Abstract
The kinetics of the isothermal reduction of iron ore–coke, iron ore–charcoal, and iron ore–biomass (straw) composite pellets were studied at 900–1200 °C. Compared with the other two composite pellets, the composite pellet using biomass as a reducing agent showed a more rapid reduction rate at a relatively low temperature. With an increase in the temperature, the reduction rates of the three different composite pellets tended to be equal. The reducing reactions of the three different composite pellets were all mainly controlled by gasification diffusion. The reduction rates can be described by the interface reaction kinetic model (). The apparent activation energies of the gasification diffusion of coke, charcoal, and biomass composite pellets at 900–1200 °C were calculated using the Arrhenius equation, and they were 95.81, 71.67, and 58.69 kJ/mol, respectively. The biomass composite pellets exhibited a lower apparent activation energy than the composite pellets with other reduction agents.
1. Introduction
Iron production is one of the largest industries in terms of energy consumption, pollution, and greenhouse gas emissions due to the use of one of the most indispensable raw reductants for blast furnaces—metallurgical coke/coal—causing coal shortages and serious pollution [1,2,3,4,5,6]. Therefore, the incorporation of renewable energy sources into existing and emerging metallurgical operations is desirable. Recently, renewable biomass-derived reducing agents have gained attention as possible substitutes for fossil fuel-based reducing agents in ironmaking blast furnaces. Biomass is characterized by lower contents of harmful elements (S, P) than fossil-based fuel and abundant raw material sources, therefore providing a very desirable reductant to perform the reduction of iron ore [7,8,9,10,11].
The reduction of iron oxides is performed using either charcoal, coke, or coal at present [12,13,14,15,16,17]. The reduction mechanisms of iron ore using these carbonaceous materials have been extensively studied [18,19,20]. In addition, the reduction kinetics and thermodynamics have been also studied extensively [14,15,21]. However, biomass materials as potential reductants and energy sources in carbon iron ore composite pellets have been largely neglected. Therefore, an attempt was made to use biomass as a reducing agent to reduce iron ores pellets in this paper. Furthermore, the effects of reducing agent type and temperature on the reduction behaviors of composite pellets were investigated. A kinetic model is proposed to describe the reducing process. The objective of this work is to explore the potential role of biomass as a reducing agent in the reduction of iron ore pellets through a comparison with kinetic behaviors of iron ore pellets when using coke, charcoal, and biomass (carbonized straw) as reducing agents and to determine the activation energies for the reduction of iron ore pellets using these reductants.
2. Materials and Methods
2.1. Materials
In this study, Brazil iron ore was used as an iron source and bentonite obtained from Xinyang, Henan, China, was employed as a binder; their chemical compositions are shown in Table 1. The biomass used in this work was obtained by carbonizing straw (Bishan, Chongqing, China) at fixed temperature of 900 °C in an inert gas—namely, N2 atmosphere. The properties of coke, charcoal, and biomass used as reducing agents are listed in Table 2. The particle sizes of the above material and reducing agent powders are less than 0.074 mm.

Table 1.
Chemical compositions of iron ore and bentonite (mass percent, %).

Table 2.
Proximate analysis of reducing agent (mass percent, %).
2.2. Experimental Procedure
Iron ore and all the reducing agents were ground to sizes smaller than 0.074 mm. Micro-balling was conducted in a laboratory-scale balling disc pelletizer. The mixing materials composed of iron ore, binder, and reducing agent were prepared using an additional moisture concentration of 7.5 ± 0.5 wt%. The bentonite addition amount was fixed at 2 wt%. To completely reduce the iron ore, a surplus carbon source was added and the molar ratio of reducible oxygen to fixed carbon was equal to 1.2. Green pellets were prepared using the above mixtures. After being sieved, green pellets with a particle size of 10−16 mm were obtained. Then, these pellets were dried in the oven at 105 °C for 2 h to remove the moisture completely.
A series of reduction experiments of iron ore composite pellets with different carbonaceous materials was performed under isothermal conditions to investigate the reduction behaviors of the carbon iron ore composite pellets. According to the previous reference [17], determining the reduction degree of a pellet at any given time is difficult. To evaluate the degree of reduction, a pseudo kinetic parameter, the fraction of reaction (m), was introduced. This is defined as the ratio of mass loss at a given time to the maximum mass loss of pellets, and it can be calculated using the following equation [17]:
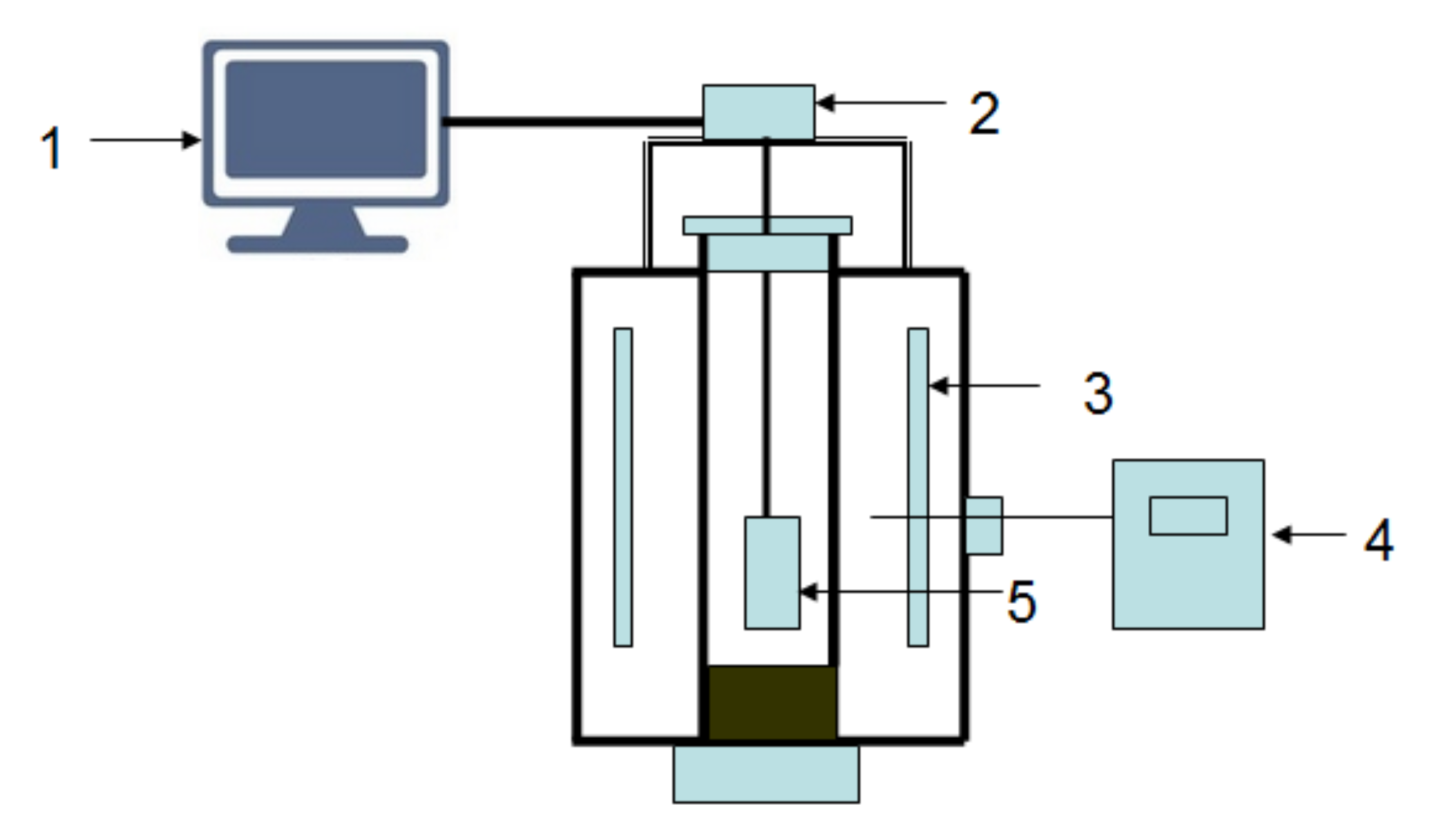
The mass loss in the pellet originated from the following three processes: the reduction of carbon, the removal of oxygen from iron ore, and the expulsion of volatile matter. The maximum mass losses of 1 g composite pellets using coke, charcoal, and biomass as reducing agents are 0.356, 0.378 and 0.374 g, respectively. The thermogravimetric method was adopted to measure the mass changes of composite pellets. Figure 1 shows the schematic diagram of experimental apparatus. It is a shaft furnace connected to a control part, and the accuracy of the electronic scale (FA3004, Shanghai Liang Ping Instruments Co., Ltd., Shanghai, China) is 0.0001 g. Before the reduction experiment, the pellet sample was dried adequately at 105 °C in a drying oven for 24 h. The mass of the experimental pellet sample was about 50 g in each reduction experiment. The experimental pellet sample was contained in a basket which was hung by a pothook connected to an electronic scale. The basket was made of Fe-Cr-Al alloy wire, which did not disturb the gas flow around the sample. The basket was kept inside the shaft furnace at given temperatures of 900, 1000, 1100 and 1200 °C for desired times of 15, 30, 45, 60, and 90 min. The mass changes of the samples were measured by the electronic scale and were recorded by a computer. A continuous N2 flow at a rate of 5 L/min was introduced into the furnace to control the atmosphere. After isothermal reduction, the samples were rapidly taken out and cooled down with N2. The phase compositions of the composite pellets after the reduction were analyzed by X-ray diffraction (XRD) (D/max 2500 PC, Rigaku, Tokyo, Japan) using Cu Kα radiation. The microstructure observation of the composite pellets was conducted using scanning electron microscopy (SEM) (JSM-7800F, JEOL, Tokyo, Japan).
