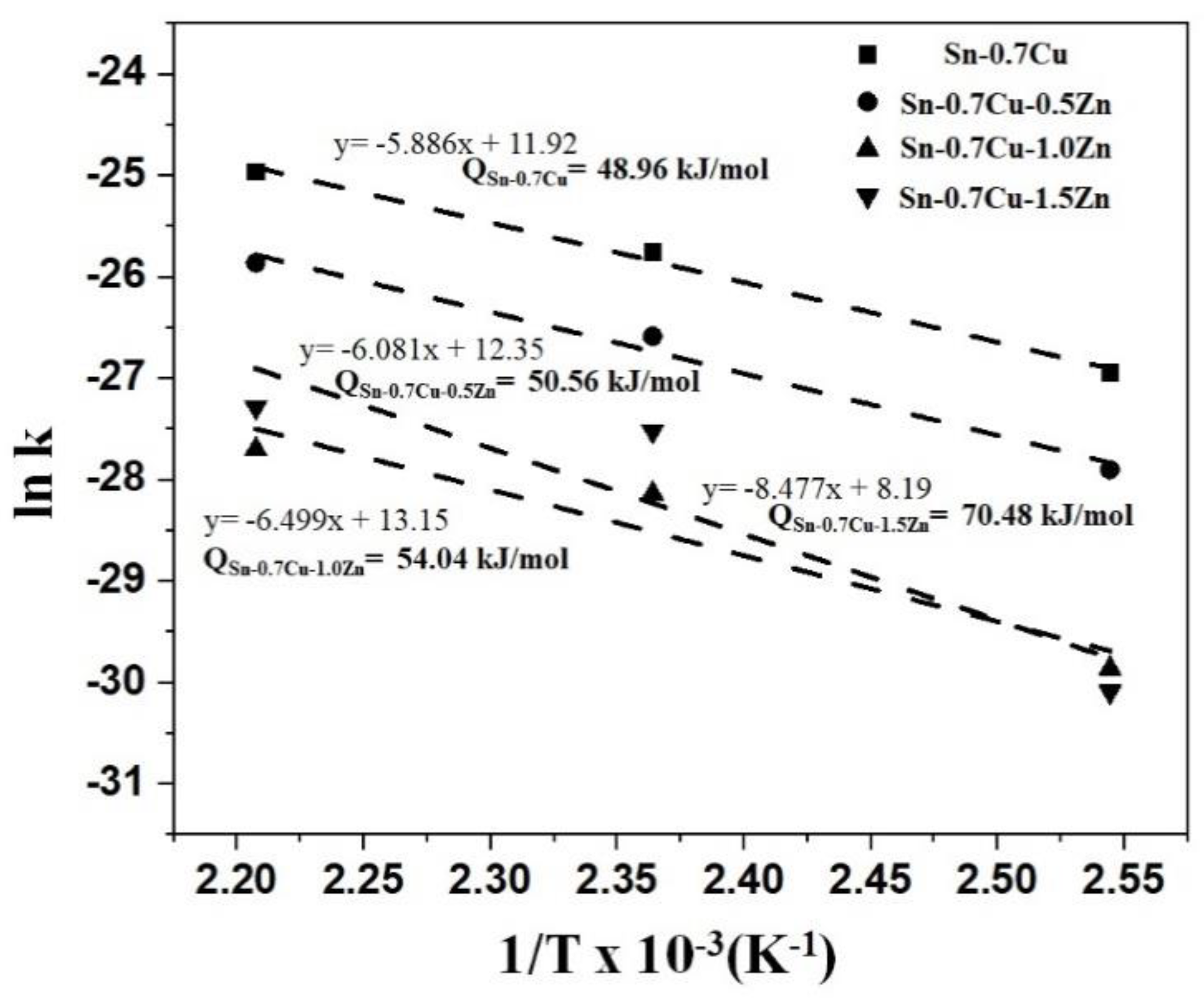
3.2. Interfacial IMC and Activation Energy
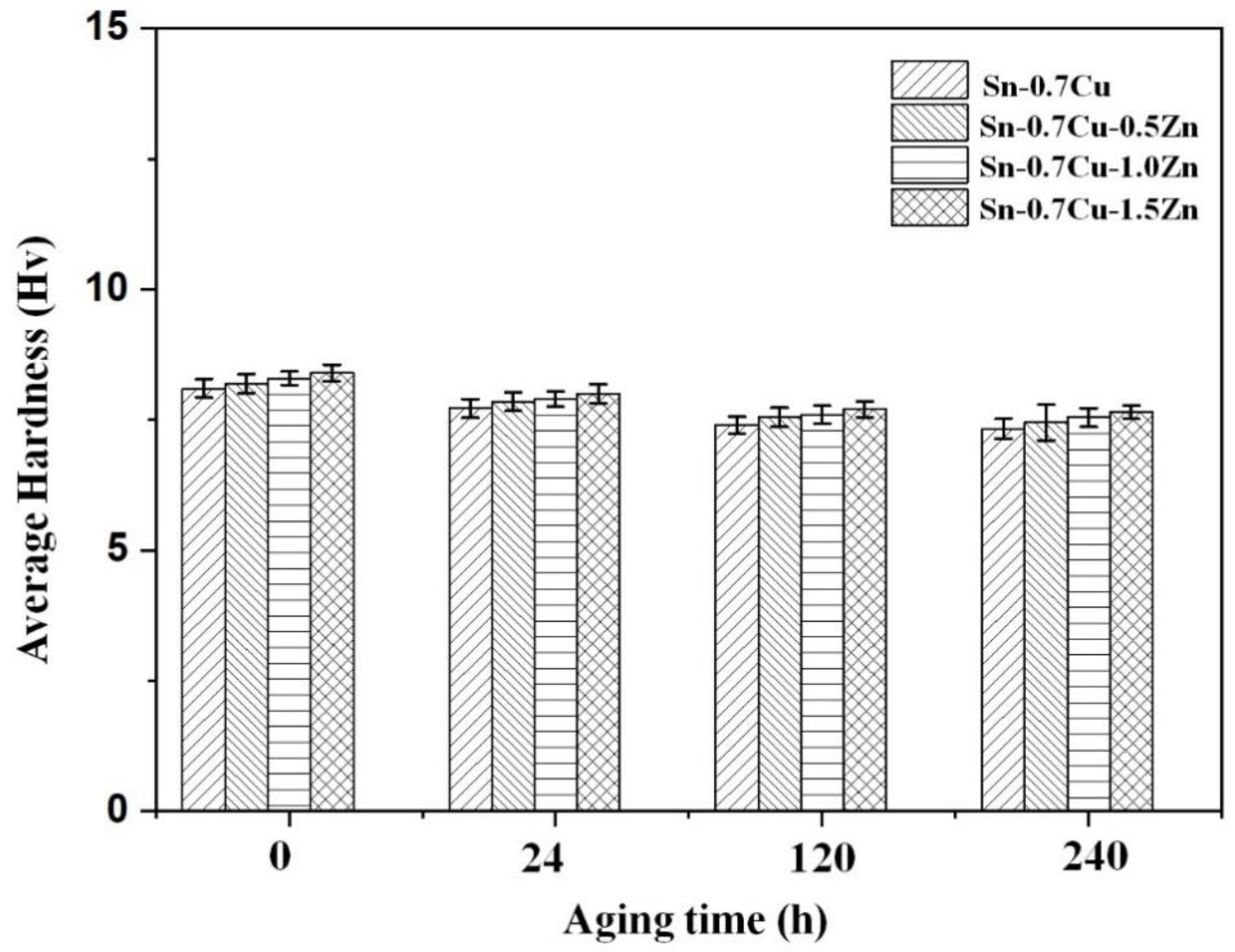
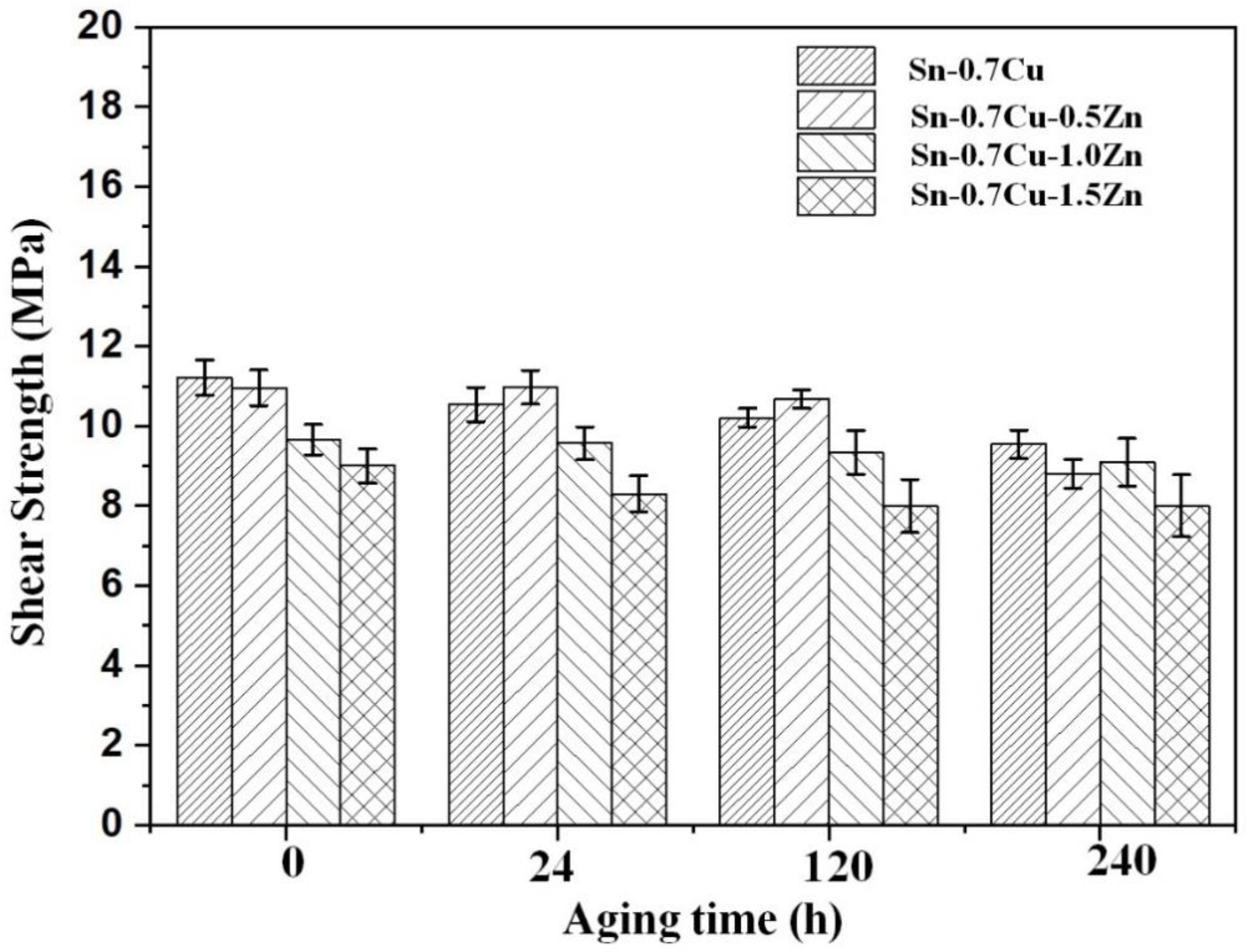
3.3. Mechanical Properties
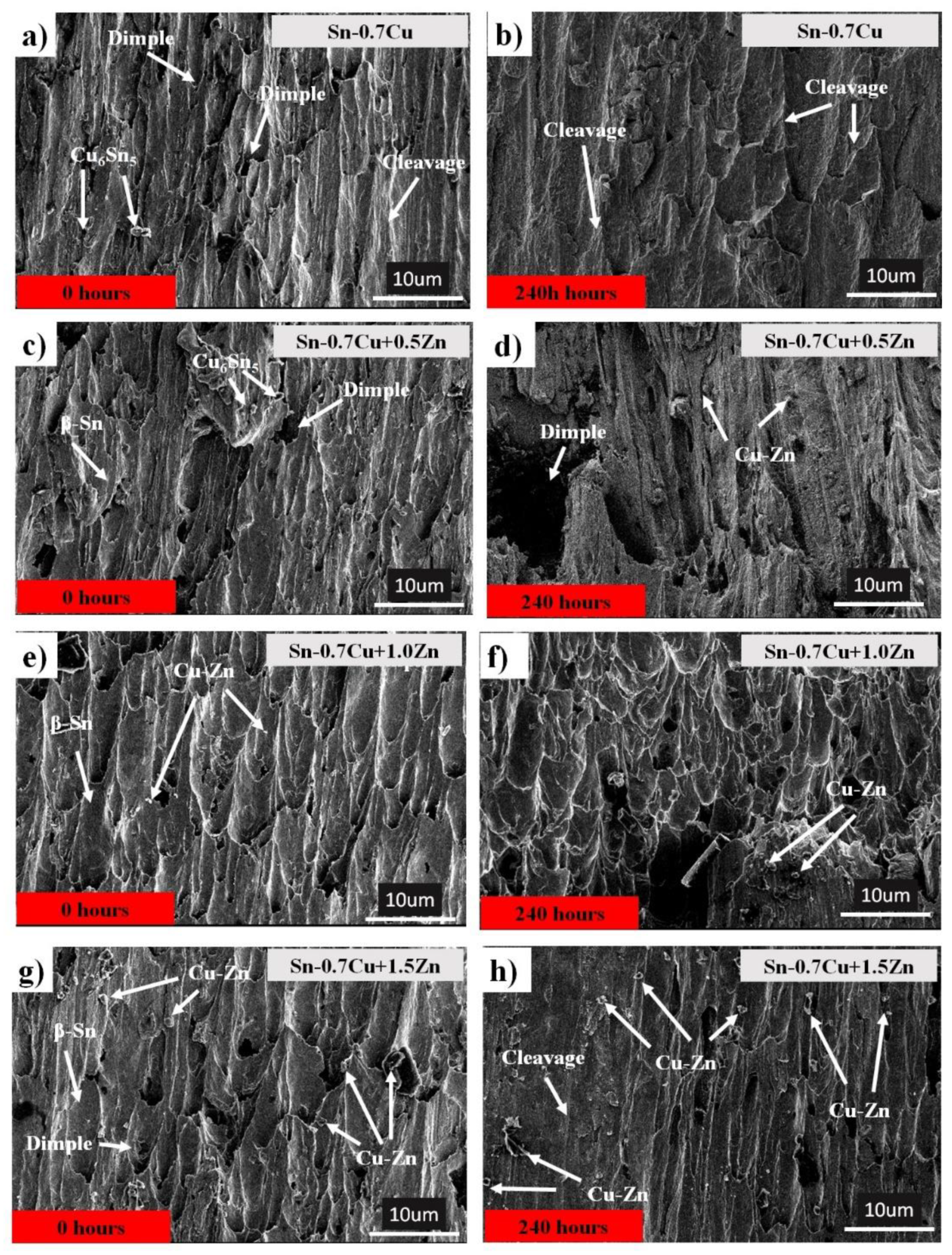
3.4. Shear Fracture Surface Morphology
4. Conclusions
- The addition of Zn into Sn-0.7Cu decreases the area fraction of the eutectic Cu6Sn5 as it is reflowed and continued decreasing as it undergoes further annealing process. However, the addition of 1.0 wt.% and 1.5 wt.% of Zn maintained the area eutectic of Cu6Sn5 even after completing the annealing process.
- Zn’s minor addition significantly suppressed the growth of the interfacial IMC after the reflow and annealing process.
- The interfacial IMC layer of the as-reflowed Sn-0.7Cu was determined to be Cu6Sn5, and with the addition of 1.0 wt.% of Zn, changed to the Cu-Zn phase as-reflowed and after the annealing process.
- The activation energy for the intermetallic layers’ growth was determined to be 48.96 kJ mol−1 and 70.48 kJ mol−1 for the Sn-0.7Cu system and Sn-0.7Cu-1.5Zn systems, respectively.
- The addition of Zn increases the hardness of Sn-0.7Cu but will decrease the hardness after the annealing process. The shear strength of the Sn-0.7Cu alloys decreases with addition of Zn and continue to decrease after annealing process due to the increased presence of the Cu-Zn phase, leading to increased brittleness.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Said, R.M.; Salleh, M.A.A.M.; Derman, M.N.; Ramli, M.I.I.; Nasir, N.M.; Saud, N. Isothermal Aging Affect to the Growth of Sn-Cu-Ni-1 wt.% TiO2 Composite Solder Paste. Key Eng. Mater. 2016, 700, 123–131. [Google Scholar] [CrossRef]