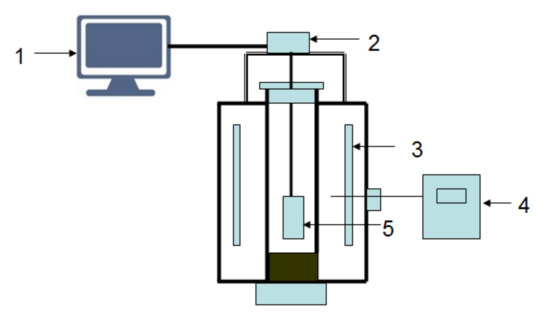
Figure 1.
Schematic diagram of experimental apparatus. 1—computer; 2—electronic scales; 3—heater; 4—control apparatus; 5—pellet.
3. Results and Discussion
3.1. Reduction Mechanism Analysis
It is well known that the reduction of iron oxides in a direct reduction system occurs mainly with gaseous phase of CO [22,23,24,25,26,27]. At relatively low temperatures, only a negligible amount of reduction will occur in solid–solid reactions between carbon particles and iron oxide particles, since such reactions are very slow. It is noteworthy that the solid–solid reactions also play a certain role in the reduction of iron ore when the temperature is relatively high (above 1200 °C). It is generally accepted that the iron ore (Fe2O3) undergoes stepwise reductions by CO gas produced through the reaction of carbon with carbon dioxide. Carbon dioxide is initially generated from the decomposition of the volatile and the chemical reaction of carbon with iron ore. After this, more carbon dioxide is produced via the reduction of iron ore by CO. Hence, the reductant CO triggers the reduction process in a series of interrelated reactions as follows:
C + CO2 ⇌ 2CO,
3Fe2O3 + CO → 2Fe3O4 + CO2,
Fe3O4 + CO → 3FeO + CO2,
FeO + CO → Fe + CO2,
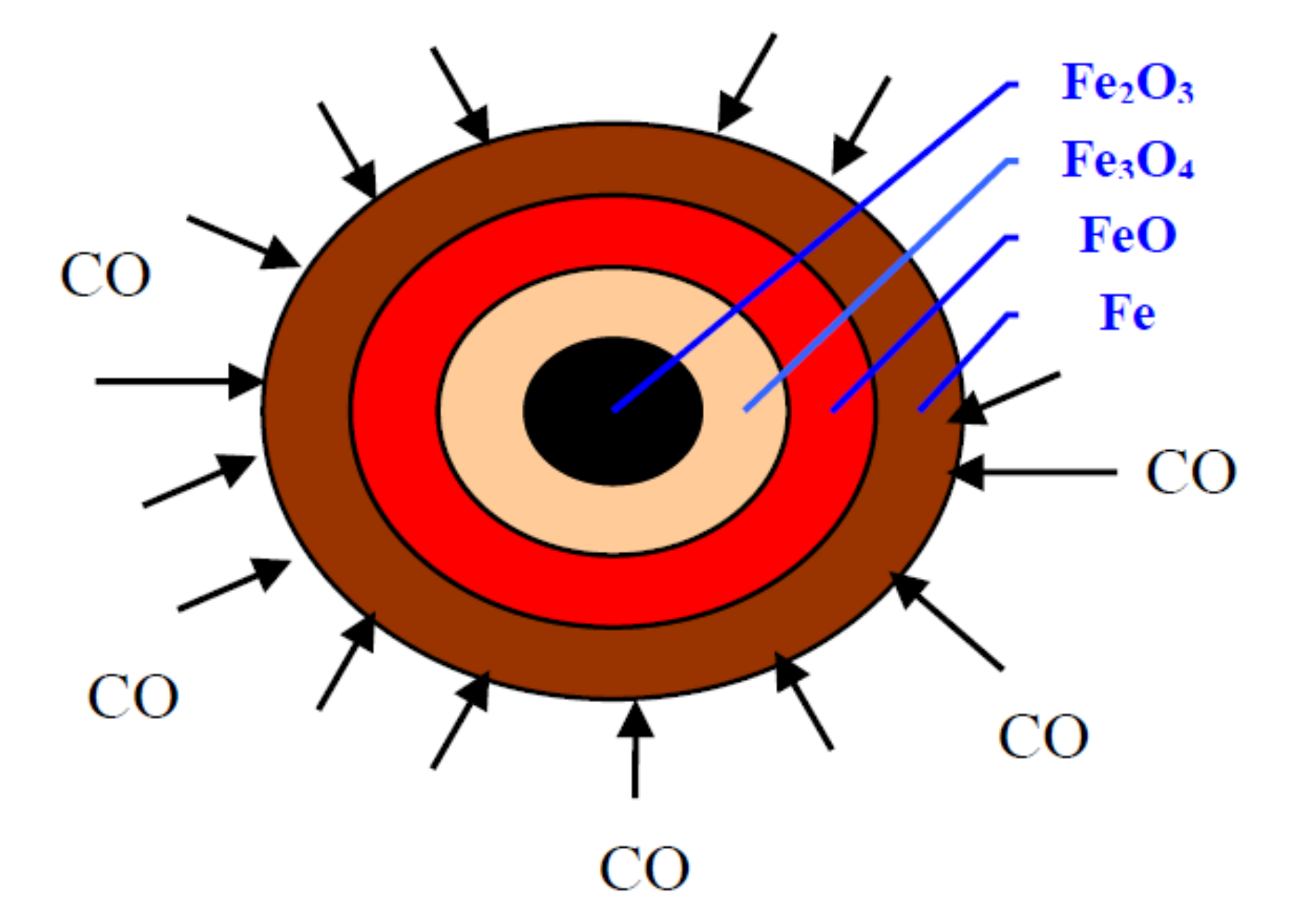
The reduction of an iron ore-carbon composition pellet can be described more intuitively by the spherical model, as shown in Figure 2.
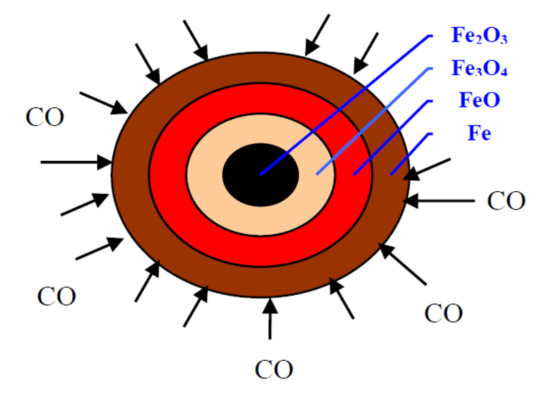
Figure 2.
Spherical model of the reduction of iron ore pellet with CO.
3.2. Comparison of the Reduction Behaviors of Carbon Iron Ore Composite Pellets with Different Reducing Agents
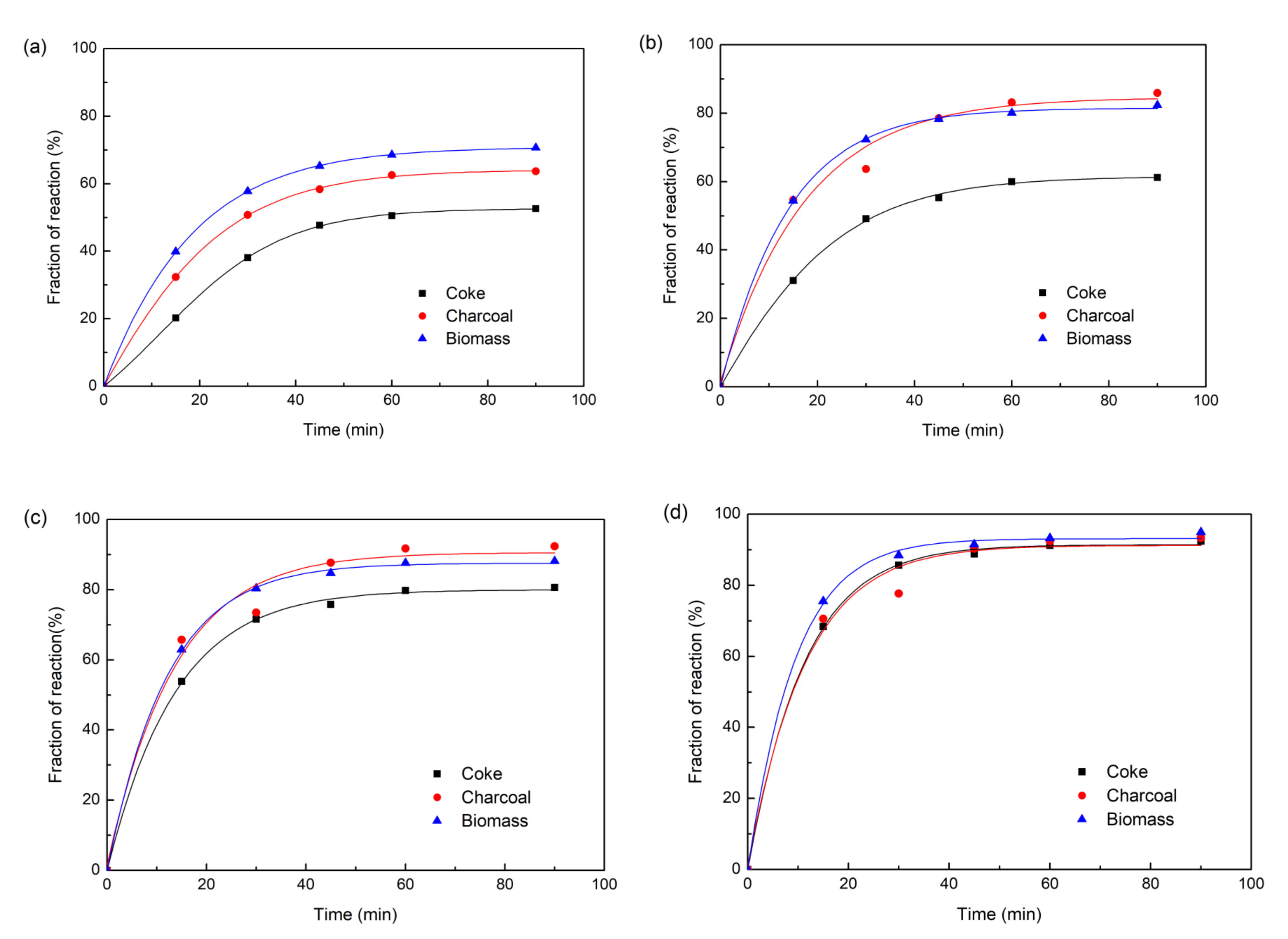
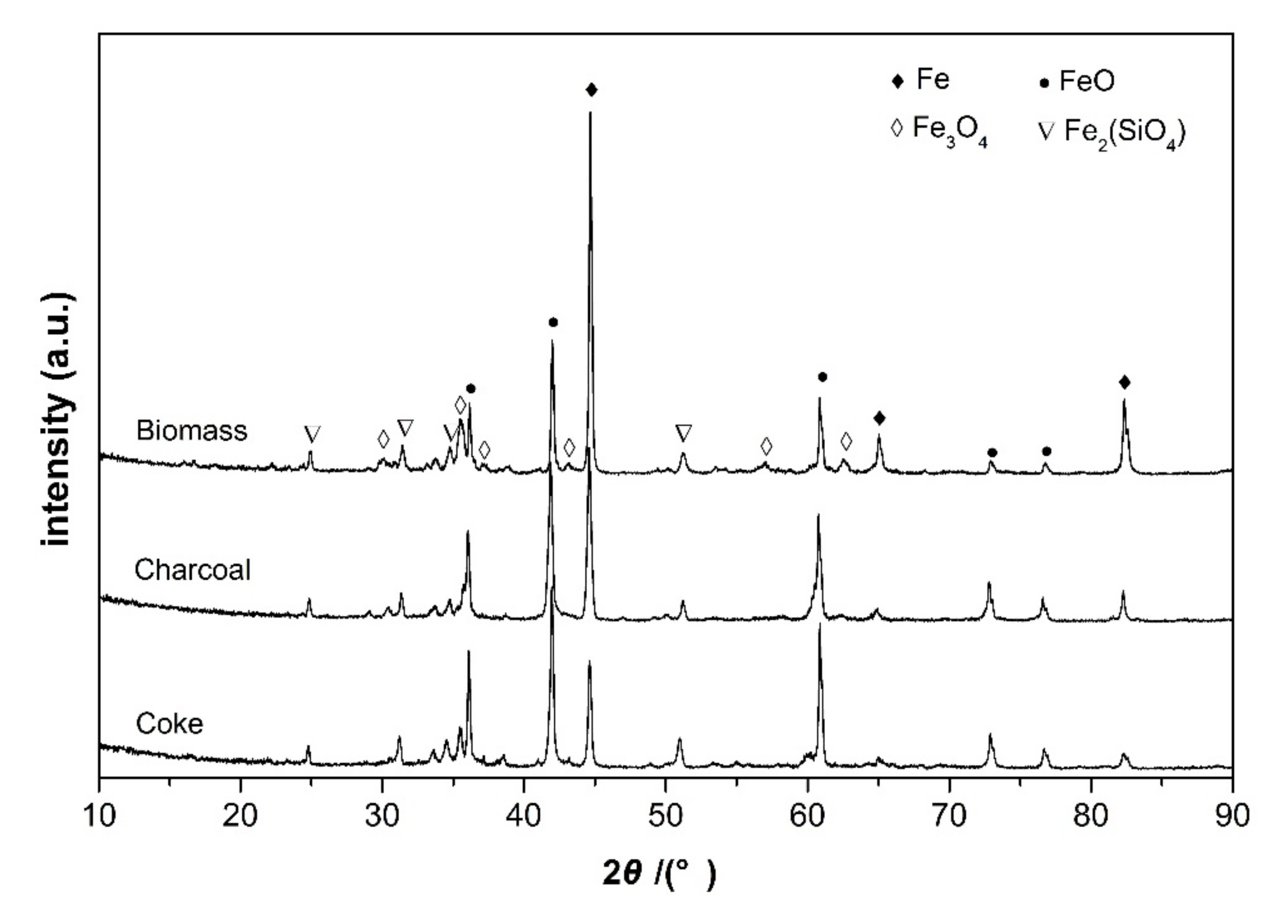
The reduction behaviors of carbon iron ore composite pellets with different reducing agents were comparatively investigated under varying temperatures from 900 to 1200 °C. The reduction curves are shown in Figure 3a–d by plotting the fraction of reaction (m) against the reduction time. Obviously, the fractions of reaction of the three different composite pellets all increased exponentially with the reduction time at all the investigated temperatures. At 900 °C, the composite pellet using straw biomass as a reducing agent shows more rapid reduction kinetics than those using coke or charcoal as reducing agents. After 90 min, the fraction of reaction of composite pellet using straw biomass as a reducing agent exceeds 70%, while the composite pellet using coke as a reducing agent exhibits a fraction of reaction of about 50%. Figure 4 shows the X-ray diffraction patterns of the three composite pellets with different reducing agents after being reduced at 900 °C for 90 min. For all the three composite pellets, the main phase components are Fe, FeO, Fe3O4, and Fe2(SiO4), while hematite (Fe2O3) peaks are absent. Moreover, the iron peaks of the iron ore-biomass composite pellet are even more intensive than those of the other two pellets, which is in accordance with the above results concerning the fraction of reaction. The faster reduction of the iron ore–biomass composite pellet compared to the other two pellets at relatively low temperatures could be related to the higher reactivity of biomass. With the temperature increasing to 1000 °C and 1100 °C, the reduction rates of the composite pellet using straw biomass as a reducing agent and that using charcoal as a reducing agent were almost equal, while the reduction rate of the composite pellet using coke as a reducing agent was relatively slow, which can be attributed to the microstructure difference of the three composite pellets after the reaction. Figure 5 shows the microstructures of the three composite