- Çadırlı, E.; Böyük, U.; Engin, S.; Kaya HA SA, N.; Maraşlı, N.; Arı, M. Investigation of microhardness and thermo-electrical properties in the Sn–Cu hypereutectic alloy. J. Mater. Sci. Mater. Electron. 2010, 21, 468–474. [Google Scholar] [CrossRef]
- Mayappan, R.; Ahmad, Z.A. Effect of Bi addition on the activation energy for the growth of Cu5Zn8 intermetallic in the Sn–Zn lead-free solder. Intermetallics 2010, 18, 730–735. [Google Scholar] [CrossRef]
- Zhao, J.; Qi, L.; Wang, X.-M.; Wang, L. Influence of Bi on microstructures evolution and mechanical properties in Sn–Ag–Cu lead-free solder. J. Alloy. Compd. 2004, 375, 196–201. [Google Scholar] [CrossRef]
- Chou, C.Y.; Chen, S.W. Phase equilibria of the Sn–Zn–Cu ternary system. Acta Mater. 2006, 54, 2393–2400. [Google Scholar] [CrossRef]
- Wang, F.J.; Yu, Z.S.; Qi, K. Intermetallic compound formation at Sn–3.0 Ag–0.5 Cu–1.0 Zn lead-free solder alloy/Cu interface during as-soldered and as-aged conditions. J. Alloy. Compd. 2007, 438, 110–115. [Google Scholar] [CrossRef]
- Siahaan, E.; Soegijono, B. The Effect of Zinc Composition in Melting Point and Microstructure of Lead-Free Solder. Int. J. Basic Appl. Sci. 2014, 14, 17–20. [Google Scholar]