pellets after being reduced at 1100 °C for 60 min. It can be seen from Figure 5 that the reduced iron ore–biomass (straw) and iron ore–charcoal composite pellets both exhibit a loose structure with many fine pores, while the reduced iron ore–coke composite pellet possesses a relatively dense structure, which can be attributed to the fact that biomass and charcoal possess looser structures than coke. Obviously, looser structures are conductive to the diffusion of reducing gas in the pellets, thereby bringing about more rapid reduction kinetics in pellets. When the temperature was further increased to 1200 °C, the three different composite pellets showed nearly the same reduction rate.
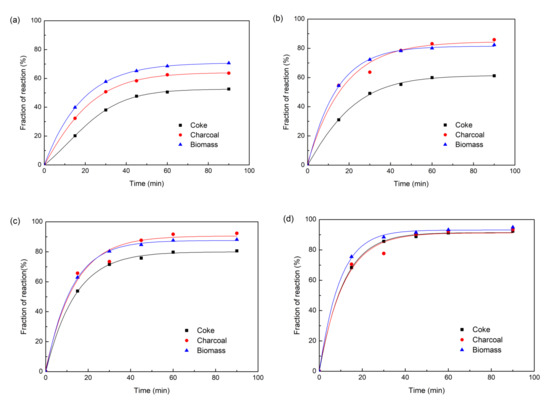
Figure 3.
The fraction of reaction of the three composite pellets with different reducing agents: (a) 900 °C, (b) 1000 °C, (c) 1100 °C, (d) 1200 °C.
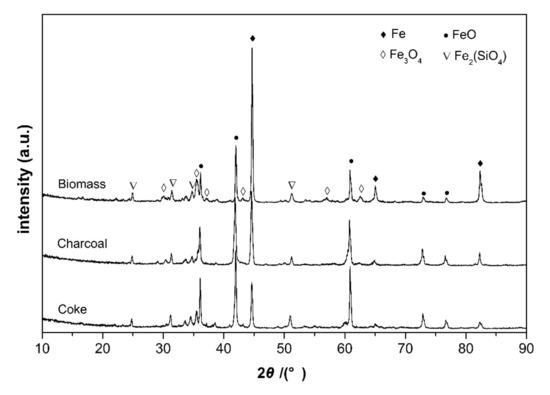
Figure 4.
XRD patterns of the three composite pellets with different reducing agents after being reduced at 900 °C for 90 min.
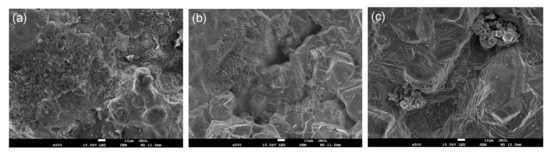
Figure 5.
SEM micrographs of the three composite pellets with different reducing agents after being reduced at 1100 °C for 60 min: (a) biomass, (b) charcoal, (c) coke.
3.3. Kinetic Analysis
The reaction kinetics of the iron ore reduction determines the rate at which iron oxide is converted to metallic iron by the removal of oxygen. As it is known, the reduction rate of iron ore highly influences the economic feasibility and competitiveness of the process technology involved. Thus, the reaction rate of the reduction process is of prime importance. The reduction of iron oxide involves multiple kinetic steps, and the slowest step among them controls the overall reaction rate. The different rate-controlling factors which control the overall rate of reduction are given below [28]:
The reaction rate is controlled by a chemical reaction at particle surface, given by:
A gas diffusion controlled reduction can be expressed by the interface reaction kinetic Jander equation:
A shrinking core model can be used to describe the boundary layer-controlled reduction:
where t is the reduction time (min), is the rate constant of the reduction model, and m is the fraction of reaction (%).
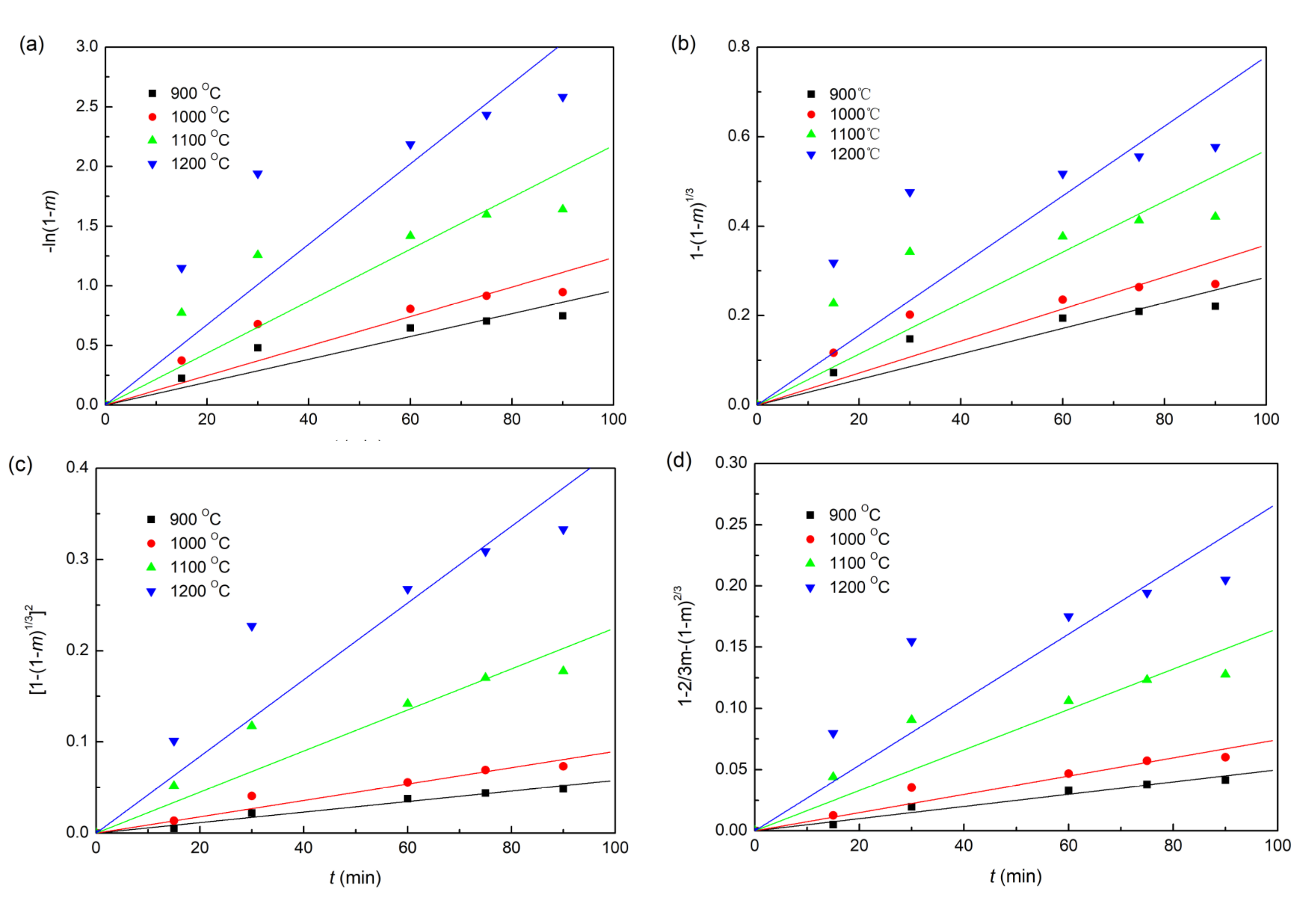
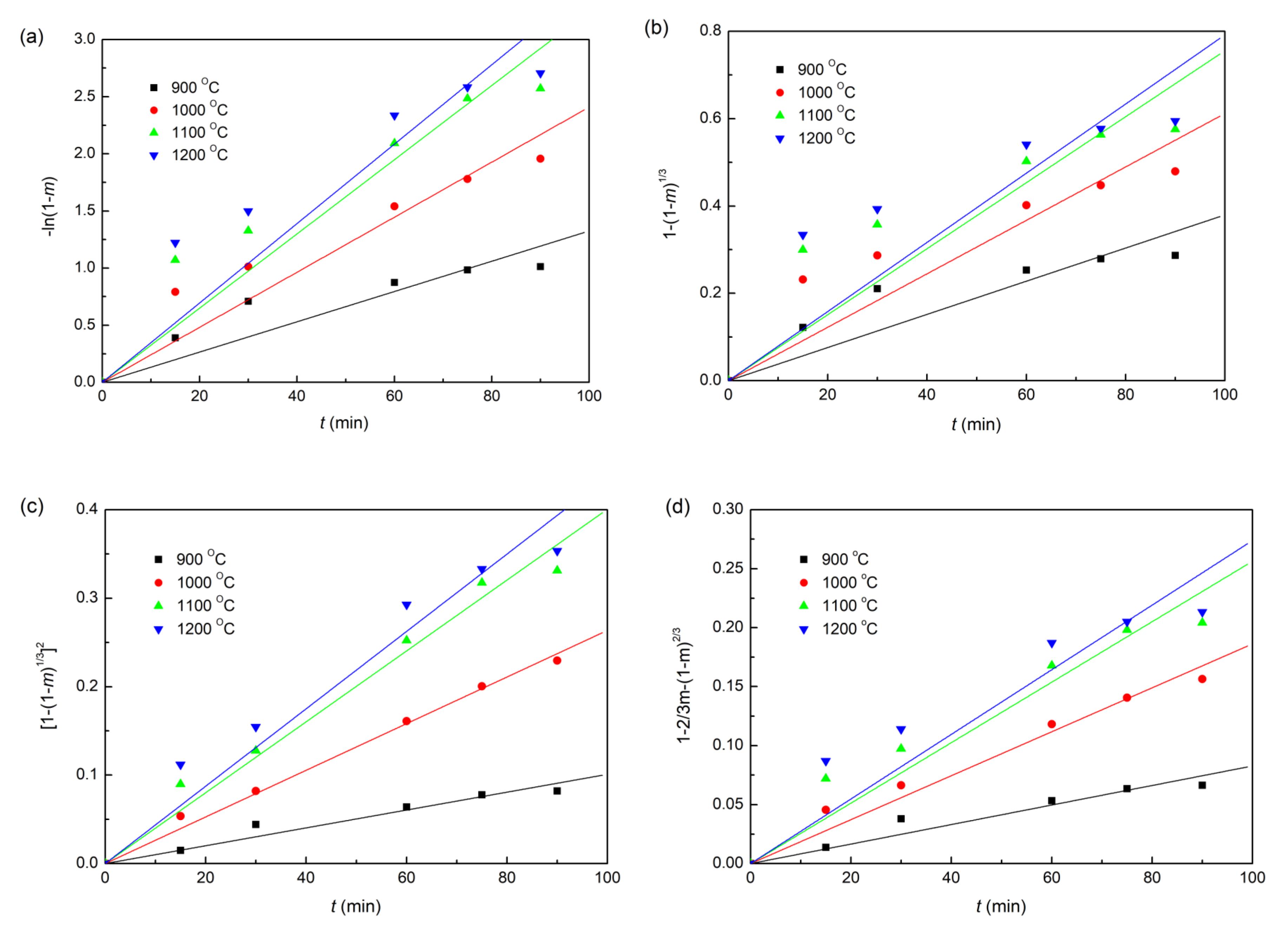
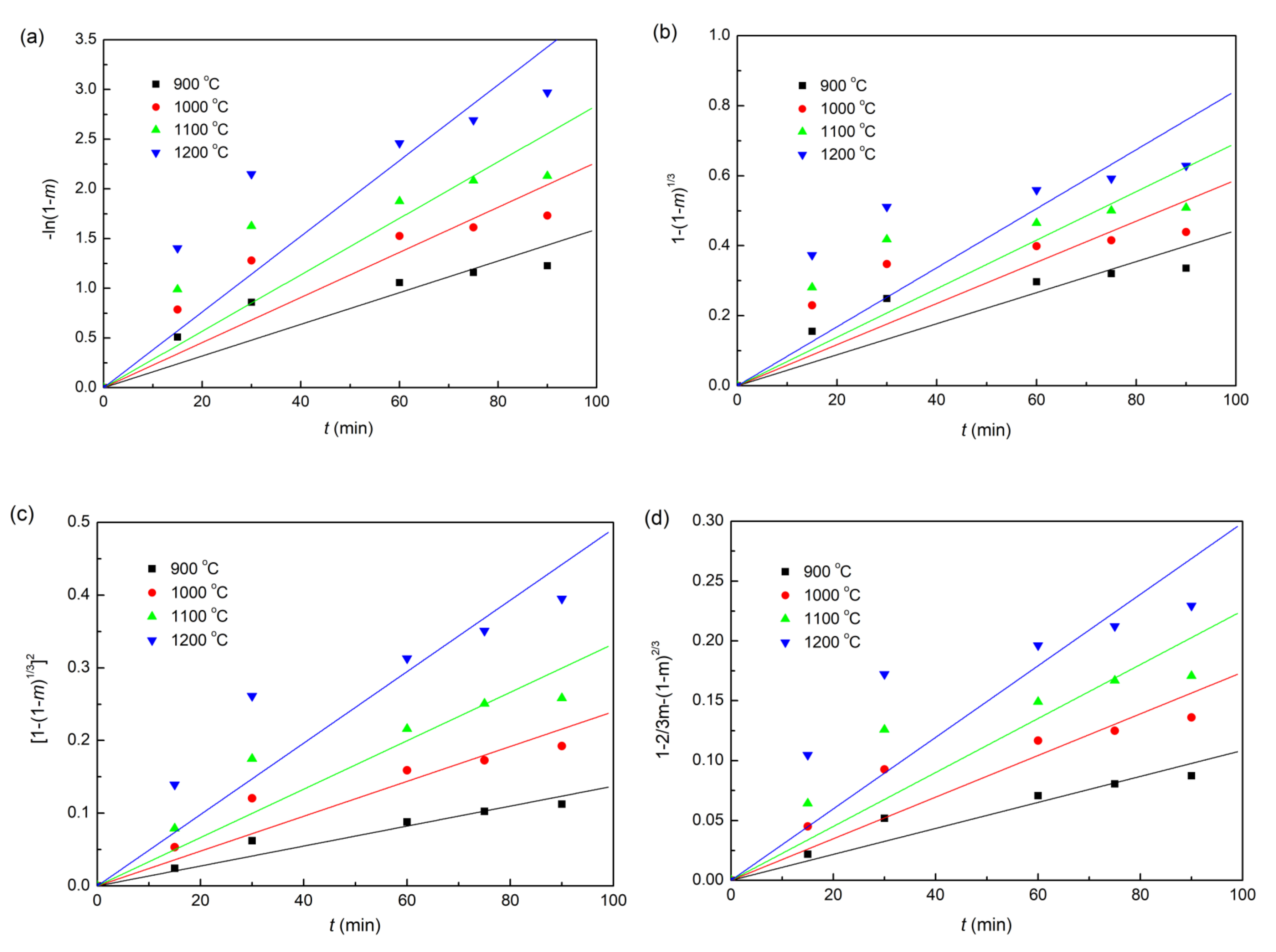
The plots of fitting the experimental data with coke, charcoal, and straw biomass as reducing agents to the linear forms of the Equations (6)–(9) reduction kinetic models are shown in Figure 6, Figure 7 and Figure 8, respectively. In addition, the correlation coefficients (r2) and linear regression equations are listed in Table 3. Obviously, for all the three different composite pellets, the experimental data agree well with the diffusion controlled model ([1−(1−m)1/3]2 = kt), with a high r2 compared with other three kinetic models, as listed in Table 3. These results indicate clearly that the reduction reactions in all the three studied composite pellets belong to the diffusion controlled reaction, and the reduction rates can be described by the interface reaction kinetic model (). This kind of reaction kinetic model also well describes the reduction of carbon ZnO composite pellets containing graphite [29].
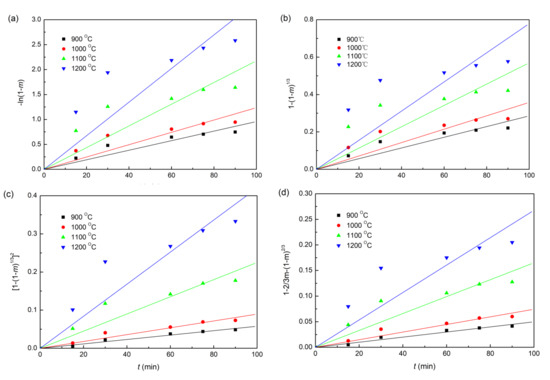
Figure 6.
Linearized form plots of a kinetic model for the reduction of iron ore in iron ore–coke composite pellets: (a) ; (b) ; (c) ; (d) .