- De Sousa, I.; Henderson, D.W.; Patry, L.; Kang, S.; Shih, D.-Y. The Influence of Low Level Doping on the Thermal Evolution of SAC Alloy Solder Joints with Cu Pad Structures. In Proceedings of the 56th Electronic Components and Technology Conference 2006, San Diego, CA, USA, 30 May–2 June 2006; p. 8. [Google Scholar]
- Wang, H.; Wang, F.; Gao, F.; Ma, X.; Qian, Y. Reactive wetting of Sn0. 7Cu–xZn lead-free solders on Cu substrate. J. Alloy. Compd. 2007, 433, 302–305. [Google Scholar] [CrossRef]
- Gao, Y.; Hui, J.; Sun, X.; Zhao, F.; Zhao, J.; Cheng, C.; Luo, Z.; Wang, L. Role of Zinc on Shear Property Evolution between Sn-0.7Cu Solder and Joints. Procedia Eng. 2011, 16, 807–811. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, R.; Hang, C.; Niu, L.; Wang, C. Relationship between morphologies and orientations of Cu6Sn5 grains in Sn3.0Ag0.5Cu solder joints on different Cu pads. Mater. Charact. 2014, 88, 58–68. [Google Scholar] [CrossRef]
- Wang, K.K.; Gan, D.; Hsieh, K.C. Orientation relationships, interfaces, and microstructure of η-Cu6Sn5 formed in the early-stage reaction between Cu and molten Sn. Thin Solid Film. 2010, 519, 1380–1386. [Google Scholar] [CrossRef]
- Cho, M.G.; Kang, S.K.; Seo, S.K.; Shih, D.Y.; Lee, H.M. Interfacial Reactions and Microstructures of Sn-0.7 Cu-x Zn Solders with Ni-P UBM During Thermal Aging. J. Electron. Mater. 2009, 38, 2242–2250. [Google Scholar] [CrossRef]
- Cai, C.; An, R.; Wang, C.; Tian, Y. Suppressing Kirkendall Void Density in Circuit Interconnections by Strain Annealing. arXiv 2017, arXiv:1712.06856. [Google Scholar]