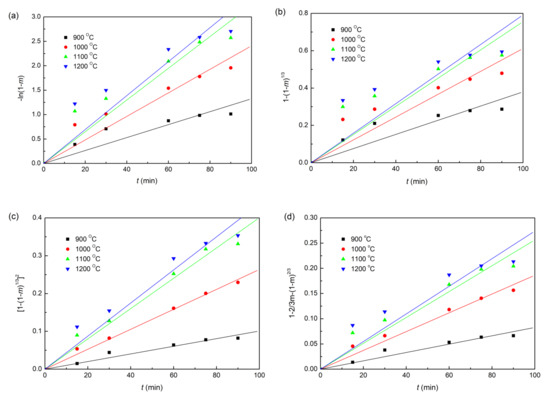
Figure 7.
Linearized form plots of kinetic model for reduction of iron ore in iron ore–charcoal composite pellet: (a) ; (b) ; (c) ; (d) .
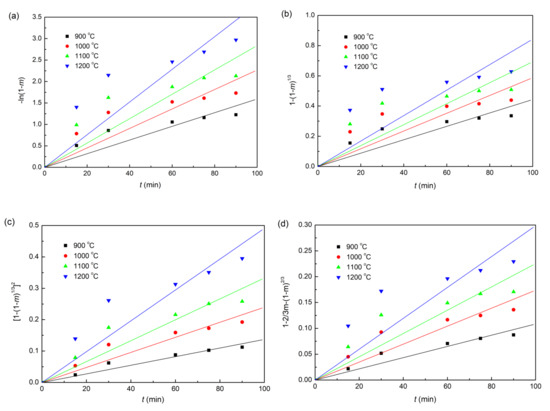
Figure 8.
Linearized form plots of kinetic model for the reduction of iron ore in iron ore–biomass composite pellet: (a) ; (b) ; (c) ; (d) .

Table 3.
Lines of kinetic model parameters for various kinetic reduction models.
3.4. Evaluation of the Apparent Activation Energies of Composite Pellets with Different Reducing Agents
In the present work, an integration method was chosen to calculate the apparent activation energy () value [30]. As is known, the integration method does not refer to any particular kinetic model and gives values at different levels of fractional reaction. The reaction rate is related to the temperature and degree of reaction by a differential equation, presented as:
where is the extent of conversion (dimensionless); t and are time (min) and temperature (K), respectively; is the reaction model; is the temperature dependent rate constant expressed as the Arrhenius below:
where is the pre-exponential factor (s−1) and and are the activation energy (J/mol) and gas constant (8.314 J/mol K), respectively.