- Zhang, L.; Xue, S.B.; Zeng, G.; Gao, L.L.; Ye, H. Interface reaction between SnAgCu/SnAgCuCe solders and Cu substrate subjected to thermal cycling and isothermal aging. J. Alloy. Compd. 2012, 510, 38–45. [Google Scholar] [CrossRef]
- Kim, D.-G.; Jung, S.-B. Interfacial reactions and growth kinetics for intermetallic compound layer between In–48Sn solder and bare Cu substrate. J. Alloy. Compd. 2005, 386, 151–156. [Google Scholar] [CrossRef]
- Shen, J.; Zhao, M.; He, P.; Pu, Y. Growth behaviors of intermetallic compounds at Sn–3Ag–0.5 Cu/Cu interface during isothermal and non-isothermal aging. J. Alloy. Compd. 2013, 574, 451–458. [Google Scholar] [CrossRef]
- Shen, J.; Cao, Z.; Zhai, D.; Zhao, M.; He, P. Effect of isothermal aging and low density current on intermetallic compound growth rate in lead-free solder interface. Microelectron. Reliab. 2014, 54, 252–258. [Google Scholar] [CrossRef]
- Yu, S.-P.; Hon, M.-H.; Wang, M.-C. The adhesion strength of A lead-free solder hot-dipped on copper substrate. J. Electron. Mater. 2000, 29, 237–243. [Google Scholar] [CrossRef]
- Hamada, N.; Uesugi, T.; Takigawa, Y.; Higashi, K. Effect of Addition of Small Amount of Zinc on Microstructural Evolution and Thermal Shock Behavior in Low-Ag Sn–Ag–Cu Solder Joints during Thermal Cycling. Mater. Trans. 2013, 54, 796–805. [Google Scholar] [CrossRef]
- Zeng, G.; McDonald, S.D.; Mu, D.; Terada, Y.; Yasuda, H.; Gu, Q.; Salleh, M.M.; Nogita, K. The influence of ageing on the stabilisation of interfacial (Cu,Ni)6(Sn,Zn)5 and (Cu,Au,Ni)6Sn5 intermetallics in Pb-free Ball Grid Array (BGA) solder joints. J. Alloy. Compd. 2016, 685, 471–482. [Google Scholar] [CrossRef]
- Zeng, G.; McDonald, S.D.; Gu, Q.; Terada, Y.; Uesugi, K.; Yasuda, H.; Nogita, K. The influence of Ni and Zn additions on microstructure and phase transformations in Sn–0.7 Cu/Cu solder joints. Acta Mater. 2015, 83, 357–371. [Google Scholar] [CrossRef]
- Wang, F.; Ma, X.; Qian, Y. Improvement of microstructure and interface structure of eutectic Sn–0.7 Cu solder with small amount of Zn addition. Scr. Mater. 2005, 53, 699–702. [Google Scholar] [CrossRef]
- Deng, X.; Sidhu, R.S.; Johnson, P.; Chawla, N. Influence of reflow and thermal aging on the shear strength and fracture behavior of Sn-3.5Ag solder/Cu joints. Met. Mater. Trans. A 2005, 36, 55–64. [Google Scholar] [CrossRef]
- Nur Nadirah, M.K.; Nurulakmal, M.S. Effect of Zn addition on bulk microstructure of lead-free solder SN100C. Am. Inst. Phys. Conf. Ser. 2017, 1901, 060005. [Google Scholar]
- Ng, W.; Zeng, G.; Nishimura, T.; Sweatman, K.; McDonald, S.D.; Nogita, K. The Beneficial Effect of Zn Additions on The Microstructure of SnCu and SnCuNi Solder Joints to Cu Substrates. In Proceedings of the International Conference on Electronic Packaging and iMAPS All Asia Conference (ICEP-IAAC), Kyoto, Japan, 14–17 April 2015; pp. 809–813. [Google Scholar]
- Hu, X.; Xu, T.; Keer, L.M.; Li, Y.; Jiang, X. Microstructure evolution and shear fracture behavior of aged Sn3Ag0.5Cu/Cu solder joints. Mater. Sci. Eng. A 2016, 673, 167–177. [Google Scholar] [CrossRef]