The activation energy expresses the energy barrier that must be overcome to activate the bond redistribution steps required to convert reactants into products [31]. The pre-exponential factor represents the occurrence frequency of the reaction situation. Combined with the integrating equation (Equation (10)), E and A can be calculated. For the interface reaction kinetic equation (), the equation is expressed as:
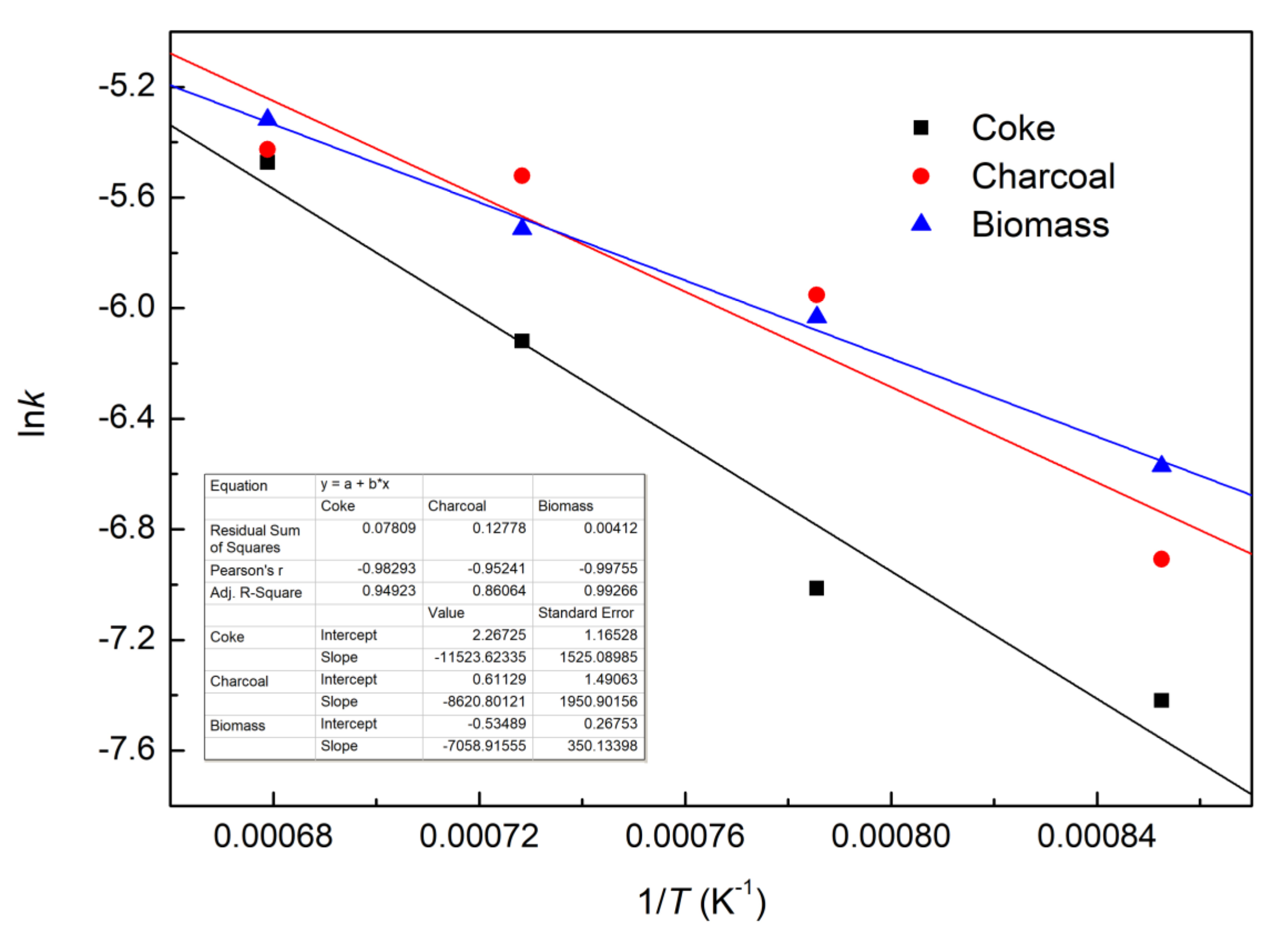
The curves of lnk vs. 1/T for each of the three composite pellets with different reducing agents are plotted in Figure 9 using the estimated kinetic parameters from Table 3. The values of pre-exponential factors and apparent activation energies of the reduction for the three different composite pellets were calculated from the intercept and slopes of the fitted curves in Figure 9, and the results were listed in Table 4. It can be seen that the iron ore–biomass composite pellet presents the lowest apparent activation energy of reduction.
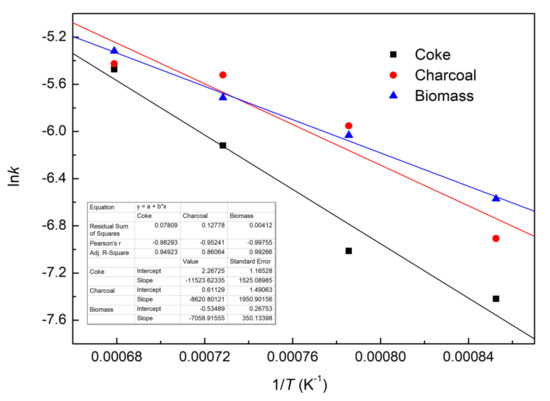
Figure 9.
Plots of lnk vs. 1/T.

Table 4.
Pre-exponential factors and apparent activation energies of different reducing agents.
4. Conclusions
The kinetics of the isothermal reduction of iron ore composite pellets using coke, charcoal, and biomass (straw) as reducing agents were studied at 900–1200 °C. The following results were found:
(1) The effect of the reducing agent on the reduction rate is remarkable when the temperature is relatively low. Due to the higher reactivity and looser structure of straw biomass, the composite pellet using straw biomass as a reducing agent shows a more rapid reduction rate. After reducing at 900 °C for 90 min, composite pellets using coke, charcoal, and biomass as reducing agents present fractions of reaction (m) of 52.66%, 63.66%, and 70.66%, respectively. As the temperature increased, the effect of the reducing agent on the reduction rate became weaker.
(2) The reduction reactions of the three composite pellets belong to gasification diffusion-controlled reaction, and the reduction rates can be described by the interface reaction kinetic model (). The apparent activation energies of coke, charcoal, and straw composite pellets at 900–1200 °C were calculated based on the Arrhenius equation, and they are 95.81, 71.67, and 58.69 kJ/mol, respectively. The activation energy of the straw composite pellet is less than that using other reduction agents.
(3) The reduction of iron ores pellets were deoxidized more easily by straw biomass reduction agents compared with other two ones; biomass waste may be a promising reduction agent for the reduction of iron ore composite pellets.