- Hu, X.; Xu, T.; Keer, L.M.; Li, Y.; Jiang, X. Shear strength and fracture behavior of reflowed Sn3.0Ag0.5Cu/Cu solder joints under various strain rates. J. Alloy. Compd. 2017, 690, 720–729. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramli, M.I.I.; Salleh, M.A.A.M.; Said, R.M.; Abdullah, M.M.A.B.; Halin, D.S.C.; Saud, N.; Nabiałek, M. The Effect of Thermal Annealing on the Microstructure and Mechanical Properties of Sn-0.7Cu-xZn Solder Joint. Metals 2021, 11, 380. https://doi.org/10.3390/met11030380
Ramli MII, Salleh MAAM, Said RM, Abdullah MMAB, Halin DSC, Saud N, Nabiałek M. The Effect of Thermal Annealing on the Microstructure and Mechanical Properties of Sn-0.7Cu-xZn Solder Joint. Metals. 2021; 11(3):380. https://doi.org/10.3390/met11030380
Chicago/Turabian StyleRamli, Mohd Izrul Izwan, Mohd Arif Anuar Mohd Salleh, Rita Mohd Said, Mohd Mustafa Al Bakri Abdullah, Dewi Suriyani Che Halin, Norainiza Saud, and Marcin Nabiałek. 2021. "The Effect of Thermal Annealing on the Microstructure and Mechanical Properties of Sn-0.7Cu-xZn Solder Joint" Metals 11, no. 3: 380. https://doi.org/10.3390/met11030380
APA StyleRamli, M. I. I., Salleh, M. A. A. M., Said, R. M., Abdullah, M. M. A. B., Halin, D. S. C., Saud, N., & Nabiałek, M. (2021). The Effect of Thermal Annealing on the Microstructure and Mechanical Properties of Sn-0.7Cu-xZn Solder Joint. Metals, 11(3), 380. https://doi.org/10.3390/met11030380