Author Contributions
Conceptualization, X.Y. and S.L.; methodology, X.Y. and S.L.; validation, X.Y., S.L., and M.Z.; investigation, X.Y., F.L., and D.Z.; data curation, X.Y., F.L., and D.Z.; writing—original draft preparation, X.Y.; writing—review and editing, S.L.; funding acquisition, X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Project Supported by the Scientific and Technological Research Program of Chongqing Municipal Education Commission (Grant No.KJQN201801507), the Venture & Innovation Support Program for Chongqing Overseas Returnees(cx2020088), the graduate science and technology innovation training program project of Chongqing University of Science & Technology (Grant No.YKJCX2020203) and the Chongqing Science and Technology Commission in China (Grant No.cstc2019jcyj-msxmX0132).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zandi, M.; Martinez-Pacheco, M.; Fray, T.A.T. Biomass for iron ore sintering. Miner. Eng. 2010, 23, 1139–1145. [Google Scholar] [CrossRef]
- Suopajarvi, H.; Pongracz, E.; Fabritius, T. The potential of using biomass-based reducing agents in the blast furnace: A review of thermochemical conversion technologies and assessments related to sustainability. Renew. Sus. Energy Rev. 2013, 25, 511–528. [Google Scholar] [CrossRef]
- Valeev, D.; Zinoveev, D.; Kondratiev, A.; Lubyanoi, D.; Pankratov, D. Reductive smelting of neutralized red mud for iron recovery and produced pig iron for heat-resistant castings. Metals 2020, 10, 32. [Google Scholar] [CrossRef]
- Castro, J.A.D.; Medeiros, G.A.D.; Oliveira, E.M.D.; Campos, M.F.D.; Nogami, H. The mini blast furnace process: An efficient reactor for green pig iron production using Charcoal and Hydrogen-Rich Gas: A Study of Cases. Metals 2020, 10, 1501. [Google Scholar] [CrossRef]
- Sohn, H.Y. Energy Consumption and CO2 emissions in ironmaking and development of a novel flash technology. Metals 2020, 10, 54. [Google Scholar] [CrossRef]
- Li, S.; Pan, J.; Zhu, D.; Guo, Z.; Shi, Y.; Chou, J.; Wu, J. An innovative technique for comprehensive utilization of high aluminum iron ore via pre-reduced-smelting separation-alkaline leaching process: Part I: Pre-reduced-smelting separation to recover iron. Metals 2020, 10, 57. [Google Scholar] [CrossRef]
- Strezov, V. Iron ore reduction using sawdust: Experimental analysis and kinetic modelling. Renew. Energy 2006, 31, 1892–1905. [Google Scholar] [CrossRef]
- Gan, M.; Fan, X.; Ji, Z.; Jiang, T.; Chen, X.; Yu, Z.; Li, G.; Yin, L. Application of biomass fuel in iron ore sintering: Influencing mechanism and emission reduction. Ironmak. Steelmak. 2015, 42, 27–33. [Google Scholar] [CrossRef]
- Castro, J.A.; Medeiros, G.A.; Oliveira, E.M.; Silva, L.M.; Nogami, H. A comprehensive modeling as a tool for developing new mini blast furnace technologies based on biomass and hydrogen operation. Sustain. Metall. 2020, 6, 281–293. [Google Scholar] [CrossRef]
- Rejdak, M.; Robak, J.; Czardybon, A.; Karina, L.; Fudała, P. Research on the production of composite fuel on the basis of fine-grained coal fractions and biomass—The impact of process parameters and the type of binder on the quality of briquettes produced. Minerals 2020, 10, 31. [Google Scholar] [CrossRef]
- El-Tawil, A.A.; Ökvist, L.S.M.; Ahmed, H.; Björkman, B. Devolatilization kinetics of different types of bio-coals using thermogravimetric analysis. Metals 2019, 9, 168. [Google Scholar] [CrossRef]
- Fruehan, R.J. Rate of reduction of iron oxides by carbon. Metall. Mater. Trans. B 1977, 8, 279–286. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Z.; Tian, J.; Li, W.; Sun, J. Mechanisms of reduction in iron ore-coal composite pellets. Ironmak. Steelmak. 1997, 24, 457–460. [Google Scholar]
- Liu, G.S.; Strezov, V.; Lucas, J.A.; Wibberley, L.J. Thermal investigations of direct iron ore reduction with coal. Thermochim. Acta 2004, 410, 133–140. [Google Scholar] [CrossRef]
- Strezov, V.; Liu, G.S.; Lucas, J.A.; Wibberley, L.J. Computational calorimetric study of the iron ore reduction reactions in mixtures with coal. Ind. Eng. Chem. Res. 2005, 44, 621–626. [Google Scholar] [CrossRef]
- Mousa, E.; Lundgren, M.; Ökvist, L.S.; From, L.E.; Robles, A.; Hällsten, S.; Sundelin, B.; Friberg, H.; El-Tawil, A. Reduced carbon consumption and CO2 emission at the blast furnace by use of briquettes containing torrefied sawdust. J. Sustain. Metall. 2019, 5, 391–401. [Google Scholar] [CrossRef]
- Ding, Y.G.; Wang, J.S.; She, X.F.; Wang, G.; Xue, Q.G. Reduction Characteristics and Kinetics of Bayanobo Complex Iron Ore Carbon Bearing Pellets. J. Iron Steel Res. Int. 2013, 20, 28–33. [Google Scholar] [CrossRef]
- Shi, J.Y.; Donskoi, E.; Mcelwain, D.L.S.; Wibberley, L.J. Modelling the reduction of an iron ore-coal composite pellet with conduction and convection in an axisymmetric temperature field. Math. Comput. Model. 2005, 42, 45–60. [Google Scholar] [CrossRef]
- Mae, K.; Inaba, A.; Hanaki, K.; Okuma, O. Production of iron/carbon composite from low rank coal as a recycle material for steel industry. Fuel 2005, 84, 227–233. [Google Scholar] [CrossRef]
- Pandey, B.K.; Sharma, T. Reducing agents and double-layered iron ore pellets. Int. J. Miner. Process. 2000, 59, 295–304. [Google Scholar] [CrossRef]
- Chowdhury, G.M.; Roy, G.G.; Roy, S.K. Reduction kinetics of iron ore-graphite composite pellets in a packed-bed reactor under inert and reactive atmospheres. Metall. Mater. Trans. B 2008, 39, 160–178. [Google Scholar] [CrossRef]
- Ueda, S.; Watanabe, K.; Yanagiya, K.; Inoue, R.; Ariyama, T. Improvement of reactivity of carbon iron ore composite with biomass char for blast furnac. ISIJ Int. 2009, 49, 1505–1512. [Google Scholar] [CrossRef]
- Fu, J.X.; Zhang, C.; Hwang, W.S.; Liau, Y.T.; Lin, Y.T. Exploration of biomass char for CO2 reduction in RHF process for steel production. Int. J. Green Gas Con. 2012, 8, 143–149. [Google Scholar] [CrossRef]
- Wu, Y.; Fang, M.; Lan, L.D.; Zhang, P.; Rao, K.V.; Bao, Z.Y. Rapid and direct magnetization of goethite ore roasted by biomass fuel. Sep. Purif. Technol. 2012, 94, 34–38. [Google Scholar] [CrossRef]
- El-Tawil, A.A.; Ahmed, H.M.; Ökvist, L.S.; Björkman, B. Self-reduction behavior of bio-coal containing iron ore composites. Metals 2020, 10, 133. [Google Scholar] [CrossRef]
- Tang, H.; Yun, Z.; Fu, X.; Du, S. Modeling and experimental study of ore-carbon briquette reduction under CO–CO2 atmosphere. Metals 2018, 8, 205. [Google Scholar] [CrossRef]
- Tang, H.; Sun, Y.; Rong, T. Experimental and numerical investigation of reaction behavior of carbon composite briquette in blast furnace. Metals 2020, 10, 49. [Google Scholar] [CrossRef]
- Habashi, F. Principles of Extractive Metallurgy, General Principles; Gordon and Breach: New York, NY, USA, 1969; pp. 153–163. [Google Scholar]
- Guo, X.Z.; Zhang, B.H.; Yang, H.B. A kinetic of the reduction of ZnO pellets containing graphite. J. Chongqing Univ. (Nat. Sci. Ed.) 2002, 25, 86–88. (In Chinese) [Google Scholar]
- Ding, L. Kinetics and mechanism of reduction of carbon–chromite composite pellets. Ironmak. Steelmak. 1997, 24, 224–229. [Google Scholar]
- Galwey, A.K.; Brown, M.E. Application of the Arrhenius equation to solid state kinetics: Can this be justified. Thermochim. Acta 2002, 386, 91–98. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).